Abstract
Streptococcus pneumoniae is an important human pathogen causing both mucosal (otitis media and pneumonia) and systemic (sepsis and meningitis) diseases. Due to increasing rates of antibiotic resistance, there is an urgent need to improve prevention of pneumococcal disease. Two currently licensed vaccines have been successful in reducing pneumococcal disease, but there are limitations with their use and effectiveness. Another approach for prevention is the use of live attenuated vaccines. Here we investigate the safety and protection induced by live attenuated strains of S. pneumoniae containing combinations of deletions in genes encoding three of its major virulence determinants: capsular polysaccharide (cps), pneumolysin (ply), and pneumococcal surface protein A (pspA). Both the cps and ply/pspA mutants of a virulent type 6A isolate were significantly attenuated in a mouse model of sepsis. These attenuated strains retained the ability to colonize the upper respiratory tract. A single intranasal administration of live attenuated vaccine without adjuvant was sufficient to induce both systemic and mucosal protection from challenge with a high dose of the parent strain. Immunization with cps mutants demonstrated cross-protective immunity following challenge with a distantly related isolate. Serum and mucosal antibody titers were significantly increased in mice immunized with the vaccine strains, and this antibody is required for full protection, as μMT mice, which do not make functional, specific antibody, were not protected by immunization with vaccine strains. Thus, colonization by live attenuated S. pneumoniae is a potentially safe and less complex vaccine strategy that may offer broad protection.
Streptococcus pneumoniae is a leading human pathogen causing diseases ranging from otitis media to pneumonia, bacteremia, and meningitis that result in an estimated 1.6 million deaths per year worldwide, more than half of which are young children in developing countries (18). The surface of the nasal mucosa is the major reservoir for S. pneumoniae, and at this site it resides primarily in a commensal relationship with its human host. Carriage is extremely common, with >50% of children acquiring at least one isolate during their first year, although an individual may harbor multiple strains simultaneously or sequentially. Each carriage episode lasts for days to months, but by age 3 carriage steadily declines until adulthood, when rates plateau at 10 to 20%. The global decrease in carriage and disease rates that occurs after early childhood suggests that protective immunity may be induced by prior carriage events and develops in a serotype-independent manner (19, 20).
Increasing rates of resistance of S. pneumoniae to antibiotics highlight the priority of preventing pneumococcal disease (14, 19). There are currently two commercially available vaccines against S. pneumoniae, both of which are based on the polysaccharide capsule, which is the major determinant necessary for causing disease. Pneumococcal isolates express at least 90 structurally unique capsular polysaccharides, its serotype-determining antigen. Pneumovax is a 23-valent polysaccharide vaccine which contains the most common types causing pneumococcal infection and is effective in adults but not in early childhood. Prevnar is a 7-valent polysaccharide conjugate vaccine (containing types 4, 6B, 9V, 14, 18C, 19F, and 23F) which is effective in young children (4) but has been shown to induce selective pressure and gradual replacement with nonvaccine types (serotype replacement) (37). Moreover, its effectiveness against the most frequent manifestations of infection, mucosal infection (pneumonia and otitis media), seems far more limited than for invasive disease (8, 29), and the conjugate vaccine is complex and costly, making it inaccessible for populations in greatest need. It is therefore desirable to find new and innovative strategies for vaccine development.
A main focus of current research is aimed at component vaccines comprised of protein antigens, with particular attention to virulence factors with antigenic determinants widely shared among strains. Among these are the pneumolysin toxoid (32), pneumococcal surface protein A (PspA) (5), neuraminidase (NanA) (38), pneumococcal histidine triad proteins A, B, and D (PhtA, PhtB, and PhtD) (1), and pneumococcal surface adhesion molecule A (PsaA) (35). Inclusion of a combination of two or more antigens will most likely be necessary to confer broad protection against the highly heterogeneous pneumococcal population (31). Another strategy uses killed whole bacteria and is able to confer broad protection in mouse models after multiple intranasal doses administered with an adjuvant (22). An alternative approach is the development of live attenuated vaccines. Live attenuated vaccines have been highly successful for prevention of viral disease but have received relatively little attention for bacterial disease; exceptions include Mycobacterium bovis BCG vaccine against tuberculosis (43) and Salmonella enterica serovar Typhi Ty21a vaccine against typhoid (12). Here we describe a live attenuated vaccine for S. pneumoniae that can elicit an immune response sufficient to reduce colonization and protect against otherwise-lethal challenge in a serotype-independent manner.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. pneumoniae strains were grown in tryptic soy broth as described elsewhere (42). Strains used in vivo were selected because of their ability to colonize efficiently the murine nasopharynx and included a type 6A strain (a mouse virulent, clinical isolate) and a type 4 strain (clinical isolate, genome sequence strain) (15, 26, 36). All strains were passaged intranasally in mice prior to preparation of frozen stocks.
The type 6A and 4 isolates were compared by multilocus sequence typing (21) as previously described (7). The isolates represent two sequence types (6A ST460 and 4 ST1982), with different alleles at 7/7 loci analyzed. Thus, the type 6A and 4 isolates represent different clonal complexes as defined by eBURST (9).
The cps operon was deleted from spontaneously streptomycin-resistant mutants (200 μg/ml) of type 6A and 4 isolates using the bicistronic positively and negatively selectable Janus cassette as previously described (34, 39). Loss of capsule was confirmed by quelling with type-specific antisera (Staten Seruminstitut, Copenhagen, Denmark).
A pneumolysin-negative S. pneumoniae strain was constructed using a previously described insertion-duplication mutant in strain D39 (type 2) (3). Strain 6Aply was generated by transformation with chromosomal DNA from D39ply with selection for erythromycin resistance (1 μg/ml) followed by serial back-transformation (6). Loss of pneumolysin expression was confirmed by Western blotting using a monoclonal antibody to pneumolysin (Novocastra, Newcastle upon Tyne, United Kingdom).
The 6Acps/ply strain was constructed by transformation of the 6Acps strain with lysates from 6Aply with selection for erythromycin resistance as above and confirmed by Western blotting.
The 6ApspA strain was constructed by amplifying a 1.3-kb fragment of the pspA gene from the 6A strain using the following primers: LSM13, 5′-GCAAGCTTATGATATAGAAATTTGTAAC-3′, and SKH2, 5′-CCACATACCGTTTTCTTGTTTCCAGCC-3′ (13). The PCR product was cloned into the TOPO PCR2.1 plasmid and transformed into an Escherichia coli TOP10F′ strain using the TOPO TA cloning kit (Invitrogen, Carlsbad, CA) and confirmed by sequencing. A 390-bp deletion was made in this 1.3-kb fragment using inverse PCR with the primers pspa390del6AF, 5′-ACGCGTCGACGATTCAGAAGATTATGCTA-3′, and pspa390del6AR, 5′-ACGCGTCGACTCCTCTGTTGCCTTAGCTA-3′, and a spectinomycin resistance cassette (aad9; GenBank accession number U30830) (33) was inserted into the plasmid cut with SalI. After confirmation by PCR, 6ApspA and 6Aply/pspA were generated by transformation with the plasmid DNA, with selection for spectinomycin resistance (200 μg/ml).
Mouse model of nasopharyngeal colonization.
Six-week-old female C57BL/6 (wild type), B6.129-S2-Igh-6tm1Cgn/J (μMT; Jackson Laboratories, Bar Harbor, ME), and B6.129-H2-Ab1tm1GruN12 (MHC II−/−; Taconic, Germantown, NY) mice were housed in accordance with Institutional Animal Care and Use Committee protocols. μMT mice contain a targeted mutation in the heavy chain locus of immunoglobulin M (IgM) and do not produce mature B cells or antibody (16). Major histocompatibility complex class II (MHC II)-deficient mice exhibit a depletion of CD4+ T cells through the disruption of the H2-Ab1 gene (10). Mice were colonized using a previously described model of nasopharyngeal colonization with S. pneumoniae (27). Briefly, mice were inoculated intranasally without anesthesia with 10 μl containing 1 ×107 to 5 ×107 CFU of phosphate-buffered saline (PBS)-washed, mid-log-phase S. pneumoniae applied atraumatically to the nares. At the time indicated, the animal was sacrificed, the trachea cannulated, and 200 μl of PBS instilled. Lavage fluid was collected from the nares for determination of viable counts of bacteria in serial dilutions plated on selective medium containing neomycin (5 μg/ml for the type 4 isolate and 20 μg/ml for the type 6A isolate) to inhibit the growth of contaminants. The lower limit of detection for bacteria in lavage culture was 20 CFU/ml. Nasal lavage fluid was stored at −20°C for determination of antibody concentrations by enzyme-linked immunosorbent assay (ELISA).
S. pneumoniae challenge.
Mice were challenged intranasally with 1 ×107 to 5 × 107 CFU of S. pneumoniae parent isolate at 5 weeks postimmunization with an attenuated mutant. Five weeks was chosen as we have previously shown that these strains are cleared from the upper respiratory tract by 4 weeks postinoculation (30). Where a two-dose immunization is indicated, the second dose was given 2 weeks after the first dose with challenge 5 weeks after the second dose. Mice were observed for signs of sepsis over a 9-day period postchallenge, and animals showing signs of sepsis were euthanized and the spleen was cultured to confirm the presence of pneumococci. After 9 days the remaining animals were euthanized and nasal washes were obtained for quantitative culture. The vaccine strain given up to 65 days earlier was never detected by selective plating of nasal washes, including in those obtained from immunodeficient mice. Blood was also collected from cardiac punctures, and serum was stored at −20°C for ELISA.
Measurement of IgG and IgA levels by ELISA.
Whole bacteria were used as the solid-phase antigen to determine the antibody titer to pneumococci. PBS-washed whole bacteria, diluted with coating buffer (0.015 M Na2CO3, 0.035 M NaHCO3) to a final optical density of 0.1, were fixed by overnight incubation at 4°C on Immulon 2HB 96-well plates (Thermo, Milford, MA). Plates were washed with PBS Brij (0.05%). After blocking with 1% bovine serum albumin (Sigma) for 1 h and washing, serum or nasal washes in 1% bovine serum albumin were added to the plate in 10-fold serial dilutions overnight at 4°C. For serum samples, antigen-specific antibodies were detected by goat anti-mouse IgG (1/4,000; heavy and light chains)-alkaline phosphatase (Sigma) for 1. 5 h and developed with p-nitrophenyl phosphate (Sigma), and the absorbance at 415 nm was recorded after a standardized period of 1 h.
For nasal wash samples, a goat anti-mouse IgA was used and developed as described for serum. Endpoint titers were determined in triplicate by calculating the sample dilution at which the absorbance was equal to 0.1. Protein concentration in the nasal wash samples was determined by using the BCA protein assay kit (Pierce, Rockford, IL) to correct for variation in dilution in nasal washes and used to calculate the geometric mean titer (GMT) of antibody per μg of total protein.
Statistical analysis.
Colonization density was expressed as the log CFU/ml for calculation of means ± standard errors of the means. Statistics were carried out on ELISA data using the Mann-Whitney test. Statistical comparisons of survival and colonization among groups were made by the nonparametric test indicated (GraphPad Prism 4).
RESULTS
Evaluation of attenuation and colonization of vaccine strains.
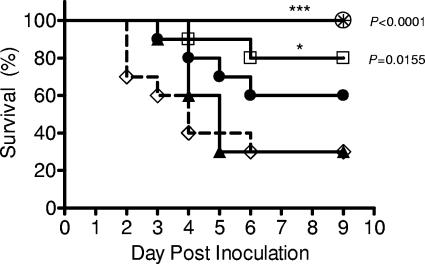
To generate live attenuated vaccine candidates, genes of each of the three major virulence determinants of S. pneumoniae (cps, ply, and pspA) were independently interrupted in a type 6A isolate capable of inducing sepsis following intranasal challenge. To establish which strains were attenuated in the mouse model, the survival of C57BL/6 mice was monitored after a high dose of these mutant strains (107 CFU/mouse) given intranasally. The 6Aply mutant remained as virulent as the parent strain, while 6ApspA displayed partial attenuation and 6Acps was completely avirulent (Fig. 1). Because of the limited attenuation of the vaccine strains lacking the proteinaceous virulence determinants, a double mutant was constructed (6Aply/pspA) and was significantly, but still not completely, attenuated.
FIG. 1.
Attenuation of S. pneumoniae: comparison of the level of attenuation of various S. pneumoniae type 6A live vaccines over a 9-day period postinoculation. Data are based on a minimum of 10 animals in each group. Statistical differences compared to the parent strain were determined by the Kaplan-Meier log-rank test. ⋄, 6A; ▴, 6Aply; •, 6ApspA; □, 6Aply/pspA; ○, 6Acps; asterisk, PBS control.
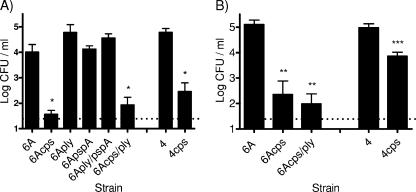
Since the ability of live vaccine strains to induce protection would be enhanced by their persistence on the mucosal surface, the effect of attenuating mutations was determined by comparing quantitative colonization of the upper respiratory tract. At day 9 postinoculation the 6Aply, 6ApspA, and 6Aply/pspA mutants displayed similar colonization densities compared to the parent strain (Fig. 2A). In contrast, colonization by the cps mutants was not significantly above the limit of detection by day 9. A similar result was seen with a cps mutant in a type 4 isolate. To further investigate the unencapsulated mutants, the density of colonization was also determined at day 2 postinoculation, and the cps mutants of both serotypes demonstrated colonization, albeit at a reduced density compared to the corresponding parent strains (Fig. 2B).
FIG. 2.
Colonization of attenuated S. pneumoniae: comparison of the abilities of various live vaccine strains to colonize the mouse nasopharynx at day 9 (A) and day 2 (B) postinoculation. Density of pneumococci in upper respiratory tract lavage fluid is shown by the mean log CFU/ml ± the standard error of the mean. n = 4 to 9 mice per group per time point. The dotted line indicates the limit of detection. Statistical differences were determined using the Mann-Whitney test with comparison to the corresponding parent strain. *, P < 0. 0286; **, P = 0.004; ***, P = 0.0008.
Protection and cross-protection of vaccine strains.
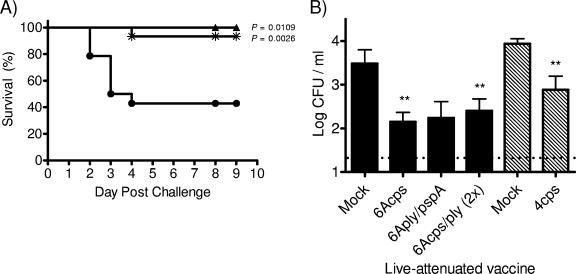
The ability to protect against sepsis was assessed for the vaccine strains that showed the greatest attenuation (6Acps and 6Aply/pspA). Colonization by live attenuated vaccine strains was used to immunize mice, and 5 weeks postimmunization the previously colonized mice were challenged intranasally with a high dose of the parent strain (107 CFU/mouse). Both 6Acps and double mutant (6Aply/pspA) vaccine strains induced significant protection (Fig. 3A).
FIG. 3.
Protection induced by live attenuated vaccine strains. (A) Survival rates of C57BL/6 mice immunized with 6Aply/pspA (▴; n = 8), 6Acps (*; n = 15), or vehicle-only control (•; n = 14) following intranasal challenge with the 6A parent strain. Statistical differences compared to the vehicle control were determined by the Kaplan-Meier log-rank test. (B) Colonization density at day 9 postinoculation of mice challenged with the type 6A parent strain (solid bars) or type 4 parent strain (hatched bars) after immunization with the indicated live vaccine. The 6Acps/ply vaccine was delivered in two doses prior to challenge. Values represent the means of 6 to 14 mice/group ± the standard errors of the means. The dotted line indicates the limit of detection. Colonization density was analyzed in surviving mice. Eight mice in the mock-immunized group and one animal in the 6Acps group that did not survive were not included in the analysis. Statistical differences were determined using the Mann-Whitney test with comparisons to the corresponding mock-immunized group. **, P < 0.0083.
Mucosal protection induced by prior colonization by vaccine strains was also assessed. The density of colonization of challenge strains was determined 9 days postinoculation with the parent isolate. Following challenge with either the type 4 or 6A parent isolate, the density of colonization was significantly reduced for mice that were immunized with the corresponding cps vaccine strains (Fig. 3B). Combinations of mutations in 6A vaccine strains, including ply/pspA and cps/ply, induced mucosal protection against the 6A parent isolate, but for the latter mutant a significant effect was only seen after two doses of the live attenuated vaccine. These data suggest that the attenuated vaccine strains can induce mucosal and systemic protection.
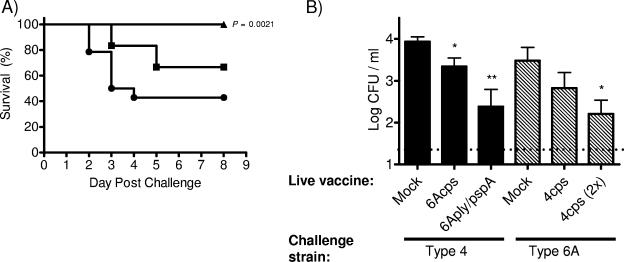
The cps mutant was able to protect significantly from sepsis and also showed the greatest mucosal protection; therefore, a type 4 cps mutant was used to assess whether immunity induced by one strain could protect against another (cross-protection). Colonization of the type 4 cps mutant was used to immunize mice before challenge with a high dose of the 6A isolate (107 CFU/mouse). With a single dose of the type 4 cps vaccine strain, mice may have partial protection from sepsis; however, with two doses of this vaccine strain there was complete protection from sepsis with the type 6A challenge (Fig. 4A). Again, the mucosal protection was investigated. A single dose of the type 4cps mutant partially reduced colonization by the 6A isolate, with further reduction following a second dose (Fig. 4B). Single-dose 6A vaccine strains (6Acps and 6Aply/pspA) were also used to investigate cross-protection from colonization with a high-dose type 4 challenge. This type 4 isolate causes septic infection at a low rate in our mouse model, and so mucosal and not systemic protection was evaluated. Both vaccine strains were proficient at reducing colonization by a distantly related isolate. These results suggest that the live attenuated strains are able to elicit protective immunity that is serotype independent, although the full extent of cross-protection is yet to be determined.
FIG. 4.
Cross-protection induced by live attenuated vaccine strains. (A) Survival rates of C57BL/6 mice immunized with 4cps in a single dose (▪; n = 12) versus two doses (▴; n = 12), compared to the vehicle-only control (•; n = 15), following intranasal challenge with the type 6A isolate. Statistical differences compared to the vehicle control were determined by the Kaplan-Meier log-rank test. (B) Colonization density at day 9 postinoculation of mice challenged with the type 4 parent isolate (solid bars) or type 6A parent isolate (hatched bars) after immunization with the indicated live vaccine of the other serotype. One dose of the vaccine strain was administered except where a second dose is indicated (2×). Values represent the means of 6 to 12 mice/group ± the standard errors of the means. The dotted line indicates the limit of detection. Colonization density was analyzed in surviving mice. Eight mice in the mock-immunized group and four animals in the 4cps single-dose group that did not survive were not included in the analysis. Statistical differences were determined using the Mann-Whitney test with comparisons to the corresponding mock-immunized group. *, P < 0.0278; **, P = 0.0028.
Mechanism of protection of vaccine strains.
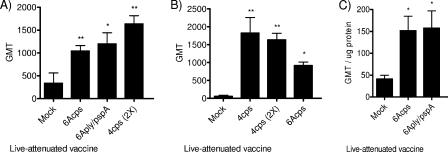
The roles of humoral and cell-mediated immunity were investigated to determine the mechanism of protection from the live attenuated vaccine strains. To assess the humoral immune response, the IgG titers to whole pneumococci were determined in serum for groups in which there was protection from either a type 6A or 4 challenge (Fig. 5A and B, respectively). The levels of pneumococcus-specific IgG in serum were significantly increased after immunization with vaccine strains (cps and ply/pspA strains). The levels of IgA were then quantified in nasal wash samples to determine the antibody titer on the mucosal surface. These values were corrected for variation in dilution of nasal wash samples by determination of total protein. The mice immunized with vaccine strains displayed significant increases in IgA titers (Fig. 5C).
FIG. 5.
ELISA titers of IgG and IgA: levels of IgG in serum against type 6A (A) or type 4 (B) (whole bacteria) from mice immunized with the indicated live vaccine. Values shown are the GMT ± standard errors of the means (SEMs) following one or two (2X) doses of the vaccine strain. Symbols in panel A: *, P = 0.0293; **, P < 0.01. Symbols in panel B: *, P = 0.0121; **, P = 0.007. (C) Levels of IgA against type 6A (whole bacteria) in nasal wash samples from mice immunized with the indicated live vaccine. Values shown are GMT/μg total protein ± SEM. *, P < 0.02. Statistical differences were determined using the Mann-Whitney test, with comparisons to the mock-immunized control group.
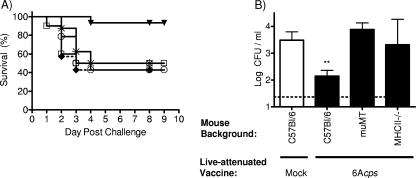
Having demonstrated an increase in antibody titer due to immunization, we then investigated if protection from sepsis is dependent on humoral or cell-mediated immunity. μMT mice (which are unable to generate specific antibody) and MHCII−/− mice (exhibiting a depletion of CD4+ T cells) were immunized with the 6Acps vaccine strain to see if protection was sustained. Neither the μMT nor the MHCII−/− mice were protected against challenge with the type 6A isolate (Fig. 6A). This suggests that the protection from sepsis is antibody dependent and requires CD4+ T cells. Antibody dependence in mucosal protection was also investigated. The reduction in colonization seen with the wild-type C57BL/6 mice following immunization was not seen with the μMT nor the MHCII−/− mice (Fig. 6B). This suggests that mucosal protection following immunization is also antibody dependent and requires CD4+ T cells.
FIG. 6.
Mechanism of protection. (A) Survival rates of μMT mice (□; n = 10), MHC II−/− mice (*; n = 8), and wild-type C57BL/6 mice (▾; n = 15) immunized with 6Acps, μMT mice mock immunized with PBS (⧫; n = 7), or C57BL/6 mice mock immunized with PBS (○; n = 14) and challenged with the 6A parent strain. (B) Colonization density at day 9 postinoculation of mice challenged with the 6A parent strain after immunization with 6Acps. Values represent the means of 8 to 15 mice/group ± the standard errors of the means. Statistical differences were determined using the Mann-Whitney test with comparisons to the wild-type C57BL/6 mice. **, P = 0.0083.
DISCUSSION
This study addressed whether protective immunity can be induced through colonization with live attenuated pneumococci. Our approach took advantage of previous studies that defined pneumococcal virulence determinants to eliminate these alone and in combination to generate mutants that are highly attenuated for causing disease but remain capable of colonizing sufficiently to induce protective immunity (2, 30, 41). The response to attenuated strains could be useful in accelerating the immunity that occurs during early childhood but could do so with safe mutants rather than potentially virulent isolates as occurs naturally. A further benefit of this approach is that it could provide more effective mucosal immunity than current strategies based on systemic immunization and, furthermore, it could induce protection with a potentially inexpensive and easily administered preparation. Beyond the potential advantages of this approach for children in developing countries, live attenuated vaccines could also be useful in boosting preexisting natural immunity later in life.
Our findings provide additional evidence that upper airway colonization may be an immunizing event. Prior studies have differed in showing whether model murine colonization with natural isolates protects from subsequent intranasal challenge with the same isolate (22, 27). The attenuated strains used in this study may be cleared more efficiently compared to wild-type isolates and may, as a result, be more effective at inducing protective immune responses. Encapsulation, for example, may obscure the immune response to underlying surface antigens. In this regard, the cps mutants tested showed limited and transient colonization that, nevertheless, was sufficient to confer significant protection. Moreover, our results demonstrate that colonization by cps mutants induces mucosal and systemic protection that does not depend on a response to capsular polysaccharide (serotype-independent protection). Serotype-independent protection against pneumococcal colonization induced by prior exposure in a mouse model was demonstrated with constructs of different types in the same genetic background (40). Our observation demonstrates that an epitope(s) shared among two isolates, which are not closely related as assessed by multilocus sequence type analysis, can also induce protective immunity. The identification of the noncapsular polysaccharide structure(s) inducing serotype-independent immunity is a topic of ongoing investigation. Based on the efficacy of mutants lacking PspA and Ply, these well-studied immunogenic proteins do not appear to be required for this effect, although in experimental human carriage studies antibodies to PspA correlated with susceptibility to colonization (26). The immune response to common epitopes may underlie the broad, cross-serotype decrease in pneumococcal carriage and disease that occurs beyond the first years of life—an event that may be due to prior colonization by a limited array of strains and types (20). The scope of cross-protection would need to be examined with a larger array of isolates to determine the number of live attenuated vaccine strains required to stimulate broad immunity.
Of the live attenuated vaccine strains tested, those lacking capsular polysaccharide and the combination of Ply and PspA were both significantly attenuated and able to induce protective immunity. The ply/pspA vaccine strain, however, was not fully attenuated in this model, indicating that additional steps might be required before a strain with this combination of mutations could be used safely. In contrast, the cps mutant was attenuated in an otherwise highly virulent mouse isolate. Although there have been occasional reports of infection caused by nonencapsulated or nontypeable pneumococcal isolates, these are generally limited to superficial sites and unencapsulated mutants are completely avirulent in models of infection (11, 24, 25). An added margin of safety may be provided by inclusion of more than a single attenuating mutation in virulence determinants. A combination of cps and ply mutations was examined in this study and shown to be as effective as the single cps mutation in inducing mucosal protection when provided in a two-dose regimen. Such a step would also reduce the chances of reversion to a more virulent phenotype, although by incorporating a mutation(s) in previously identified genes required for natural competence it should be possible to minimize this possibility (17).
Colonization with live attenuated pneumococci induced increased levels of antipneumococcal serum IgG (and mucosal IgA). This serum IgG response could account for the observed protection from systemic infection and offers the possibility of long-acting immunity. The lack of protection seen following immunization of μMT but not immunocompetent parental mice demonstrates the importance of antibody in mucosal and systemic protection. Surprisingly, a similar requirement for humoral immunity was not observed in the clearance of colonization in naïve C57BL/6 mice or in protection from colonization induced by prior exposure to encapsulated pneumococci (27, 40). This difference in the requirement for antibody offers further evidence that the immune response to live attenuated strains and wild-type isolates is distinct. Malley et al. have previously shown that intranasal vaccination with killed whole pneumococci given in multiple doses with an adjuvant generates protection from colonization in a serotype-independent manner (22). This effect was also shown to occur in mice that failed to produce specific antibody, suggesting different protective immune responses to live attenuated and killed strains (23). While both intranasal vaccine approaches are potentially less complex and more broadly acting than currently available products, the antibody-dependent effects demonstrated in our study included protection from systemic infection and did not require use of a pharmacological adjuvant. Current efforts are focused on defining the specific antigens that induce cross-protective immunity.
Prior exposure to live attenuated mutants, including cps, ply/pspA, and cps/ply, was shown here to decrease the density of colonization by wild-type isolates when challenged intranasally 5 weeks later. Since nasal colonization is the reservoir for pneumococcal transmission, this effect on carriage suggests that a live attenuated vaccine could, like the conjugate vaccine, have the potential to induce herd immunity (28). In addition, because of the effect on carriage, previously described experimental human carriage studies offer a means of testing the safety and efficacy of immunization using live attenuated pneumococci in the natural host (26).
Acknowledgments
We thank Jason Stewart at Columbus Childrens Research Institute for technical assistance and M. Sebert for critical review.
This work was supported by grants from the U.S. Public Health Service to J.N.W. (AI44231 and AI38446) and to the Bacterial Respiratory Pathogen Research Unit (NO1 AI30040).
Editor: A. Camilli
Footnotes
Published ahead of print on 5 March 2007.
REFERENCES
- 1.Adamou, J. E., J. H. Heinrichs, A. L. Erwin, W. Walsh, T. Gayle, M. Dormitzer, R. Dagan, Y. A. Brewah, P. Barren, R. Lathigra, S. Langermann, S. Koenig, and S. Johnson. 2001. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect. Immun. 69:949-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balachandran, P., A. Brooks-Walter, A. Virolainen-Julkunen, S. Hollingshead, and D. Briles. 2002. Role of pneumococcal surface protein C in nasopharyngeal carriage and pneumonia and its ability to elicit protection against carriage of Streptococcus pneumoniae. Infect. Immun. 70:2526-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry, A., and J. Paton. 2000. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative virulence proteins. Infect. Immun. 68:133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. Hansen, L. Elvin, K. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 5.Briles, D., S. Hollingshead, J. Paton, E. Ades, L. Novak, F. van Ginkel, and W. J. Benjamin. 2003. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J. Infect. Dis. 188:339-348. [DOI] [PubMed] [Google Scholar]
- 6.Dawid, S., A. M. Roche, and J. N. Weiser. 2007. The blp bacteriocins of Streptococcus pneumoniae mediate intraspecies competition both in vitro and in vivo. Infect. Immun. 75:443-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049-3060. [DOI] [PubMed] [Google Scholar]
- 8.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Kayhty, P. Karma, R. Kohberger, G. Siber, P. Makela, et al. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 9.Feil, E. J., B. C. Li, D. M. Aanensen, W. P. Hanage, and B. G. Spratt. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grusby, M. J., R. S. Johnson, V. E. Papaioannou, and L. H. Glimcher. 1991. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science 253:1417-1420. [DOI] [PubMed] [Google Scholar]
- 11.Hanage, W., T. Kaijalainen, A. Saukkoriipi, J. Rickcord, and B. Spratt. 2006. A successful, diverse disease-associated lineage of nontypeable pneumococci that has lost the capsular biosynthesis locus. J. Clin. Microbiol. 44:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hohmann, E. L., C. A. Oletta, K. P. Killeen, and S. I. Miller. 1996. phoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J. Infect. Dis. 173:1408-1414. [DOI] [PubMed] [Google Scholar]
- 13.Hollingshead, S., R. Becker, and D. Briles. 2000. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 68:5889-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs, M. R. 2004. Streptococcus pneumoniae: epidemiology and patterns of resistance. Am. J. Med. 117(Suppl. 3A):3S-15S. [DOI] [PubMed] [Google Scholar]
- 15.Kim, J., and J. Weiser. 1998. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 177:368-377. [DOI] [PubMed] [Google Scholar]
- 16.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 17.Lee, M. S., B. A. Dougherty, A. C. Madeo, and D. A. Morrison. 1999. Construction and analysis of a library for random insertional mutagenesis in Streptococcus pneumoniae: use for recovery of mutants defective in genetic transformation and for identification of essential genes. Appl. Environ Microbiol. 65:1883-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine, O. S., K. L. O'Brien, M. Knoll, R. A. Adegbola, S. Black, T. Cherian, R. Dagan, D. Goldblatt, A. Grange, B. Greenwood, T. Hennessy, K. P. Klugman, S. A. Madhi, K. Mulholland, H. Nohynek, M. Santosham, S. K. Saha, J. A. Scott, S. Sow, C. G. Whitney, and F. Cutts. 2006. Pneumococcal vaccination in developing countries. Lancet 367:1880-1882. [DOI] [PubMed] [Google Scholar]
- 19.Lipsitch, M. 1999. Bacterial vaccines and serotype replacement: lessons from Haemophilus influenzae and prospects for Streptococcus pneumoniae. Emerg. Infect. Dis. 5:336-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipsitch, M., C. Whitney, E. Zell, T. Kaijalainen, R. Dagan, and R. Malley. 2005. Are anticapsular antibodies the primary mechanism of protection against invasive pneumococcal disease? PLoS Med. 2:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malley, R., M. Lipsitch, A. Stack, R. Saladino, G. Fleisher, S. Pelton, C. Thompson, D. Briles, and P. Anderson. 2001. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect. Immun. 69:4870-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malley, R., K. Trzcinski, A. Srivastava, C. Thompson, P. Anderson, and M. Lipsitch. 2005. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. USA 102:4848-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin, M., J. H. Turco, M. E. Zegans, R. R. Facklam, S. Sodha, J. A. Elliott, J. H. Pryor, B. Beall, D. D. Erdman, Y. Y. Baumgartner, P. A. Sanchez, J. D. Schwartzman, J. Montero, A. Schuchat, and C. G. Whitney. 2003. An outbreak of conjunctivitis due to atypical Streptococcus pneumoniae. N. Engl. J. Med. 348:1112-1121. [DOI] [PubMed] [Google Scholar]
- 25.Mato, R., I. Sanches, C. Simas, S. Nunes, J. Carrico, N. Sousa, N. Frazao, J. Saldanha, A. Brito-Avo, J. Almeida, and H. Lencastre. 2005. Natural history of drug-resistant clones of Streptococcus pneumoniae colonizing healthy children in Portugal. Microb. Drug Resist. 11:309-322. [DOI] [PubMed] [Google Scholar]
- 26.McCool, T., T. R. Cate, G. Moy, and J. Weiser. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 195:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCool, T., and J. Weiser. 2004. Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infect. Immun. 72:5807-5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musher, D. M. 2006. Pneumococcal vaccine-direct and indirect (“herd”) effects. N. Engl. J. Med. 354:1522-1524. [DOI] [PubMed] [Google Scholar]
- 29.Musher, D. M., A. M. Rueda-Jaimes, E. A. Graviss, and M. C. Rodriguez-Barradas. 2006. Effect of pneumococcal vaccination: a comparison of vaccination rates in patients with bacteremic and nonbacteremic pneumococcal pneumonia. Clin. Infect. Dis. 43:1004-1008. [DOI] [PubMed] [Google Scholar]
- 30.Nelson, A. L., A. M. Roche, J. M. Gould, K. Chim, A. J. Ratner, and J. N. Weiser. 2007. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect. Immun. 75:83-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogunniyi, A. D., M. Grabowicz, D. E. Briles, J. Cook, and J. C. Paton. 2007. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect. Immun. 75:350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogunniyi, A. D., M. C. Woodrow, J. T. Poolman, and J. C. Paton. 2001. Protection against Streptococcus pneumoniae elicited by immunization with pneumolysin and CbpA. Infect. Immun. 69:5997-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, C. J., L. A. Rollins, and A. C. Parker. 1995. Nucleotide sequence determination and genetic analysis of the Bacteroides plasmid, pBI143. Plasmid 34:211-222. [DOI] [PubMed] [Google Scholar]
- 34.Sung, C., H. Li, J. Claverys, and D. Morrison. 2001. An rpsL cassette, janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67:5190-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talkington, D. F., B. G. Brown, J. A. Tharpe, A. Koenig, and H. Russell. 1996. Protection of mice against fatal pneumococcal challenge by immunization with pneumococcal surface adhesin A (PsaA). Microb. Pathog. 21:17-22. [DOI] [PubMed] [Google Scholar]
- 36.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 37.Toltzis, P., and M. R. Jacobs. 2005. The epidemiology of childhood pneumococcal disease in the United States in the era of conjugate vaccine use. Infect. Dis. Clin. North Am. 19:629-645. [DOI] [PubMed] [Google Scholar]
- 38.Tong, H. H., D. Li, S. Chen, J. P. Long, and T. F. DeMaria. 2005. Immunization with recombinant Streptococcus pneumoniae neuraminidase NanA protects chinchillas against nasopharyngeal colonization. Infect. Immun. 73:7775-7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trzcinski, K., C. Thompson, and M. Lipsitch. 2003. Construction of otherwise isogenic serotype 6B, 7F, 14, and 19F capsular variants of Streptococcus pneumoniae strain TIGR4. Appl. Environ. Microbiol. 69:7364-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trzcinski, K., C. Thompson, R. Malley, and M. Lipsitch. 2005. Antibodies to conserved pneumococcal antigens correlate with, but are not required for, protection against pneumococcal colonization induced by prior exposure in a mouse model. Infect. Immun. 73:7043-7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Rossum, A., E. Lysenko, and J. Weiser. 2005. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect. Immun. 73:7718-7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiser, J. N., R. Austrian, P. K. Sreenivasan, and H. R. Masure. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 62:2582-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young, D. B. 2003. Building a better tuberculosis vaccine. Nat. Med. 9:503-504. [DOI] [PubMed] [Google Scholar]