Abstract
Relative resistance to African trypanosomiasis is based on the development of a type I cytokine response, which is partially dependent on innate immune responses generated through MyD88 and Toll-like receptor 9 (TLR9). Therefore, we asked whether enhancement of the immune response by artificial stimulation with CpG oligodeoxynucleotide (ODN), a TLR9 agonist, would result in enhanced protection against trypanosomes. In susceptible BALB/c mice, relative resistance to infection was significantly enhanced by CpG ODN treatment and was associated with decreased parasite burden, increased cytokine production, and elevated parasite-specific B- and T-cell responses. In relatively resistant C57BL/6 mice, survival was not enhanced but early parasitemia levels were reduced 100-fold and the majority of the parasites were nondividing, short stumpy (SS) forms. CpG ODN treatment of lymphocyte-deficient C57BL/6-scid and BALB/cByJ-scid mice also enhanced survival and reduced parasitemia, indicating that innate resistance to trypanosome infection can be enhanced. In C57BL/6-scid and BALB/cByJ-scid mice, the parasites were also predominantly SS forms during the outgrowth of parasitemia. However, the effect of CpG ODN treatment on parasite morphology was not as marked in gamma interferon (IFN-γ)-knockout mice, suggesting that downstream effects of IFN-γ production may play a discrete role in parasite cell differentiation. Overall, these studies provide the first evidence that enhancement of resistance to African trypanosomes can be induced in susceptible animals in a TLR9-dependent manner and that CpG ODN treatment may influence the developmental life cycle of the parasites.
The innate immune system plays an integral role in generating and directing an immune response to infectious microbes. Pattern recognition receptors are molecules involved in the detection of conserved microbial molecules termed pathogen-associated molecular patterns. One class of pattern recognition receptor is the Toll-like receptors (TLRs), which recognize a variety of pathogen-associated molecular patterns, including lipopolysaccharide, CpG DNA, and flagellin. The microbial molecules recognized early during infection ultimately shape the subsequent adaptive immune responses, a phenomenon which highlights the importance of innate recognition during infection (16, 17, 31).
In experimental African trypanosomiasis, inbred strains of mice differ in their level of relative resistance to infection. C57BL/6 and C57BL/10 strains display the highest degree of relative resistance, while BALB/c and C3H mice exhibit intermediate and susceptible phenotypes, respectively (24). The differences in resistance among inbred strains are not linked to major histocompatibility complex class II haplotype or parasite-specific antibody responses (4, 25) but are linked to type I cytokine responses, specifically gamma interferon (IFN-γ) (13, 29, 35, 36). The production of IFN-γ is partially dependent on MyD88 and TLR9, a pattern recognition molecule that recognizes CpG motifs in microbial DNA and synthetic CpG oligodeoxynucleotide (ODN) (7).
CpG ODN, when used as an adjuvant, has provided protection against several microbial infections, including those by several intracellular bacteria and the protozoan parasites Leishmania major and Plasmodium yoelii (8-10, 18, 20, 22, 42). CpG ODN-mediated protection from microbial challenge is commonly associated with a strong polarized Th1-cell response, including increased production of IFN-γ and Th1-associated antibody isotypes (immunoglobulin G2a [IgG2a], IgG2b, and IgG3) and a reduction in IgG1 production (6, 10, 15, 18, 19, 21, 22, 27, 32, 40). Previous studies have demonstrated that various adjuvants, including trehalose dimycolate and muramyl dipeptide, Propionibacterium acnes, Mycobacterium bovis BCG, and Bordetella pertussis increased resistance to trypanosome infection (1, 2, 34). Enhanced resistance was associated with an early reduction in parasitemia and a decrease in the proportion of dividing parasites. The prevailing hypothesis was that activated macrophages were capable of producing factors that limited the early outgrowth of parasites. More recent studies suggest that IFN-γ-mediated activation of macrophages may be centrally important in these effects (13). A number of factors produced by macrophages, including nitric oxide, reactive oxygen species, and tumor necrosis factor alpha, have all been shown to kill trypanosomes in vitro (14, 28).
In this study, we have used the TLR9 agonist CpG ODN as a tool to boost the innate immune response of infected animals. We show that CpG ODN treatment significantly enhances resistance to Trypanosoma brucei rhodesiense in susceptible mouse strains. The increase in resistance is associated with decreased parasite levels, an increase in nondividing SS forms, increased Th1 cytokine production, higher parasite-specific antibody levels, and elevated T-cell cytokine production in response to parasite antigen. Although survival is not enhanced in resistant mice by CpG ODN treatment, parasite levels are reduced 100-fold during the first wave of parasitemia and the parasites are also predominantly SS forms in these animals. Further investigation revealed that reductions in parasitemia and induction of parasite differentiation also occurred in SCID mice. In the absence of IFN-γ, however, resistance, parasitemia levels, and parasite differentiation were not affected to the same degree as in wild-type mice. Thus, the downstream effects of IFN-γ production may play a central role in both CpG ODN-induced enhancement of host resistance to trypanosomes and effects on the parasite developmental life cycle.
MATERIALS AND METHODS
Infections and CpG ODN treatment.
Frozen stabilates of the T. brucei rhodesiense clone LouTat 1 were expanded in cyclophosphamide (300 mg/kg of body weight; Sigma-Aldrich, St. Louis, MO)-immunosuppressed Swiss Webster mice (Jackson Laboratory, Bar Harbor, ME). For infections, age- and sex-matched C57BL/6, C57BL/6-scid, BALB/cJ, BALB/cByJ, BALB/cByJ-scid, C57BL/6 IFN-γ-knockout (GKO), and BALB/c GKO mice (Jackson Laboratory) were injected with 1 × 105 trypanosomes intraperitoneally (i.p.). CpG ODN-treated animals were injected i.p. with 50 μg CpG ODN 1826 (Coley Pharmaceuticals, Ottawa, Canada) in 300 μl phosphate-buffered saline (PBS) 1 day prior to and at the time of infection. As controls, mice received non-CpG-containing ODN 2138 (Coley Pharmaceuticals) or PBS alone. All mice were housed in university-approved facilities and were handled strictly according to National Institutes of Health and University of Wisconsin—Madison Research Animal Resource Center guidelines.
Assessment of parasitemia and parasite morphology.
To monitor parasitemia, tail blood was diluted in PBS with glucose with heparin (1× PBS, 10 U/ml heparin, 1% glucose) and parasites were counted using a hemacytometer. For morphological classification of parasites, blood smears were hematoxylin and eosin stained and examined by light microscopy. Parasite morphology was assigned according to the parameters described by Wijers (41). Trypanosomes were classified as either long slender (LS) (30), intermediate (I), or SS. Briefly, LS parasites have elongated nuclei and “bodies” and a long free flagellum, while SS parasites have thicker, shorter “bodies,” oval nuclei, and no free flagellum. Parasites categorized as I had characteristics that were between the extreme phenotypes of the LS and SS parasites. One hundred parasites were counted per slide. If fewer than 100 parasites were present, the entire slide was counted. Additionally, the stained blood smears were used to analyze the trypanosome cell cycle as an assessment of cell division capabilities. Parasites of each morphological classification were analyzed by enumerating the number of nuclei and kinetoplasts present in each trypanosome (23, 26). All SS parasites contained one nucleus and one kinetoplast (1N1K), whereas LS parasites were composed of 87% 1N1K, 9% 1N2K, and 4% 2N2K cells. Microscopy was performed using a Zeiss Axioplan IIi at ×63 magnification. Images were obtained using a Zeiss AxioCam black-and-white charge-coupled device camera and OpenLabs 4.0 software (Improvision, Inc., Lexington, MA).
Measurement of serum cytokine levels.
Mice were infected and treated with CpG ODN as described above. Two days postinfection, the mice were sacrificed and serum was collected. Interleukin-12p70 (IL-12p70) and IFN-γ levels were determined by enzyme-linked immunosorbent assay (ELISA) (BD Biosciences, San Jose, CA).
Measurement of parasite-specific antibody.
Soluble variant surface glycoprotein (VSG) was prepared from trypanosomes as we have previously described (3). Plasma samples were collected from infected and CpG ODN-treated infected mice (12). Briefly, tail blood was diluted in PBS-glucose plus heparin. Plasma was obtained by centrifugation (4,000 rpm for 4 min at 4°C). Parasite-specific antibody responses were measured by ELISA, as previously described (36). Briefly, 96-well plates were coated with 4 μg/ml VSG. Optimal dilutions of plasma samples or serum were made, and isotype-specific antibody responses were measured (Zymed, San Francisco, CA).
Measurement of T-cell cytokine production.
T-cell cytokine responses to VSG were assessed as previously described (13, 35, 36). Briefly, whole-spleen-cell cultures were established from CpG ODN-treated and control mice 7 days postinfection. The cultures were incubated in the presence of RPMI 1640 medium alone, 2.5 μg/ml concanavalin A (ConA), or 50 μg/ml VSG for 24 h at 37°C in 5% CO2. Cell-free supernatants were analyzed for IL-2, IL-4, and IFN-γ levels by cytokine-specific ELISA (BD Biosciences).
Statistics.
Statistical analyses were performed with Prism 4 for Macintosh (GraphPad Software, Inc., San Diego, CA). P values less than 0.05 were considered significant.
RESULTS
Resistance to trypanosome infection is enhanced in CpG ODN-treated susceptible mice.
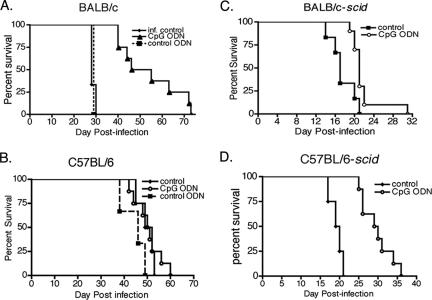
To determine whether administering CpG ODN as a TLR9 agonist increased resistance to trypanosome infection, relatively resistant C57BL/6 and susceptible BALB/c mice were treated and infected. Mice were given 50 μg CpG ODN 1826, non-CpG ODN 2138, or PBS i.p. on the day prior to and at the time of infection with 1 × 105 LouTat 1 parasites. The survival of CpG ODN-treated BALB/c mice was greatly enhanced with a mean survival time of 54 ± 5 days in comparison to untreated and ODN 2138 (non-CpG-containing ODN)-treated mice with mean survival times of 29 days (Fig. 1A). The difference in survival time was significant as assessed by log rank analysis (P = 0.0005). In contrast, the survival of relatively resistant C57BL/6 mice was not extended by CpG ODN treatment (Fig. 1B). Thus, CpG ODN is an efficacious adjuvant and CpG ODN treatment selectively promotes resistance in a susceptible mouse strain.
FIG. 1.
CpG ODN treatment enhances resistance to trypanosome infection in susceptible wild-type and SCID mice. BALB/c (A), C57BL/6 (B), BALB/cByJ-scid (C), and C57BL/6-scid (D) mice were injected i.p. with 50 μg CpG ODN 1826 (CpG ODN), control ODN 2138 (control ODN), or PBS (control) 1 day prior to and at the time of infection. Mice were infected with 1 × 105 trypanosomes in 300 μl PBS. Survival times of CpG ODN-treated and control-treated animals were recorded and subjected to the log rank test for significance. Survival time was significantly increased in CpG ODN-treated BALB/c (P = 0.0005), BALB/c-scid (P = 0.0068), and C57BL/6-scid (P = 0.0021) mice. The data presented are representative of three independent experiments.
Parasitemia levels are dramatically decreased in CpG ODN-treated BALB/c and C57BL/6 mice.
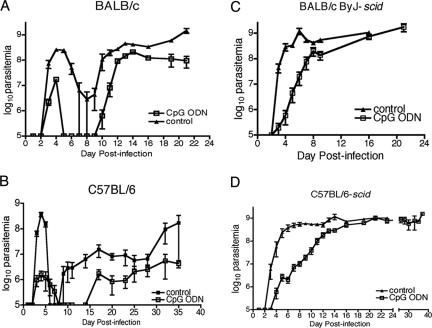
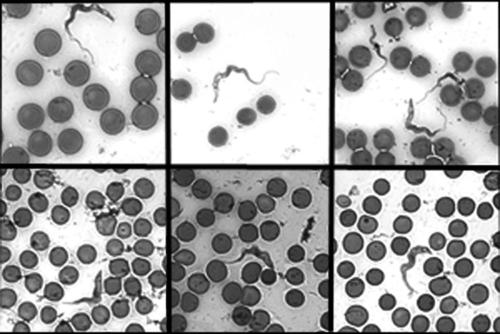
The effect of CpG ODN treatment on the control of parasitemia was monitored over the course of infection. Tail blood samples were taken from CpG ODN-treated and infected control C57BL/6 and BALB/c mice. The samples were diluted to enumerate parasitemia with a hemacytometer and also to make blood smears. Blood smears were stained with hematoxylin and eosin, and parasite morphology was observed by light microscopy according to the parameters described by Wijers (41). In both strains of mice CpG ODN treatment decreased the magnitude of the first wave of parasitemia with a 10-fold reduction in BALB/c mice and a 100-fold decrease in C57BL/6 mice (Fig. 2A and B). The degree of reduction of parasitemia was correlated with the number of dividing LS parasites present. Detectable parasites in treated C57BL/6 mice were predominantly nondividing SS parasites (Table 1) in comparison to those of control-treated mice, which were nearly all LS parasites (Fig. 3). The prevalence of SS forms during the outgrowth of parasites in treated BALB/c mice was not as dramatic as that in the C57BL/6 mice but was greater than that found in control-treated animals (Table 1). These results demonstrate that CpG ODN administration affects parasite differentiation, and the degree to which this occurs correlates with the degree of parasite reduction during the first wave of parasitemia.
FIG. 2.
CpG ODN treatment reduces parasite burden in susceptible and SCID mice. BALB/c (A), C57BL/6 (B), BALB/cByJ-scid (C), and C57BL/6-scid (D) mice were treated and infected according to the legend to Fig. 1. Parasitemia was measured from tail blood using a hemacytometer. The limit of detection was 1 × 106 parasites/ml. The mean parasitemias ± standard errors of the means of six animals in each group are presented. The data are representative of three independent experiments.
TABLE 1.
Parasites in CpG ODN-treated animals are predominantly nondividing SS forms during the initial outgrowtha
| Mouse strain | CpG ODN | Day of parasite outgrowthb | Morphology (mean % ± SEM)
|
||
|---|---|---|---|---|---|
| LS | I | SS | |||
| C57BL/6 | − | 3 | 100 ± 0 | 0 | 0 |
| + | 4 | 19 ± 8 | 29 ± 7 | 53 ± 14 | |
| C57BL/6-scid | − | 3 | 99 ± 0 | 1 ± 0 | 0 |
| + | 5 | 3 ± 3 | 20 ± 5 | 77 ± 5 | |
| C57BL/6 GKO | − | 3 | 83 ± 2 | 16 ± 0.3 | 2 ± 2 |
| + | 3 | 53 ± 3 | 44 ± 6 | 3 ± 3 | |
| BALB/c | − | 3 | 93 ± 2 | 6 ± 2 | 1 ± 0.5 |
| + | 3 | 68 ± 7 | 17 ± 2 | 14 ± 4 | |
Mice were treated with 50 μg CpG ODN or PBS i.p. on the day prior to and at the time of infection with 1 × 105 LouTat 1 parasites i.p. Blood smears were made from diluted tail blood samples and stained with hematoxylin and eosin. Parasite morphology was determined according to the characteristics described by Wijers (41). Three mice of each treatment group for each strain were monitored.
The day of parasite outgrowth was determined by the first mean parasitemia level greater than 1 × 106 parasites/ml (the limit of detection).
FIG. 3.
CpG ODN treatment induces parasite differentiation. Hematoxylin- and eosin-stained blood smears from infected (top row) and CpG ODN-treated infected (bottom row) C57BL/6 mice from day 5 postinfection were analyzed by light microscopy at ×63 magnification. Parasite morphology was determined according to the parameters described by Wijers (41). Images are representative of parasites from each treatment group. The top and bottom rows represent three independent observations each.
CpG ODN treatment increases parasite-specific adaptive immune responses.
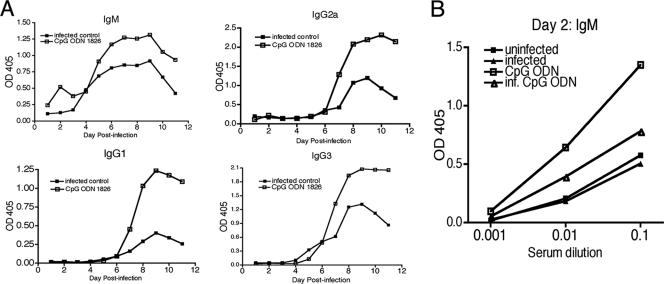
CpG ODN treatment has been shown to influence the amount and isotype (Th1 associated) of antibody produced during infection with other parasites (27). For this reason, the antibody response to VSG was measured in BALB/c mice. Plasma samples were collected from mice and analyzed for isotype-specific antibody production by ELISA. CpG ODN treatment increased the production of all antibody isotypes typically induced by trypanosome infection, IgM, IgG1, IgG2a, and IgG3 (Fig. 4A) (36). IgM levels were increased as early as day 2 postinfection. Further analysis of day 2 antibody levels from serum samples demonstrated that VSG-specific IgM was induced in CpG-treated animals in the presence or absence of an infection (Fig. 4B). The analysis of antibody responses in CpG ODN-treated mice indicates that the antibody response to trypanosome infection is enhanced, but the isotypes produced were not shifted towards IgG2a, IgG2b, and IgG3 and away from IgG1. CpG ODN treatment also enhanced background levels of naturally occurring VSG-specific IgM in mice that have never been exposed to trypanosomes (33).
FIG. 4.
CpG ODN treatment enhances parasite-specific antibody production. Plasma samples were collected from tail blood from BALB/c mice that were treated and infected according to the legend to Fig. 1. VSG-specific isotype-specific antibody levels were measured from 1:10-diluted plasma samples by ELISA. (A) The relative levels of antibody in each treatment group over the first 11 days of infection are depicted. (B) Early IgM production was measured from day 2 serum samples. Data are representative of three independent experiments.
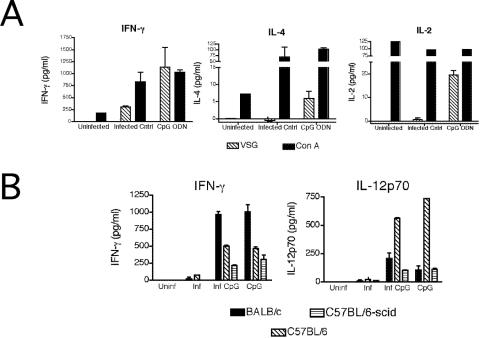
Changes in antibody in CpG ODN-treated mice were accompanied by the induction of VSG-specific Th1-cell responses. Given that trypanosome infection induces a polarized Th1-cell response in resistant mice (35), T-cell responses were measured in CpG ODN-treated susceptible BALB/c mice to determine if the VSG-specific Th1 response was enhanced by treatment. T-cell cytokine responses were measured from whole-spleen-cell cultures from day 7 postinfection. The cells were incubated in the presence of a mitogen, ConA, as a nonspecific stimulus or the parasite antigen VSG for 24 h. Cytokine production was measured by ELISA. Supernatant fluid obtained from T cells from CpG ODN-treated mice stimulated in vitro with VSG contained more IL-2 and IFN-γ in response to parasite antigen than did similar preparations of cells of untreated infected animals. IL-4 was detected at levels near the lower limit of detection (Fig. 5A). Thus, CpG ODN treatment increases T-cell production of type I cytokines in response to parasite antigen.
FIG. 5.
CpG ODN treatment increases type I cytokine production. (A) Whole-spleen cultures from uninfected, infected, and infected CpG ODN-treated BALB/c mice were prepared on day 7 postinfection. The cultures were incubated in medium alone, 2.5 μg/ml ConA, or 50 μg/ml VSG for 24 h. IFN-γ, IL-12p70, and IL-4 cytokine-specific ELISAs were performed on cell-free supernatants. The data are presented as the mean cytokine production over medium-alone levels and representative of three independent experiments. (B) BALB/c, C57BL/6, and C57BL/6-scid mice were treated with 50 μg CpG ODN on day −1 and day 0 (CpG), treated with CpG ODN and infected (inf. CpG), infected only (inf.), or left untreated (uninf.). On day 2, serum was collected and IFN-γ and IL-12p70 levels were measured. The mean cytokine production levels from three independent experiments are presented.
CpG ODN mediates enhanced survival and affects parasite growth rate and morphology in the absence of the adaptive immune system.
In order to assess the specific adaptive versus innate immune responses to changes observed following CpG ODN treatment, SCID mice on the BALB/cByJ and C57BL/6 genetic backgrounds were treated with CpG ODN and infected. Resistance was significantly increased in both strains, with mean survival times increasing from 19 ± 1 days to 29 ± 1 days in C57BL/6-scid mice (P = 0.0002) and increasing from 17 ± 1 days to 22 ± 1 days in BALB/cByJ-scid mice (P = 0.007) (Fig. 1C and D). Parasitemia was monitored throughout the course of infection. It was noteworthy that CpG ODN-treated SCID mice exhibited a slower outgrowth of parasites than the untreated controls did (Fig. 2C and D). Typically, parasites in SCID mice are detectable by day 3 postinfection and rapidly increase to peak levels by day 4 to 5 and then the numbers plateau since there is no antibody-mediated clearance from the blood. In CpG ODN-treated mice, the first detectable mean parasitemia level (above the detection limit of 1 × 106 parasites/ml) was not observed until day 5 and the subsequent outgrowth was much slower. Mice exhibited a plateau in parasite number on day 12 postinfection for C57BL/6-scid mice and day 8 for BALB/cByJ-scid mice (Fig. 2C and D). During delayed trypanosome outgrowth, a greater percentage of the trypanosomes exhibited the SS morphology (Table 1). These results indicate that an enhanced innate immune response is able to control parasite growth in the absence of parasite-specific B- and T-cell responses and that this is associated with an increase in nondividing parasites.
CpG ODN treatment increases type I cytokine production.
Since CpG ODN-mediated protection against other parasitic protozoans is dependent on type I cytokines, the induction of IFN-γ and that of IL-12p70 were measured (10, 40). BALB/c, C57BL/6, and C57BL/6-scid mice were treated with CpG ODN only, treated with CpG ODN and infected, infected only, or left untreated. On day 2 postinfection, serum was collected and analyzed for cytokine levels by ELISA. IL-12p70 and IFN-γ levels were essentially undetectable in infected mice on day 2 postinfection. In contrast, CpG treatment, in the presence and absence of infection, induced readily detectable levels of both cytokines (Fig. 5B). These results suggest that CpG ODN treatment augments type I cytokine production early during infection.
IFN-γ production following CpG ODN treatment is an essential component in the observed changes in parasitemia, parasite morphology, and survival time.
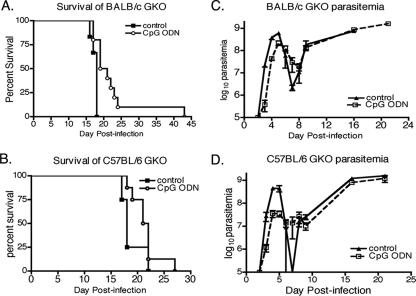
To further explore the role of IFN-γ in CpG ODN-induced effects on the course of trypanosome infection, GKO mice with the BALB/c and C57BL/6 genetic backgrounds were CpG ODN treated and infected. In both strains of mice, the levels of parasitemia were much higher than those seen during infections of wild-type mice (Fig. 6C and D). The difference in the morphology of parasites in CpG ODN-treated C57BL/6 GKO mice was also not as dramatic as that observed in wild-type C57BL/6 and C57BL/6-scid mice (Table 1). CpG ODN treatment induced a significant increase in survival time for BALB/c GKO mice (P = 0.006) but not C57BL/6 GKO mice (P = 0.13). The mean survival time for BALB/c GKO mice was only minimally increased from 17.5 ± 0.3 days to 22.4 ± 2.4 days (Fig. 6A and B). These results suggest that the downstream effects of IFN-γ are an essential component of CpG ODN-induced enhancement of relative resistance in susceptible mice, the early reduction of parasitemia, and the induced changes in parasite morphology.
FIG. 6.
Effects of CpG ODN treatment on parasitemia and resistance are reduced in the absence of IFN-γ. GKO mice on the BALB/c (A and C) and C57BL/6 (B and D) genetic backgrounds were CpG ODN treated and infected according to the legend for Fig. 1, and survival times were recorded (A and B). Parasitemia levels (C and D) were recorded as described in the legend to Fig. 2. Resistance was significantly increased in CpG ODN-treated BALB/c GKO mice (P = 0.006). Data are representative of three independent experiments.
DISCUSSION
The development of an innate immune response leading to the production of type I cytokines has been shown to be important for relative resistance to Trypanosoma brucei rhodesiense infection (7, 13, 29, 35, 36). In this study we sought to determine whether enhanced stimulation of the innate immune response through TLR9, a receptor known to play a role in resistance to trypanosome infection, would improve the immune response and provide increased resistance to infection. We found that CpG ODN treatment prior to infection nearly doubles the survival time of intermediately susceptible BALB/c mice, with increases from approximately 30 to 60 days. Interestingly, treatment of relatively resistant C57BL/6 mice, which survive on an average of 60 days postinfection, did not display increased resistance as measured by survival time (24). This suggests that CpG ODN treatment primarily enhances those specific elements of early resistance that distinguish resistant from susceptible mice. These results thus provide a novel experimental approach to identifying these critical elements.
Although changes in resistance to infection were most marked in relatively susceptible mice, CpG ODN treatment did decrease the first wave of parasitemia in both susceptible and resistant mouse strains. In this case, CpG ODN treatment had a stronger effect in C57BL/6 mice, which had a 100-fold decrease in parasitemia in comparison to a 10-fold reduction in BALB/c mice. Thus, the degree to which parasites were controlled by CpG ODN treatment was not a predictor of subsequent increased resistance in wild-type mice.
Since CpG ODN treatment is known to increase antibody production, we predicted that effects on B cells may be partly responsible for the observed enhanced resistance to infection and the reduction in parasitemia. The isotypes of VSG-specific antibodies that are detectable during trypanosome infection (IgM, IgG1, IgG2a, and IgG3) were all increased in CpG-ODN-treated infected mice (13, 35, 36). Unexpectedly and unlike in other studies of CpG ODN-induced effects on adaptive immunity, no reduction in the amount of VSG-specific IgG1 produced was observed. Since the IgG1 produced during infection is IL-4 dependent and T cell independent, CpG ODN treatment may not affect the T-cell-independent component of the VSG-specific B-cell response (36). Interestingly, early anti-VSG IgM production was not dependent on trypanosome infection and was produced in response to CpG ODN treatment alone. Previous studies have described the existence of VSG-specific antibodies in the serum of animals that were never exposed to trypanosomes (33). It appears that CpG ODN treatment may increase the level of such antibodies and may play a role in the early reduction of parasitemia in CpG ODN-treated animals.
In addition to enhanced B-cell responses, we also detected elevated type I cytokine levels in serum and from VSG-specific T cells, plus enhanced innate resistance in SCID mice. Treated SCID mice survived significantly longer than untreated controls and were able to control parasites during the early part of infection, providing clear evidence for a central role of the innate immune system in the observed effects. However, CpG ODN treatment of SCID mice with a susceptible genetic background did not enhance survival to the same degree as in wild-type BALB/c mice. Thus, both innate and adaptive immune responses appear to play a significant role in the observed CpG ODN-induced resistance in susceptible mice, with enhanced innate responses likely supporting enhanced adaptive immunity.
Finally, CpG ODN treatment induced alterations in the physiology of parasites early during infection. Trypanosomes undergo a complex life cycle with distinct life cycle stages in both the tsetse fly and the bloodstream of an infected host. In the bloodstream, parasites are of two predominant morphologies, the rapidly dividing LS form and the nondividing SS form, which is preadapted for uptake into the tsetse fly (38, 41). The parasites observed in CpG ODN-treated C57BL/6 mice were predominantly nondividing SS forms. Trypanosomes in CpG ODN-treated BALB/c mice were composed of more SS and I forms than were those in untreated mice, but a predominance of SS forms similar to that in the treated C57BL/6 mice was not observed. The outgrowth of parasites in SCID mice was delayed by several days in both strains and was associated with increases in SS forms. This demonstrates that the CpG ODN-induced changes in parasite morphology occur in the absence of adaptive immunity and that the reduction in dividing LS parasites may explain reductions in the first wave of parasitemia. CpG ODN treatment of mice lacking IFN-γ demonstrated that the induction of nondividing SS parasites is due in part to the downstream effects induced by IFN-γ. This may also explain why parasitemia levels in untreated GKO mice are higher than those in wild-type mice.
The profound effect of CpG ODN treatment on the morphology of the parasites provides potential insights into host-parasite interactions that may govern the progression of disease. On the one hand, the relative level and strength of the immune response may provide a signal to the parasite that stimulates differentiation into the vector-transmissible form, thus increasing the odds that transmission will occur before parasites are eliminated. An alternative explanation is that the immune system is partially able to eliminate parasites by inducing parasite differentiation and subsequent cell death in the presence of IFN-γ. Earlier studies using P. acnes as an adjuvant showed that macrophages make factors that affect the growth of parasites in vitro (2). We have shown that adjuvant-induced parasite differentiation is partially dependent on the presence of IFN-γ. Since IFN-γ does not have any detectable direct effects on parasite growth or morphology, it is likely that IFN-γ may induce the production of several factors that can affect these elements. Previous in vitro studies led to the identification of a low-molecular-weight molecule, stumpy inducible factor, capable of triggering the transformation of LS to SS forms (39). However, relatively little is known about the factors that influence the transformation of the parasite in the bloodstream, including whether or not the host immune response can influence differentiation (37). Our results demonstrate that CpG ODN-enhanced immune responses can induce parasite differentiation. Thus, CpG ODN treatment may provide a way to identify molecules that are capable of inducing parasite differentiation in vitro.
CpG ODN-enhanced immunity to trypanosome infection is multifaceted, with innate and adaptive immune responses playing discrete roles. Current paradigms of host resistance suggest that critical cytokines and other factors are produced during CpG ODN enhancement of resistance to trypanosomes. In addition to molecules that we have emphasized in this study, CpG ODN treatment has been shown to enhance the production of several trypanolytic molecules, including nitric oxide, reactive oxygen species, and tumor necrosis factor alpha (13, 21, 25). These molecules may be key components of the observed enhanced resistance following CpG ODN treatment. CpG ODN treatment also triggers the production of type I IFNs. Type I IFNs are produced during trypanosome infection, but the importance of type I IFNs in resistance to infection is unknown and remains to be fully tested (5).
Overall, these studies reveal that an enhanced immune response has several beneficial impacts on immunity to trypanosome infection. Resistance is enhanced in susceptible mice and is associated with enhanced nonspecific and specific immune responses. CpG ODN treatment altered the initial developmental outgrowth of parasites in both wild-type and SCID mice, which demonstrates that enhanced innate immune responses can control parasite levels. Reduced parasite levels were associated with an increase in the number of nondividing SS parasites observed early during infection. Thus, enhanced innate immunity may control parasitemia by affecting the physiology of the parasite as well as altering specific factors associated with resistance.
Acknowledgments
These studies were supported by USPHS grants AI048242, AI051421 (D.M.P.), and AI22441 (J.M.M.). T.H.H. is supported by NIH Cellular and Molecular Parasitology Training Program grant USPHS AI007414.
We thank Vicki Leatherberry and Jim Schrader for their assistance with animal infections. We also thank Jay Bangs, Rebecca Lopez, Brian Leppert, Bailey Freeman, and Nicole Cooney for their comments and discussions during the completion of the manuscript.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 5 March 2007.
REFERENCES
- 1.Askonas, B. A., and G. J. Bancroft. 1984. Interaction of African trypanosomes with the immune system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 307:41-49. [DOI] [PubMed] [Google Scholar]
- 2.Black, S. J., M. Murray, S. Z. Shapiro, R. Kaminsky, N. K. Borowy, R. Musanga, and F. Otieno-Omondi. 1989. Analysis of Propionibacterium acnes-induced non-specific immunity to Trypanosoma brucei in mice. Parasite Immunol. 11:371-383. [DOI] [PubMed] [Google Scholar]
- 3.Coller, S. P., J. M. Mansfield, and D. M. Paulnock. 2003. Glycosylinositolphosphate soluble variant surface glycoprotein inhibits IFN-gamma-induced nitric oxide production via reduction in STAT1 phosphorylation in African trypanosomiasis. J. Immunol. 171:1466-1472. [DOI] [PubMed] [Google Scholar]
- 4.De Gee, A. L., R. F. Levine, and J. M. Mansfield. 1988. Genetics of resistance to the African trypanosomes. VI. Heredity of resistance and variable surface glycoprotein-specific immune responses. J. Immunol. 140:283-288. [PubMed] [Google Scholar]
- 5.DeGee, A. L., J. M. Mansfield, and G. Sonnenfeld. 1986. Treatment of trypanosome-infected mice with exogenous interferon, interferon inducers, or antibody to interferon. J. Parasitol. 72:792-794. [PubMed] [Google Scholar]
- 6.Dittmer, U., and A. R. Olbrich. 2003. Treatment of infectious diseases with immunostimulatory oligodeoxynucleotides containing CpG motifs. Curr. Opin. Microbiol. 6:472-477. [DOI] [PubMed] [Google Scholar]
- 7.Drennan, M. B., B. Stijlemans, J. Van den Abbeele, V. J. Quesniaux, M. Barkhuizen, F. Brombacher, P. De Baetselier, B. Ryffel, and S. Magez. 2005. The induction of a type 1 immune response following a Trypanosoma brucei infection is MyD88 dependent. J. Immunol. 175:2501-2509. [DOI] [PubMed] [Google Scholar]
- 8.Elkins, K. L., T. R. Rhinehart-Jones, S. Stibitz, J. S. Conover, and D. M. Klinman. 1999. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162:2291-2298. [PubMed] [Google Scholar]
- 9.Finlay, B. B., and R. E. Hancock. 2004. Can innate immunity be enhanced to treat microbial infections? Nat. Rev. Microbiol. 2:497-504. [DOI] [PubMed] [Google Scholar]
- 10.Gramzinski, R. A., D. L. Doolan, M. Sedegah, H. L. Davis, A. M. Krieg, and S. L. Hoffman. 2001. Interleukin-12- and gamma interferon-dependent protection against malaria conferred by CpG oligodeoxynucleotide in mice. Infect. Immun. 69:1643-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosskinsky, C. M., and B. A. Askonas. 1981. Macrophages as primary target cells and mediators of immune dysfunction in African trypanosomiasis. Infect. Immun. 33:149-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris, T. H., N. M. Cooney, J. M. Mansfield, and D. M. Paulnock. 2006. Signal transduction, gene transcription, and cytokine production triggered in macrophages by exposure to trypanosome DNA. Infect. Immun. 74:4530-4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hertz, C. J., H. Filutowicz, and J. M. Mansfield. 1998. Resistance to the African trypanosomes is IFN-gamma dependent. J. Immunol. 161:6775-6783. [PubMed] [Google Scholar]
- 14.Hertz, C. J., and J. M. Mansfield. 1999. IFN-gamma-dependent nitric oxide production is not linked to resistance in experimental African trypanosomiasis. Cell. Immunol. 192:24-32. [DOI] [PubMed] [Google Scholar]
- 15.Ishii, K. J., I. Gursel, M. Gursel, and D. M. Klinman. 2004. Immunotherapeutic utility of stimulatory and suppressive oligodeoxynucleotides. Curr. Opin. Mol. Ther. 6:166-174. [PubMed] [Google Scholar]
- 16.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987-995. [DOI] [PubMed] [Google Scholar]
- 17.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 18.Klinman, D. M. 2004. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 4:249-258. [DOI] [PubMed] [Google Scholar]
- 19.Klinman, D. M., K. M. Barnhart, and J. Conover. 1999. CpG motifs as immune adjuvants. Vaccine 17:19-25. [DOI] [PubMed] [Google Scholar]
- 20.Klinman, D. M., J. Conover, and C. Coban. 1999. Repeated administration of synthetic oligodeoxynucleotides expressing CpG motifs provides long-term protection against bacterial infection. Infect. Immun. 67:5658-5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieg, A. M. 2006. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 5:471-484. [DOI] [PubMed] [Google Scholar]
- 22.Krieg, A. M., L. Love-Homan, A. K. Yi, and J. T. Harty. 1998. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J. Immunol. 161:2428-2434. [PubMed] [Google Scholar]
- 23.Kumar, P., and C. C. Wang. 2006. Dissociation of cytokinesis initiation from mitotic control in a eukaryote. Eukaryot. Cell 5:92-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine, R. F., and J. M. Mansfield. 1981. Genetics of resistance to African trypanosomes: role of the H-2 locus in determining resistance to infection with Trypanosoma rhodesiense. Infect. Immun. 34:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine, R. F., and J. M. Mansfield. 1984. Genetics of resistance to the African trypanosomes. III. Variant-specific antibody responses of H-2-compatible resistant and susceptible mice. J. Immunol. 133:1564-1569. [PubMed] [Google Scholar]
- 26.Li, Z., and C. C. Wang. 2003. A PHO80-like cyclin and a B-type cyclin control the cell cycle of the procyclic form of Trypanosoma brucei. J. Biol. Chem. 278:20652-20658. [DOI] [PubMed] [Google Scholar]
- 27.Lin, L., A. J. Gerth, and S. L. Peng. 2004. CpG DNA redirects class-switching towards “Th1-like” Ig isotype production via TLR9 and MyD88. Eur. J. Immunol. 34:1483-1487. [DOI] [PubMed] [Google Scholar]
- 28.Magez, S., M. Radwanska, A. Beschin, K. Sekikawa, and P. De Baetselier. 1999. Tumor necrosis factor alpha is a key mediator in the regulation of experimental Trypanosoma brucei infections. Infect. Immun. 67:3128-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansfield, J. M., and D. M. Paulnock. 2005. Regulation of innate and acquired immunity in African trypanosomiasis. Parasite Immunol. 27:361-371. [DOI] [PubMed] [Google Scholar]
- 30.Mansfield, J. M., and J. H. Wallace. 1974. Suppression of cell-mediated immunity in experimental African trypanosomiasis. Infect. Immun. 10:335-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medzhitov, R., and C. Janeway, Jr. 2000. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 173:89-97. [DOI] [PubMed] [Google Scholar]
- 32.Miyagi, K., K. Kawakami, Y. Kinjo, K. Uezu, T. Kinjo, K. Nakamura, and A. Saito. 2005. CpG oligodeoxynucleotides promote the host protective response against infection with Cryptococcus neoformans through induction of interferon-gamma production by CD4+ T cells. Clin. Exp. Immunol. 140:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller, N., J. M. Mansfield, and T. Seebeck. 1996. Trypanosome variant surface glycoproteins are recognized by self-reactive antibodies in uninfected hosts. Infect. Immun. 64:4593-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray, M., and W. I. Morrison. 1979. Non-specific induction of increased resistance in mice to Trypanosoma congolense and Trypanosoma brucei by immunostimulants. Parasitology 79:349-366. [DOI] [PubMed] [Google Scholar]
- 35.Schleifer, K. W., H. Filutowicz, L. R. Schopf, and J. M. Mansfield. 1993. Characterization of T helper cell responses to the trypanosome variant surface glycoprotein. J. Immunol. 150:2910-2919. [PubMed] [Google Scholar]
- 36.Schopf, L. R., H. Filutowicz, X. J. Bi, and J. M. Mansfield. 1998. Interleukin-4-dependent immunoglobulin G1 isotype switch in the presence of a polarized antigen-specific Th1-cell response to the trypanosome variant surface glycoprotein. Infect. Immun. 66:451-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seed, J. R., and J. B. Sechelski. 1989. Mechanism of long slender (LS) to short stumpy (SS) transformation in the African trypanosomes. J. Protozool. 36:572-577. [DOI] [PubMed] [Google Scholar]
- 38.Seed, J. R., and M. A. Wenck. 2003. Role of the long slender to short stumpy transition in the life cycle of the African trypanosomes. Kinetoplastid Biol. Dis. 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vassella, E., B. Reuner, B. Yutzy, and M. Boshart. 1997. Differentiation of African trypanosomes is controlled by a density sensing mechanism which signals cell cycle arrest via the cAMP pathway. J. Cell Sci. 110:2661-2671. [DOI] [PubMed] [Google Scholar]
- 40.Walker, P. S., T. Scharton-Kersten, A. M. Krieg, L. Love-Homan, E. D. Rowton, M. C. Udey, and J. C. Vogel. 1999. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12- and IFN-gamma-dependent mechanisms. Proc. Natl. Acad. Sci. USA 96:6970-6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wijers, D. J. 1959. Polymorphism in Trypanosoma gambiense and Trypanosoma rhodesiense, and the significance of the intermediate forms. Ann. Trop. Med. Parasitol. 53:59-68. [DOI] [PubMed] [Google Scholar]
- 42.Zimmermann, S., O. Egeter, S. Hausmann, G. B. Lipford, M. Rocken, H. Wagner, and K. Heeg. 1998. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 160:3627-3630. [PubMed] [Google Scholar]