Abstract
Shigella flexneri is a facultative intracellular organism that causes bacillary dysentery. The Shigella IpaB protein activates caspase 1 in macrophages, which eventually leads to apoptosis. In contrast, epithelial cells infected with Shigella undergo a stress response but do not die. Therefore, the objective of this study was to determine if Shigella has the ability to inhibit apoptosis in epithelial cells. A modified gentamicin protection assay was used to investigate if HeLa cells infected with S. flexneri are able to resist the induction of apoptosis following treatment with 4 μM of staurosporine. Nuclear staining and immunofluorescence revealed that infected cells remained healthy while uninfected cells appeared apoptotic. Only uninfected cells had detectable levels of activated caspase 3 upon immunofluorescence, and this was verified by Western blot analysis. Despite interfering with caspase 3 activation, Shigella-infected cells treated with staurosporine did have cytochrome c release and caspase 9 activation, indicating that Shigella protects epithelial cells from apoptosis by inhibiting caspase 3 activation. Analysis of S. flexneri mutants showed that invasion and a functional type III secretion system were required to block apoptosis. In addition, a mutant with a deletion in mxiE, which encodes a transcriptional activator for genes induced intracellularly, failed to inhibit apoptosis. Therefore, protection of epithelial cells from apoptosis by S. flexneri is regulated by one or more of the bacterial genes under the control of mxiE. We believe that S. flexneri, like other pathogens, inhibits apoptosis in epithelial cells but causes apoptosis in macrophages to ensure survival inside the host.
Shigella flexneri is a gram-negative, facultative intracellular organism, the causative agent of bacillary dysentery, and generates a significant global burden (19). Infection with Shigella causes an intense acute inflammatory reaction that leads to the destruction of the colonic epithelium. Clinical symptoms include watery diarrhea, severe abdominal pain, and bloody, mucoid stools (14). These symptoms of dysentery are due to the penetration of Shigella into colonic epithelial cells, which provide an intracellular environment for the bacteria to multiply and spread to adjacent cells. Entry into epithelial cells is mediated by the Ipa proteins encoded on the 220-kb virulence plasmid. Secretion of these proteins is dependent on a type III secretion system (T3SS), which is encoded by 20 genes in the mxi-spa locus of the virulence plasmid (30).
Previous studies have shown that resident macrophages undergo apoptosis after phagocytosis of Shigella (5, 12, 36). Apoptosis, also known as programmed cell death, is a typical mechanism used during fetal development and in adult cell maintenance to eliminate cells without causing an inflammatory response. It has now been recognized that numerous bacteria and viruses exploit or interact with the apoptotic pathway to enhance the infection process (4, 8, 24). Apoptosis consists of extrinsic and intrinsic pathways that can be utilized by cells. One feature that the two pathways have in common is the use of an effector cysteine aspartate-specific protease (caspase 3) that cleaves substrates like protein kinases, signal transduction proteins, and chromatin-modifying proteins such as poly(ADP-ribose) polymerase and DNA repair proteins, leading to cell death (28). Other important players in these pathways include prosurvival proteins, proapoptotic proteins, initiator caspases (e.g., caspase 8 and caspase 9), and cytochrome c release from the mitochondria (1).
Shigella was first recognized to be involved in apoptosis through its induction of the pathway in macrophages (36). Caspase 1 is activated in macrophages infected with Shigella through the binding of the Shigella effector IpaB. Caspase 1, also known as interleukin-1β-converting enzyme, is responsible for activating the proinflammatory cytokines interleukin-1β and interleukin-18. Caspase 1 is considered an initiator caspase; however, the role of caspase 1 in apoptosis has not been defined. There is some controversy as to whether macrophages undergo apoptosis or necrosis or if caspase 1 is even required for the killing of the macrophages (18, 25, 33). The fact of the matter is that S. flexneri is able to induce cell death in macrophages. In contrast, epithelial cells infected with S. flexneri undergo a stress response but do not die. Stress was measured by examining deoxynucleoside triphosphate levels and the ability to synthesize proteins and transport hexose. Although infected epithelial cells do not die, analysis for apoptosis has not been done (21).
The goal of this study was to determine if S. flexneri infection of epithelial cells protects the cells from apoptosis. We hypothesize that S. flexneri inhibits apoptosis in epithelial cells in order to ensure the bacterium's intracellular survival and replication. We found that S. flexneri is able to protect HeLa cells from staurosporine (STS)-induced apoptosis by preventing the activation of caspase 3 despite the presence of cytochrome c release and caspase 9 activation. Analysis of a ΔmxiE mutant revealed that this mutant was unable to protect epithelial cells from apoptosis to the same extent as wild-type S. flexneri. The protein products of the MxiE-regulated genes are effectors secreted through the T3SS, which are expressed during intracellular growth (15, 20). Therefore, one or several of the genes regulated by MxiE are important for protecting Shigella-infected epithelial cells from apoptosis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains of S. flexneri used are listed in Table 1. Bacteria were routinely cultured at 37°C either in Luria-Bertani broth (LB) with aeration or on tryptic soy broth plates with 1.5% agar and 0.025% Congo red (Sigma). Antibiotics were used at the indicated concentrations: kanamycin, 50 μg/ml; streptomycin, 50 μg/ml; chloramphenicol, 5 μg/ml; and ampicillin, 100 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| S. flexneri strains | ||
| 2457T | Wild-type serotype 2a | 11 |
| M90T | Wild-type serotype 5 | 23 |
| BS543 | 2457T/ΔicsA | 29 |
| BS547 | 2457T/ΔmxiM1::aphA3 | 31 |
| BS567 | M90T/ΔipaB2::aphA3 | 23 |
| BS611 | 2457T/ΔmxiE2::aphA3 | 15 |
| BS758 | BS543/ΔmxiE2::aphA3 | This study |
| BS766 | 2457T transformed with pKM208 | This study |
| BS826 | 2457T/ΔipgD | This study |
| BS828 | BS543/ΔipgD | This study |
| Plasmids | ||
| pKD3 | oriR6K bla cat | 7 |
| pKM208 | Temperature-sensitive red-, gam-, lacI-expressing plasmid driven by Ptac promoter; bla | 7 |
| pCP20 | FLP+ λ cI857+ λ ρR Reptsbla cat | 6 |
Mutant construction.
BS758 was constructed by transduction of BS543 with P1L4 grown on BS611 with selection for kanamycin resistance. BS828 was constructed using the λ red linear recombination method as previously described (7) with the following modifications. PCR was used to amplify a chloramphenicol resistance cassette gene (cat) from pKD3 (Table 1) with 5′ and 3′ overhangs identical to the 5′ and 3′ regions of ipgD internal to the start and stop codons, respectively (Table 2). BS766 was grown overnight at 30°C, subcultured into LB, and grown to mid-log phase at 30°C. Isopropyl-β-d-thiogalactopyranoside (IPTG; 1 mM) was added to the medium to induce expression of the λ red recombination genes, and the bacteria were grown at 37°C for 30 min. The bacteria were then heat shocked at 42°C for 15 min, electroporated with the cleaned PCR product, recovered in SOC medium, and plated on tryptic soy broth plates containing Congo red and chloramphenicol. Positive recombinants were purified and screened via PCR using two sets of primers (Table 2). One set amplified ipgD in the wild-type control while also amplifying the cat gene in the mutants so that the sizes of the products could be compared. The second primer set amplified half of ipgD in the wild-type control and resulted in no product in the mutants because ipgD was not present. Next, this mutant was used as the donor strain for transduction of 2457T and BS543 using P1L4, and positive transductants were selected via chloramphenicol resistance. After PCR confirmation of the deletion in the wild-type and BS543 backgrounds, the cat cassette was removed by transforming pCP20 (Table 1) and incubating restreaked colonies at 42°C to generate BS826 and BS828. Control experiments were performed to ensure that BS826 and BS828 were invasive in HeLa cells.
TABLE 2.
Primers used in this study
| Purpose | Forward primer
|
Reverse primer
|
||
|---|---|---|---|---|
| Designation | Sequence | Designation | Sequence | |
| Amplify cat cassette for ipgD deletion | ipgDcatF | ACTAATTTGGGATTGCATCAGGTTTCATTTCAAAGCGGAGATTCCTATAAAGTGTAGGCTGGAGCTGCTTC | ipgDcatR | GACGAATACCCTTTCACCATATTCCATATTTTTGGGTCCCCTATTCTTTCCATATGAATACCTCCTTAGT |
| Confirm ipgD deletion | ipgDF | AGAGAACCCTGTTGAATAAG | ipgDR | ATTAGCACATCATCATCAAG |
| ipgDcenF | TTATATCAGCCTATGGATTG | ipgDR | ATTAGCACATCATCATCAAG | |
Apoptosis assay.
The HeLa cell invasion assay was performed as previously described (13), but modifications were made to measure the effect of STS on infected cells. Bacteria were subcultured from an overnight culture at 37°C into LB, grown at 37°C with shaking to mid-log phase, and standardized to an optical density at 600 nm of 0.72 in every experiment. The bacteria were then washed with 1× phosphate-buffered saline (PBS) prewarmed to 37°C, resuspended in prewarmed 1× Dulbecco's minimal essential medium (DMEM), and applied to semiconfluent monolayers of HeLa cells in six-well tissue culture plates. Next, the plates were centrifuged at 3,000 rpm for 10 min at 37°C to facilitate the invasion process by allowing the bacteria to make contact with the HeLa cells. The plates were incubated at 37°C with 5% CO2 for 30 min. The cells were then washed with 1× PBS, prewarmed DMEM plus 50 μg/ml gentamicin was added, and the plates were incubated for 3 hours at 37°C with 5% CO2. Cells were then washed again, and DMEM plus 50 μg/ml gentamicin and 4 μM STS (Sigma and Calbiochem) was added for an additional 3 hours. After the 6-hour invasion assay, cells were either fixed for immunofluorescence or scraped for Western blot analysis. For the Western blot assays, the initial DMEM incubation was extended to an hour and the incubation in DMEM plus 50-μg/ml gentamicin was reduced to 2.5 hours to increase the percentage of the monolayer that became infected.
Immunofluorescence.
After infection, cells (or uninfected controls) were fixed with 3% formaldehyde (36% stock; Sigma) and 0.2% glutaraldehyde (25% stock; Sigma) in 1× PBS for 5 min at 4°C. To visualize nuclei, 5 mg/ml of 4,6-diamido-2-phenylindole (DAPI; Molecular Probes) was diluted 1:1,000 in 1× PBS and added to the monolayers for 20 min at room temperature in the dark.
For the activated caspase 3 staining, monolayers were blocked with 10% natural goat serum (NGS) and 0.1% saponin in 1× PBS for 20 min at room temperature. After washing, a rabbit anti-human cleaved caspase 3 antibody (Cell Signaling Technology) was added in a 1:500 dilution in 1× PBS with 10% NGS and 0.1% saponin for 1 h. After another round of washing, cells were incubated with the secondary antibody, a goat anti-rabbit immunoglobulin G antibody conjugated to Alexa 594 (Invitrogen), at a 1:1,000 dilution in 1× PBS with 10% NGS and 0.1% saponin for 45 min (32). DAPI staining was performed following the antibody staining.
For the cytochrome c staining, the STS incubation time in the apoptosis assay was adjusted to 2 hours in order to detect optimal levels of cytochrome c release. All other aspects of the assay remained the same. The staining procedures were followed as described in the protocol provided by Molecular Probes.
For all immunofluorescence experiments, antifade reagent (Molecular Probes) was added before coverslips were applied after the staining procedure. Samples were stored in the dark at 4°C and analyzed with an Olympus BX60 fluorescence microscope with an attached digital camera using ×100 magnification.
Protein sample preparation and Western blot analysis.
Infected monolayers and uninfected controls were washed with 1× PBS after the modified invasion assay, and lysis solution consisting of 1% Triton X-100 and 1% protease inhibitor cocktail (Sigma) was immediately added to the monolayers. Cells were scraped on ice and then sonicated. Proteins in the whole-cell lysates were concentrated by trichloroacetic acid precipitation. Protein pellets were resuspended in 100 μl of sodium dodecyl sulfate (SDS) loading buffer. One microliter of each sample was diluted in 100 μl 1× PBS to measure the final protein concentration at an optical density reading of 280 nm on a Nanodrop ND-1000 spectrophotometer in which samples were blanked against 1 μl of SDS loading buffer diluted in 100 μl 1× PBS.
Each sample was resolved by SDS-polyacrylamide gel electrophoresis (PAGE), and Coomassie blue staining verified equal loading of total protein for all samples. Proteins were transferred to a nitrocellulose membrane and blocked with 5% dry milk in Tris-buffered saline (TBS). Caspases were detected by applying anti-caspase 3 antibody or an anti-caspase 9 antibody (Cell Signaling Technology) at a concentration of 1:1,000 in TBS-Tween (TBST) with 5% dry milk overnight at 4°C. After washing, donkey anti-rabbit immunoglobulin G antibody conjugated to horseradish peroxidase (Amersham Biosciences) was added at a 1:1,000 concentration in TBST with 5% dry milk for 1 h. Prior to use in immunodetection, antibodies were adsorbed separately against wild-type S. flexneri to remove some cross-reactivity of the antibodies to the bacteria. Briefly, an acetone powder was prepared from a fresh culture of S. flexneri 2457T. Each antibody was diluted 1:1,000 in TBST and incubated with the powder for 30 min at 4°C with gentle rotation. Afterwards, each antibody dilution was centrifuged at 10,000 × g for 10 min at 4°C. Each supernatant was removed and added to the 5% dry milk to be applied to the nitrocellulose membrane for the appropriate incubation times.
Western blots were developed using the Visualizer developing system (Upstate Cell Signaling Solutions) according to the protocol provided, and the blots were imaged using a Fuji Intelligent Dark Box with Fujinon lens and Image Reader LAS-3000 software (Fuji) on the chemiluminescence setting in increment mode at 10-second intervals. Densitometry comparisons were made using the Image Gauge V4.22 software provided, which determined the amount of inactivated and activated caspase 3 for each treatment condition.
Statistical analysis.
The data presented in Table 3 were analyzed using the Fisher exact test on the Strata Statistics/Data Analysis program, version 8.2 for Windows. The results for the three separate experiments were analyzed and then combined using the method of Fisher (10). The densitometry results for the Western blots were analyzed using Tukey's analysis of variance post hoc test on the SPSS program, version 12.0.1 for Windows.
TABLE 3.
Cell counts of apoptosis assay experiments
| Expt | No. of cells
|
|||
|---|---|---|---|---|
| Infected
|
Uninfected
|
|||
| Healthyb | Apoptoticc | Healthyb | Apoptoticc | |
| Firsta | 102d | 0 | 0 | 219 |
| Second | 125d | 0 | 0 | 224 |
| Third | 89d | 0 | 0 | 224 |
| Avg | 105d | 0 | 0 | 222 |
At least 300 cells were analyzed for each experiment.
Defined as having a normal appearance of the nuclei (normal shape and visible nucleolus) in addition to having a normal appearance of the cytoplasm compared to control HeLa cells without STS treatment.
Defined as having a characteristic apoptotic nucleus (DNA fragmentation and chromatin condensation) in addition to having cytoplasmic shrinkage compared to control HeLa cells with STS treatment.
P value of <0.001 using Fisher's exact test. Results for the three separate experiments were combined using the method of Fisher (10).
RESULTS
S. flexneri inhibits STS-induced apoptosis.
The apoptosis assay was used to determine if S. flexneri has the ability to inhibit STS-induced apoptosis. STS inhibits protein kinases such as protein kinase C. Inhibition of protein kinase C allows the proapoptotic protein Bad to permeabilize the mitochondrial membrane, which leads to cytochrome c release and activation of the caspase cascade. A 3-hour incubation of 4 μM STS was optimal for inducing apoptosis in uninfected HeLa cells (data not shown). Therefore, the gentamicin assay was modified as described in Materials and Methods. In addition, an ΔicsA mutant of S. flexneri (BS543), which invades and replicates intracellularly but is unable to spread from cell to cell, was used to keep the bacteria in the same HeLa cell throughout the course of the modified assay. MIC experiments confirmed that 2457T and BS543 were able to replicate in the presence of up to 8 μM STS in LB (data not shown).
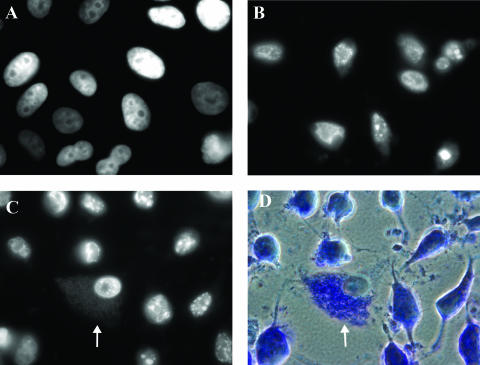
Upon DAPI staining of the infected monolayer, the nuclei of infected cells appeared healthy with a normal shape and a visible nucleolus (Fig. 1C) similar to what is seen upon DAPI staining of healthy, normal HeLa cells (Fig. 1A). However, the nuclei of uninfected cells in the infected monolayer had the characteristic appearance of apoptotic nuclei, namely, DNA fragmentation and chromatin condensation as seen in the control experiments of an uninfected monolayer induced to undergo apoptosis using 4 μM STS for 3 h (Fig. 1B). In addition, the cytoplasm of uninfected cells in the infected monolayer appeared to be shrinking while the cytoplasm of infected cells was able to maintain its shape (Fig. 1D). These results were reproducible in three separate experiments, and cell counts were performed as a method of quantitation. Out of a total of 983 cells, all uninfected cells (667) appeared apoptotic while all infected cells (316) appeared healthy (Table 3). In addition, cell counts were performed in experiments in which the STS incubation times were changed to 2 h or 4 h in the apoptosis assay. In these experiments, S. flexneri was still able to consistently protect HeLa cells from STS-induced apoptosis (data not shown).
FIG. 1.
S. flexneri protects HeLa cells from STS-induced apoptosis. (A) DAPI-stained image of a monolayer of normal, healthy HeLa cells. (B) DAPI-stained monolayer of HeLa cells induced to undergo apoptosis using 4 μM STS for 3 h. (C) DAPI-stained monolayer infected with S. flexneri strain BS543 and treated with 4 μM STS. The arrow points to an infected cell in which the bacteria are also visible in the cytoplasm upon DAPI staining. This image is representative of cells observed in three independent experiments. (D) Phase-contrast view of panel C with Giemsa staining. The arrow points to the infected cell.
S. flexneri prevents the activation of caspase 3 in the presence of STS.
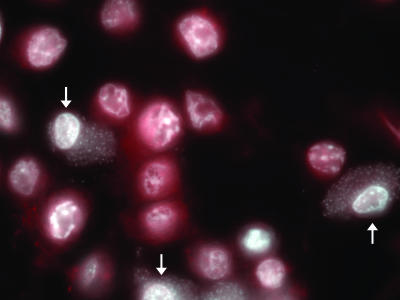
As a method to confirm the above observations, infected monolayers were stained for activated caspase 3. STS-treated uninfected cells stained positive for activated caspase 3 (red) in addition to possessing apoptotic nuclei (Fig. 2). However, STS-treated infected cells did not stain for activated caspase 3. This observation was consistent in three repeated experiments.
FIG. 2.
Activated caspase 3 is not present in Shigella-infected cells after STS treatment. Immunofluorescence analysis of an infected monolayer stained for activated caspase 3 (red) in addition to DAPI staining (white) after STS treatment in the modified assay. The arrows point to infected cells. This image is representative of cells observed in three independent experiments.
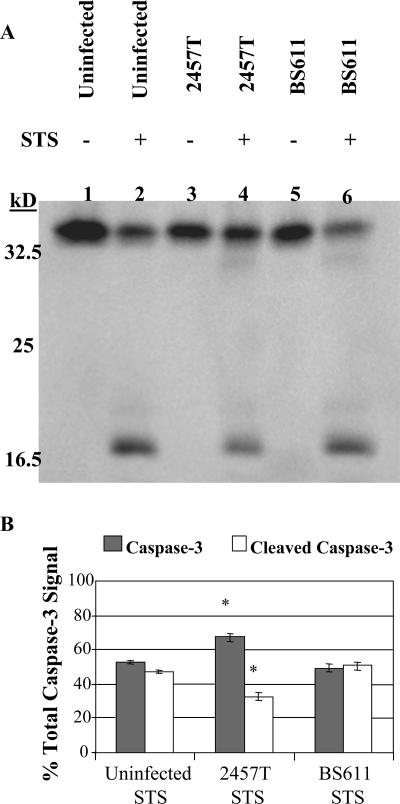
In order to confirm the immunofluorescence results, Western blot experiments were performed to measure the effect of Shigella infection on STS-induced activation of caspase 3. The apoptosis assay was adjusted to increase the percentage of the monolayer that became infected upon invasion with S. flexneri. Therefore, wild-type S. flexneri 2a (2457T) was used because the ability to spread from cell to cell was required to achieve optimal infection of the monolayer. In addition, the DMEM incubation was slightly increased as described in Materials and Methods. An anti-caspase 3 antibody, which recognizes the full-length (35-kDa), inactive form of caspase 3 in addition to the activated (17-kDa), cleaved form of the protein, was used. As seen in Fig. 3A, there was more inactive caspase 3 and there was less activated caspase 3 in the sample treated with STS and infected with 2457T (lane 4) than in the uninfected cells receiving STS treatment (lane 2). Densitometry analysis of the Western blot showed a significant induction in activated caspase 3 in uninfected cells treated with STS compared to infected cells treated with STS (Fig. 3B). These results verified that S. flexneri was able to inhibit STS-induced apoptosis in infected HeLa cells by affecting the activation of caspase 3.
FIG. 3.
Western blot analysis for the activation of caspase 3. (A) Whole-cell lysates were separated by SDS-PAGE and analyzed by immunoblotting with an anti-caspase 3 antibody, which recognizes the full-length, inactive form of caspase 3 (35 kDa) and the large fragment resulting from cleavage during activation (17 kDa). Lane 1, uninfected HeLa cells; lane 2, uninfected HeLa cells with STS treatment; lane 3, 2457T-infected cells; lane 4, 2457T-infected cells with STS treatment; lane 5, BS611-infected cells; lane 6, BS611-infected cells with STS treatment. (B) Results of densitometry analysis of the percentage of the total caspase 3 detected in the Western blot are divided into the inactive form (caspase 3) and the activated form (cleaved caspase 3). The average percentage of each form is shown, with error bars representing the standard deviations from three independent experiments. *, P < 0.001 using Tukey's analysis of variance post hoc test, showing a significant difference between the 2457T STS treatment group and the other two treatment groups.
S. flexneri does not prevent cytochrome c release or caspase 9 activation in the presence of STS.
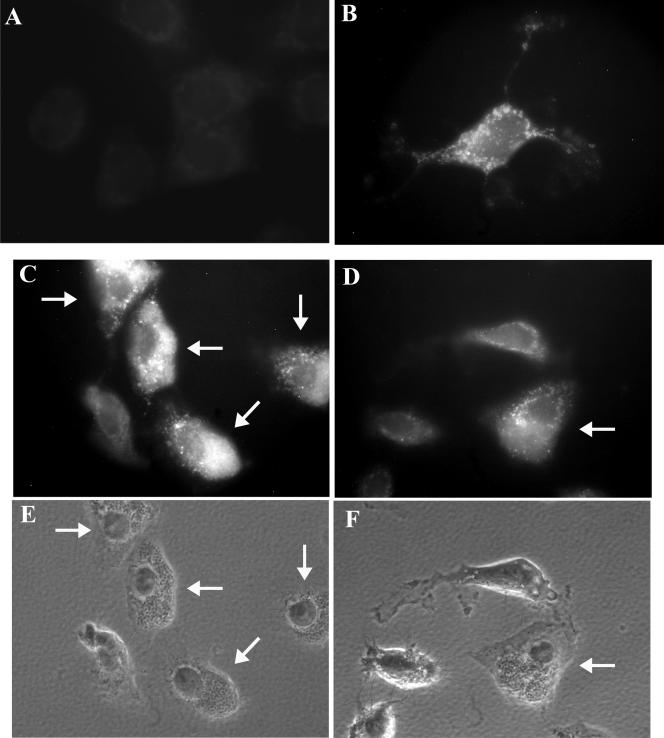
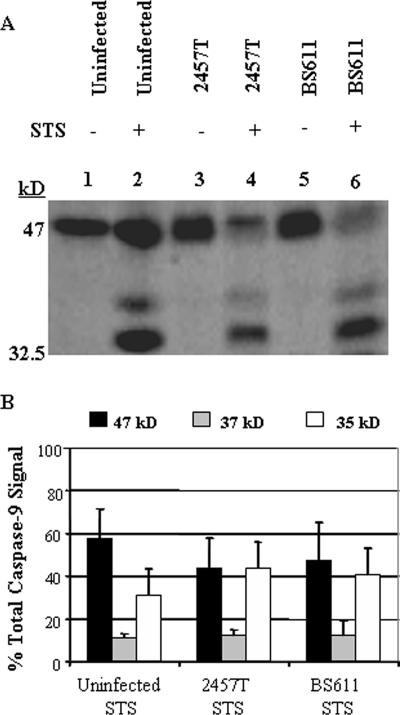
In order to determine at which point in the apoptotic pathway S. flexneri inhibits apoptosis, we next determined if cytochrome c release occurred in infected cells. When stained for cytochrome c release, uninfected, healthy cells without STS treatment displayed weak, background staining since cytochrome c remains inside the mitochondria under normal conditions (Fig. 4A). However, STS treatment resulted in a very bright, positive signal for cytochrome c release in uninfected cells (Fig. 4B). When the staining procedure was applied to a Shigella-infected monolayer after STS treatment, infected cells also had a very bright signal for cytochrome c release (Fig. 4C and D). This observation was consistent in three repeated experiments. Western blot analysis for caspase 9 activation was next performed using an anti-caspase 9 antibody, which recognizes the inactive form of the protein (47 kDa) in addition to the two cleaved products (37 and 35 kDa). As seen in Fig. 5, there is the same level of caspase 9 activation in all monolayers treated with STS. Densitometry analysis revealed that there was no significant difference in caspase 9 activation between Shigella-infected and uninfected monolayers treated with STS. Taken together, the results demonstrate that S. flexneri has the ability to protect HeLa cells from STS-induced apoptosis by inhibiting the activation of caspase 3 while not affecting any other aspect of the apoptosis pathway.
FIG. 4.
Cytochrome c release occurs in infected cells after STS treatment. (A to D) Staining for cytochrome c release. (A) Healthy HeLa cells (no infection or STS treatment). (B) An uninfected HeLa cell after 2 h of STS treatment. (C and D) Infected monolayer after 2 h of STS treatment. Arrows point to infected cells. Images are representative fields from three experiments. Control experiments demonstrated that the cytochrome c antibody did not cross-react with the S. flexneri surface or secreted proteins and that cytochrome c release does not occur in infected cells without STS treatment (data not shown). (E and F) Phase-contrast images of panels C and D, respectively, showing bacteria inside infected cells.
FIG. 5.
Western blot analysis of caspase 9 activation. (A) Whole-cell lysates were separated by SDS-PAGE and analyzed by immunoblotting with an anti-caspase 9 antibody, which recognizes the full-length, inactive form of caspase 9 (47 kDa) and the two fragments resulting from cleavage during activation (37 and 35 kDa). Lane 1, uninfected HeLa cells; lane 2, uninfected HeLa cells with STS treatment; lane 3, 2457T-infected cells; lane 4, 2457T-infected cells with STS treatment; lane 5, BS611-infected cells; lane 6, BS611-infected cells with STS treatment. (B) Results of densitometry analysis of the percentage of the total caspase 9 detected in the Western blot are divided into the inactive form (47 kDa) and the two activated forms (37 and 35 kDa). The average percentage of each form is shown, with error bars representing the standard deviations from three independent experiments.
HeLa cell protection from STS-induced apoptosis requires S. flexneri invasion, secretion, and MxiE-regulated gene products.
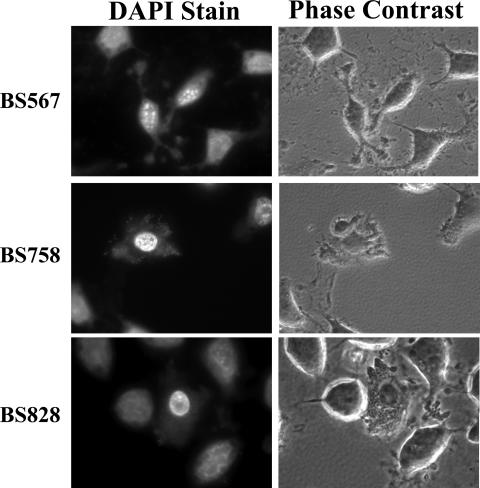
We first determined if invasion and a functional T3SS were important for protection by analyzing a ΔmxiM mutant (BS547), which has a dysfunctional T3SS, is unable to translocate the Ipa invasins, and is therefore noninvasive. Since this mutant is noninvasive, gentamicin was removed from the medium. The ΔmxiM mutant was unable to protect HeLa cells from apoptosis (data not shown). Additional experiments were performed with a ΔipaB mutant (BS567) since this mutant is noninvasive but still has a functional T3SS. As seen in Fig. 6, although the bacteria were present in the extracellular environment, the HeLa cells still became apoptotic after the STS treatment. As a result of the mutant analysis, we concluded that S. flexneri needed to express a functional T3SS inside of the host cytosol in order to protect HeLa cells from STS-induced apoptosis.
FIG. 6.
S. flexneri mutants analyzed in the apoptosis assay with DAPI-stained images on the left and the corresponding phase-contrast views on the right. All images are representative of three independent experiments.
Based on the above observations, analysis was performed on a ΔmxiE mutant because MxiE regulates the expression of genes induced intracellularly. Some of the protein products of these genes are known to be secreted via the T3SS into the cytosol of the host cell. As seen in Fig. 6, BS758 (ΔmxiE in an invasive but nonspreading ΔicsA background) was unable to protect HeLa cells from STS-induced apoptosis. Upon DAPI staining, distorted nuclei of the infected cells were evident and the nucleoli were no longer visible despite the presence of intracellular bacteria. In addition, the cytoplasm of the cells appeared deformed. The effect of the ΔmxiE mutation in the icsA+ background (BS611) on infected HeLa cells receiving STS treatment was analyzed in the Western blot assay for caspase 3 levels. The same level of activation of caspase 3 was seen in STS-treated cells infected with this mutant as was seen in uninfected STS-treated HeLa cells (Fig. 3, lanes 2 and 6). Therefore, one or more of the genes regulated by mxiE are required to protect HeLa cells from STS-induced apoptosis.
Finally, BS828, a ΔipgD mutant, was analyzed, given the fact that IpgD has been shown to lead to the activation of Akt, which is a prosurvival gene (26). As shown in Fig. 6, there was no difference between HeLa cells infected with BS828 and HeLa cells infected with BS543 (ipgD+) in the apoptosis assay (Fig. 1). BS828-infected cells exposed to STS retained normal nuclei with visible nucleoli upon DAPI staining. Analysis for caspase 3 activation was performed via Western blot analysis using the ΔipgD mutation in the icsA+ background (BS826). Again, there was no difference between HeLa cells infected with a ΔipgD mutant and HeLa cells infected with wild-type bacteria (data not shown). These results indicate that IpgD is not essential for S. flexneri to protect epithelial cells from STS-induced apoptosis.
DISCUSSION
Our goal in this study was to determine if S. flexneri infection has the ability to protect epithelial cells from apoptosis given the fact that previous work noted that HeLa cells infected with S. flexneri undergo a stress response yet do not die as a result of infection (21). We found that S. flexneri infection of HeLa cells resulted in inhibition of STS-induced apoptosis. Upon immunofluorescence analysis of 2457T-infected monolayers treated with STS, there was no detectable signal for activated caspase 3. This phenotype correlated with the consistent observations seen upon DAPI staining in which the nuclei of infected cells that were treated with STS had a healthy appearance. The Western blot results support the immunofluorescence images since 2457T-infected monolayers had significantly reduced levels of caspase 3 activation in the presence of STS compared to those for uninfected cells (Fig. 3, lane 4). Since 100% of the monolayer cannot become infected by 2457T, the uninfected HeLa cells in the monolayer became apoptotic upon STS treatment and contributed to the level of activated caspase 3 observed. A similar observation was made for monolayers of Salmonella enterica-infected epithelial cells (17). Therefore, these results verify that S. flexneri has the ability to inhibit the activation of caspase-3 in the presence of STS in order to protect HeLa cells from apoptosis. It should be noted that there is no caspase 3 activation in 2457T-infected monolayers without STS treatment (lane 3), which confirms that Shigella does not induce apoptosis upon normal infection of epithelial cells.
In order to identify the point of interaction that the bacterium has with the apoptosis pathway, we tested for the presence or absence of cytochrome c release. By determining if cytochrome c release occurred, we would be able to focus upstream or downstream of this point in the apoptotic pathway, since proapoptotic proteins must be activated in order for cytochrome c release to occur. When we examined cytochrome c staining in infected monolayers treated with STS, we found that cytochrome c release does occur. The healthy, uninfected cells that did not receive STS treatment in control experiments displayed only weak staining, due to the fact that cytochrome c remains inside the mitochondria. The uninfected and infected cells treated with STS had strong signals, which remained in the cytoplasm of the cells, indicating that cytochrome c was released from the mitochondria. The staining patterns were similar to what was seen by Willhite et al. when they performed similar staining for their studies with Helicobacter pylori (35).
The next step was to determine if caspase 9 activation occurred, which would allow us to identify the point in the apoptosis pathway that is affected by S. flexneri infection. Western blot analysis revealed that monolayers infected with 2457T and treated with STS had the same levels of caspase 9 activation as did uninfected monolayers treated with STS. Densitometry analysis revealed that there was no significant difference in the activation of caspase 9 between the two treatments. Therefore, S. flexneri is protecting HeLa cells from STS-induced apoptosis by interfering with caspase 3 activation. Interestingly, microarray analysis by Pedron et al. found significant up-regulation of the inhibitor of apoptosis protein 1 (IAP-1) upon Shigella infection of epithelial cells (26). This up-regulation of IAP-1 correlates with the observations reported here, since IAP-1 inhibits caspase 3 activation directly. A similar observation was made in Neisseria gonorrhoeae infection of human urethral epithelium. Up-regulation of the antiapoptotic genes bfl-1 and cox-2 and the c-IAP-2 gene was found to be dependent on the Neisseria protein porin IB (2, 3). Therefore, up-regulation of IAP-1 by S. flexneri is a reasonable mechanism for the inhibition of apoptosis by the bacteria in epithelial cells.
Our observations suggest that S. flexneri could also inhibit the extrinsic pathway of apoptosis. If S. flexneri inhibits just the activation of caspase 3, it is possible that the bacteria can inhibit apoptosis that is induced via a pathway that is independent of cytochrome c release from the mitochondria. For example, the tumor necrosis factor family causes apoptosis through use of death receptors and the activation of caspase 8. This is typically seen when activated T cells use their Fas ligand to bind to the Fas receptor on infected target cells in order to induce apoptosis. Caspase 8 can activate caspase 3 either directly or indirectly through the activation of the proapoptotic protein Bid (28, 34). Thus, by inhibiting the activation of caspase 3, S. flexneri can protect the epithelial cells from several different apoptotic pathways to increase its survival inside the host.
To help determine the requirements for S. flexneri to protect HeLa cells from STS-induced apoptosis, the ΔipaB mutant and the ΔmxiM mutant were analyzed. The ΔipaB mutant is noninvasive, while the ΔmxiM mutant has a dysfunctional T3SS in addition to the noninvasive phenotype. Both mutants were unable to protect HeLa cells from STS-induced apoptosis, and therefore, it is shown that invasion is required for protection. The ΔipaB mutant has a functional T3SS, and some effectors are still secreted by the bacteria upon contact with host cells (23). The data suggested that certain effectors of the T3SS needed to be inside the cytoplasm of the HeLa cells in order to protect these cells from STS-induced apoptosis.
Based on the results of the mutant analysis, we tested a ΔmxiE mutant since MxiE-regulated genes are expressed when the bacteria are present in the intracellular environment of the host cell. Some of these gene products are also secreted via the T3SS into the cytoplasm of the host cell (15, 20). DAPI staining of ΔmxiE-infected cells treated with STS showed an intermediate level of protection in that the nuclei were not healthy but also were not completely apoptotic. The shape of the nuclei and the cytoplasm appeared distorted. Western blot analysis confirmed the DAPI staining and showed that caspase 3 is activated in HeLa cells infected with the ΔmxiE mutant to the same extent as seen in the uninfected controls. These observations suggest that one or several of the MxiE-regulated genes are important for protection from apoptosis. Experiments are currently in progress to determine which MxiE-regulated genes are required for protection from STS-induced apoptosis. In addition, we cannot yet exclude the possibility that a gene not regulated by MxiE has a role in protection from apoptosis.
It was recently reported by Pendaries et al. (27) that the IpgD protein from S. flexneri increases phosphatidylinositol 5-monophosphate production during infection in epithelial cells. The phosphatidylinositol 5-monophosphate in turn leads to the eventual activation of the prosurvival protein Akt (27). The authors stated that IpgD was responsible for the inhibition of STS-induced apoptosis. We constructed a ΔipgD mutant and found that it was still able to protect HeLa cells from STS-induced apoptosis. Supporting this observation is the analysis of cytochrome c release in infected cells. Akt is responsible for preventing the proapoptotic protein Bad from permeabilizing the mitochondrial membrane, which prevents cytochrome c release and activation of the caspase cascade (16). The presence of cytochrome c release and caspase 9 activation in 2457T-infected cells treated with STS in our assays indicates that any Akt activation by IpgD is not sufficient to inhibit STS-induced apoptosis. Salmonella enterica has also been observed to inhibit apoptosis by utilizing SopB to activate Akt (17). IpgD is the Shigella homolog to SopB, but there are important differences between the influences of these effectors on Akt. First, SopB sustains Akt activation for 4 hours postinfection. IpgD on the other hand activates Akt for 1 hour postinfection. The prolonged activation of Akt by Salmonella would result in a longer prosurvival effect on infected epithelial cells than would activation of Akt by Shigella. Second, a Salmonella ΔsopB mutant is unable to protect epithelial cells from the intrinsic pathway of apoptosis when camptothecin is used to induce apoptosis during infection. Our Shigella ΔipgD mutant was able to protect epithelial cells from the intrinsic pathway when STS was used during infection. Therefore, we believe that, although Akt may have some prosurvival effects on S. flexneri-infected epithelial cells, this is not the main mechanism of inhibiting STS-induced apoptosis. Based on our observations, we believe that the mechanism of apoptosis inhibition by S. flexneri works by blocking caspase 3 activation.
In conclusion, we have found that S. flexneri is able to protect epithelial cells from STS-induced apoptosis by inhibiting the activation of caspase 3 despite the release of cytochrome c and the activation of caspase 9. Both bacterial invasion and a functional T3SS are essential for protection, and we believe that the presence of certain T3SS effectors inside the cytoplasm of the host cell is required for protection. One or several of these effectors are the proteins whose expression is regulated by MxiE, given that the ΔmxiE mutant was unable to protect HeLa cells from STS-induced apoptosis. Since S. flexneri is a pathogen that must find an intracellular niche inside the host to ensure survival, one mechanism to ensure survival is the induction of apoptosis in macrophages that would otherwise kill the bacteria. More importantly, the ability of S. flexneri to inhibit apoptosis in epithelial cells ensures bacterial replication and survival in an intracellular niche within the host. Other pathogens such as Salmonella, Chlamydia trachomatis, and Neisseria also inhibit apoptosis in order to ensure survival in vivo (2, 3, 9, 17, 22). These organisms affect the ability of proapoptotic proteins to permeabilize the mitochondrial membrane and therefore prevent cytochrome c release. This outcome is distinct from what we report for Shigella-infected cells, since cytochrome c release and subsequent caspase 9 activation are detected with STS treatment and yet caspase 3 activation is inhibited. Therefore, S. flexneri is unique in that it protects epithelial cells from late-stage apoptosis by interfering with caspase 3 activation.
Acknowledgments
This work was supported by grant AI24656 from the National Institute of Allergy and Infectious Diseases.
We also thank Rachel Binet, Reinaldo Fernandez, and Daniel Zurawski for technical assistance and Cara Olsen for her help with the statistical analysis.
The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the Uniformed Services University of the Health Sciences.
Editor: D. L. Burns
Footnotes
Published ahead of print on 5 March 2007.
REFERENCES
- 1.Ashe, P. C., and M. D. Berry. 2003. Apoptotic signaling cascades. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 27:199-214. [DOI] [PubMed] [Google Scholar]
- 2.Binnicker, M. J., R. D. Williams, and M. A. Apicella. 2003. Infection of human urethral epithelium with Neisseria gonorrhoeae elicits an upregulation of host anti-apoptotic factors and protects cells from staurosporine-induced apoptosis. Cell. Microbiol. 5:549-560. [DOI] [PubMed] [Google Scholar]
- 3.Binnicker, M. J., R. D. Williams, and M. A. Apicella. 2004. Gonococcal porin IB activates NF-κB in human urethral epithelium and increases the expression of host antiapoptotic factors. Infect. Immun. 72:6408-6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne, G. I., and D. M. Ojcius. 2004. Chlamydia and apoptosis: life and death decisions of an intracellular pathogen. Nat. Rev. Microbiol. 2:802-808. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Y., M. R. Smith, K. Thirumalai, and A. Zychlinsky. 1996. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 15:3853-3860. [PMC free article] [PubMed] [Google Scholar]
- 6.Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9-14. [DOI] [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFilippis, R. A., E. C. Goodwin, W. Lingling, and D. MiMaio. 2003. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J. Virol. 77:1551-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong, F., M. Pirbhai, Y. Xiao, Y. Zhong, Y. Wu, and G. Zhong. 2005. Degradation of the proapoptotic proteins Bik, Puma, and Bim with Bcl-2 domain 3 homology in Chlamydia trachomatis-infected cells. Infect. Immun. 73:1861-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher, R. A. 1925. Statistical methods for research workers, 13th ed. Oliver & Loyd, London, England.
- 11.Formal, S. B., G. J. Dammin, E. H. LeBrec, and H. Schneider. 1958. Experimental Shigella infections: characteristics of a fatal infection produced in guinea pigs. J. Bacteriol. 75:604-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilbi, H., J. E. Moss, D. Hersh, Y. Chen, J. Arondel, S. Banerjee, R. A. Flavell, J. Yuan, P. J. Sansonetti, and A. Zychlinsky. 1998. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 273:32895-32900. [DOI] [PubMed] [Google Scholar]
- 13.Hromockyj, A. E., and A. T. Maurelli. 1989. Identification of Shigella invasion genes by isolation of temperature-regulated inv::lacZ operon fusions. Infect. Immun. 57:2963-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennison, A. V., and N. K. Verma. 2004. Shigella flexneri infection: pathogenesis and vaccine development. FEMS Microbiol. Rev. 28:43-58. [DOI] [PubMed] [Google Scholar]
- 15.Kane, C. D., R. Schuch, W. A. Day, Jr., and A. T. Maurelli. 2002. MxiE regulates intracellular expression of factors secreted by the Shigella flexneri 2a type III secretion system. J. Bacteriol. 184:4409-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy, S. G., E. S. Kandel, T. K. Cross, and N. Hay. 1999. Akt/protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol. Cell. Biol. 19:5800-5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knodler, L. A., B. B. Finlay, and O. Steele-Mortimer. 2005. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J. Biol. Chem. 280:9058-9064. [DOI] [PubMed] [Google Scholar]
- 18.Koterski, J. F., M. Nahvi, M. M. Venkatesan, and B. Haimovich. 2005. Virulent Shigella flexneri causes damage to mitochondria and triggers necrosis in infected human monocyte-derived macrophages. Infect. Immun. 73:504-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotloff, K. L., J. P. Winickoff, B. Ivanoff, J. D. Clemens, D. L. Swerdlow, P. J. Sansonetti, G. K. Adak, and M. M. Levine. 1999. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull. W. H. O. 77:651-666. [PMC free article] [PubMed] [Google Scholar]
- 20.Le Gall, T., M. Mavris, M. C. Martino, M. L. Bernardini, E. Denamur, and C. Parsot. 2005. Analysis of virulence plasmid gene expression defines three classes of effectors in the type III secretion system of Shigella flexneri. Microbiology 151:951-962. [DOI] [PubMed] [Google Scholar]
- 21.Mantis, N., M. C. Prevost, and P. J. Sansonetti. 1996. Analysis of epithelial cell stress response during infection by Shigella flexneri. Infect. Immun. 64:2474-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massari, P., C. A. King, A. Y. Ho, and L. M. Wetzler. 2003. Neisserial PorB is translocated to the mitochondria of HeLa cells infected with Neisseria meningitidis and protects cells from apoptosis. Cell. Microbiol. 5:99-109. [DOI] [PubMed] [Google Scholar]
- 23.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarre, W. W., and A. Zychlinsky. 2000. Pathogen-induced apoptosis of macrophages: a common end for different pathogenic strategies. Cell. Microbiol. 2:265-273. [DOI] [PubMed] [Google Scholar]
- 25.Nonaka, T., T. Kuwabara, H. Mimuro, A. Kuwae, and S. Imajoh-Ohmi. 2003. Shigella-induced necrosis and apoptosis of U937 cells and J774 macrophages. Microbiology 149:2513-2527. [DOI] [PubMed] [Google Scholar]
- 26.Pedron, T., C. Thibault, and P. J. Sansonetti. 2003. The invasive phenotype of Shigella flexneri directs a distinct gene expression pattern in the human intestinal epithelial cell line Caco-2. J. Biol. Chem. 278:33878-33886. [DOI] [PubMed] [Google Scholar]
- 27.Pendaries, C., H. Tronchere, L. Arbibe, J. Mounier, O. Gozani, L. Cantley, M. J. Fry, F. Gaits-Iacovoni, P. J. Sansonetti, and B. Payrastre. 2006. PtdIns(5)P activates the host cell PI3-kinase/Akt pathway during Shigella flexneri infection. EMBO J. 25:1024-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed, J. C. 2000. Warner-Lambert/Parke-Davis Award lecture: mechanisms of apoptosis. Am. J. Pathol. 157:1415-1429.11073801 [Google Scholar]
- 29.Sandlin, R. C., and A. T. Maurelli. 1999. Establishment of unipolar localization of IcsA in Shigella flexneri 2a is not dependent on virulence plasmid determinants. Infect. Immun. 67:350-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sansonetti, P. J., G. Tran Van Nhieu, and C. Egile. 1999. Rupture of the intestinal epithelial barrier and mucosal invasion by Shigella flexneri. Clin. Infect. Dis. 28:466-475. [DOI] [PubMed] [Google Scholar]
- 31.Schuch, R., and A. T. Maurelli. 1999. The Mxi-Spa type III secretory pathway of Shigella flexneri requires an outer membrane lipoprotein, MxiM, for invasin translocation. Infect. Immun. 67:1982-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steele-Mortimer, O., J. H. Brumell, L. A. Knodler, S. Meresse, A. Lopez, and B. B. Finlay. 2002. The invasion-associated type III secretion system of Salmonella enterica serovar Typhimurium is necessary for intracellular proliferation and vacuole biogenesis in epithelial cells. Cell. Microbiol. 4:43-54. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki, T., K. Nakanishi, H. Tsutsui, H. Iwai, S. Akira, N. Inohara, M. Chamaillard, G. Nuñez, and C. Sasakawa. 2005. A novel caspase-1/toll-like receptor 4-independent pathway of cell death induced by cytosolic Shigella in infected macrophages. J. Biol. Chem. 280:14042-104050. [DOI] [PubMed] [Google Scholar]
- 34.Thorburn, A. 2004. Death receptor-induced cell killing. Cell. Signal 16:139-144. [DOI] [PubMed] [Google Scholar]
- 35.Willhite, D. C., T. L. Covers, and S. R. Blanke. 2003. Cellular vacuolation and mitochondrial cytochrome c release are independent outcomes of Helicobacter pylori vacuolating cytotoxin activity that are each dependent on membrane channel formation. J. Biol. Chem. 278:48204-48209. [DOI] [PubMed] [Google Scholar]
- 36.Zychlinsky, A., M. C. Prevost, and P. J. Sansonetti. 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358:167-169. [DOI] [PubMed] [Google Scholar]