Abstract
Impaired erythropoiesis causes anemia during genetic disorders, chronic disease, and infection. In studies of the underlying mechanisms researchers have increasingly focused on gamma interferon (IFN-γ). Here, we identified a previously unrecognized role for interleukin-15 (IL-15) in red blood cell homeostasis and demonstrated that IFN-γ and signal transducer and activator of transcription protein 1-dependent pathways up-regulate expression of IL-15 in vivo. These findings identified new therapeutic targets for anemia.
Anemia is a condition in which the blood becomes deficient in red blood cells (RBC) or hemoglobin, which reduces the supply of oxygen to tissues and organs. Clinically significant anemia accompanies a variety of illnesses characterized by acute or chronic immune activation, including cancer, cardiac disease, autoimmunity, and infection (14, 22, 25). During life-threatening illnesses, anemia often correlates with an increased risk of early death (2, 9, 16, 23).
Given these important clinical implications, the causes of anemia have been studied extensively. To maintain RBC homeostasis, the bone marrow continually produces new RBC through a process called erythropoiesis, while cells of the reticuloendothelial system phagocytose senescent RBC and recycle iron, an essential component of oxygen-carrying hemoglobin. Anemia often results from both a decrease in erythropoiesis and an increase in the rate at which RBC are lost from the circulation (14, 22, 25).
In studies of the mechanisms promoting anemia during genetic disorders, chronic disease, and infection researchers have increasingly focused on roles for gamma interferon (IFN-γ) (14, 25). This pleiotropic cytokine performs myriad host-protective functions during infection and immunity (21), but it also suppresses the growth of erythroid CFU (CFU-E) in vitro (15, 27). The specific pathways linking IFN-γ to suppressed erythropoiesis in vivo are poorly defined, thereby hampering the development of palliative therapies.
The binding of IFN-γ to its receptor activates signal transducer and activator of transcription protein 1 (STAT1). STAT1 participates in the suppression of erythropoiesis by IFN-γ (7, 18). However, STAT1 activates a large number of IFN-γ-regulated genes (20), so the development of therapeutic strategies that target the erythropoiesis-suppressing activities of IFN-γ without compromising its critical host-protective functions requires further delineation of the relevant intermediate pathways.
Toxoplasma gondii is an obligate intracellular protozoan parasite (13). This common pathogen causes significant disease upon transplacental transmission to a developing fetus and in immunocompromised adults. Following ingestion of encysted organisms, T. gondii parasites invade the intestinal wall, disseminate, and elicit a robust immune response. IFN-γ plays a critical role in restraining parasite replication but also causes significant collateral pathology (4, 8, 11).
We previously demonstrated that anemia accompanies the acute phase of T. gondii infection and that depletion of IFN-γ suppresses this anemia (8). We also demonstrated that mice lacking the capacity to produce fibrin, a product of the coagulation pathway, display exacerbated anemia and profound hepatic hemorrhage during acute toxoplasmosis (8). Together, these findings suggested that hemorrhagic blood loss caused by IFN-γ accounts for the exacerbated anemia in fibrin-deficient mice. However, a contemporaneous report suggested that decreased erythropoiesis contributes to anemia during acute toxoplasmosis (19).
Reticulocytopenia during acute toxoplasmosis.
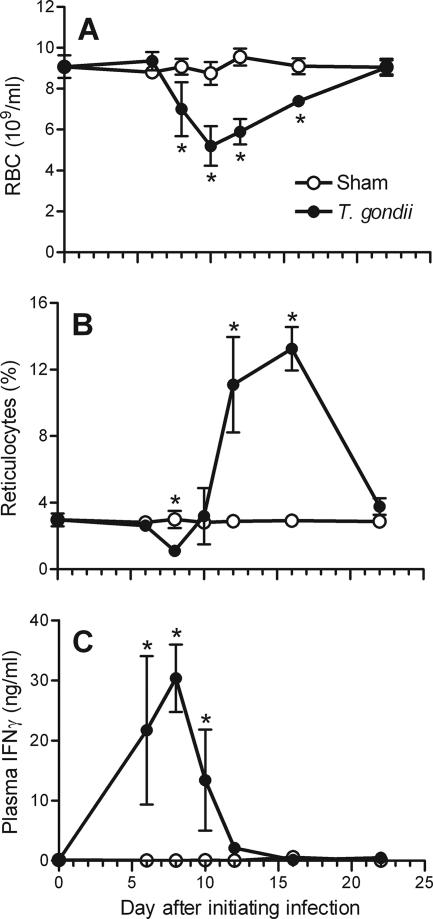
Peroral infection of wild-type C57BL/6 mice with 10 cysts of T. gondii strain ME49 (8) transiently reduced the number of circulating RBC (Fig. 1A), as determined with a Coulter Counter (Beckman Coulter). Anemia became evident by day 8 after the initiation of T. gondii infection, peaked at day 10, and resolved by day 22. Consistent with anemias commonly associated with inflammation and chronic disease, the toxoplasmosis-associated anemia was normochromic and normocytic, as determined by the ADVIA 120 hematology system (Bayer Diagnostics, Tarrytown, NY) (data not shown).
FIG. 1.
Kinetic analysis of anemia, reticulocytes, and IFN-γ levels during acute T. gondii infection. Wild-type C57BL/6 mice were infected with 10 ME49 cysts. The numbers of RBC (A), the percentages of circulating reticulocytes (B), and the plasma IFN-γ levels (C) were evaluated on the days indicated. Asterisks depict significant differences between sham-infected control mice and T. gondii-infected mice (P < 0.02, as determined by Student's t test). The data are means and standard deviations of four mice per group.
We next assessed whether suppressed erythropoiesis and/or impaired release of newly formed RBC (reticulocytes) from bone marrow contributes to anemia during T. gondii infection. Reticulocytes retain significant levels of RNA for 24 to 48 h after they are released from the bone marrow. To quantify reticulocytes, blood was stained with the RNA-binding dye thiazole orange (Retic-Count; BD Biosciences) and analyzed by flow cytometry, using forward and side scatter to gate on RBC (24). Consistent with previous reports (12), approximately 3% of the circulating RBC in naïve mice stained positive with thiazole orange (Fig. 1B). The levels of reticulocytes remained at this basal level through day 6 after the initiation of T. gondii infection but then abruptly and dramatically declined to 37% of the basal level on day 8 (P < 0.001) (Fig. 1B). The reticulocyte levels rebounded by day 10 and were dramatically higher than the levels observed in sham-infected mice on days 12 and 18, before they returned to baseline level on day 22 (Fig. 1B). The reticulocytosis observed on days 12 and 18 postinfection coincided with the alleviation of anemia and was similar to the reticulocytosis observed during malarial infection in C57BL/6 mice (1). Thus, acute toxoplasmosis causes reticulocytopenia, which presumably results from suppressed erythropoiesis and/or impaired release of reticulocytes from bone marrow.
Suppression of reticulocytopenia by IFN-γ, IFN-γR, and STAT1.
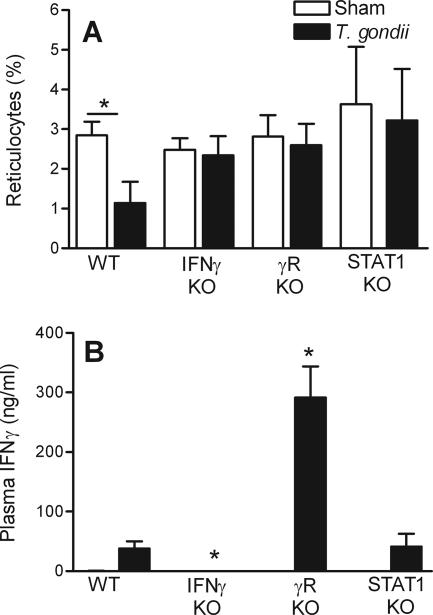
IFN-γ causes anemia during T. gondii infection (8). The plasma levels of IFN-γ, as measured by a sandwich enzyme-linked immunosorbent assay (BD Biosciences), peaked at the time of maximal reticulocytopenia (Fig. 1C). As shown in Fig. 2A, a genetic deficiency in either IFN-γ or the IFN-γ receptor (IFN-γR) prevented the reticulocytopenia at day 8 after the initiation of T. gondii infection, despite a 1,000-fold increase in the parasite burden (8). We concluded that IFN-γ suppresses erythropoiesis and/or impairs release of reticulocytes from bone marrow during acute toxoplasmosis.
FIG. 2.
IFN-γ, IFN-γR, and STAT1 expression are required for infection-stimulated reticulocytopenia. Wild-type (WT) C576BL/6 mice or C576BL/6 mice deficient (KO) in IFN-γ, IFN-γR (γR), or STAT1 were infected with 10 ME49 cysts, and blood samples were collected 8 days later. The percentages of circulating reticulocytes (A) and the plasma IFN-γ levels (B) for sham-infected mice and T. gondii-infected mice were determined. The asterisks in panel A indicate differences between sham-infected mice and T. gondii-infected mice (P < 0.001, as determined by Student's t test). The asterisks in panel B indicate significant reductions when wild-type infected mice and deficient infected mice were compared (P < 0.001, as determined by Student's t test). The data are means and standard deviations of at least five mice per group.
STAT1 deficiency did not significantly affect the levels of IFN-γ during acute toxoplasmosis (Fig. 2B). Nevertheless, STAT1 deficiency prevented the infection-stimulated reticulocytopenia (Fig. 2A). Consistent with previous studies in which distinct models were used (7, 18), we concluded that STAT1 acts downstream of IFN-γ to suppress erythropoiesis and/or impair release of reticulocytes from bone marrow.
IL-15 suppresses erythropoiesis.
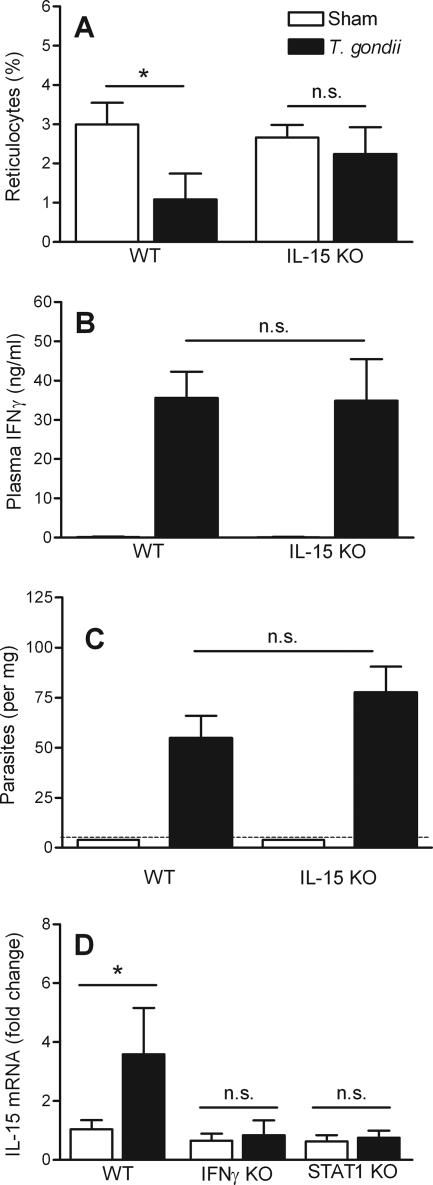
As recently reported (10), we observed similar levels of IFN-γ in wild-type and interleukin-15 (IL-15)-deficient mice on day 8 after the initiation of T. gondii infection (Fig. 3B). The parasite burden also was not significantly affected by IL-15 deficiency (Fig. 3C). Nevertheless, IL-15 deficiency prevented the infection-stimulated reticulocytopenia (Fig. 3A). Thus, IL-15 suppresses erythropoiesis and/or impairs release of reticulocytes from bone marrow during acute toxoplasmosis without significantly affecting IFN-γ levels.
FIG. 3.
Infection-stimulated reticulocytopenia is IL-15 dependent. Wild-type (WT) C576BL/6 mice or C576BL/6 mice deficient (KO) in IL-15, IFN-γ, or STAT1 were infected with 10 ME49 cysts, and samples were collected 8 days later. The percentages of circulating reticulocytes (A), the plasma IFN-γ levels (B), the hepatic parasite burdens (C), and the hepatic IL-15 mRNA levels (D) were determined for sham-infected and T. gondii-infected mice. The dashed line in panel C indicates the limit of detection. The asterisks indicate significant differences for the comparisons indicated, as determined by Student's t test (P < 0.0001; n.s., not significant). The data are means and standard deviations of at least seven mice per group.
In previous studies researchers found that IFN-γ and STAT1 promote IL-15 expression in vitro (6, 17, 26). We determined the levels of hepatic IL-15 mRNA by real-time PCR and normalized these levels to the levels of glyceraldehyde-3-phosphate dehydrogenase (8). Acute T. gondii infection significantly increased the expression of IL-15 in wild-type mice (Fig. 3C) but not in mice lacking the capacity to express IFN-γ, IFN-γR, or STAT1 (Fig. 3D and data not shown). To our knowledge, this is the first demonstration of IFN-γ-dependent up-regulation of IL-15 expression in vivo. These observations suggest that IFN-γ suppresses erythropoiesis by up-regulating STAT1-dependent expression of IL-15.
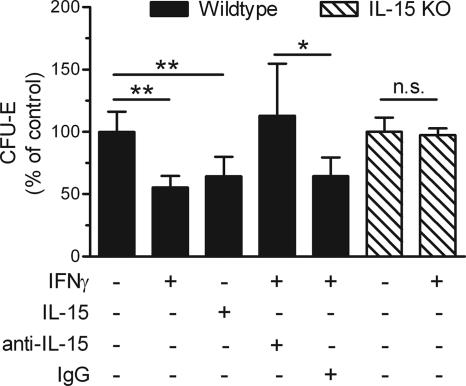
To further decipher the relationships between IFN-γ and IL-15, we investigated their effects on erythropoiesis in vitro. Bone marrow cells from wild-type mice were cultured for 2 to 3 days in methylcellulose medium supplemented with erythropoietin (StemCell Technologies, Vancouver, BC, Canada), which promotes the growth of erythroid progenitor cells. Consistent with previous reports (15, 27), supplementing the medium with IFN-γ (100 ng/ml; Peprotech, Rocky Hill, NJ) suppressed CFU-E growth (Fig. 4). Remarkably, supplementing the medium with IL-15 (50 to 100 ng/ml; Peprotech) likewise suppressed CFU-E growth (Fig. 4). To investigate whether IFN-γ suppressed erythropoiesis via IL-15, we neutralized IL-15 activity in cultures supplemented with IFN-γ. Addition of rabbit anti-murine IL-15 (10 μg/ml; Peprotech), but not addition of control IgG, prevented IFN-γ from suppressing CFU-E growth (Fig. 4). Furthermore, IFN-γ did not suppress CFU-E growth in cell cultures derived from IL-15-deficient mice (Fig. 4). Together, these data establish that IL-15 plays a primary role in the suppression of CFU-E growth by IFN-γ.
FIG. 4.
Suppression of CFU-E growth by IFN-γ is IL-15 dependent. Bone marrow cells from wild-type (WT) or IL-15-deficient (KO) mice were cultured in triplicate. The indicated cytokines and antibodies were added to the medium at the initiation of culture. The asterisks depict significant differences for the comparisons indicated, as determined by Student's t test (two asterisks, P < 0.005; one asterisk, P < 0.03; n.s., not significant). The data are averages of data pooled from two independent experiments. The absolute number of CFU-E in control cultures ranged from 232 to 860. IgG, immunoglobulin G.
Accumulating data suggest that IFN-γ plays important roles in promoting numerous forms of anemia by suppressing erythropoiesis. Here, we advanced this field by demonstrating, for the first time, a critical role for IL-15 in the suppression of erythropoiesis by IFN-γ. Our findings indicate that IFN-γ acts via STAT1 to induce IL-15, which then suppresses erythropoiesis. Notably, membrane-bound IL-15 is constitutively overexpressed by stromal bone marrow cells in patients afflicted with aplastic anemia (26). Future research will need to focus on the functional significance of IL-15 expression in this scenario and during other human anemias. It also will be important to determine precisely how IL-15 suppresses erythropoiesis. Recent studies have demonstrated that IFN-γ suppresses the growth of CFU-E in vitro at least in part by inducing expression of apoptosis-promoting receptors (3, 5). Our preliminary data indicate that antagonism of IL-15 in cultures of IFN-γ-treated bone marrow likewise alters expression of apoptosis-related genes (data not shown). These findings suggest that IL-15 participates in the IFN-γ-mediated apoptosis of erythroid progenitor cells.
Our data show that IL-15 is a key mediator of IFN-γ-dependent suppression of erythropoiesis. Therefore, targeting IL-15 and IL-15-dependent pathways provides a novel therapeutic opportunity for preventing IFN-γ-mediated anemia while maintaining the myriad protective attributes of this pleiotropic cytokine.
Acknowledgments
We are grateful to Andrea Cooper, Shabaana Khader, and Deborah Brown for critical reviews of the manuscript. We are indebted to the employees of the Trudeau Institute Animal Breeding and Maintenance Facilities for dedicated care of the mice used in these studies. Breeding stock of C57BL/6 backcrossed mice deficient in STAT1 were kindly provided by Christine Biron (Brown University).
This work was supported by the Trudeau Institute and by Public Health Service grants HL72937 (to S.T.S.), AI46571 (to L.L.J.), AI61587 (to L.L.J.), and AI49823 (to I.K.M.).
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 16 February 2007.
REFERENCES
- 1.Chang, K. H., M. Tam, and M. M. Stevenson. 2004. Modulation of the course and outcome of blood-stage malaria by erythropoietin-induced reticulocytosis. J. Infect. Dis. 189:735-743. [DOI] [PubMed] [Google Scholar]
- 2.Claster, S. 2002. Biology of anemia, differential diagnosis, and treatment options in human immunodeficiency virus infection. J. Infect. Dis. 185(Suppl. 2):S105-S109. [DOI] [PubMed] [Google Scholar]
- 3.Dai, C. H., J. O. Price, T. Brunner, and S. B. Krantz. 1998. Fas ligand is present in human erythroid colony-forming cells and interacts with Fas induced by interferon gamma to produce erythroid cell apoptosis. Blood 91:1235-1242. [PubMed] [Google Scholar]
- 4.Denkers, E. Y., and R. T. Gazzinelli. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11:569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felli, N., F. Pedini, A. Zeuner, E. Petrucci, U. Testa, C. Conticello, M. Biffoni, A. Di Cataldo, J. A. Winkles, C. Peschle, and R. De Maria. 2005. Multiple members of the TNF superfamily contribute to IFN-gamma-mediated inhibition of erythropoiesis. J. Immunol. 175:1464-1472. [DOI] [PubMed] [Google Scholar]
- 6.Gysemans, C. A., L. Ladriere, H. Callewaert, J. Rasschaert, D. Flamez, D. E. Levy, P. Matthys, D. L. Eizirik, and C. Mathieu. 2005. Disruption of the gamma-interferon signaling pathway at the level of signal transducer and activator of transcription-1 prevents immune destruction of beta-cells. Diabetes 54:2396-2403. [DOI] [PubMed] [Google Scholar]
- 7.Halupa, A., M. L. Bailey, K. Huang, N. N. Iscove, D. E. Levy, and D. L. Barber. 2005. A novel role for STAT1 in regulating murine erythropoiesis: deletion of STAT1 results in overall reduction of erythroid progenitors and alters their distribution. Blood 105:552-561. [DOI] [PubMed] [Google Scholar]
- 8.Johnson, L. L., K. N. Berggren, F. M. Szaba, W. Chen, and S. T. Smiley. 2003. Fibrin-mediated protection against infection-stimulated immunopathology. J. Exp. Med. 197:801-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, G. R. 1983. The anemia of chronic disease. Semin. Hematol. 20:61-80. [PubMed] [Google Scholar]
- 10.Lieberman, L. A., E. N. Villegas, and C. A. Hunter. 2004. Interleukin-15-deficient mice develop protective immunity to Toxoplasma gondii. Infect. Immun. 72:6729-6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liesenfeld, O., H. Kang, D. Park, T. A. Nguyen, C. V. Parkhe, H. Watanabe, T. Abo, A. Sher, J. S. Remington, and Y. Suzuki. 1999. TNF-alpha, nitric oxide and IFN-gamma are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 21:365-376. [DOI] [PubMed] [Google Scholar]
- 12.Manodori, A. B., and F. A. Kuypers. 2002. Altered red cell turnover in diabetic mice. J. Lab. Clin. Med. 140:161-165. [DOI] [PubMed] [Google Scholar]
- 13.McCabe, R. E., and J. S. Remington. 1990. Toxoplasma gondii, p. 2090-2103. In G. L. Mandell, R. G. Douglas, and J. E. Bennett (ed.), Principles and practices of infectious diseases, 3rd ed. Churchill Livingstone, Inc., New York, NY.
- 14.Means, R. T., Jr. 2003. Recent developments in the anemia of chronic disease. Curr. Hematol. Rep. 2:116-121. [PubMed] [Google Scholar]
- 15.Means, R. T., Jr., S. B. Krantz, J. Luna, S. A. Marsters, and A. Ashkenazi. 1994. Inhibition of murine erythroid colony formation in vitro by interferon gamma and correction by interferon receptor immunoadhesin. Blood 83:911-915. [PubMed] [Google Scholar]
- 16.Muller, H. M., J. H. Horina, D. Kniepeiss, M. B. Tripolt, V. Stadelbauer, M. Schweiger, and K. H. Tscheliessnigg. 2001. Characteristics and clinical relevance of chronic anemia in adult heart transplant recipients. Clin. Transplant 15:343-348. [DOI] [PubMed] [Google Scholar]
- 17.Musso, T., L. Calosso, M. Zucca, M. Millesimo, D. Ravarino, M. Giovarelli, F. Malavasi, A. N. Ponzi, R. Paus, and S. Bulfone-Paus. 1999. Human monocytes constitutively express membrane-bound, biologically active, and interferon-gamma-upregulated interleukin-15. Blood 93:3531-3539. [PubMed] [Google Scholar]
- 18.Pang, Q., S. Fagerlie, T. A. Christianson, W. Keeble, G. Faulkner, J. Diaz, R. K. Rathbun, and G. C. Bagby. 2000. The Fanconi anemia protein FANCC binds to and facilitates the activation of STAT1 by gamma interferon and hematopoietic growth factors. Mol. Cell. Biol. 20:4724-4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petakov, M., N. Stojanovic, G. Jovcic, D. Bugarski, V. Todorovic, and O. Djurkovic-Djakovic. 2002. Hematopoiesis during acute Toxoplasma gondii infection in mice. Haematologia 32:439-455. [PubMed] [Google Scholar]
- 20.Ramana, C. V., M. P. Gil, R. D. Schreiber, and G. R. Stark. 2002. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol. 23:96-101. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder, K., P. J. Hertzog, T. Ravasi, and D. A. Hume. 2004. Interferon-gamma: an overview of signals, mechanisms and function. J. Leukoc. Biol. 75:163-189. [DOI] [PubMed] [Google Scholar]
- 22.Spivak, J. L. 2000. The blood in systemic disorders. Lancet 355:1707-1712. [DOI] [PubMed] [Google Scholar]
- 23.Vaglio, J., D. M. Safley, M. Rahman, M. Kosiborod, P. Jones, R. Thompson, H. M. Krumholz, and J. A. Spertus. 2005. Relation of anemia at discharge to survival after acute coronary syndromes. Am. J. Cardiol. 96:496-499. [DOI] [PubMed] [Google Scholar]
- 24.Van Hove, L., W. Goossens, V. Van Duppen, and R. L. Verwilghen. 1990. Reticulocyte count using thiazole orange. A flow cytometry method. Clin. Lab. Haematol. 12:287-299. [DOI] [PubMed] [Google Scholar]
- 25.Weiss, G., and L. T. Goodnough. 2005. Anemia of chronic disease. N. Engl. J. Med. 352:1011-1023. [DOI] [PubMed] [Google Scholar]
- 26.Wenxin, L., F. Jinxiang, W. Yong, L. Wenxiang, S. Wenbiao, and Z. Xueguang. 2005. Expression of membrane-bound IL-15 by bone marrow fibroblast-like stromal cells in aplastic anemia. Int. Immunol. 17:429-437. [DOI] [PubMed] [Google Scholar]
- 27.Zoumbos, N. C., J. Y. Djeu, and N. S. Young. 1984. Interferon is the suppressor of hematopoiesis generated by stimulated lymphocytes in vitro. J. Immunol. 133:769-774. [PubMed] [Google Scholar]