Abstract
Gamma interferon (IFN-γ) is a key cytokine in host defense against intracellular mycobacterial infection. It has been believed that both CD4 and CD8 T cells are the primary sources of IFN-γ. However, the relative contributions of CD4 and CD8 T-cell subsets to IFN-γ production and the relationship between CD4 and CD8 T-cell activation have not been examined. By using a model of pulmonary mycobacterial infection and various immunodetection assays, we found that CD4 T cells mounted a much stronger IFN-γ response than CD8 T cells at various times after mycobacterial infection, and this pronounced IFN-γ production by CD4 T cells was attributed to both greater numbers of antigen-specific CD4 T cells and a greater IFN-γ secretion capacity of these cells. By using major histocompatibility complex class II-deficient or CD4-deficient mice, we found that the lack of CD4 T cells did not negatively affect primary or secondary CD8 T-cell IFN-γ responses. The CD8 T cells activated in the absence of CD4 T cells were capable of immune protection against secondary mycobacterial challenge. Our results suggest that, whereas both CD4 and CD8 T cells are capable of IFN-γ production, the former represent a much greater cellular source of IFN-γ. Moreover, during mycobacterial infection, CD8 T-cell IFN-γ responses and activation are independent of CD4 T-cell activation.
The type 1 T-cell-mediated immune response is essential to host defense against intracellular mycobacterial infection. Indeed, hosts deficient in both CD4 and CD8 T cells readily succumb to mycobacterial infection (9, 13, 26, 28). Although CD4 T cells are traditionally believed to play a major protective role, the CD4 and CD8 T-cell subsets have each been found to be able to confer immune protection (4, 5, 20, 21, 25, 26), and a lack of CD8 T cells results in weakened host defense against mycobacterial infection (9, 13, 26). Gamma interferon (IFN-γ) plays a critical role in T-cell immunity via its potent activating effects on Mycobacterium-infected macrophages and granuloma formation (2, 27, 28). Although T cells, NK cells, and macrophages are all able to produce IFN-γ (6, 24), CD4 and CD8 T cells are considered to be the primary sources of this cytokine during mycobacterial infection (20, 25, 26, 29). Recent evidence suggests that IFN-γ, but not cytotoxic activity, is required for antimycobacterial immune protection mediated by CD8 T cells (3, 11, 20). However, the relative contribution of CD4 and CD8 T-cell subsets to IFN-γ responses during mycobacterial infection has remained largely to be determined. More specifically, it remains to be determined whether CD4 and CD8 T cells produce differential quantities of IFN-γ and, if so, whether such differential IFN-γ production is attributed to differential frequencies of antigen-specific T-cell subsets, differential IFN-γ secretion capacities, or both. Furthermore, it also is not yet completely understood whether CD4 and CD8 T cells are dependent on one another for their activation and IFN-γ responses during mycobacterial infection. Further understanding of the regulation and the capacity of CD8 T-cell IFN-γ production during mycobacterial infection is important in developing effective prophylactic and therapeutic strategies designed to target CD8 T cells in CD4 T-cell-deficient hosts, such as human immunodeficiency virus-infected individuals.
We have used here a model of pulmonary mycobacterial infection to analyze and compare IFN-γ responses in purified CD4 and CD8 T-cell subsets cocultured with antigen-presenting cells (APCs) by using both enzyme-linked immunosorbent assay (ELISA) and enzyme-linked immunospot (ELISPOT) assay techniques for the determination of total IFN-γ protein released into culture media, the frequencies of IFN-γ-secreting cells, and IFN-γ secretion capacities. We have also investigated the role of CD4 T cells in CD8 T-cell activation during both primary and secondary mycobacterial infections.
MATERIALS AND METHODS
Mice.
All mouse strains used in the present study had an H-2b C57BL/6 (B6) genetic background. Major histocompatibility complex class II (MHC-II)−/−, CD4−/−, CD8−/−, and RAG2−/− mice originally purchased from Taconic Farms and bred in-house and C57BL/6 mice purchased from Harlan Breeders (Indianapolis, IN) were kept under the specific-pathogen-free conditions at the Central Animal Facility of McMaster University.
Primary and secondary pulmonary mycobacterial infection.
M. bovis BCG (Connaught strain) was prepared as previously described (26, 28). Briefly, the Mycobacterium strain was grown in Middlebrook 7H9 media (Difco) supplemented with Middlebrook OADC enrichment (Invitrogen), 20% glycerol, and 0.05% Tween 80 for 10 to 15 days, and samples were then divided into aliquots and stored at −70°C. BCG was washed twice with phosphate-buffered saline (PBS) containing 0.05% Tween 80 and resuspended in PBS. It was then passed through a 27-gauge needle 10 times to disperse clumps and then diluted with PBS to the desired concentration before use. A volume of 40 μl per mouse was used for intratracheal (i.t.) injection. For primary BCG infection, C57BL/6 and MHC II−/− mice were infected with 0.5 million CFU of BCG. For secondary mycobacterial challenge, infected mice were challenged i.t. with 1.5 million CFU of BCG 6 weeks after primary BCG infection. The antibiotic isoniazid was administered to mice 3 weeks after primary infection to minimize the level of remaining live organisms in vivo before secondary infection. Isoniazid tablets (100 mg) were dissolved in 20 ml of PBS and filtered with 0.22-μm-pore-size filters prior to injection. Each mouse was injected intraperitoneally with 50 mg of isoniazid/kg (body weight) per day for 7 consecutive days.
Isolation of whole splenocytes and lymph node cells.
Both splenocytes and lymph node cells were isolated and cultured as previously described (25, 26, 28). Briefly, spleen cells were released into RPMI media by mashing spleens between glass slides. After red blood cell lysis with a mouse erythrocyte lysing kit (R&D Systems), splenocytes were filtered and resuspended in complete RPMI (cRPMI) media (10% fetal bovine serum, 1% penicillin-streptomycin, 1% l-glutamine). Lymph node cells were released from mediastinal lymph nodes in cRPMI media.
Purification of CD4 and CD8 T cells and coculture with APCs.
Positive selections of CD4 and CD8 T cells were carried out with MACS using VarioMAC magnet, LS columns, and CD4 or CD8a microbeads (Miltenyi Biotec) according to the manufacturer's protocol, with the exception that the purification process was repeated once for the positive fractions in order to maximize the cell purity. Briefly, for every 107 total splenocytes or lymph node cells, 10 μl of magnetic beads and 90 μl of MACS buffer (0.5% bovine serum albumin and 2 mM EDTA in PBS) were used, and the mixture was incubated at 4°C for 15 min. Excess beads were washed away, and the total cells were resuspended to a 108 cells/500 μl. The cells were then loaded onto a prewashed column, and the negative fraction was allowed to flow through, followed by three 3-ml washes of the column. Finally, the positive fraction was flushed out with a plunger and centrifuged and resuspended in cRPMI for later use. The purity of purified T-cell subsets was consistently >90% as determined by fluorescence-activated cell sorting (FACS), and the viability of these subsets was >95%. All cell cultures were performed with 96-well flat-bottom polystyrene plates. A total volume of 250 μl of cRPMI medium per well was used, and the cells were incubated for 3 days at 37°C. Cell culture supernatants (200 μl) were collected from each well after incubation and stored at −20°C.
For coculture of purified T-cell subsets and APCs, a half-million purified CD4 or CD8 T cells were cocultured with M. bovis BCG-infected APCs in a total volume of 250 μl as previously detailed (29). Mycobacterium-infected APCs were always prepared the day before T-cell purification by plating freshly isolated alveolar macrophages from the lungs of naive C57BL/6 mice at concentrations of 4,000 cells per well in 100 μl of penicillin-streptomycin-free RPMI (10% fetal bovine serum, 1% l-glutamine) and infected in vitro with live BCG at a multiplicity of infection of 40 CFU. Control wells included CD4 or CD8 T cells alone, noninfected APCs, infected APCs, CD4 or CD8 T cells cultured with uninfected APCs, or CD4 or CD8 T cells with live BCG but no APCs. All of these controls yielded no or negligible levels of IFN-γ production. Coculture was carried out for 3 days before the supernatant was collected for cytokine measurement by ELISA.
Measurement of IFN-γ production by cultured CD4 and CD8 T cells.
IFN-γ levels in cell culture supernatants were assessed by ELISA kits for murine IFN-γ (R&D Systems). The sensitivity of detection was 2 pg/ml.
Determination of frequency of mycobacterial-antigen-specific, IFN-γ-releasing CD4 and CD8 T cells by ELISPOT assay.
The IFN-γ ELISPOT assay was carried out according to the manufacturer's protocol with the supplied capture and detection monoclonal antibodies (MAbs) and color development reagents (R&D Systems) on 96-well plates with nitrocellulose bottom (Millipore). The plate was coated with capture antibodies the day prior to cell plating. CD4 or CD8 T cells (0.3 or 0.15 million) were cocultured with 0.2 million APCs (gamma-irradiated splenocytes from naive C57BL/6 mice) and stimulated with 2 μg of Mycobacterium tuberculosis culture filtrate protein (CFP) in a total volume of 100 μl of cRPMI per well for 1 day at 37°C (25). Control wells included APC, APC plus CFP; CD4 or CD8 T cells alone, CD4 or CD8 T cells with APC, or CD4 or CD8 T cells with CFP but no APC. All of these controls generated a minimum number of background spots that were subtracted from the positive findings. After culture, the plate was incubated with detection antibodies and subjected to color development of the spots. IFN-γ+ spots were quantified, and the frequency of IFN-γ-secreting cells was expressed as the percent IFN-γ+ cells among the total number of plated cells. To determine the IFN-γ secretion capacity of CD4 and CD8 T subsets, the amount of IFN-γ production determined by ELISA was divided by the number of IFN-γ+ CD4 or CD8 T cells determined by ELISPOT assay for each million of cultured cells.
Determination of CD8 T-cell proliferation by [3H]thymidine incorporation assay.
Purified CD8 T cells from C57BL/6 and MHC II−/− mice after secondary mycobacterial challenge were cultured as described above. At 20 h before the termination of 3-day incubation, 1 μCi of [3H]thymidine was added to each well. Cell cultures were then subjected to freezing and thawing once. Using a Cell Harvester, cells were washed, and their contents were lysed and collected onto GFC filter plates (Perkin-Elmer). The radioactivity from lysed cell contents was measured with a Top Count radioactivity reader.
Cell surface immunostaining and FACS analysis.
All MAbs used here were purchased from BD Pharmingen. Cells were blocked for nonspecific binding of their Fc receptors with anti-CD16/CD32 antibodies for 15 min and then stained for 30 min on ice with the appropriate combinations of fluorochrome-conjugated MAbs. Fluorochrome-conjugated MAbs to CD3, CD4, CD8, CD44, CD45, CD25, and CD62L were used. Unstained cells were used as a control. The data were collected with FACScan, LSRII, or FACSCanto (all BD Biosciences) flow cytometers using CellQuest or FACSDiva software and analyzed with WinMDI or Flowjo software.
Adoptive T-cell transfer.
Several days prior to T-cell transfer, the number of Mycobacterium-specific CD8 T cells in the spleen was predetermined in BCG-infected C57BL/6 and MHC-II−/− mice by using IFN-γ ELISPOT assay. This information was then used for calculation such that RAG2−/− immunodeficient mice will receive an equal number of antigen-specific CD8 T cells isolated from both BCG-infected wild-type B6 and MHC-II−/− mice. Purified CD8 T cells from BCG-infected C57BL/6 and MHC-II−/− mice were injected intravenously into RAG2−/− immunodeficient mice. Each recipient mouse received CD8 T cells in a volume of 200 to 300 μl via the tail vein.
Statistical analysis.
Statistical analysis was evaluated by using Excel spreadsheet software (Microsoft). The data were collected and are represented as the mean of samples with or without a standard error bar. Whenever applicable, the statistical significance between differences was determined with the Student t test. A P value of ≤0.05 is considered significant.
RESULTS
IFN-γ production by whole splenocytes and purified CD4 and CD8 T-cell subsets during pulmonary mycobacterial infection.
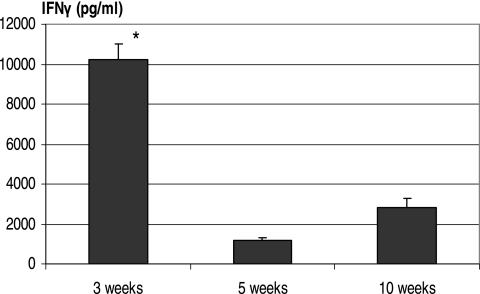
Before examining the kinetics of CD4 and CD8 T-cell IFN-γ responses, we first examined the kinetics of IFN-γ production by whole splenocytes. Not only does the spleen serve as a reliable window to lung type 1 immunity after mycobacterial infection (25, 26, 28, 29), but it is a rich source of antigen-specific T cells. Whole splenocytes were isolated at various times after pulmonary M. bovis BCG infection and stimulated with M. tuberculosis CFP. The level of IFN-γ was measured by ELISA. Splenocytes produced the greatest amounts of IFN-γ at 3 weeks after BCG infection, and the levels of IFN-γ responses markedly decreased at weeks 5 and 10 (Fig. 1).
FIG. 1.
Kinetic production of IFN-γ by whole splenic lymphocytes after pulmonary mycobacterial infection. C57BL/6 mice were infected i.t. with M. bovis BCG for 3, 5, or 10 weeks. Splenocyte culture was stimulated with M. tuberculosis CFP. The IFN-γ levels in cell culture supernatants were determined by ELISA. The data represent means ± the SEM of triplicate cultures from pooled samples of six to eight mice per time point. *, Statistically significant difference from values at 5 or 10 weeks (P < 0.001).
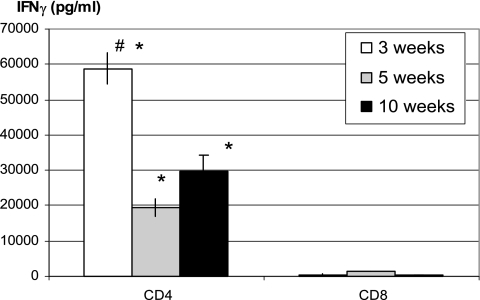
To begin examining the relative contribution of each T-cell subset to IFN-γ responses in the whole splenocytes, the same number of purified CD4 and CD8 T cells from the whole splenocytes isolated at various times was analyzed by coculture with Mycobacterium-infected APCs. The purity of isolated splenic CD4 and CD8 T cells used for such coculture was consistently greater than 90% as assessed by FACS. For instance, the purities of isolated CD4 and CD8 T cells were 92, 91, and 94% and 94, 92, and 95%, respectively, at weeks 3, 5, and 10 (Fig. 2 and 3). By using ELISA, CD4 T cells were found to produce much higher levels of IFN-γ than the same number of CD8 T cells at all time points examined upon in vitro stimulation with Mycobacterium-infected-APCs (Fig. 2). CD4 T cells isolated at 3 weeks postinfection produced the highest amounts of IFN-γ, in keeping with high levels of IFN-γ responses by whole splenocytes at this time (Fig. 1).
FIG. 2.
Comparison of kinetic production of IFN-γ by CD4 and CD8 T-cell subsets after pulmonary mycobacterial infection. Mycobacterium-infected C57BL/6 mice were sacrificed at 3, 5, and 10 weeks. CD4 and CD8 T cells were purified from whole splenocytes using MACS columns and were cocultured with Mycobacterium-infected APCs. The IFN-γ levels in cell culture supernatants were determined by ELISA. The data represent means ± the SEM of triplicates from pooled samples of six to eight mice per time point. #, Statistically significant difference compared to the week 5 or 10 time points in CD4 T-cell responses (P < 0.003); *, statistically significant difference compared to the corresponding time points in CD8 T-cell responses (P < 0.003).
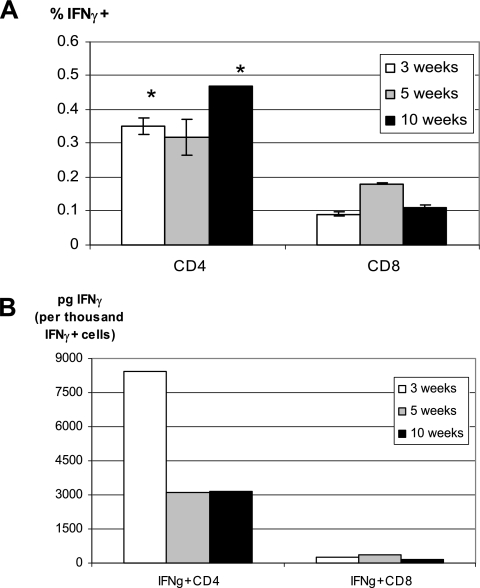
FIG. 3.
(A) Comparison of frequencies of antigen-specific, IFN-γ-releasing CD4 and CD8 T cells. The frequencies of IFNγ+ CD4 and IFN-γ+ CD8 cells were evaluated by IFN-γ ELISPOT assay where purified CD4 or CD8 cells were cultured with irradiated APCs and stimulated with M. tuberculosis CFP. CD4 and CD8 T cells were purified from the whole splenocytes isolated from Mycobacterium-infected C57BL/6 mice sacrificed at 3, 5, or 10 weeks postinfection. The data represent means ± the SEM of triplicates from pooled samples of six to eight mice. *, Statistically significance compared to the corresponding time points of CD8 T-cell responses (P < 0.02). (B) Comparison of IFN-γ secretion capacities of CD4 and CD8 T-cell subsets. IFN-γ secretion capacities were calculated based on the quantification of IFN-γ production by total CD4 and CD8 T cells and the frequencies of antigen-specific IFN-γ-releasing CD4 and CD8 T cells determined by ELISA and ELISPOT assays, respectively. The results are representative of two independent determinations.
Frequencies and IFN-γ secretion capacity of mycobacterial antigen-specific CD4 and CD8 T cells during primary pulmonary infection.
Since higher amounts of IFN-γ protein measured in culture supernatants of CD4 T cells may result from greater IFN-γ production capacity on a per-cell basis or from greater frequencies of antigen-specific T cells or both, we examined the frequency of mycobacterial antigen-specific CD4 and CD8 T cells by using ELISPOT assay. On average, there were 0.3 to 0.5% of CD4 T cells being IFN-γ positive between weeks 3 and 10 compared to 0.1 to 0.2% of CD8 T cells being positive (Fig. 3A). This suggests that a greater number of antigen-specific CD4 T cells account at least in part for much greater amounts of IFN-γ protein released from CD4 T-cell cultures (Fig. 2). Thus, based on the fact that there were always twice as many total CD4 T cells as CD8 T cells in the spleen at various times as assessed by FACS analysis (data not shown), clearly there were many more antigen-specific CD4 T cells per spleen than CD8 T-cell counterparts at all of the time points examined [for instance, there were (100 ± 4.0) × 103 and (40 ± 3.5) × 103 IFN-γ+ CD4 T cells versus (13 ± 0.2) × 103 and (13.5 ± 0.1) × 103 IFN-γ+ CD8 T cells at weeks 3 and 5, respectively].
Using the information generated by ELISA and ELISPOT assays, we also determined the relative IFN-γ secretion capacity of antigen-specific CD4 and CD8 T cells. We found that IFN-γ-positive CD4 T cells had a much greater IFN-γ secretion capacity than IFN-γ-positive CD8 T cells (Fig. 3B). This suggests that individual antigen-specific CD4 T cells likely release more IFN-γ than individual CD8 T-cell counterparts. Thus, not only was there a greater number of IFN-γ-producing CD4 T cells, but each activated CD4 T-cell secreted more IFN-γ than each activated CD8 T cell. Of note, CD4 T cells isolated at 3 weeks produced a lot more IFN-γ than CD4 T cells isolated at later time points (Fig. 2), and yet the frequency of antigen-specific CD4 T cells at weeks 3 was similar to those at later time points (Fig. 3A), suggesting that heightened IFN-γ production by CD4 T cells at weeks 3 was likely due to more pronounced T-cell activation.
Role of CD4 T cells in CD8 T-cell activation and IFN-γ responses during primary pulmonary mycobacterial infection.
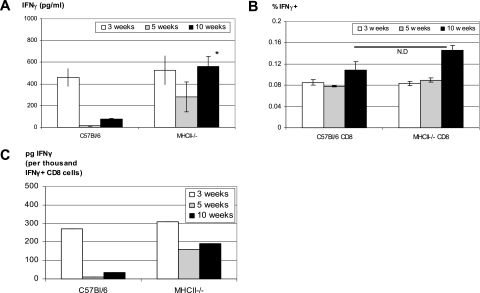
It is known that CD4 and CD8 T-cell subsets may affect each other's activation in a number of models of infectious diseases (14, 19). To investigate the potential reciprocal regulatory effect of T-cell subsets on each other's IFN-γ responses, we first examined CD4 T-cell responses in infected CD8 T-cell-deficient mice at 3 or 12 weeks postinfection. Both IFN-γ production and the frequency of antigen-specific CD4 T cells were not affected by the lack of CD8 T cells (data not shown). To investigate whether CD4 T cells were required for optimal CD8 T-cell activation, MHC-II-deficient (MHC-II−/−) mice were infected with mycobacteria, and CD8 T cells were purified at various times and compared to those isolated from infected wild-type B6 mice. Compared to CD4-deficient (CD4−/−) mice, MHC-II−/− mice represent “cleaner” and better CD4 T-cell-deficient hosts for studying CD8 T-cell responses since CD4−/− mice were found to develop aberrant MHC-II-restricted CD8+ and CD4−/CD8− T-cell populations (12, 22). Thus, MHC-II−/− mice were used as a primary CD4 T-cell-deficient host or tool in our study. We found that at 3 weeks the level of IFN-γ production by CD8 T cells of MHC-II−/− mice was comparable to that by wild-type CD8 T cells, while at later times it appears even higher by CD8 T cells in MHC-II−/− mice than in wild-type B6 mice (Fig. 4A). Furthermore, the frequency of antigen-specific CD8 T cells examined at various time points was also similar between CD4 T-cell deficient and wild-type hosts (Fig. 4B). IFN-γ secretion capacity of CD8 T cells in CD4 T-cell-deficient hosts was found to be even greater at weeks 5 and 10 (Fig. 4C), thus explaining the somewhat higher levels of IFN-γ production by these cells at later times (Fig. 4A). Similar results were also obtained with CD8 T cells purified from infected CD4-deficient mice (data not shown). Upon examination of T-cell activation and the memory surface markers CD45, CD25, CD44, and CD62L, we found that the percentage of CD3+ CD8+ CD45+ or CD3+ CD8+ CD25+ (activation), CD3+ CD8+/CD44+ (effector memory/activation), or CD3+ CD8+/CD44+ CD62L+ (central memory) T cells from the spleen of infected MHC-II−/− mice was comparable to that in infected wild-type mice (Table 1). Although there were small but significant differences in the proportion of CD8 and CD45+ CD8 T cells, the mean fluorescence intensity, which takes into consideration of average fluorescent staining intensity of all positive cells, is not different for all of the comparison groups between MHC-II−/− and B6 mice (data not shown). These results together suggest that during mycobacterial infection CD4 T-cell help is not required for the differentiation and activation of CD8 T cells.
FIG. 4.
(A) Comparison of kinetic production of IFN-γ by CD8 T cells generated in CD4 T-cell-efficient and CD4 T-cell-deficient hosts after pulmonary mycobacterial infection. C57BL/6 wild-type and MHC-II−/− mice were infected i.t. with mycobacteria for 3, 5, or 10 weeks. CD8 T cells were purified by MACS columns from whole splenocytes and cocultured with Mycobacterium-infected APCs. The levels of IFN-γ released into the culture supernatants were determined by ELISA. The data represent means ± the SEM of triplicates from pooled samples of four to eight mice per time point. *, Statistically significant difference from the corresponding time point of the B6 control (P = 0.007). (B) Comparison of the frequencies of antigen-specific, IFN-γ-releasing CD8 T cells generated in CD4 T-cell-efficient and CD4 T-cell-deficient hosts after pulmonary mycobacterial infection. The frequencies of antigen-specific IFN-γ-releasing CD8 T cells were evaluated by IFN-γ ELISPOT assay in which CD8 T cells were cocultured with irradiated APCs and stimulated with M. tuberculosis CFP. The data represent means ± the SEM of triplicates from pooled samples of four to eight mice per time point. The difference between B6 and MHC-II−/− CD8 T cells at all corresponding time points is not significant. N.D, no difference. (C) Comparison of IFN-γ secretion capacities of CD8 T cells generated in CD4 T-cell-efficient and -deficient hosts. The IFN-γ secretion capacities were calculated based on the quantification of IFN-γ production by total CD8 T cells, and the frequencies of antigen-specific IFN-γ-releasing CD8 T cells were determined by ELISA and ELISPOT assays, respectively. The results are representative of two independent determinations.
TABLE 1.
Comparison of activation phenotypes of CD3+ CD8+ T cells in wild-type and MHC-II−/− mice
| Mouse type | Mean % T cells ± SEMa
|
||||
|---|---|---|---|---|---|
| CD3+ CD8+ | CD45+ CD3+ CD8+ | CD25+ CD3+ CD8+ | CD44+ CD3+ CD8+ | CD62L+ CD44+ CD3+ CD8+ | |
| Wild type (C57BL/6) | 25.2 ± 2.6 | 69.02 ± 2.6 | 15.5 ± 0.45 | 84.72 ± 1.3 | 59.16 ± 1.2 |
| MHC-II−/− | 18.63 ± 0.4* | 51.17 ± 1.5* | 13.3 ± 0.45 | 80.84 ± 1.9 | 59.19 ± 1.6 |
Mice were infected i.t. with M. bovis BCG. Total splenic cells were obtained at 5 weeks postinfection and analyzed for the phenotype of CD8 T cells by FACS. The numbers in column 2 represent the proportion of CD3+ CD8+ T cells out of all of the splenocytes. The numbers in the remaining columns represent the proportion of CD8 T cells expressing CD45, CD25, CD44, or both CD44 and CD62L out of total CD3+ CD8+ T cells. *, P ≤ 0.05 compared to B6 control. Data are compiled from individually analyzed mice (three mice/strain).
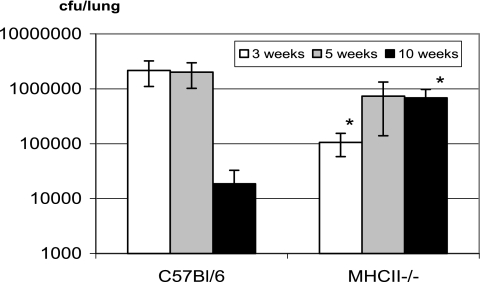
Having demonstrated that the lack of CD4 T cells had little effect on the frequency and IFN-γ production of antigen-specific CD8 T cells, we examined the level of mycobacterial infection in CD4 T-cell-deficient mice after pulmonary mycobacterial infection. Of interest, at weeks 3 and 5 postinfection the level of pulmonary mycobacterial infection in MHC-II−/− mice was similar to or even lower than that in wild-type B6 mice (Fig. 5), an observation in agreement with our previous findings (26). However, although the level of infection markedly declined in the lung of wild-type B6 mice at week 10, it remained high in the lungs of MHC-II−/− mice (Fig. 5). Similar trends were also found in the spleens of MHC-II−/− and B6 mice (data not shown). Such higher bacterial loads in the lungs of MHC-II−/− mice may have contributed to the statistically significantly higher IFN-γ responses by CD8 T cells of MHC-II−/− mice at week 10 (Fig. 4A). These data suggest that activated CD8 T cells in the absence of CD4 T cells could well control primary mycobacterial infection at least initially.
FIG. 5.
Comparison of mycobacterial infection levels in the lungs of C57BL/6 and MHC-II−/− mice. Mice were infected i.t. with M. bovis BCG for 3, 5, or 10 weeks. The titers of mycobacterial CFU in homogenized lungs were determined by colony formation assay. The data represent means ± the SD of titers from four to eight mice per time point. *, Statistically significant difference compared to the corresponding time points of B6 control lungs (P ≤ 0.03).
Role of CD4 T cells in CD8 T-cell activation and IFN-γ responses during secondary pulmonary mycobacterial infection.
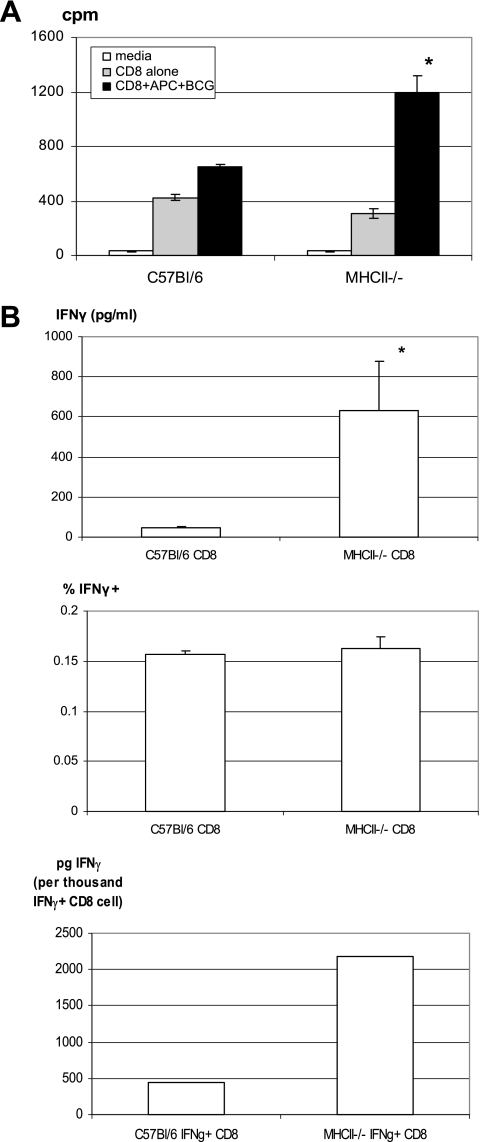
Having demonstrated that the lack of CD4 T cells had little effect on CD8 T-cell IFN-γ responses during primary mycobacterial infection, we investigated whether this might also be true of CD8 T-cell responses upon secondary pulmonary mycobacterial infection. To this end, wild-type B6 and MHC-II-deficient mice were infected with mycobacteria, and at week 3 they were treated daily with the antimycobacterial antibiotic isoniazid for a period of 7 days to reduce the primary infection in both B6 and MHC-II−/− mice. This therapeutic regimen was found to effectively reduce the level of infection by about 150 times in the lung (785,250 to 900,000 CFU without isoniazid versus 5,400 to 6,975 CFU with isoniazid treatment) and to clear infection in the spleen in both mouse strains when assessed shortly prior to reinfection. At week 6 after primary infection, these mice were then reinfected i.t. with a high dose of mycobacteria. Mice were sacrificed 7 days after secondary infection, and CD8 T cells were purified from the spleens and local draining lymph nodes and analyzed for their activities after secondary mycobacterial infection. Purified CD8 T cells from reinfected MHC-II−/− mice proliferated ex vivo at a rate similar to or even higher than that by the cells of wild-type B6 mice (Fig. 6A). Furthermore, the production of IFN-γ by CD8 T cells and the frequencies of antigen-specific, IFN-γ-producing CD8 T cells of MHC-II−/− mice were also similar to or even greater than those of wild-type B6 CD8 T cells in the spleen (Fig. 6B). The IFN-γ secretion capacity of CD8 T cells from MHC- II−/− mice was found to be greater than that of the wild-type counterparts (Fig. 6B). Both the frequencies of IFN-γ-producing CD8 T cells and the IFN-γ secretion capacity of these cells were also found to be almost identical between wild-type B6 and CD4-deficient (CD4−/−) mice (data not shown). That the IFN-γ secretion capacity of CD8 T cells from MHC-II−/− mice was greater than that of CD4−/− mice, relative to B6 wild-type control, likely reflects a greater extent of compensation by CD8 T cells of MHC-II−/− mice since these cells are fully dependent on the MHC-I pathway for activation. In comparison, CD4−/− mice develop aberrant MHC-II-restricted CD8+ and CD4− CD8− T-cell populations (12, 22). We also examined the activation and/or memory phenotypes of CD8 T cells from reinfected MHC- II−/− mice and found that the proportion of effector memory CD8 T cells (CD44+ CD62L−) was slightly higher both in the spleen and in the draining lymph nodes of MHC- II−/− mice (data not shown). The mycobacterial titers in the lung at 7 days after secondary mycobacterial challenge were also very similar between wild-type B6 and MHC-II−/− or CD4−/− mice (data not shown). These data together suggest that lack of CD4 T cells does not negatively affect CD8 T-cell activation and IFN-γ responses upon secondary mycobacterial exposure in previously infected hosts.
FIG. 6.
Comparison of secondary responses of memory CD8 T cells generated in CD4 T-cell-efficient and CD4 T-cell-deficient hosts. C57BL/6 wild-type and MHC-II−/− mice were infected with mycobacteria and at 6 weeks after infection were challenged i.t, with a high dose of mycobacteria. Seven days after challenge mice were sacrificed, and CD8 T cells were purified by MACS columns. (A) Purified CD8 T cells from the spleen were stimulated with Mycobacterium-infected-APCs for 3 days with addition of [3H]thymidine for the last 20 h of culture for determination of T-cell proliferation. The radioactivity of [3H]thymidine incorporated into proliferating cells was expressed as counts per minute (cpm), and the data represent means ± the SEM of triplicates from pooled samples of six to eight mice per group. *, Statistically significant difference compared to antigen-stimulated B6 control (P = 0.013). (B) Purified CD8 T cells from the spleen of B6 and MHC-II−/− mice were further evaluated to determine the IFN-γ production (top panel), the percent frequencies of antigen-specific IFN-γ-releasing CD8 T cells (middle panel), and the IFN-γ secretion capacities (bottom panel) as described in Fig. 4. *, Statistically significant difference compared to B6 control (P = 0.02). The data represent means ± the SEM of triplicates from pooled samples of six to eight mice. The results are representative of two independent determinations.
Immune protection by activated CD8 T cells generated in CD4 T-cell-deficient environment.
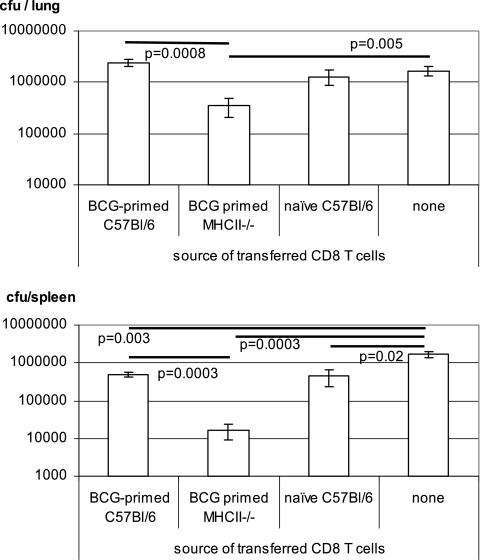
Having shown that the lack of CD4 T cells did not result in any deficiencies in both primary and secondary CD8 T-cell activation and IFN-γ responses, we examined whether CD8 T cells primed in the absence of CD4 T cells could provide immune protection against mycobacterial challenge upon adoptive transfer to an immune deficient host. To this end, MHC-II−/− and wild-type B6 mice were infected with M. bovis BCG, and splenic CD8 T cells were purified at 6 weeks. The frequencies of antigen-specific CD8 T cells were predetermined by ELISPOT assay, and this information was used to allow the transfer of an equal number of antigen-specific CD8 T cells derived from both wild-type B6 and MHC-II−/− mice. Purified CD8 T cells were transferred intravenously to naive immunodeficient RAG2−/− mice, which were then challenged i.t. with M. bovis BCG. The level of mycobacterial infection in the lungs and spleens of Mycobacterium-infected RAG2−/− mice was determined at 5 weeks postchallenge. The CD8 T cells from BCG-primed MHC-II−/− mice offered significant protection to BCG-challenged RAG2−/− mice in both the lungs and the spleens, and this level of protection was even better than that achieved by CD8 T cells from BCG-primed C57BL/6 mice (Fig. 7). The better protection provided by CD8 T cells from MHC- II−/− mice was likely due to their increased IFN-γ production capacity and secondary responses (Fig. 4C and 6). Although an assessment at a further time point beyond 5 weeks after mycobacterial challenge will be helpful, our current data support the conclusion that CD8 T cells generated in a CD4 T-cell-deficient environment are at the least as capable of protection as those generated in a wild-type host (protection at a single time point, 4 to 6 weeks, postchallenge, is also frequently used in vaccine studies as an index of protective potential of the endogenous or transferred antigen-specific T cells in question).
FIG. 7.
Immune protection of immunodeficient mice by adoptively transferred CD8 T cells generated in Mycobacterium-infected C57BL/6 wild-type and MHC-II−/− mice. B6 and MHC-II−/− mice were infected with mycobacteria for 6 weeks before their splenic CD8 T cells were purified for adoptive transfer. After T-cell transfer, RAG2−/− mice were challenged with mycobacteria and sacrificed at 5 weeks postchallenge for quantification of the CFU in the lungs and spleens. The data represent means ± the SEM of about five mice per group.
DISCUSSION
Type 1 immune cytokine IFN-γ plays a critical role in antimycobacterial host defense via its potent activating effects on macrophage mycobactericidal activities including NO production (2, 22, 27). Although cells including T cells, NK cells, and macrophages are able to produce IFN-γ (6, 24), CD4 T cells are believed to be the primary cellular source of this cytokine during mycobacterial infection. In addition to their cytotoxic-T-lymphocyte activities, CD8 T cells are also able to produce IFN-γ (19). Indeed, recent studies have clearly shown that both CD4 and CD8 cells contribute to immunity against mycobacterial infection. In this regard, the immunoprotective role by CD8 T cells is primarily via its ability to produce IFN-γ and not its cytotoxic-T-lymphocyte activities (3, 10, 11, 20). Nevertheless, there has been a lack of studies directly comparing the two T-cell subsets for their relative contributions to IFN-γ responses during pulmonary mycobacterial infection. Furthermore, it is not yet completely understood whether the absence of one T-cell subset has an impact on the activation and effector functions of the other (17-19). It is particularly relevant to investigate whether CD4 cells are required for optimal primary and secondary responses of CD8 T cells. The answers to these questions may enhance our understanding of T-cell biology and help with the development of antipulmonary mycobacterial vaccines and immunotherapeutics that aim to activate CD8 T cells in human immunodeficiency virus-infected people who may suffer various degrees of CD4 T-cell deficiency.
Although we and others have previously shown that both CD4 and/or CD8 T cells in wild-type mice are capable of IFN-γ secretion in the course of mycobacterial infection, the evidence of IFN-γ responses in these T-cell subsets was obtained primarily by examining unpurified total mononuclear cell populations with a single technique such as mRNA detection, intracellular cytokine immunostaining, or ELISPOT assay (7, 16, 23, 25, 29). Similar approaches were also used to examine CD8 T-cell activation and IFN-γ responses in CD4 T-cell- or MHC-II-deficient mice (1, 15, 25, 26). These approaches unfortunately provide only qualitative information and do not allow a quantitative assessment and comparison. For instance, similar percentages of IFN-γ-secreting CD4 and CD8 T-cell subsets determined in total mononuclear cells by using intracellular cytokine immunostaining techniques do not show whether these T-cell subsets can secrete similar amounts of IFN-γ or whether there are similar numbers of IFN-γ-secreting CD4 and CD8 T cells in the organ examined. An adequate quantitative assessment entails the purification of CD4 and CD8 T-cell subsets, the coculture of purified T cells with mycobacterial-antigen-loaded APCs, and analysis of T-cell IFN-γ responses by using a combination of several techniques for quantification of total IFN-γ production, frequencies of IFN-γ-secreting T cells, and the capacities of IFN-γ secretion on a per-cell basis.
We used here a model of pulmonary mycobacterial infection with live attenuated M. bovis BCG and, at various times postinfection, we quantified and compared the IFN-γ responses in purified CD4 and CD8 T-cell subsets cocultured with Mycobacterium-infected APC by using both ELISA and ELISPOT techniques for the determination of total IFN-γ protein released into culture media, the frequencies of IFN-γ-secreting cells and IFN-γ secretion capacities. We find that CD4 T cells upon antigen restimulation are able to release much more IFN-γ than the same number of CD8 T cells at various times after mycobacterial infection. Higher levels of IFN-γ production by CD4 T cells are attributed both to greater numbers of antigen-specific CD4 T cells and to the greater IFN-γ secretion capacity of these cells. We further demonstrated that a lack of CD4 T cells does not negatively affect primary or secondary CD8 T-cell IFN-γ responses, as demonstrated by IFN-γ production and the frequency of antigen-specific IFN-γ-secreting CD8 T cells. Our results thus establish that whereas both CD4 and CD8 T cells are capable of IFN-γ production, the former represent a much greater cellular source of IFN-γ. Moreover, during mycobacterial infection, CD8 T-cell activation is independent of CD4 T-cell activation.
In fact, we found that not only did CD8 T cells activated in the absence of CD4 T cells not demonstrate an impaired IFN-γ response but they also mounted a somewhat higher level of IFN-γ response, particularly at later time points after infection, due primarily to enhanced IFN-γ secretion capacity. These findings suggest first that CD8 T-cell activation in mycobacterial infection is independent of CD4 T cells and second that, perhaps by compensation for the lack of CD4 T cells, CD8 T cells may undergo a greater extent of proliferation and activation. Indeed, we detected two to three times more total splenic CD8 T cells in MHC-II−/− mice than in wild-type B6 mice at various times postinfection (data not shown). This fact emphasizes further the importance of comparing CD8 T-cell responses in wild-type and CD4 T-cell deficient hosts by using quantitative methods, i.e., the use of purified CD4 and CD8 T-cell subsets and multiple immunoassays, as we have shown in the present study. Although the mechanisms for this remain to be understood, the lack of CD4 T regulatory subsets may contribute to heightened CD8 T-cell activation (8). By using a model of parenteral BCG vaccination with CD4-deficient mice and an intracellular IFN-γ immunostaining method, we have also recently observed a greater proportion of activated CD8 T cells, although such activated CD8 T cells initially displayed a delayed movement from draining lymph nodes to the systemic sites (25). It should be noted that, in the present study, the detailed kinetic quantitative comparison in IFN-γ responses of CD4 and CD8 T-cell subsets was made possible in a model by using live attenuated M. bovis BCG and, due to some differences between BCG and M. tuberculosis, the study was not intended to completely examine the events in pulmonary tuberculosis infection. In future studies, it would be of interest to compare CD8 T-cell activation in both M. bovis BCG- and M. tuberculosis-infected mice. However, to support our current conclusions, we have recently shown that IFN-γ-releasing CD8 T cells activated in CD4−/− mice by BCG exposure were able to provide robust immunoprotection from pulmonary M. tuberculosis challenge (25). Furthermore, although again only the qualitative methods were used, previous studies have also shown that the percentage of IFN-γ-positive CD8 T cells in the lungs or peripheral organs of MHC-II−/− or CD4−/− mice was similar to that in wild-type hosts after M. tuberculosis infection (1, 17, 25). In the present study, we have further demonstrated that CD8 T cells activated in the absence of CD4 T cells, upon adoptive transfer to immunodeficient RAG2−/− mice, could confer protection from mycobacterial challenge both in the lung and in the spleen. Of note, as a control, CD8 T cells generated in wild-type mice, upon adoptive transfer, conferred protection in the spleen but not in the lung. This is likely due to the fact that, as we have shown, CD8 T cells generated in a CD4-deficient environment underwent a greater extent of activation than those generated in a wild-type CD4-efficient environment and that the number of antigen-specific CD8 T cells transferred was limited. Our finding is in fact in agreement with a report by Feng and Britton that purified CD8 T cells from wild-type hosts, upon transfer to immunodeficient mice, provided much less protection from M. bovis BCG challenge in the lung than in the spleen (5).
We believe that our findings enhance our understanding of the mechanisms of T-cell activation and the relative contributions of CD4 and CD8 T-cell subsets to host defense against mycobacterial infection. Such understanding will help with the future development of antimycobacterial vaccines and therapeutics.
Acknowledgments
We are grateful to Jonathan Bramson for his support of this project. We thank Chuyan Ying for help with the transgenic mice. We also acknowledge the provision of M. tuberculosis culture filtrate proteins from Colorado State University through funds from the National Institutes of Health.
This study is supported by research funds from the Canadian Institutes of Health Research.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 16 February 2007.
REFERENCES
- 1.Caruso, A. M., N. Serbina, E. Klein, K. Triebold, B. R. Bloom, and J. L. Flynn. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-γ, yet succumb to tuberculosis. J. Immunol. 162:5407-5416. [PubMed] [Google Scholar]
- 2.Cooper, A. M., et al. 1993. Disseminated tuberculosis in interferon γ gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper, A. M., C. D'Souza, A. A. Frank, and I. M. Orme. 1997. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect. Immun. 65:1317-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derrick, S. C., C. Repique, P. Snoy, A. L. Yang, and S. Morris. 2004. Immunization with a DNA vaccine cocktail protects mice lacking CD4 cells against an aerogenic infection with Mycobacterium tuberculosis. Infect. Immun. 72:1685-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng, C. G., and W. J. Britton. 2000. CD4+ and CD8+ T cells mediate adoptive immunity to aerosol infection of Mycobacterium bovis bacillus Calmette-Guérin. J. Infect. Dis. 181:1846-1849. [DOI] [PubMed] [Google Scholar]
- 6.Fruch, D. M., T. Fukao, C. Bogdan, H. Schindler, J. J. O'Shea, and S. Koyasu. 2001. IFN-gamma production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 22:556-560. [DOI] [PubMed] [Google Scholar]
- 7.Fulton, S. A., T. D. Martin, R. W. Redline, and H. W. Boom. 2000. Pulmonary immune responses during primary Mycobacterium bovis Calmette-Guerin bacillus infection in C57BL/6 mice. Am. J. Respir. Cell Mol. Biol. 22:333-343. [DOI] [PubMed] [Google Scholar]
- 8.Kursar, M., K. Bonhagen, J. Fensterle, A. Kohler, R. Hurwitz, T. Kamradt, S. H. Kaufmann, and H. W. Mittrucker. 2002. Regulatory CD4+ CD25+ T cells restrict memory CD8+ T-cell responses. J. Exp. Med. 196:1585-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ladel, C. H., S. Daugelat, and S. H. Kaufmann. 1995. Immune response to Mycobacterium bovis bacillus Calmette-Guerin infection in major histocompatibility complex class I- and II-deficient knockout mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur. J. Immunol. 25:377-384. [DOI] [PubMed] [Google Scholar]
- 10.Laochumroonvorapong, P., J. Wang, C. C. Liu, W. Ye, A. L. Moreira, K. B. Elkon, V. H. Freedman, and G. Kaplan. 1997. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect. Immun. 65:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazarevic, V., D. Nolt, and J. L. Flynn. 2005. Long-term control of Mycobacterium tuberculosis is mediated by dynamic immune responses. J. Immunol. 175:1107-1117. [DOI] [PubMed] [Google Scholar]
- 12.Locksley, R. M., S. L. Reiner, F. Hatam, D. R. Littman, and N. Killeen. 1993. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science 216:1448-1451. [DOI] [PubMed] [Google Scholar]
- 13.Mogues, T., M. E. Goodrich, L. Ryan, R. LaCourse, and R. J. North. 2001. The relative importance of T-cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocha, B., and C. Tanchot. 2004. Towards a cellular definition of CD8+ T-cell memory: the role of CD4+ T-cell help in CD8+ T-cell responses. Curr. Opin. Immunol. 16:259-263. [DOI] [PubMed] [Google Scholar]
- 15.Saunders, B. M., A. A. Frank, I. M. Orme, and A. M. Cooper. 2002. CD4 is required for the development of a protective granulomatous response to pulmonary tuberculosis. Cell. Immunol. 216:65-72. [DOI] [PubMed] [Google Scholar]
- 16.Serbina, N. V., and J. L. Flynn. 2001. CD8+ T cells participate in the memory immune response to Mycobacterium tuberculosis. Infect. Immun. 69:4320-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serbina, N. V., V. Lazarevic, and J. L. Flynn. 2001. CD4 T cells are required for the development of cytotoxic CD8 T cells during Mycobacterium tuberculosis infection. J. Immunol. 167:6991-7000. [DOI] [PubMed] [Google Scholar]
- 18.Shedlock, D. J., J. K. Whitmire, J. Tan, A. S. MacDonald, R. Ahmed, and H. Shen. 2003. Role of CD4 T-cell help and costimulation in CD8 T-cell responses during Listeria monocytogenes infection. J. Immunol. 170:2053-2063. [DOI] [PubMed] [Google Scholar]
- 19.Smith, S. M., and H. M. Dockrell. 2000. Role of CD8 T cells in mycobacterial infections. Immunol. Cell Biol. 78:325-333. [DOI] [PubMed] [Google Scholar]
- 20.Tascon, R. E., E. Stavropoulos, K. V. Lukacs, and M. J. Colston. 1998. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect. Immun. 66:830-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner, J., A. A. Frank, and I. M. Orme. 2002. Old mice express a transient early resistance to pulmonary tuberculosis that is mediated by CD8 T cells. Infect. Immun. 70:4628-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyznik, A. J., J. C. Sun, and M. J. Bevan. 2004. The CD8 population in CD4-deficient mice is heavily contaminated with MHC class II-restricted T cells. J. Exp. Med. 199:559-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakeham, J., J. Wang, J. Magram, K. Croitoru, R. Harkness, Dunn, A. P. Zganiacz, and Z. Xing. 1998. Lack of both types 1 and 2 cytokines, tissue inflammatory responses, and immune protection during pulmonary infection by Mycobacterium bovis bacillus Calmette-Guérin in IL-12-deficient mice. J. Immunol. 160:6101-6111. [PubMed] [Google Scholar]
- 24.Wang, J., J. Wakeham, R. Harkness, and Z. Xing. 1999. Macrophages are a significant source of type 1 cytokines during mycobacterial infection. J. Clin. Investig. 103:1023-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, J., P. M. Santosuosso, Ngai, A. Zganiacz, and Z. Xing. 2004. Activation of CD8 T cells by mycobacterial vaccination protects against pulmonary tuberculosis in the absence of CD4 T cells. J. Immunol. 173:4590-4597. [DOI] [PubMed] [Google Scholar]
- 26.Xing, J. Z., Wang, K. Croitoru, and J. Wakeham. 1998. Protection by CD4 or CD8 T cells against pulmonary Mycobacterium bovis bacillus Calmette-Guérin infection. Infect. Immun. 66:5537-5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing, Z., A. Zganiacz, and M. Santosuosso. 2000. Role of IL-12 in macrophage activation during intracellular infection: IL-12 and mycobacteria synergistically release TNF-α and nitric oxide from macrophages via IFN-γ induction. J. Leukoc. Biol. 68:897-902. [PubMed] [Google Scholar]
- 28.Xing, Z., A. Zganiacz, J. Wang, and S. K. Sharma. 2001. Enhanced protection against fatal mycobacterial infection in SCID beige mice by reshaping innate immunity with IFN-γ transgene. J. Immunol. 167:375-383. [DOI] [PubMed] [Google Scholar]
- 29.Zganiacz, A., M. Santosuosso, J. Wang, T. Yang, L. Chen, M. Anzulovic, S. Alexander, B. Gicquel, Y. Wan, J. Bramson, M. Inman, and Z. Xing. 2004. TNFα is a critical negative regulator of type I immune activation during intracellular bacterial infection. J. Clin. Investig. 113:401-413. [DOI] [PMC free article] [PubMed] [Google Scholar]