Abstract
Infection with Leishmania major is enhanced when the sand fly Lutzomyia longipalpis salivary peptide maxadilan (MAX) is injected along with the parasite. Here we determined the effect that MAX has on the secretion of cytokines and nitric oxide (NO) and on parasite survival in macrophages (MΦs). The cytokines produced by MΦs can enhance a type 1 response, which will increase NO and the killing of intracellular pathogens such as L. major, or a type 2 response, leading to antibody production that is ineffective against intracellular pathogens such as L. major. A mouse macrophage cell line (RAW 264.7) was stimulated with various concentrations of MAX and lipopolysaccharide (LPS), and the supernatants were collected after 1, 2, and 3 days. Supernatants were assayed for interleukin-12p70 (IL-12p70), IL-10, IL-6, transforming growth factor β (TGF-β), NO, and tumor necrosis factor alpha (TNF-α). Our results indicate that the addition of MAX upregulates the cytokines associated with a type 2 response (IL-10, IL-6, and TGF-β) but downregulates type 1 cytokines (IL-12p70 and TNF-α) and NO. MAX was also added to L. major-infected mouse peritoneal exudate cells (PECs), and the parasite load increased significantly. The enhanced parasite load correlated with decreased NO production by PECs that were stimulated with LPS and gamma interferon in the presence of MAX. The ability of MAX to foster a type 2 response, to enhance parasite survival, and to decrease NO argues that MAX may be crucial for the early survival of Leishmania in the vertebrate host, and therefore, MAX holds considerable promise as an antigenic component for a vaccine against Leishmania.
Leishmaniasis is a parasitic disease caused by members of the genus Leishmania. Leishmania major is transmitted to its host in vector saliva when the sand fly probes for a blood meal. The parasite evades host immunity likely through vector salivary factors and survival inside parasitophorous vacuoles in macrophages (MΦs) (30). Leishmaniasis is estimated to be responsible for more than 80,000 deaths per year and is found in portions of 88 countries within Central America, South America, Africa, India, the Middle East, Asia, and southern Europe and around the Mediterranean, with 2 million new cases registered per year (8). The high incidence of this disease, with its disfiguring open cutaneous or mucosal sores and fatal visceral infections, led the World Health Organization to include it among the six most serious vector-borne diseases for which an effective vaccine should be developed (http://www.who.int/en/).
Vaccination is frequently the most efficacious and cost-effective method for preventing disease. In the case of leishmaniasis, however, inoculation with live, wild-type L. major remains the only truly successful vaccine strategy for humans. The safety concerns surrounding the development of ulcerating primary lesions that are slow to heal have made this procedure a nonviable option (17). There are 20 different species of Leishmania that are transmitted by at least 30 different sand fly species, making vaccines targeting the parasites extremely complex, and thus far this approach has not been successful (http://www.who.int/en/). Chemotherapies for leishmaniasis are expensive and toxic, and the emergence of drug resistance demands a vaccine or an alternative control measure.
The role of sand fly saliva in the transmission of the disease was originally investigated by injecting mice with L. major parasites in the presence of homogenized salivary glands from Lutzomyia longipalpis (29). This procedure resulted in cutaneous lesions from L. major infection that were on average 5 to 10 times larger and contained as much as 5,000-fold more parasites than those of mice injected with the parasite alone (29). The enhancing effects of sand fly saliva on leishmaniasis are associated with its ability to selectively inhibit several MΦ functions, including antigen presentation and nitric oxide (NO) production and thus the ability of MΦs to kill intracellular L. major (26, 33). However, the molecule(s) responsible for these effects is unknown.
The search for the sand fly salivary molecule that could mediate such immunomodulation resulted in the isolation and characterization of the most potent vasodilatory peptide known to date, maxadilan (MAX) (6, 10, 11). MAX, a 63-amino-acid peptide, exacerbates infection with L. major to the same degree as whole saliva, and vaccinating against MAX protects mice against infection with L. major (18). Antibodies against MAX block vasodilation and the amount of blood ingested and the number of eggs laid by sand flies (16).
Since (i) MAX exacerbates infection with L. major (18), (ii) MAX appears to foster a type 2 immune response (26), and (iii) type 2 immune responses exacerbate infection with L. major (1, 20, 22), we hypothesized that MAX would promote a type 2 response in the parasite's principal host cell type, the MΦ. To test this hypothesis, we investigated the effects of MAX on cytokine secretion, NO production, and parasite survival in both RAW 264.7 cells (an Abelson's leukemia virus-transformed MΦ cell line derived from BALB/c mice) and peritoneal exudate cells (PECs) from C3H mice. We found that MAX promoted a type 2 response but inhibited a type 1 response, which correlated with a reduced production of NO and increased survival of L. major in MΦs.
MATERIALS AND METHODS
RAW cells.
RAW 264.7 MΦs from the American Type Culture Collection, Manassas, VA, were cultured in Dulbecco's modified Eagle's medium (14) containing 5% fetal bovine serum (Gemini, West Sacramento, CA). MΦs were plated at 5 × 105 cells per ml onto 24-well tissue culture plates (catalog number 3526; Corning Inc., Corning, NY), and plates were incubated at 37°C for 48 h. Plated cells were rinsed, and dilutions of MAX (0, 2, 10, and 50 ng of MAX1.1/ml; Global Peptides, Fort Collins, CO) were added. The cells were incubated for 1 h at 37°C, and lipopolysaccharide (LPS) dilutions (0, 10, 50, 100, and 5,000 ng/ml) were added. The LPS used was Escherichia coli 055:B5 LPS (Difco, Detroit, MI). Supernatants were collected at 24, 48, and 72 h, and enzyme-linked immunosorbent assays (ELISAs) were performed to determine cytokine profiles.
Synthetic MAX.
The MAX utilized in the experiments described herein was synthesized as a 63-amino-acid polypeptide by Global Peptides (Fort Collins, CO) by using a proprietary synthetic procedure.
ELISAs.
ELISAs were performed according to the protocols of the OptEIA ELISA kit manufacturer (BD Biosciences, San Jose, CA) by using supernatants collected at 1, 2, and 3 days for the analysis of interleukin-6 (IL-6), IL-10, IL-12p70, tumor necrosis factor alpha (TNF-α), and transforming growth factor β (TGF-β). Results were graphed and recorded using an Emax Precision microplate reader (Molecular Devices Corp., Sunnyvale, CA) and the SoftMax Pro (Molecular Devices Corp., Sunnyvale, CA).
NO assay.
NO assays were performed using the Griess reaction with a reagent obtained from Sigma (St. Louis, MO; catalog number G4410-10G) as described previously (31). Results were recorded on an Emax ELISA plate reader by using SoftMax Pro.
Mice.
C3H/HeN mice were originally obtained as breeding pairs from Jackson Laboratories (Bar Harbor, ME). The animals were then bred at the Laboratory Animal Resources facility at Colorado State University, Fort Collins, CO. The maintenance and care of all experimental animals complied with National Institutes of Health guidelines (under pathogen-free conditions) for the humane use of laboratory animals and institutional policies. Female mice (6 to 8 weeks of age) were used in all experiments.
L. major parasites.
L. major promastigotes (LV39, RHO/SU/59/P, Neal, or P strain) were maintained as described previously (28). Briefly, when used in experiments, the parasites were harvested from stationary-phase cultures (23). The virulence of L. major parasites was maintained by infecting mice with the parasites and reisolating these virulent parasites from the infected mice.
Mouse PEC cultures.
PECs were derived as described elsewhere (27). Briefly, C3H/HeN mice were injected intraperitoneally with a 3% thioglycolate solution. Four days later, the cells were harvested from the peritoneums and used in experiments. Typically, the purity of macrophages in these cultures is >95% (3). Mouse gamma interferon (IFN-γ) used was a recombinant protein (catalog number 554587; BD Biosciences Pharmingen, San Diego, CA).
When used to generate NO, PECs were cultured in 24-well plates. Cultures consisted of 3 × 105 PECs in 1 ml of Dulbecco's modified Eagle's medium-5% fetal bovine serum. PECs were pretreated for 2 h with 3 ng of MAX/ml (determined to be optimal in pilot experiments) before stimulation with IFN-γ and/or LPS (from E. coli 055:B5) at the optimal dose for each. The doses of IFN-γ and LPS used to stimulate PECs, as well as the days that culture supernatants were assayed for NO, are indicated below.
When infected with L. major, PECs were cultured in 24-well plates that contained sterile glass coverslips to which the cells adhere (27). Cultures consisted of 3 × 105 PECs in 1 ml of medium. The PECs were infected with 3 L. major parasites/PEC, and the cultures were incubated overnight. At this time, extracellular parasites were washed from the PECs and the PECs were pretreated for 2 h with 3 ng of MAX/ml before stimulation with IFN-γ and LPS. On days 1, 2, and 3, cells were harvested and stained with Dip Quick stain according to the instructions of the manufacturer (Jorgenson Laboratories, Loveland, CO) and the numbers of intracellular L. major parasites were determined by light microscopy.
Finally, in some experiments, NO levels in cultures of infected PEC MΦs were determined. This experimental approach was examined in order to determine whether the inhibition of NO production directly correlated with the inability of the infected MΦs to kill L. major.
Statistical analyses.
Statistical analyses were performed using Simple Interactive Statistical Analysis (Quantitative Skills; http://home.clara.net/sisa/index.htm). All data were analyzed using paired two-tailed t tests. A P value of <0.05 was considered significant. In all experiments, the means shown are an average of results for three assay wells, and all experiments were performed by three independent researchers at least twice and as many as five times total.
RESULTS
MAX inhibits production of NO by RAW 264.7 cells treated with MAX and 100 ng of LPS/ml.
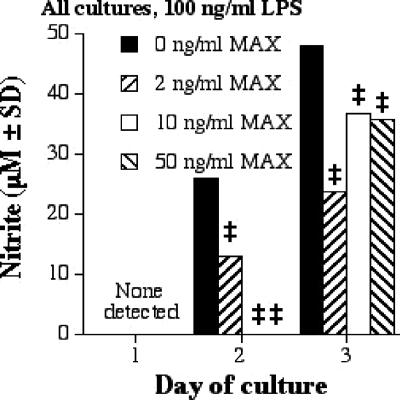
The production of NO correlates with the killing of L. major (6). Figure 1 shows that MAX decreased NO production by RAW 264.7 cells stimulated with 100 ng of LPS/ml. At 1 day poststimulation, NO could not be detected; however, a significant inhibition of NO was achieved on days 2 and 3 (Fig. 1; Table 1). Similar levels of NO inhibition at other concentrations of LPS were seen.
FIG. 1.
NO production by RAW 267.4 cells stimulated with 100 ng of LPS/ml and various concentrations of MAX (0, 2, 10, and 50 ng/ml). Cells were plated at 5 × 105 cells per ml; supernatants were collected after 1, 2, and 3 days; and a NO assay was performed. Significant differences in levels of NO production are indicated in the figure (comparing MAX-treated cells to untreated cells) as follows: ‡, P = 0.001. SD, standard deviation.
TABLE 1.
Effects of LPS and MAX on cytokine and NO secretion and parasite survivala
| Cell type and molecule or factor assessed | Corresponding figure no. | Amt of LPS (ng/ml) | Day | % Inhibition (↓) or augmentation (↑) | Amt of MAXb (ng/ml) | P value |
|---|---|---|---|---|---|---|
| RAW 264.7 cells | ||||||
| NO | 1 | 100 | 1 | NA | 0-50 | NA |
| 1 | 100 | 2 | 99.5↓ | 0-50 | <0.001 | |
| 1 | 100 | 3 | 50↓ | 0-2 | <0.001 | |
| IL-10 | 2 | 5,000 | 1 | 33↑ | 0-50 | <0.046 |
| 2 | 5,000 | 2 | >100↑ | 0-50 | <0.001 | |
| 2 | 5,000 | 3 | NA | 0-50 | NA | |
| IL-6 | 3a | 0 | 1 | NA | 0-50 | NA |
| 3a | 0 | 2 | 35↑ | 0-50 | NS | |
| 3a | 0 | 3 | 30↑ | 0-10 | <0.05 | |
| 3b | 5,000 | 1 | 47↑ | 0-2 | <0.005 | |
| 3b | 5,000 | 2 | 74↑ | 0-50 | <0.002 | |
| 3b | 5,000 | 3 | 45↑ | 0-50 | <0.03 | |
| TGF-β | 4 | 5,000 | 1 | 17↑ | 0-50 | NS |
| 4 | 5,000 | 2 | 16↑ | 0-50 | NS | |
| 4 | 5,000 | 3 | 47↑ | 0-50 | <0.02 | |
| TNF-α | 5 | 0 | 1 | 90↓ | 0-50 | <0.005 |
| 5 | 0 | 2 | 75↓ | 0-50 | <0.03 | |
| 5 | 0 | 3 | 91↓ | 0-2 | <0.05 | |
| IL-12p70 | 6 | 0 | 1 | 87↓ | 0-50 | <0.05 |
| 6 | 0 | 2 | 68↓ | 0-50 | <0.03 | |
| 6 | 0 | 3 | NA | 0-50 | NA | |
| PECs | ||||||
| NOc | 7 | 100 | 1 | NA | 3 | NA |
| 7 | 100 | 2 | 68↓ | 3 | <0.004 | |
| 7 | 100 | 3 | 93↓ | 3 | <0.002 | |
| Intracellular L. major parasitesc | 8 | 100 | 1 | 49↑ | 3 | <0.005 |
| 8 | 100 | 2 | 60↑ | 3 | <0.01 | |
| 8 | 100 | 3 | 78↑ | 3 | <0.002 |
NA, not applicable; NS, not significant.
Comparisons were made between cultures treated with no MAX and those treated with the concentration of MAX that had the most significant cytokine effect.
Comparisons were made between cultures treated with either IFN-γ and LPS or IFN-γ, LPS, and MAX.
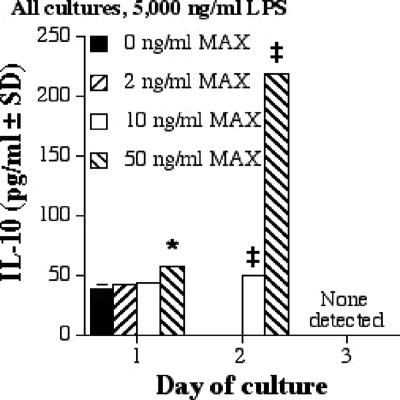
MAX enhances IL-10 production by RAW 264.7 cells treated with 5,000 ng of LPS/ml.
RAW cells were stimulated with a range of concentrations (0, 2, 20, and 50 ng/ml) of MAX and 5,000 ng of LPS/ml, and ELISAs were conducted to determine the amounts of type 1 (TNF-α and IL-12p70) and type 2 (IL-10, IL-6, and TGF-β) cytokines secreted. IL-10 has been linked to decreases in the production of IL-12 and TNF-α, ultimately promoting the progression of leishmaniasis in BALB/c mice (9). At 5,000 ng of LPS/ml, IL-10 production increased as the MAX concentration increased (Fig. 2; Table 1). Other doses of LPS corresponded to similar trends (data not shown).
FIG. 2.
IL-10 production by RAW 267.4 cells stimulated with 5,000 ng of LPS/ml and various concentrations of MAX (0, 2, 10, and 50 ng/ml). Cells were plated at 5 × 105 cells per ml; supernatants were collected after 1, 2, and 3 days; and ELISAs were performed. Significant differences in levels of IL-10 production are indicated in the figure (comparing MAX-treated cells to untreated cells) as follows: *, P < 0.05; ‡, P = 0.001. SD, standard deviation.
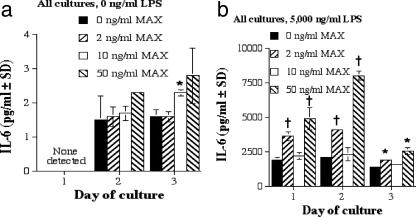
MAX enhances IL-6 production by RAW 264.7 cells treated with LPS at 0 and 5,000 ng/ml.
IL-6 is a proinflammatory cytokine that has been shown to suppress IFN-γ and TNF-α activation of murine MΦs necessary for killing of Leishmania amazonensis (7). As seen in Fig. 3a, IL-6 production by RAW 267.4 cells was significantly enhanced as a function of increasing concentrations of MAX and LPS. Furthermore, an even greater amount of IL-6 secretion occurred in the presence of 5,000 ng of LPS/ml (Fig. 3b). These data support the idea that MAX not only affects the levels of IL-6 secretion in resting MΦs but also augments IL-6 production by activated cells. Results from three experiments are summarized in Table 1.
FIG. 3.
IL-6 production by RAW 267.4 cells. (a) IL-6 production by RAW 267.4 cells with 0 ng of LPS/ml and various concentrations of MAX (0, 2, 10, and 50 ng/ml). (b) IL-6 production by RAW 267.4 cells stimulated with 5,000 ng of LPS/ml and various concentrations of MAX (0, 2, 10, and 50 ng/ml). Cells were plated at 5 × 105 cells per ml; supernatants were collected after 1, 2, and 3 days; and ELISAs were performed. Significant differences in levels of IL-6 production are indicated in the figure (comparing MAX-treated cells to untreated cells) as follows: *, P < 0.05; †, P = 0.005. SD, standard deviation.
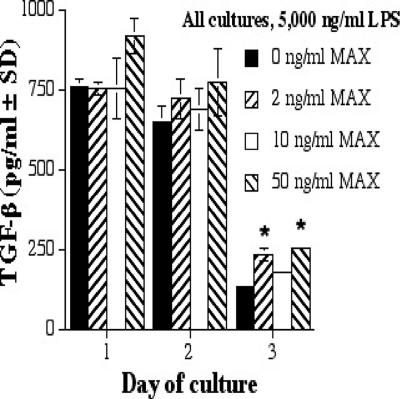
MAX augments TGF-β production by RAW 264.7 cells treated with 5,000 ng of LPS/ml.
TGF-β is a type 2-associated cytokine that is a potent suppressor of nitric oxide synthetase 2 expression in mouse MΦs (12, 13). At 5,000 ng of LPS/ml, TGF-β production was enhanced as the concentration of MAX increased (Fig. 4; Table 1). A common finding was that TGF-β production increased as LPS and MAX levels increased (data not shown).
FIG. 4.
TGF-β production by RAW 267.4 cells with 5,000 ng of LPS/ml and various concentrations of MAX (0, 2, 10, and 50 ng/ml). Cells were plated at 5 × 105 cells per ml; supernatants were collected after 1, 2, and 3 days; and ELISAs were performed. Significant differences in levels of IL-6 production are indicated in the figure (comparing MAX-treated cells to untreated cells) as follows: *, P < 0.05. SD, standard deviation.
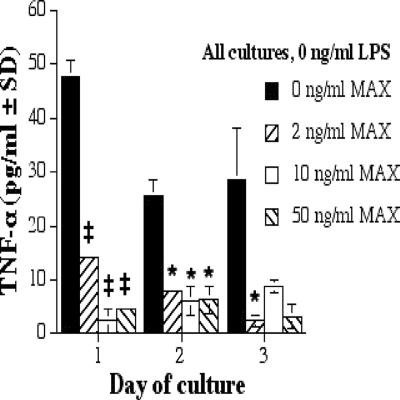
MAX inhibits TNF-α production by RAW 264.7 cells.
TNF-α is a type 1 inflammatory cytokine that is a member of a group of molecules that stimulate the acute-phase reaction. TNF-α secretion correlates with increased production of NO (19). TNF-α was inhibited as the MAX concentration increased in the absence of LPS (Fig. 5; Table 1).
FIG. 5.
TNF-α production by RAW 267.4 cells with 0 ng of LPS/ml and various concentrations of MAX (0, 2, 10, and 50 ng/ml). Cells were plated at 5 × 105 cells per ml; supernatants were collected after 1, 2, and 3 days; and ELISAs were performed. Significant differences in levels of TNF-α production are indicated in the figure (comparing MAX-treated cells to untreated cells) as follows: *, P < 0.05; ‡, P = 0.001. SD, standard deviation.
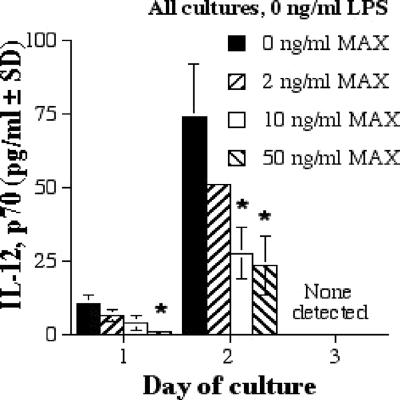
MAX downregulates IL-12p70 production by RAW 264.7 cells.
We also chose to look at IL-12p40 and the dimerized form, IL-12p70, because it has been suggested that they are differentially expressed in Leishmania infections (2, 5). Moreover, IL-12p70 protects against intracellular pathogens and is considered a key cytokine in type 1-induced inflammation (1, 20, 22). In RAW 264.7 cells, IL-12p70 production decreased as the MAX level increased in the absence of LPS (Fig. 6; Table 1). IL-12p70 production was not influenced by MAX in the presence of LPS, and IL-12p40 was not affected by any concentration of LPS or MAX (data not shown).
FIG. 6.
IL-12p70 production by RAW 267.4 cells with 0 ng of LPS/ml and various concentrations of MAX (0, 2, 10, and 50 ng/ml). Cells were plated at 5 × 105 cells per ml; supernatants were collected after 1, 2, and 3 days; and ELISAs were performed. Significant differences in levels of IL-12 production are indicated in the figure (comparing MAX-treated cells to untreated cells) as follows: *, P < 0.05. SD, standard deviation.
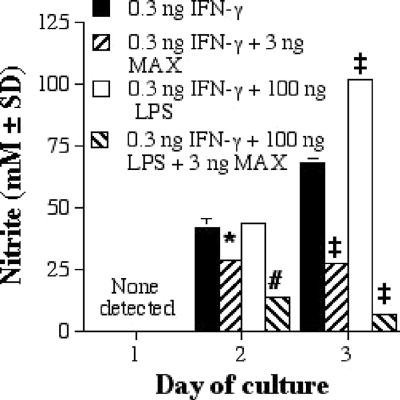
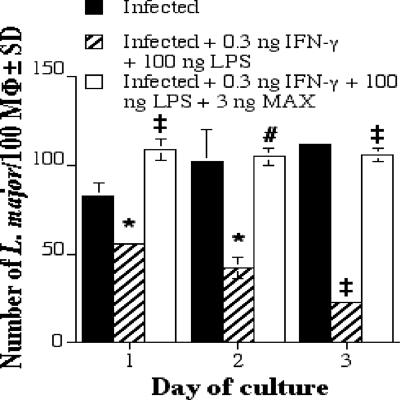
MAX inhibits the destruction of L. major within PEC MΦs, and this inhibition correlates with an inability of the MΦs to produce NO.
In the experiments described above, RAW 264.7 cells were used because these cells are an easily cultured and controlled homogeneous cell line and because these cells have been used with the human MAX homolog, pituitary adenylate cyclase-activating peptide (PACAP), to address similar questions regarding cytokine production. However, to confirm and extend the results presented here and to make them directly comparable to findings of previous studies from this laboratory (6, 21, 24), we felt it important to repeat the most significant result presented above (the inhibition of NO production) by using mouse PEC MΦs and to determine whether this inhibition of NO production resulted in an inhibition of the killing of L. major by PEC MΦs. We found that MAX inhibited the IFN-γ (with or without LPS)-dependent secretion of NO by mouse PECs (Fig. 7; Table 1). Moreover, we found that MAX also significantly enhanced the intracellular L. major burden of PEC MΦs (Fig. 8; Table 1).
FIG. 7.
Nitrite (mM) production by mouse PECs incubated with MAX and stimulated with IFN-γ with or without LPS. Cells were plated at 3 × 105 PECs per ml; supernatants were collected at 1, 2, and 3 days; and a NO assay was performed. Significant differences in levels of NO production are indicated in the figure as follows: *, P < 0.05; #, P < 0.01; ‡, P = 0.001. SD, standard deviation.
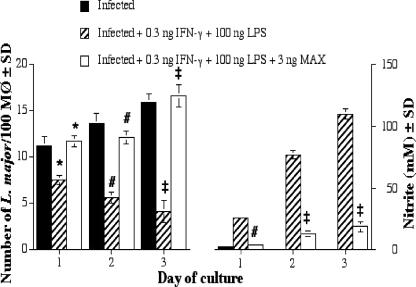
FIG. 8.
The number of L. major parasites per 100 mouse PEC MΦs. Cells were plated at 3 × 105 PECs per ml; coverslips were harvested at 1, 2, and 3 days; and the coverslips were stained. The numbers of intracellular L. major parasites were determined by light microscopy. Significant differences in parasite survival rates are indicated in the figure as follows: *, P < 0.05; #, P < 0.01; ‡, P = 0.001. SD, standard deviation.
To extend these observations, we also simultaneously monitored parasite burdens within PEC MΦs and NO production by those MΦs after stimulation with IFN-γ and LPS in cultures treated or not treated with MAX. We found that the inability of MΦs to kill L. major correlated with the inability of the cells to produce NO (Fig. 9). Both of these effects were significant (0.05 > P > 0.0005) (Fig. 9).
FIG. 9.
The inability of MAX-treated PEC MΦs to kill L. major correlates with an inability of the cells to produce NO. Cells were plated at 3 × 105 PECs per ml, and coverslips and culture supernatants were harvested at 1, 2, and 3 days. The numbers of intracellular L. major parasites were determined by light microscopy, and NO assays were run on the supernatants. Significant differences in parasite survival rates and levels of NO production are indicated in the figure as follows: *, P < 0.05; #, P < 0.01; ‡, P = 0.001. SD, standard deviation.
Collectively, these results are consistent with our hypothesis that MAX increases type 2 cytokines (IL-6, IL-10, and TGF-β), decreases type 1 cytokines (IL-12p70 and TNF-α), inhibits NO production, and ultimately allows for the survival of intracellular parasites such as L. major.
DISCUSSION
Infected sand flies transmit Leishmania within their saliva as they feed on the vertebrate host for a blood meal. Sand fly salivary components have been shown to modulate a large number of host immune system defenses—innate, inflammatory, and adaptive (26). In addition, arthropods have developed salivary vasodilators, anticoagulants, and anticomplement factors that enhance their ability to obtain a blood meal (26). As a result of these observations, two laboratories have almost simultaneously hypothesized and reported that immunizing against components of sand fly salivary glands can protect against infection with Leishmania in conjunction with sand fly saliva (18, 32).
In this report, we have assessed the effects of MAX on the host immune response. We report here that MAX can modulate a number of immune system functions that are likely to be important to the host's successful response to infection with Leishmania. Specifically, we have analyzed the effect of MAX on MΦs, the principal host cell for Leishmania.
We found in all experimental approaches examined the same all-encompassing result: the effects of MAX are to increase type 2 cytokine production while inhibiting type 1 cytokines, which results in decreased NO production by MΦs and, as a result, an increased parasite load within MΦs (Table 1). It is also interesting that MAX was almost always able to significantly inhibit or augment the production of cytokines or NO at all time points tested, even at time points when the maximum levels of cytokines or NO were produced. For example, as shown in Fig. 1, NO was inhibited throughout the experiment, including at 72 h when maximum levels of NO were secreted. Similar results were seen in vivo since the ability of MAX to exacerbate infection with L. major was still quite apparent even when MAX was injected 96 h before the parasite was injected (25). Thus, MAX clearly has pronounced and persistent effects both in vitro and in vivo. This overall effect of MAX may explain its exacerbative effects on infection with L. major in mice (18) since type 2 responses always lead to exacerbative effects on infection with L. major while type 1 immune responses lead to the resolution of infection (1, 20, 22).
MAX makes up 1 to 2% of the total protein in New World sand fly saliva and exhibits a range of immunomodulatory activities by acting as a PACAP homolog and interacting with the PACAP type 1 receptor on MΦs and other cells (6, 24). Interestingly, PACAP has several immunomodulatory effects on the RAW 264.7 cells used in this study. For example, similar to MAX, PACAP has repeatedly been shown to promote the outgrowth of type 2 cells and cytokines (4).
In this study, we completed our survey of the effects of MAX on murine MΦs. Ongoing experiments in the laboratory are extending these observations to the effects of MAX on human cells, which are very similar to those of MAX on murine cells, but the effects are also unique.
Given that MAX has such dramatic effects on MΦs, the obvious extension of these studies is to examine the effect of MAX on the antigen-presenting cell that is most involved in the activation of host adaptive T-cell immunity, the dendritic cell. Such studies are also already under way in our laboratory.
MAX must be considered as a component of vaccines that target leishmanial diseases given that MAX has been shown to exacerbate infections with several species of Leishmania (18, 26). MAX is also capable of acting as a protective immunogen that can be used to vaccinate against challenge with whole sand fly saliva and L. major (18), and we have found here that MAX detrimentally modulates the response of antigen-presenting cells of the vertebrate host. There is, however, a significant caveat with this approach: MAX is polymorphic (15), and therefore, vaccines utilizing this peptide must attempt to identify common epitopes of the peptide that will circumvent such polymorphism.
In conclusion, we demonstrated that MAX deviates the vertebrate immune system towards type 2 immunity, an activity previously attributed to several other arthopod salivary molecules (26). The mechanism behind this common effect of arthropod saliva is unknown (26). However, regardless of what the consequences of arthropod saliva are, the potential for neutralizing these effects by targeting the molecules in arthropod saliva that are responsible offers a new and exciting approach towards controlling arthropod-borne diseases.
Acknowledgments
This research was supported by an RO1 AI65784 grant.
We thank Bill Wheat, Keith Nelson, Jeanette Bishop, and Santiago Mejia for their help with experiments as well as with the writing of the manuscript.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 5 March 2007.
REFERENCES
- 1.Belkaid, Y. 2003. The role of CD4(+)CD25(+) regulatory T cells in Leishmania infection. Expert. Opin. Biol. Ther. 3:875-885. [DOI] [PubMed] [Google Scholar]
- 2.Belkaid, Y., B. Butcher, and D. L. Sacks. 1998. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. Eur. J. Immunol. 28:1389-1400. [DOI] [PubMed] [Google Scholar]
- 3.Chakkalath, H. R., C. M. Theodos, J. S. Markowitz, M. J. Grusby, L. H. Glimcher, and R. G. Titus. 1995. Class II major histocompatibility complex-deficient mice initially control an infection with Leishmania major but succumb to the disease. J. Infect. Dis. 171:1302-1308. [DOI] [PubMed] [Google Scholar]
- 4.Delgado, M., J. Leceta, and D. Ganea. 2002. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide promote in vivo generation of memory Th2 cells. FASEB J. 16:1844-1846. [DOI] [PubMed] [Google Scholar]
- 5.Gorak, P. M., C. R. Engwerda, and P. M. Kaye. 1998. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 28:687-695. [DOI] [PubMed] [Google Scholar]
- 6.Hall, L. R., and R. G. Titus. 1995. Sand fly vector saliva selectively modulates macrophage functions that inhibit killing of Leishmania major and nitric oxide production. J. Immunol. 155:3501-3506. [PubMed] [Google Scholar]
- 7.Hatzigeorgiou, D. E., S. He, J. Sobel, K. H. Grabstein, A. Hafner, and J. L. Ho. 1993. IL-6 down-modulates the cytokine-enhanced antileishmanial activity in human macrophages. J. Immunol. 151:3682-3692. [PubMed] [Google Scholar]
- 8.Hsia, R., N. E. Wang, and J. Halpern. 2 November 2006, revision date. Leishmaniasis. eMedicine. http://www.emedicine.com/emerg/topic296.htm.
- 9.Kane, M. M., and D. M. Mosser. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 166:1141-1147. [DOI] [PubMed] [Google Scholar]
- 10.Lerner, E. A., J. M. Ribeiro, R. J. Nelson, and M. R. Lerner. 1991. Isolation of maxadilan, a potent vasodilatory peptide from the salivary glands of the sand fly Lutzomyia longipalpis. J. Biol. Chem. 266:11234-11236. [PubMed] [Google Scholar]
- 11.Lerner, E. A., and C. B. Shoemaker. 1992. Maxadilan. Cloning and functional expression of the gene encoding this potent vasodilator peptide. J. Biol. Chem. 267:1062-1066. [PubMed] [Google Scholar]
- 12.Mabley, J. G., J. M. Cunningham, N. John, M. A. Di Matteo, and I. C. Green. 1997. Transforming growth factor beta 1 prevents cytokine-mediated inhibitory effects and induction of nitric oxide synthase in the RINm5F insulin-containing beta-cell line. J. Endocrinol. 155:567-575. [DOI] [PubMed] [Google Scholar]
- 13.MacMicking, J., Q. W. Xie, and C. Nathan. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15:323-350. [DOI] [PubMed] [Google Scholar]
- 14.Maryanski, J. L., J. Van Snick, J. C. Cerottini, and T. Boon. 1982. Immunogenic variants obtained by mutagenesis of mouse mastocytoma P815. III. Clonal analysis of the syngeneic cytolytic T lymphocyte response. Eur. J. Immunol. 12:401-406. [DOI] [PubMed] [Google Scholar]
- 15.Milleron, R. S., J. P. Mutebi, S. Valle, A. Montoya, H. Yin, L. Soong, and G. C. Lanzaro. 2004. Antigenic diversity in maxadilan, a salivary protein from the sand fly vector of American visceral leishmaniasis. Am. J. Trop. Med. Hyg. 70:286-293. [PubMed] [Google Scholar]
- 16.Milleron, R. S., J. M. Ribeiro, D. Elnaime, L. Soong, and G. C. Lanzaro. 2004. Negative effect of antibodies against maxadilan on the fitness of the sand fly vector of American visceral leishmaniasis. Am. J. Trop. Med. Hyg. 70:278-285. [PubMed] [Google Scholar]
- 17.Modabber, F. 1995. Vaccines against leishmaniasis. Ann. Trop. Med. Parasitol. 89(Suppl. 1):83-88. [DOI] [PubMed] [Google Scholar]
- 18.Morris, R. V., C. B. Shoemaker, J. R. David, G. C. Lanzaro, and R. G. Titus. 2001. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J. Immunol. 167:5226-5230. [DOI] [PubMed] [Google Scholar]
- 19.Ritter, U., J. Mattner, J. S. Rocha, C. Bogdan, and H. Korner. 2004. The control of Leishmania (Leishmania) major by TNF in vivo is dependent on the parasite strain. Microbes Infect. 6:559-565. [DOI] [PubMed] [Google Scholar]
- 20.Rogers, K. A., G. K. DeKrey, M. L. Mbow, R. D. Gillespie, C. I. Brodskyn, and R. G. Titus. 2002. Type 1 and type 2 responses to Leishmania major. FEMS Microbiol. Lett. 209:1-7. [DOI] [PubMed] [Google Scholar]
- 21.Rogers, K. A., and R. G. Titus. 2003. Immunomodulatory effects of Maxadilan and Phlebotomus papatasi sand fly salivary gland lysates on human primary in vitro immune responses. Parasite Immunol. 25:127-134. [DOI] [PubMed] [Google Scholar]
- 22.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 23.Sacks, D. L., S. Hieny, and A. Sher. 1985. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J. Immunol. 135:564-569. [PubMed] [Google Scholar]
- 24.Soares, M. B., R. G. Titus, C. B. Shoemaker, J. R. David, and M. Bozza. 1998. The vasoactive peptide maxadilan from sand fly saliva inhibits TNF-alpha and induces IL-6 by mouse macrophages through interaction with the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor. J. Immunol. 160:1811-1816. [PubMed] [Google Scholar]
- 25.Theodos, C. M., and R. G. Titus. 1993. Salivary gland material from the sand fly Lutzomyia longipalpis has an inhibitory effect on macrophage function in vitro. Parasite Immunol. 15:481-487. [DOI] [PubMed] [Google Scholar]
- 26.Titus, R. G., J. V. Bishop, and J. S. Mejia. 2006. The immunomodulatory factors of arthropod saliva and the potential for these factors to serve as vaccine targets to prevent pathogen transmission. Parasite Immunol. 28:131-141. [DOI] [PubMed] [Google Scholar]
- 27.Titus, R. G., A. Kelso, and J. A. Louis. 1984. Intracellular destruction of Leishmania tropica by macrophages activated with macrophage activating factor/interferon. Clin. Exp. Immunol. 55:157-165. [PMC free article] [PubMed] [Google Scholar]
- 28.Titus, R. G., G. C. Lima, H. D. Engers, and J. A. Louis. 1984. Exacerbation of murine cutaneous leishmaniasis by adoptive transfer of parasite-specific helper T cell populations capable of mediating Leishmania major-specific delayed-type hypersensitivity. J. Immunol. 133:1594-1600. [PubMed] [Google Scholar]
- 29.Titus, R. G., and J. M. Ribeiro. 1988. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science 239:1306-1308. [DOI] [PubMed] [Google Scholar]
- 30.Titus, R. G., C. M. Theodos, A. H. Shankar, and L. R. Hall. 1994. Interactions between Leishmania major and macrophages. Immunol. Ser. 60:437-459. [PubMed] [Google Scholar]
- 31.Urioste, S., L. R. Hall, S. R. Telford III, and R. G. Titus. 1994. Saliva of the Lyme disease vector, Ixodes dammini, blocks cell activation by a nonprostaglandin E2-dependent mechanism. J. Exp. Med. 180:1077-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valenzuela, J. G., Y. Belkaid, M. K. Garfield, S. Mendez, S. Kamhawi, E. D. Rowton, D. L. Sacks, and J. M. Ribeiro. 2001. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J. Exp. Med. 194:331-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waitumbi, J., and A. Warburg. 1998. Phlebotomus papatasi saliva inhibits protein phosphatase activity and nitric oxide production by murine macrophages. Infect. Immun. 66:1534-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]