Abstract
Transcutaneous immunization allows safe delivery of native heat-labile enterotoxin (LT) from Escherichia coli via application of a simple patch. Physical disruption of the stratum corneum can improve the efficiency of delivery. In the current study, the stratum corneum was disrupted using an electrocardiogram prep pad prior to patch application. The effects were quantified using transepidermal water loss (TEWL) and were correlated with the immune responses. Sixty adults received 50 μg of LT from three lots of LT (20 adults per group) administered in a patch on days 0 and 21. The immunizations were well tolerated. There were no differences in the anti-LT immunoglobulin G (IgG) titers between the three LT lots; the seroconversion rate was 100%, and the mean anti-LT IgG titer was 12,185 enzyme-linked immunosorbent assay units (EU) (a 24-fold increase). TEWL measurements obtained at the time of the second immunization were found to correlate with the day 42 individual increases in the anti-LT IgG titer (r = 0.59, P < 0.001). In a comparative assessment of the immune responses, sera after an LT+ ST+ (E2447A) oral ETEC challenge, which induced moderate to severe diarrhea in 81% of the recipients, had anti-LT IgG titers of 3,245 EU (a 10.8-fold increase). Similarly, the anti-LT IgG titer after administration of an oral cholera toxin B subunit-containing cholera vaccine, which cross-reacts with LT and protects against LT and LT/heat-stable toxin ETEC disease in the field, was 6,741 EU (a 3.3-fold increase). This study confirmed that a well-tolerated regimen for stratum corneum disruption before vaccine patch application results in robust immunity comparable to natural immunity and vaccine-induced immunity and that the magnitude of stratum corneum disruption correlates with the immune response.
Enterotoxigenic Escherichia coli (ETEC) produces a toxin-mediated, secretory diarrhea that is common in warm climates and is associated with fecal contamination of food and water. It is estimated that ETEC strains cause more than 200 million cases of diarrhea per year and an estimated 380,000 deaths in children less than 5 years old per year (32). ETEC is the most common cause of traveler's diarrhea and is responsible for 30 to 50% of all traveler's diarrhea (1).
ETEC disease is mediated by two toxins: the heat-labile enterotoxin (LT) of E. coli, an 86,000-Da protein, and the heat-stable protein (ST), an 18-amino-acid, highly folded peptide. LT has a pentameric B subunit that binds to GM1 ganglioside receptors in the intestinal epithelium, resulting in entry into the cell, and an enzymatically active A subunit that is cleaved by a trypsin-like enzyme, producing an A1 fragment. The ADP-ribosylating activity of A1 leads to activation of adenyl cyclase, increased levels of cAMP, efflux of chloride ions, and watery diarrhea (9). Cholera toxin (CT) exhibits more than 82% amino acid homology with LT, has a strikingly similar AB5 structure, and produces diarrhea by a nearly identical pathogenic pathway (31). The second ETEC toxin, ST, binds to a separate receptor but causes a similar disruption of chloride channels in the cell and secretory diarrhea. The two ETEC pathogenic factors, LT and ST, are logical vaccine targets; however, ST is poorly immunogenic due to its small size. By contrast, native LT is highly immunogenic but has been difficult to deliver as a vaccine antigen because of its toxicity. LT is expressed in up to 66% of ETEC strains in areas where ETEC is endemic, either alone or in combination with ST (36), and thus is responsible for a significant worldwide disease burden for both travelers and children in the developing world.
It is clear from many lines of evidence that immune responses to ETEC can confer protection against diarrheal disease. In developing countries, ETEC illness decreases over time (2), and in long-term travelers illness decreases with the length of stay in an area where ETEC is endemic (6). Furthermore, an experimental challenge with an ETEC strain protects against rechallenge (7, 22). Development of a vaccine for ETEC is complicated by the large number of antigenic targets and varied distribution of serotypes with numerous O and H antigens (15, 26, 35). The other potential antigens that have been identified include colonization factor antigens and fimbrial adhesions, which attach to the intestinal epithelium. Attempts have been made to develop a multivalent colonization factor antigen-based vaccine, but such vaccines require up to six antigens to achieve coverage for the majority of ETEC strains. LT (either alone or with ST) is a highly immunogenic antigen that is expressed by 50 to 60% of ETEC strains in areas where ETEC is endemic and thus is a potential vaccine candidate that could protect against heterogeneous ETEC strains. Each year, there are an estimated 10 million cases of LT-related ETEC traveler's diarrhea and more than 200 million cases of weanling diarrhea that may be related to LT (32).
Antitoxin antibodies have been shown to play a key role in protection against ETEC disease in the field (4, 23, 24, 27; A. L. Bourgeois, J. Halpern, B. Gustafsson, A. M. Svennerholm, O. Torres, J. Belkind-Gerson, and D. A. Sack, presented at the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 2005). In these previous studies, the homologous and cross-reacting cholera toxin B subunit (CTB) was used as an oral vaccine antigen because the native LT cannot be safely delivered orally or nasally. Transcutaneous immunization (TCI) (delivery of vaccine antigens to the skin) is an immunization method that allows safe delivery of the native LT to the immune system. The skin is densely populated with antigen-presenting cells that can be readily accessed using transcutaneous delivery methods. LT delivered to the outer layers of the skin can safely induce antitoxin antibodies. In previous studies, patches were applied to the skin without physical disruption of the stratum corneum (12, 13). In a pilot study, several classic methods for skin pretreatment suggested that stratum corneum disruption allows more efficient delivery (11), but the relationship between stratum corneum disruption and delivery was not studied. Here, we present data describing standard dermatologic methods for quantitation of stratum corneum disruption (8) after a medical-grade electrocardiogram (EKG) skin pretreatment pad was used to partially remove the stratum corneum. The subsequent immune responses to LT delivered by TCI were compared to anti-LT responses after a challenge with live infectious ETEC and after administration of an oral cholera vaccine that conferred protection in the field (4, 27).
MATERIALS AND METHODS
In this open-label, randomized study we evaluated the safety and immunogenicity of TCI using three different manufactured lots of LT. LT was obtained from Berna Biotech (Berne, Switzerland). Group 1 in this study received resuspended lyophilized LT, while groups 2 and 3 received buffered aqueous LT.
The study was conducted by 3ClinicalResearch in Berlin, Germany. A local independent ethics committee, Landesaertzekammer Brandenburg (Berlin, Germany), reviewed and approved the final protocol and associated documents. Recruitment occurred in May 2004, and vaccinations were given in May and June 2004.
Selection of participants.
Sixty healthy adult males and females who were 18 to 40 years old inclusive were recruited from Berlin and the surrounding environs. Subjects signed written informed consent prior to enrollment in the study. Subjects were not eligible for participation if any clinically significant abnormalities were found in their medical histories (including immunocompromising conditions), during physical examination, or in blood and urine tests (e.g., serum chemistry, hematology, and urinalysis). A randomization list with block sizes of two, generated by Amarex Inc., Germantown, MD, was used to assign study participants to groups that received one of the three lots of LT by TCI. Group allocation, based on time of arrival at the clinic, was performed by the study site nurse, and group assignments remained the same throughout the duration of the study.
Exclusion criteria.
The exclusion criteria included clinically significant abnormalities in hematology, serum chemistry, urinalysis, or a physical exam for allergies to any component of the vaccine (including adhesives); receipt of an investigational product within 30 days of the first vaccination; treatment with investigational ETEC LT, LT (R192G), or Nasalflu; receipt of CT or cholera vaccine (e.g., Orochol or Dukoral); a history of traveler's diarrhea within the previous 2 years; a history of acute or chronic skin disease; active skin allergy; recent or regular use of oral or systemic steroid medications; evidence of immunosuppression; recent or regular use of immunosuppressive oral or systemic steroid medications (inhaled steroids were allowed); concomitant immunosuppressive therapy (e.g., systemic treatment for cancer, diabetes, end-stage renal disease, etc.); use of topical steroid medications; a positive test for human immunodeficiency virus type 1 or human immunodeficiency virus type 2 antibodies, HBsAg, or hepatitis C virus antibodies; abnormal coagulation; acute skin infection, sunburn, or skin abnormalities on the upper arms, including fungal infections, severe acne, or active contact dermatitis; a fever of >37.5°C during screening or at the time of planned vaccination; alcohol or substance abuse; blood or blood product donation in the previous month; pregnancy or breast feeding; employment at the investigational site or a family member of the investigational site staff; and a history of achlorhydria. Women who were not postmenopausal or surgically sterile were required to have a negative pregnancy test at the time of screening and within 24 h of each vaccination and were required to use an effective form of birth control for the duration of the study.
Vaccination procedure.
Eligible subjects received two TCI vaccinations consisting of 50 μg LT 21 days apart. A 5- by 5-cm area on the deltoid was marked at four corners with a marking pen. A medical-grade, nonwoven, abrasive EKG prep pad (Jupiter Medical, Florida) was dipped into a 10% glycerol-70% isopropanol solution and then stroked 15 times over the marked area. LT suspended in phosphate-buffered saline (50 μg in 150 μl) was pipetted onto a 5-cm2 gauze patch, covered with an occlusive overlay, and secured with a Tegaderm patch. Patches were worn for 6 to 8 h.
Subjects were observed for any acute reactions, and patch adhesion was checked 30 min after vaccination. Subjects were then released from the clinic with a diary card to record local and systemic reactions that occurred during the 3 weeks following vaccination. The solicited adverse events included pain, pruritus, erythema, and swelling at the vaccination site, fever (body temperature of >37.5°C), headache, malaise, and diarrhea.
LT potency.
For the LT lots used in this study, an IOMAI LT stability program regularly assessed the functional activity of the LT, which induces rounding and nonadherence of Y-1 adrenal cells. This assay was performed by adding a suspension of Y-1 cells to serially diluted samples of LT in a 96-well tissue culture plate, which was then incubated for 1 day at 37°C in the presence of 5% CO2. Cells were stained with neutral red, and the tissue culture plates were washed to remove cells affected by LT. After washing, neutral red was released from the cells by addition of an extraction solution, and the plates were read with a 96-well UV-visible microtiter plate reader. The results were then plotted, and four-parameter dose-response curves were generated. The 50% effective dose (ED50) was determined by using optical densities at 530 nm (OD530) that correlated with the numbers of adherent cells in wells after washing. Finally, LT potency was expressed as the log10 ED50 per μg of LT. No potency differences were observed in the three lots.
Challenge study.
In an unrelated vaccine trial, 16 unvaccinated control subjects were challenged with E24377A, an LT+ ST+ strain isolated from a traveler returning from Egypt with ETEC disease (3). For this study, the subjects were admitted to the General Clinical Research Center at Johns Hopkins Hospital. The next morning following a light breakfast and a 90-min fast, the subjects drank 120 ml of sodium bicarbonate buffer (to neutralize stomach acidity), followed 1 min later by 30 ml of sodium bicarbonate buffer containing 109 CFU of E. coli E24377A. The subjects then fasted for an additional 90 min. All stools were collected, graded, and weighed. Sera used for antibody measurement were obtained before and 7 and 28 days after challenge and were frozen at −20°C.
Laboratory measurement.
All anti-LT immunoglobulin G (IgG) analyses were conducted at IOMAI Corporation's Department of Research, Gaithersburg, MD. Serial threefold dilutions of patients' sera were added to microtiter wells coated with antigen. After incubation overnight (18 to 24 h), the wells were washed extensively, and peroxidase conjugated anti-IgG or anti-IgA was added to the wells to detect the presence of antigen-specific IgG and IgA antibodies. Following a second incubation, the microtiter wells were washed again, and the peroxidase substrate ABTS [2,2′-azino-di-(3-ethylbenzthiazoline-6-sulfonate)] was added. Cleavage of the ABTS substrate by the peroxidase resulted in the development of a blue-green reactant with an OD that was measured at 405 nm. Titers were expressed as the reciprocal of the highest dilution which resulted in an OD405 of 1.0. Based on the assay coefficient of variation, seroconversion was defined as a ≥2-fold increase in the titer for IgG or a fourfold increase in the titer for IgA.
Toxin-neutralizing antibody.
LT at a concentration of 5 ng/ml was preincubated with serial twofold dilutions of human serum from days 0 and 42 for 30 min at 37°C. Next, Y-1 cells were added to the plates containing the LT-serum reaction mixture, and the plates were incubated overnight (15 to 18 h). The following day, Y-1 cells were stained by adding a 0.01% neutral red solution in Dulbecco's phosphate-buffered saline. After incubation with the neutral red solution for 3 h at 37°C, the plates were washed three times with Dulbecco's phosphate-buffered saline to remove excess neutral red stain. Cells susceptible to LT became rounded and detached easily from the plate following the wash. The neutral red stain was eluted from the remaining viable cells by addition of extraction buffer (1% acetic acid in 50% ethanol), and OD530 were determined. ED50s were expressed the reciprocal of the serum dilution which resulted in a 50% reduction in toxin activity. Cross-neutralization of LT antisera with CT was performed in the same way, except that CT was preincubated with human sera instead of LT.
TEWL measurement.
All water loss measurements were obtained using the DermaLab modular system with transepidermal water loss (TEWL) probes, which were manufactured by Cortex Technology (Hadsund, Denmark). This instrument is based on the vapor pressure gradient estimation method designed by Nilsson. The DermaLab TEWL probes contain two sensors that measure the temperature and relative humidity at two fixed points along the axis normal to the skin surface, which allows the device to electronically derive a value that corresponds to evaporative water loss expressed in g/m2/h.
The data from the DermaLab modular system were completely computerized and continuously transferred to a personal computer through a serial port using an RS-232C cable and associated cyberDERM, Inc. software for the evaporimeters. The application program C1BASIX1 was used to capture the water loss data from the attached evaporimeter at a sampling rate of 4 inputs/s. These inputs were graphed as a real-time display on the computer monitor. The extracted value indicated the average evaporative water loss rate collected over a 20-s interval once steady-state conditions were achieved. The values were directly transferred to an Excel file using a DDE link.
TEWL measurements were obtained using the DemaLab system on the day 42 visit prior to vaccination. The DermaLab instrument probe was placed lightly on the skin within a marked area for 1 to 2 min. The skin within the marked area was then swabbed with a nonwoven abrasive pad soaked in saline and blotted dry. After 30 min, the probe was placed on the skin again within the pretreated, marked area for 1 to 2 min. Water loss values were electronically recorded using a spreadsheet format based on Excel software. The net TEWL was calculated by subtracting the individual baseline value from the posttreatment value.
Statistical methods.
All analyses were performed with the SAS statistical software, version 8.2 or later, and the StatXact software, version 5 or later. Statistical analyses of serum LT IgG titers and fold ratios were performed with the log10-transformed data. Safety data were analyzed for all subjects in the intention-to-treat sample for the interim analysis following the day 42 visit. Stop rules were based on the occurrence of severe or serious adverse events that could be related to the vaccination. Adverse events were tabulated using the MedDRA code by severity (none, mild, moderate, and severe), and data for different groups were compared by using exact Cochran-Mantel-Haenszel tests implemented in the StatXact software.
In this phase I/II study, a group size of 20 was used, as this was considered to be an appropriate compromise between safety risks and the ability to interpret the immunogenicity and safety findings and to evaluate the differences between three lots of LT. On a log-transformed scale, geometric mean titers (GMT) and fold increases for LT IgG were compared using one-way analysis of variance, as well as a generalized linear model (GLM) adjusted for age, gender, and baseline titer (after day 0). For LT IgG seroconversion (≥2-fold increase), the chi square test with the Cochran-Mantel-Haenszel option was utilized for unadjusted comparisons of the three treatment groups. Logistic regression models were used for covariate-adjusted analysis of the three treatment groups. Two-sided statistical tests with a type I error rate of 0.05 were used to determine the statistical significance for all inferential tests.
RESULTS
The immunizations were well tolerated, and the safety profiles were excellent. There were no serious adverse events in this study and no differences in the adverse events between the groups receiving the three different lots of LT. The investigator elected to exclude one subject (in group 1) from the second vaccination at day 21 due to tachycardia and erythema of the face and neck experienced by the subject after the first vaccination.
The percentages of subjects reporting one or more events (local or systemic events) were not statistically significantly different for the three treatment groups after each of the vaccinations. For local (patch site) adverse events as a whole, there were no statistically significant differences between the LT groups after the first vaccination and after the second vaccination; 3.3% and 5% of the participants, respectively, reported a moderate local rash or pruritus at the site of patch application during the study period. Systemic adverse events were infrequent; 1.7% of the participants reported moderate headaches, and 1.7% of the participants reported diarrhea.
Immunogenicity.
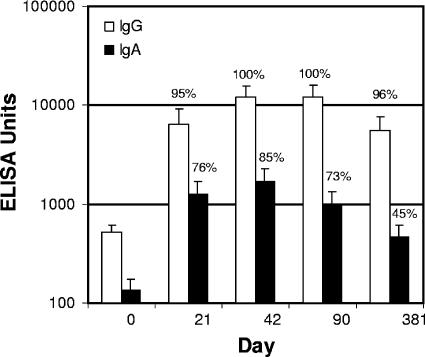
Sixty subjects, randomly sorted into three groups, were immunized transcutaneously on days 0 and 21 with 50 μg obtained from three separate lots of LT. Anti-LT IgG responses were evaluated by performing enzyme-linked immunosorbent assays (ELISA) (Table 1). The day 0 GMT ranged from 369 to 655 ELISA units (EU) for the three individual groups, and the average GMT was 500 EU for the combined subject population. There was a significant difference between groups 1 and 2 at day 0. However, there were no differences in the serum anti-LT IgG responses between groups after day 0, meeting the study's primary objective. The magnitude and kinetics of the serum IgG and IgA immune responses are shown in Fig. 1. The GMT at day 21 for the combined subject population following a single dose of 50 μg LT was 6,297 EU (range for groups, 5,217 to 8,553 EU; 95% serconversion) and increased to 12,185 EU (range for groups, 10,939 to 12,988 EU; 100% seroconversion) after a second dose, indicating that the second immunization provided a boost, and little change in the titers was seen through day 90.
TABLE 1.
LT IgG GMT for subjects immunized transcutaneously
| Study day | GMT (EU)
|
|||
|---|---|---|---|---|
| Group 1 (n = 20) | Group 2 (n = 20) | Group 3 (n = 20) | All treatments (n = 60) | |
| 0 | 369 | 656a | 514 | 499 |
| 21 | 5,217 | 8,553 | 5,595 | 6,297 |
| 42 | 12,734 | 12,988 | 10,939 | 12,185 |
| 90 | 10,772 | 14,350 | 11,222 | 12,016 |
P = 0.008 for a pairwise comparison of group 1 and group 2 using the covariate-adjusted and unadjusted generalized linear model.
FIG. 1.
Immunogenicity of LT delivered by TCI. Following pretreatment of the skin with a nonwoven abrasive EKG prep pad to disrupt the stratum corneum, a wet patch containing 50 μg of one of three lots of LT was applied to the pretreated area on days 0 and 21. Serum samples obtained on days 0, 21, 42, 90, and 381 were analyzed to determine the presence of anti-LT IgG and IgA by ELISA. There were no significant differences between the anti-LT immune responses of the three groups (P < 0.05). The serum antibody levels for the combined treatment groups are expressed in ELISA units; the bars indicate geometric means, and the error bars indicate 95% confidence intervals. The percentages are percentages of seroconversion (a twofold increase compared with the baseline for IgG and a fourfold increase for IgA).
IgG seroconversion (twofold) was achieved by all subjects by day 42, and the levels remained high through day 90 (Fig. 1). The mean fold increase in the GMT for all subjects was 12-fold at day 21, and it was more than 24-fold at days 42 and 90. At day 381, 96% of the subjects exhibited positive seroconversion with an average 10.5-fold increase in the anti-LT IgG titer compared with the titer at day 0. At day 42 there was a fourfold increase in the IgA titer in 85% of the subjects, and at day 381 45% of the subjects still exhibited a fourfold increase compared with the baseline value.
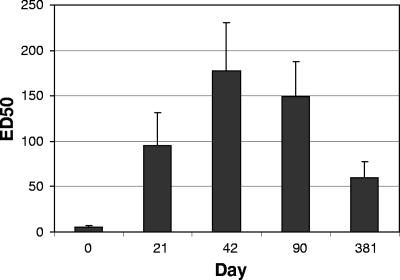
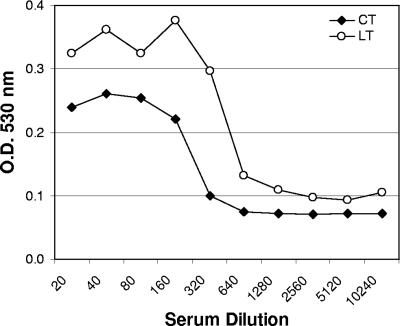
Inhibition of LT toxicity by sera of subjects demonstrated the functional activity of anti-LT antibody. As shown in Fig. 2, toxin-neutralizing activity was observed in the sera from the majority of the subjects on day 21 and in the sera from all of the subjects by day 42. Overall, the neutralizing titers increased from the baseline GMT on day 0 (<5 ED50) to 103 ED50 on day 21 and continued to increase to 180 ED50 on day 42. Studies have shown that anti-CTB antibodies can cross-neutralize LT, resulting in protection against LT-expressing ETEC in the field. In order to assess whether the anti-LT antibodies have similar effects on cholera, the same neutralizing assay was performed with preincubation of pooled sera with CT. As shown in Fig. 3, neutralizing antibodies against CT were readily observed using sera from subjects immunized with LT.
FIG. 2.
LT toxin neutralization by LT immune sera. Serum samples obtained on days 0, 21, 42, 90, and 381 from subjects vaccinated on days 0 and 21 with 50 μg of LT by TCI were tested to determine their abilities to neutralize LT in a Y-1 serum neutralization assay. The serum antibody levels for the combined treatment groups are expressed as average ED50s, and the error bars indicate 95% confidence intervals.
FIG. 3.
Comparison of neutralization of LT and CT toxins by LT immune sera. Pooled serum samples obtained on days 0 and 42 from subjects vaccinated on days 0 and 21 with 50 μg of LT by TCI were tested to determine their abilities to neutralize CT in a Y-1 serum neutralization assay. Toxin activity is indicated by the reduction in OD530, which reflects the number of neutral red-stained cells remaining after treatment. Toxin neutralization of both LT and CT by serum is shown.
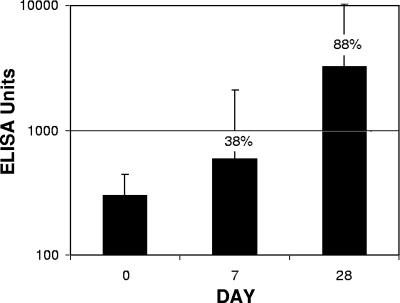
The immune response to TCI was compared to the serum anti-LT response observed in a live infectious challenge study not related to the current clinical trial. Sixteen subjects received 2 × 109 virulent ETEC E24377A LT+ ST+ organisms. Moderate to severe diarrhea subsequently developed in 13 of the 16 subjects (81%). As shown in Fig. 4, the day 28 anti-LT IgG GMT following the live oral challenge was 3,245 EU (seroconversion rate, 88%; mean increase, 11-fold), compared to a day 21 GMT of 6,297 EU (seroconversion rate, 95%; mean increase, 12-fold) for TCI after a single patch application (Fig. 1).
FIG. 4.
Anti-LT serum IgG induced following a live infectious challenge. Subjects received an oral infectious challenge consisting of 1 × 109 viable ETEC E2447A LT+ ST+ bacteria. Serum samples obtained on day 0, as well as on days 7 and 28 following the oral challenge, were analyzed to determine the presence of anti-LT IgG by ELISA. The serum antibody levels are expressed in ELISA units; the bars indicate geometric means, and the error bars indicate 95% confidence intervals. The percentages are percentages of seroconversion.
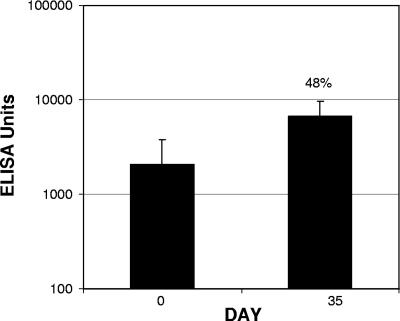
Sera from subjects immunized with a single dose of an oral whole-cell cholera vaccine containing 1 mg of CTB (sera kindly provided by David Taylor, Johns Hopkins Bloomberg School of Public Health) were analyzed by ELISA using LT as the antigen. CTB exhibits a high level of homology with LT, and anti-CTB has been shown to cross protect against ETEC disease, presumably through homologous antitoxin immunity. As shown in Fig. 5, the day 0 titer was 2,054 EU, suggesting that there had been preexisting exposure to ETEC or cholera in some subjects. The postimmunization, day 35 anti-LT IgG GMT was 6,741 EU, representing a 3.3-fold increase in the anti-LT IgG response.
FIG. 5.
Serological recognition of LT by sera from CTB-vaccinated subjects. Sera obtained from subjects 35 days after vaccination with a single dose of an oral whole-cell cholera vaccine containing 1 mg CTB and killed V. cholerae cells were analyzed to determine their recognition of LT, using an ELISA. The serum antibody levels are expressed in ELISA units; the bars indicate geometric means, and the error bars indicate 95% confidence levels. The percentage is the percentage of seroconversion.
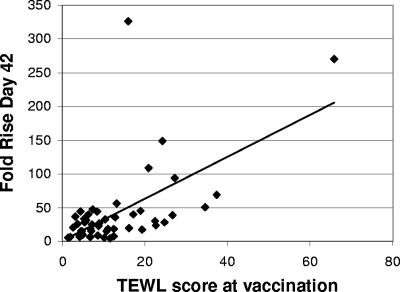
TEWL was measured as described above after pretreatment and before patch application for 51 of 59 subjects at the time of the second immunization (Table 2). Although the pretreatment was performed using a simple, hand-held abrasive pad, we detected no difference in the stratum corneum disruption, as indicated by TEWL measurement, between the three groups (Table 2). Overall, a net increase in TEWL of 12.9 g/m2/h was observed after 15 strokes of the medical-grade, nonwoven, abrasive EKG prep pad. The individual net TEWL measurements were plotted against the increases in the day 42 anti-LT IgG responses in the same subjects, as shown in Fig. 6. There was a significant correlation between the posttreatment TEWL and the increase in the antibody response at day 42 (r = 0.59, P < 0.0001).
TABLE 2.
TEWL resultsa
| Group | n | TEWL(g/m2/h)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline
|
After pretreatment of skin
|
Net
|
||||||||
| n | Mean (SD) | Range | n | Mean (SD) | Range | n | Mean (SD) | Range | ||
| 1 | 18 | 18 | 7.6 (2.2) | 4.2-11.2 | 17 | 21.9 (16.1) | 8.8-74.2 | 17 | 14.2 (15.6) | 3.2-65.9 |
| 2 | 17 | 16 | 7.2 (2.1) | 3.0-11.2 | 16 | 20.3 (9.4) | 10.0-45.4 | 16 | 13.2 (9.2) | 4.29-37.4 |
| 3 | 16 | 17 | 6.9 (2.1) | 2.0-10.8 | 17 | 18.2 (9.3) | 7.3-34.7 | 17 | 11.3 (8.7) | 1.4-27.1 |
| All | 51 | 51 | 7.3 (2.1)b | 2.0-11.22 | 50 | 20.1 (11.9)b | 7.3-74.2 | 50 | 12.9 (11.5) | 1.4-65.9 |
Baseline TEWL, TEWL after pretreatment of the skin at day 42, and net TEWL were determined. The net TEWL was calculated by subtracting the baseline TEWL from the TEWL after skin pretreatment. The differences between groups for baseline TEWL, TEWL after skin pretreatment, and net TEWL were not significant (P > 0.005, as determined by a t test).
The differences between the group TEWL and the combined subject TEWL after skin pretreatment and baseline TEWL were significant (P < 0.01).
FIG. 6.
Correlation between net TEWL and fold increase in the IgG titer. TEWL was measured on the skin before pretreatment and after skin pretreatment but before patch application in 51 of 59 subjects at day 42. Individual net TEWL (posttreatment TEWL − baseline TEWL) at the time of vaccination is plotted against the increase in anti-LT IgG at day 42 (r = 0.59, P = 0.001).
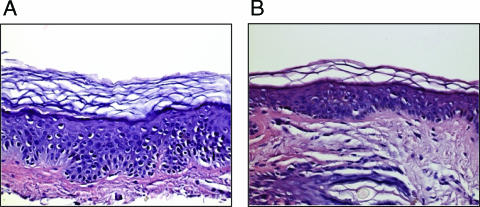
In a separate study, skin biopsy analyses were performed with three volunteers after use of the nonwoven abrasive EKG prep pad to disrupt the stratum corneum. Control stratum corneum and epidermis from skin pretreated with either 15 or 20 strokes of the nonwoven abrasive pad were removed by the shave biopsy method after intradermal lidocaine infusion. The skin was sectioned; then one half of the sample was formalin fixed for hematoxylin and eosin staining, and the second half was placed in liquid nitrogen for quantitation of the stratum corneum cell layers using dansyl chloride fixation. As shown in Table 3, the mean number of stratum corneum layers in the control skin was 19.3. Fifteen strokes with the nonwoven abrasive EKG prep pad removed 29% of the cell layers (approximately 6 of 19 cell layers), and 20 strokes removed 50% of the cell layers. As shown in Fig. 7, hematoxylin and eosin staining of normal skin and skin pretreated with 15 strokes reflected the clear but modest change in the stratum corneum thickness. Together, the data indicate that the pretreatment used in the clinical trial resulted in only modest changes in the stratum corneum and no microscopically apparent changes in the epidermis, suggesting that the procedure resulted in relatively minimal changes in the skin. Uncontrolled observations in the clinic shortly after pretreatment indicated that there was mild and transient erythema at the treatment site, consistent with the biopsy findings that there was only minimal disruption of the skin. These studies indicated that 15 strokes with an nonwoven abrasive pad like that used in the immunization protocol disrupt the stratum corneum and that only mild stratum corneum disruption is required to elicit robust immune responses to the LT doses used in the patch.
TABLE 3.
Summary of stratum corneum cell layer measurements
| Subject | No. of strokes | No. of stratum corneum cell layers removed (mean ± SD) | % Reduction |
|---|---|---|---|
| 001 | None (control) | 17 ± 2.0 | |
| 15 | 12.5 ± 0.7 | 26 | |
| 20 | 9.0 ± 1.5 | 47 | |
| 002 | None (control) | 18.8 ± 2.2 | |
| 15 | 14.1 ± 1.5 | 25 | |
| 20 | 7.9 ± 2.1 | 58 | |
| 003 | None (control) | 22.0 ± 1.8 | |
| 15 | 14.4 ± 1.7 | 35 | |
| 20 | 11.8 ± 2.4 | 46 | |
| Mean | None (control) | 19.3 | |
| 15 | 13.7 | 29 | |
| 20 | 9.6 | 50 |
FIG. 7.
Biopsy of control and nonwoven abrasive pad-treated human skin. After informed consent, a shave biopsy of human skin was obtained from a control site (A) and a site that was treated with 15 strokes of a nonwoven abrasive EKG pad (B). The skin was formalin fixed and stained using hematoxylin and eosin. The three layers of the skin, the stratum corneum, epidermis, and dermis, are visible, and in the group pretreated with the nonwoven abrasive pad a modest decrease in the thickness of the stratum corneum is visible.
DISCUSSION
LT-related ETEC disease accounts for considerable morbidity among travelers and mortality in weanling infants (32). LT is both an immunogenic and key pathogenic factor for LT-related ETEC disease and is thus an ideal vaccine antigen. However, LT is difficult to deliver by traditional vaccine delivery routes due to its strong effects on cells via the ADP-ribosyl transferase pathway. This results in diarrhea if LT is ingested as an oral antigen in buffer, or it results in inflammation in the nose if LT is given intranasally (17) or at the site of injection if LT is given parenterally (5). Delivery to the skin by TCI is a safe method for delivery and results in an ideal immune environment, resulting in strong antitoxin immune responses (11-13), as demonstrated in the present study.
The stratum corneum is the skin's principal barrier to penetration of molecules and is an effective yet fragile barrier (12). TCI is possible with minimal intervention to the skin, even with no physical stratum corneum disruption, by simple application of a wet, occlusive patch with antigen (12, 13). An occlusive patch hydrates the stratum corneum and allows small molecules (generally less than 500 Da), such as drugs used in transdermal patches, to penetrate through the skin, and this method has also been shown to allow much larger molecules (e.g., 86,000-Da LT), such as antigens, to move into the skin. However, follow-up patch vaccine delivery studies have indicated that even modest physical disruption of the stratum corneum can significantly improve the efficiency of TCI for a given dose (11). In these studies, classic methods for stratum corneum disruption, including tape stripping and EKG prep pad abrasives, were employed. Using an EKG prep pad (emery paper), a 50-fold increase in the IgG titer was achieved with a 50-μg LT patch. When hydration was used as a pretreatment, a 29-fold increase in the LT IgG response was achieved using 400 μg of LT in a patch after two doses, indicating that physical disruption of the stratum corneum resulted in almost doubling of the increase in the LT IgG response using 87.5% less LT, an approximately 10-fold improvement in efficiency (11).
In the present study, TEWL measurement was conducted after skin pretreatment, prior to application of the patch. TEWL analysis is a well-developed method that is used in both transdermal drug delivery development and skin studies, and it provides an accurate measurement of stratum corneum disruption (8). However, there have been no studies correlating TEWL and the immune response after TCI. In early pilot studies, we first demonstrated that there was a graduated increase in the TEWL based on the number of nonwoven abrasive pad strokes used on the skin (data not shown) and determined that 15 strokes were well tolerated and could consistently increase the TEWL in humans. Although we recognize that this method is not a practical bedside method for an ETEC vaccine, it is a medically accepted method for skin pretreatment that we believe can be modified so that it is suitable for use in a product. We then used this regimen as a pretreatment in a vaccination regimen with a 50-μg LT patch applied for 6 h. Immune responses, indicated by individual increases in anti-LT antibody titers, correlated with the individual TEWL measurements, suggesting that the magnitude of stratum corneum disruption and delivery are related. In a separate study, skin biopsies after the same pretreatment regimen resulted in loss of 29% of the cell layers in the stratum corneum. Together, these data indicated that stratum corneum disruption was modest but important since the increase in TEWL correlated with immune responses and, therefore, delivery.
There were no differences in the immunogenicity and safety of the three lots of LT, the primary endpoint. The immune response after pretreatment and patch application appeared to be robust, both compared to previous data (11, 12, 13) and compared to postinfection immune responses and to antitoxin responses to an oral cholera vaccine. The immune responses to LT delivered via TCI were greater than the immune responses observed for sera in subjects after oral challenge with virulent LT+ ST+ ETEC. In the challenge study, subjects' diarrhea may have been caused by exposure to both LT and ST. However, 88% of the subjects in the challenge group seroconverted to LT, indicating that there was LT exposure at the level of the gut, suggesting that the diarrhea was in part mediated by LT. In animal studies, this level of exposure and the subsequent immune responses are acutely accompanied by measurable gut fluid accumulation. The immune responses to LT in the human challenge study suggest that LT played a role in the virulent diarrhea seen in subjects and that TCI resulted in immunity that was superior to the immunity generated by LT exposure in the gut that accompanies ETEC disease (i.e., postinfection immunity). Similarly, the LT cross-reacting anti-CTB immune responses to the recombinant CTB/cholera whole-cell vaccine, which has been shown protect against LT+ ST+ ETEC in field studies, were approximately 50% of the responses produced by TCI. Although it has been shown that TCI elicits a mucosal immune response (12, 16), studies of the mucosal immunity for both live infectious challenge and recombinant CTB/cholera vaccination have not been performed, and the mucosal responses may be important contributors to protection. However, the systemic immune responses to live infectious challenge and an existing vaccine provide important benchmarks for immune responses elicited by TCI and suggest that the anti-LT IgG elicited by skin immune responses is robust.
There were several limitations in this study. The primary endpoints, safety, and immunogenicity of the three lots of LT precluded measuring TEWL for the first immunization. It was important to immunize without any additional procedures or skin interventions and to compare the immunogenicities of the different lots of LT, which could be evaluated after a single dose. TEWL measurements were obtained for the second dose. Similarly, the biopsy was separate from the immunization, although the same pretreatment protocol was used and appeared to be a highly consistent procedure, as suggested by TEWL measurements obtained for the three treatment groups. Furthermore, a control group without pretreatment would have provided additional information, but this was not within the scope of the study. However, there have been no previous correlations of TEWL, a common measure of stratum corneum disruption, and delivery of antigens to the skin, and there have been no previous comparisons of the LT immune response to the results of comparable regimens that can elicit anti-LT antibodies.
In the present study, we were able to demonstrate robust, persistent immune neutralizing antitoxin responses to LT with a two-dose regimen using TCI, suggesting that toxin immunity may be feasible as an approach to an ETEC vaccine. In early studies of enteric vaccines workers used a toxin-based vaccine strategy, which is mirrored by several current, highly successful licensed vaccines (vaccines for diphtheria, pertussis, tetanus). In animals, compelling data for protection were generated using LT (22), ST (18, 21), and CT/toxoids (14, 28-30). The concept was further explored using LT-ST conjugates, which were fully protective in challenges with strains producing both toxins (19, 20). In the past, use of these toxins as vaccine antigens presented difficulties since the native antigens can cause untoward reactions when delivery modes (e.g., intranasal, oral, or parenteral) other than TCI are used. Additionally, the chemically produced toxoid antigen for cholera failed in the field, and together these problems cast doubt on the viability of the toxin-based approach for dealing with enteric disease.
In concert with extensive preclinical data showing that antitoxin immunity is protective, experimental ETEC infections and field data have shown that active immunity and passive immunity are protective (23, 24, 34). Field data have shown that antitoxin neutralizing titers to LT play a key role in protection against ETEC disease in the face of natural exposure to repeated infections. In a 2-year follow-up study of 200 neonates, neonates infected with ETEC were protected 47% of the time from repeat LT+ ETEC infection, and the protection appeared to be based on immunity to the toxin (33). Several studies have shown the protective effects of breast milk antibodies to LT and CT against diarrheal disease (10, 24). In developing countries, ETEC illness decreases over time (2), and in long-term travelers, illness decreases with length of stay in an area where ETEC is endemic (6). Experimental challenge with an ETEC strain protected against rechallenge (7, 22), although a small challenge study cast disproportionate doubt on the potential for antitoxin immunity to be protective (22). By contrast, antitoxin neutralizing titers have been shown to play a key role in protection against ETEC disease in large field trials of an oral cholera vaccine containing CTB, which is homologous to LT and induces cross-reacting anti-LT antibodies for both LT and LT+ ST+ disease (4, 27; Bourgeois et al., 45th ICAAC). In a live-organism challenge, recipients of LT-expressing ETEC strains develop LT ELISA titers that are lower than those seen with TCI. The robust, LT-specific (holotoxin) immunity that can exceed the immunity seen after live infectious challenge and that compares well to the immunity obtained with an oral CTB-based vaccine that has shown field efficacy suggests that an anti-LT toxin vaccine could be efficacious in the field for both LT and LT+ ST+ ETEC disease.
ETEC is a burden on the health of people who live in or travel to areas where ETEC is endemic. Although some workers have suggested that an ETEC LT vaccine provides only narrow coverage against the host of enteric organisms, lessons learned from studying vaccines against other mucosal pathogens, such as diphtheria and pertussis vaccines, suggest that focused targets are more likely to succeed in vaccine development programs. The burden of disease due to LT-related ETEC is very large, both in travelers and in children in the poorest countries (25, 32). The prospect of elimination of several hundred million cases of dehydrating diarrhea in infants and hundreds of thousands of deaths with a vaccine that is needle-free, stable at room temperature, and easy to administer has led to a vigorous program to develop a patch and pretreatment regimen suitable for such an application.
Acknowledgments
We thank Wanda Hardy for assistance with manuscript preparation.
Editor: A. Camilli
Footnotes
Published ahead of print on 29 January 2007.
REFERENCES
- 1.Black, R. E. 1990. Epidemiology of travelers' diarrhea and relative importance of various pathogens. Rev. Infect. Dis. 12(Suppl. 1):S73-S79. [DOI] [PubMed] [Google Scholar]
- 2.Black, R. E., M. H. Merson, B. Rowe, P. R. Taylor, A. R. Abdul Alim, R. J. Gross, and D. A. Sack. 1981. Enterotoxigenic Escherichia coli diarrhoea: acquired immunity and transmission in an endemic area. Bull. W. H. O. 59:263-268. [PMC free article] [PubMed] [Google Scholar]
- 3.Clemens, J., S. Savarino, R. Abu-Elyazeed, M. Safwat, M. Rao, T. Wierzba, A. M. Svennerholm, J. Holmgren, R. Frenck, E. Park, and A. Naficy. 2004. Development of pathogenicity-driven definitions of outcomes for a field trial of a killed oral vaccine against enterotoxigenic Escherichia coli in Egypt: application of an evidence-based method. J. Infect. Dis. 189:2299-2307. [DOI] [PubMed] [Google Scholar]
- 4.Clemens, J. D., D. A. Sack, J. R. Harris, J. Chakraborty, P. K. Neogy, B. Stanton, N. Huda, M. U. Khan, B. A. Kay, M. R. Khan, et al. 1988. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J. Infect. Dis. 158:372-377. [DOI] [PubMed] [Google Scholar]
- 5.Craig, J. 1965. The effect of cholera stool and culture filtrates on the skin of guinea pigs and rabbits, p. 153-158. Proceedings of the Cholera Research Symposium, January 24-29, 1965, Honolulu, HI. Public Health Service publication no. 1328. U.S. Government Printing Office, Washington, DC.
- 6.Dupont, H. L., G. A. Haynes, L. K. Pickering, W. Tjoa, P. Sullivan, and J. Olarte. 1977. Diarrhea of travelers to Mexico. Relative susceptibility of United States and Latin American students attending a Mexican University. Am. J. Epidemiol. 105:37-41. [DOI] [PubMed] [Google Scholar]
- 7.Evans, D. G., D. Y. Graham, and D. J. Evans, Jr. 1984. Administration of purified colonization factor antigens (CFA/I, CFA/II) of enterotoxigenic Escherichia coli to volunteers. Response to challenge with virulent enterotoxigenic Escherichia coli. Gastroenterology 87:934-940. [PubMed] [Google Scholar]
- 8.Fluhr, J. W., K. R. Feingold, and P. M. Elias. 2006. Transepidermal water loss reflects permeability barrier status: validation in human and rodent in vivo and ex vivo models. Exp. Dermatol. 15:483-492. [DOI] [PubMed] [Google Scholar]
- 9.Freytag, L. C., and J. D. Clements. 1999. Bacterial toxins as mucosal adjuvants. Curr. Top. Microbiol. Immunol. 236:215-236. [DOI] [PubMed] [Google Scholar]
- 10.Glass, R. I., A. M. Svennerholm, B. J. Stoll, M. R. Khan, K. M. Hossain, M. I. Huq, and J. Holmgren. 1983. Protection against cholera in breast-fed children by antibodies in breast milk. N. Engl. J. Med. 308:1389-1392. [DOI] [PubMed] [Google Scholar]
- 11.Glenn, G. M., R. T. Kenney, L. R. Ellingsworth, S. A. Frech, S. A. Hammond, and J. P. Zoeteweij. 2003. Transcutaneous immunization and immunostimulant strategies: capitalizing on the immunocompetence of the skin. Expert Rev. Vaccines 2:253-267. [DOI] [PubMed] [Google Scholar]
- 12.Glenn, G. M., D. N. Taylor, X. Li, S. Frankel, A. Montemarano, and C. R. Alving. 2000. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat. Med. 6:1403-1406. [DOI] [PubMed] [Google Scholar]
- 13.Güereña-Burgueño, F., E. R. Hall, D. N. Taylor, F. J. Cassels, D. A. Scott, M. K. Wolf, Z. J. Roberts, G. V. Nesterova, C. R. Alving, and G. M. Glenn. 2002. Safety and immunogenicity of a prototype enterotoxigenic Escherichia coli vaccine administered transcutaneously. Infect. Immun. 70:1874-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmgren, J., A. M. Svennerholm, O. Ouchterlony, A. Anderson, G. Walletstrom, and U. Westerberg-Berndtsson. 1975. Antitoxic immunity in experimental cholera: protection, and serum and local antibody responses in rabbits after enteral and parenteral immunization. Infect. Immun. 12:1331-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jertborn, M., C. Ahren, J. Holmgren, and A. M. Svennerholm. 1998. Safety and immunogenicity of an oral inactivated enterotoxigenic Escherichia coli vaccine. Vaccine 16:255-260. [DOI] [PubMed] [Google Scholar]
- 16.Kenney, R., J. Yu, M. Guebre-Xabier, A. Lambert, B. Heller, L. Ellingsworth, J. Eyles, E. D. Williamson, and G. Glenn. 2004. Induction of protective immunity against lethal anthrax challenge with a patch. J. Infect. Dis. 190:774-782. [DOI] [PubMed] [Google Scholar]
- 17.Kenney, R. T., S. A. Frech, L. R. Muenz, C. P. Villar, and G. M. Glenn. 2004. Dose sparing with intradermal injection of influenza vaccine. N. Engl. J. Med. 351:2295-2301. [DOI] [PubMed] [Google Scholar]
- 18.Klipstein, F. A., R. F. Engert, and J. D. Clements. 1982. Development of a vaccine of cross-linked heat-stable and heat-labile enterotoxins that protects against Escherichia coli producing either enterotoxin. Infect. Immun. 37:550-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klipstein, F. A., R. F. Engert, and J. D. Clements. 1981. Immunization of rats with heat-labile enterotoxin provides uniform protection against heterologous serotypes of enterotoxigenic Escherichia coli. Infect. Immun. 32:1100-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klipstein, F. A., R. F. Engert, and J. D. Clements. 1981. Protection in rats immunized with Escherichia coli heat-stable enterotoxin. Infect. Immun. 34:637-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klipstein, F. A., R. F. Engert, J. D. Clements, and R. A. Houghten. 1983. Protection against human and porcine enterotoxigenic strains of Escherichia coli in rats immunized with a cross-linked toxoid vaccine. Infect. Immun. 40:924-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine, M. M., D. R. Nalin, D. L. Hoover, E. J. Bergquist, R. B. Hornick, and C. R. Young. 1979. Immunity to enterotoxigenic Escherichia coli. Infect. Immun. 23:729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long, K., E. Vasquez-Garibay, J. Mathewson, J. de la Cabada, and H. DuPont. 1999. The impact of infant feeding patterns on infection and diarrheal disease due to enterotoxigenic Escherichia coli. Salud Publica Mex. 41:263-270. [DOI] [PubMed] [Google Scholar]
- 24.Long, K. Z., J. W. Wood, E. Vasquez Gariby, K. M. Weiss, J. J. Mathewson, F. J. de la Cabada, H. L. DuPont, and R. A. Wilson. 1994. Proportional hazards analysis of diarrhea due to enterotoxigenic Escherichia coli and breast feeding in a cohort of urban Mexican children. Am. J. Epidemiol. 139:193-205. [DOI] [PubMed] [Google Scholar]
- 25.Okhuysen, P. C., Z. D. Jiang, L. Carlin, C. Forbes, and H. L. DuPont. 2004. Post-diarrhea chronic intestinal symptoms and irritable bowel syndrome in North American travelers to Mexico. Am. J. Gastroenterol. 99:1774-1778. [DOI] [PubMed] [Google Scholar]
- 26.Oyofo, B. A., D. S. Subekti, A. M. Svennerholm, N. N. Machpud, P. Tjaniadi, T. S. Komalarini, B. Setiawan, J. R. Campbell, A. L. Corwin, and M. Lesmana. 2001. Toxins and colonization factor antigens of enterotoxigenic Escherichia coli among residents of Jakarta, Indonesia. Am. J. Trop. Med. Hyg. 65:120-124. [DOI] [PubMed] [Google Scholar]
- 27.Peltola, H., A. Siitonen, H. Kyronseppa, I. Simula, L. Mattila, P. Oksanen, M. J. Kataja, and M. Cadoz. 1991. Prevention of travellers' diarrhoea by oral B-subunit/whole-cell cholera vaccine. Lancet 338:1285-1289. [DOI] [PubMed] [Google Scholar]
- 28.Peterson, D. L. 1981. Isolation and characterization of the major protein and glycoprotein of hepatitis B surface antigen. J. Biol. Chem. 256:6975-6983. [PubMed] [Google Scholar]
- 29.Pierce, N. F., W. C. Cray, Jr., and P. F. Engel. 1980. Antitoxic immunity to cholera in dogs immunized orally with cholera toxin. Infect. Immun. 27:632-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierce, N. F., W. C. Cray, Jr., J. B. Sacci, Jr., J. P. Craig, R. Germanier, and E. Furer. 1983. Procholeragenoid: a safe and effective antigen for oral immunization against experimental cholera. Infect. Immun. 40:1112-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snider, D. P. 1995. The mucosal adjuvant activities of ADP-ribosylating bacterial enterotoxins. Crit. Rev. Immunol. 15:317-348. [DOI] [PubMed] [Google Scholar]
- 32.Steffen, R., F. Castelli, H. Dieter Nothdurft, L. Rombo, and N. J. Zuckerman. 2005. Vaccination against enterotoxigenic Escherichia coli, a cause of travelers' diarrhea. J. Travel Med. 12:102-107. [DOI] [PubMed] [Google Scholar]
- 33.Steinsland, H., P. Valentiner-Branth, H. K. Gjessing, P. Aaby, K. Molbak, and H. Sommerfelt. 2003. Protection from natural infections with enterotoxigenic Escherichia coli: longitudinal study. Lancet 362:286-291. [DOI] [PubMed] [Google Scholar]
- 34.Tribble, D., and D. Taylor. 2004. ETEC and enteric vaccines, p. 275-297. In E. Jong (ed.), Traveler's vaccines. BC Decker, Inc., London, United Kingdom.
- 35.Wolf, M. K., D. N. Taylor, E. C. Boedeker, K. C. Hyams, D. R. Maneval, M. M. Levine, K. Tamura, R. A. Wilson, and P. Echeverria. 1993. Characterization of enterotoxigenic Escherichia coli isolated from U.S. troops deployed to the Middle East. J. Clin. Microbiol. 31:851-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. 1999. New frontiers in the development of vaccines against enterotoxigenic (ETEC) and enterohaemmorrhagic (EHEC) E. coli infections. Wkly. Epidemiol. Rec. 74:98-101. [PubMed] [Google Scholar]