Abstract
Clostridium perfringens type D isolates are important in biodefense and also cause natural enterotoxemias in sheep, goats, and occasionally cattle. In these isolates, the gene (etx) encoding ɛ-toxin is thought to reside on poorly characterized large plasmids. Type D isolates sometimes also produce other potentially plasmid-encoded toxins, including C. perfringens enterotoxin and beta2 toxin, encoded by the cpe and cbp2 genes, respectively. In the current study we demonstrated that the etx, cpe, and cpb2 genes are carried on plasmids in type D isolates and characterized the toxin-encoding plasmids to obtain insight into their genetic organization, potential transferability, and diversity. Southern blotting of pulsed-field gels showed that the etx gene of type D isolates can be present on at least five different plasmids, whose sizes range from 48 to 110 kb. The etx plasmids also typically carried IS1151 and tcp open reading frames (ORFs) known to mediate conjugative transfer of C. perfringens plasmid pCW3. PCR studies revealed that other than their tcp ORFs, etx plasmids of type D isolates do not carry substantial portions of the conserved or variable regions in the cpe plasmids of type A isolates. Southern blotting also demonstrated that in type D isolates the cpe and cpb2 genes are sometimes present on the etx plasmid. Collectively, these findings confirmed that the virulence of type D isolates is heavily plasmid dependent and indicated that (i) a single type D isolate can carry multiple virulence plasmids, (ii) a single type D virulence plasmid can carry up to three different toxin genes, and (iii) many etx plasmids should be capable of conjugative transfer.
The anaerobic sporeformer Clostridium perfringens is an important human and animal pathogen (16, 22, 24) that can produce up to 15 different toxins and enzymes. Production of four toxins (α, β, ɛ, and ι toxins) is used to classify C. perfringens into five different types (types A to E) (22). Type D isolates must produce both α-toxin and ɛ-toxin, which is encoded by the etx gene and is the third-most-potent clostridial toxin after the botulinum toxins and tetanus toxin (27). Because of this potency, both the CDC and U.S. Department of Agriculture have listed ɛ-toxin as an overlap class B select toxin. Beyond their biodefense significance due to their production of ɛ-toxin, type D isolates also naturally cause enterotoxemias in sheep, goats, and occasionally cattle (16).
Many C. perfringens toxins, including ɛ-toxin, can be plasmid encoded (24). However, despite their biodefense and pathogenic importance, in general, the toxin-encoding C. perfringens virulence plasmids have received only limited research attention to date. The major exceptions have been the ∼70- to 75-kb plasmids carrying the cpe gene (encoding C. perfringens enterotoxin) that are present in some type A isolates. Miyamoto et al. recently identified two major families of cpe-carrying plasmids in type A isolates, one with an IS1151 sequence (but no IS1470-like sequence) downstream of the cpe gene and the other with an IS1470-like sequence (but no IS1151 sequence) downstream of the cpe gene (18, 19). These two type A cpe plasmid families share a conserved region comprising ∼50% of each plasmid. In this conserved region are Tn916-related sequences, designated the tcp locus (1), that were recently demonstrated to mediate conjugative transfer of the C. perfringens tetracycline resistance plasmid pCW3 (1). The presence of this tcp locus in the cpe plasmids of type A isolates probably explains why some, if not all, of these cpe plasmids can transfer conjugatively (5). The variable regions of the two type A cpe plasmid families differ significantly and can carry an additional toxin gene (the cpb2 gene encoding beta2 toxin is present in these cpe plasmids with downstream IS1151 sequences), bacteriocin open reading frames (ORFs), or putative metabolic ORFs (10, 19).
By contrast, much less is known about etx genetics. The etx gene has been sequenced (12) for only a single type B strain (NCTC8533) and a single type D strain (NCTC8346), and the limited sequencing studies suggested that there may be a few type-specific differences in the etx ORF. It has also been shown that insertion sequences (IS) are present upstream and downstream of etx in one type D isolate (8, 24; GenBank accession number X60694). Initial studies of a few type D isolates suggested that the etx gene is present in a large plasmid (2, 9, 23), but the etx plasmid(s) has not been studied yet in any detail. Finally, we and other workers recently showed by using multiplex PCR that there are genotypic variations among type D isolates (26) and that some type D isolates carry up to three different potentially plasmid-borne toxin genes (etx, cpe, and cpb2).
Considering the limited information now available about etx genetics, in the current study we sought to confirm whether etx genes are typically plasmid borne in a large collection of type D animal disease isolates. If etx was confirmed to be predominantly plasmid borne, we then intended to evaluate the diversity of the type D etx plasmids and also determine the association, if any, between cpe, cpb2, and etx genes on type D virulence plasmids. Finally, to begin assessing possible mobilization of the etx gene or etx-carrying plasmids, we examined whether tcp genes or IS sequences are typically present on etx plasmids.
MATERIALS AND METHODS
Bacterial strains, media, and reagents.
As described previously (26), the 23 C. perfringens type D isolates examined in this study originated from diseased animals. The plasmid-borne toxin genotypes of these isolates (17), also demonstrated previously (26), are shown in Tables 1 and 2. All type D isolates were grown overnight at 37°C under anaerobic conditions on SFP agar (Difco Laboratories) containing 0.04% d-cycloserine (Sigma Aldrich) in order to ensure culture purity. Fluid thioglycolate medium (FTG) (Difco Laboratories) and 3% tryptic soy broth (Becton-Dickinson) containing 2% glucose (Sigma Aldrich), 1% yeast extract (Becton Dickinson), and 0.1% thioglycolate (Sigma Aldrich) (TGY) were used to grow broth cultures.
TABLE 1.
Plasmid carriage of etx, tcpF/H, and IS1151 in cpe-negative cpb2-negative type D isolates
| Isolate | Size (kb) of plasmid carrying:
|
||
|---|---|---|---|
| etx | tcpF/H | IS1151 | |
| CN462 | 48 | 48 | 48 |
| CN1020 | 48 | 48 | 48 |
| CN1184 | 48 | 48 | 48 |
| CN1634 | 75 | 48 | 75 |
| CN2062 | 48 | 48 | 48 |
| CN3693 | 48 | 48 | 48 |
| CN3793 | 48 | 48 | 48 |
| CN3977 | 48 | 48 | 48 |
| CN3978 | 48 | 48 | 48 |
| CN3841 | 48 | 48 | 48 |
| CN4029 | 75 | 75 | 75 |
| CN4031 | 75 | 75 | 75 |
| JGS1942 | 48 | 48 | 48 |
TABLE 2.
Plasmid carriage of selected genes in cpe+ and/or cpb2+ type D isolates
| Isolate | Size(s) (kb) of plasmid(s) carrying:
|
||||
|---|---|---|---|---|---|
| etx | cpe | cpb2 | tcpF/H | IS1151 | |
| CN2068 | 73 | 73 | 73 | 73 | |
| CN1183 | 75 | 75 | 75 | 75 | 75 |
| CN3842 | 85 | 85 | 85 | 85 | 85 |
| CN4003 | 75 | 110 | 45 | 75, 110 | 110 |
| CN3948 | 75 | 110 | 75 | 75, 110 | 75, 110 |
| JGS1240 | 75 | 110 | 75 | 48, 65, 75 | 48, 65, 75 |
| JGS1902 | 110 | 110 | 75 | 75, 110 | 75 |
| JGS4138 | 110 | 110 | 75 | 75, 110 | 75, 110 |
| JGS4139 | 75 | 110 | 75 | 75 | 75, 110 |
| JGS4152 | 110 | 110 | 75 | 110 | 110 |
Pulsed-field gel electrophoresis.
C. perfringens type D isolates were grown overnight at 37°C in FTG broth. A 0.1-ml aliquot of each of the starter cultures was then inoculated into 10 ml of TGY and grown overnight at 37°C. The overnight TGY cultures were used to prepare genomic C. perfringens DNA-containing agarose plugs. The bacterial cells were washed three times in TES buffer (1 M Tris, 0.5 M EDTA, 6.7% [vol/vol] 5 M sucrose; pH 8.0). Cells were resuspended in 0.4 ml of Tris-EDTA and embedded in 2% chromosome-grade agarose (Bio-Rad Laboratories) by mixing equal volumes (0.4 ml) of a cell suspension and melted agarose equilibrated to 65 to 70°C. Plugs were solidified at 4°C in 1.5-mm-thick molds (Bio-Rad Laboratories) and then cut into 2- to 3-mm slices. The agarose-embedded cells were lysed by incubation with gentle shaking of the plugs overnight at 37°C in lysis buffer (0.5 M EDTA [pH 8.0], 2.5% [vol/vol] 20% Sarkosyl, 0.25% lysozyme [Sigma], 0.2% deoxycholic acid). Finally, the plugs were incubated in 0.2% proteinase K (Gene Choice) buffer at 55°C for 2 days.
To help distinguish whether colocalizing signals obtained using probes for two different ORFs indicated that these two ORFs were present on the same plasmid or on two comigrating plasmids, restriction endonucleases ApaI, BamHI, BstXI, ClaI, KpnI, NcoI, SalI, ScaI, SmaI, StuI, and XhoI (New England Biolabs) were used to digest plugs containing DNA from two representative type D isolates (CN1183 and JGS1902). In these experiments, each set of plugs was incubated with or without a restriction enzyme in 200 μl of the appropriate buffer solution, as recommended by the enzyme manufacturer.
Pulsed-field gel electrophoresis was performed with a 1% agarose gel using a CHEF-DR II system (Bio-Rad Laboratories) and 0.5× Tris-borate-EDTA buffer at 14°C. The running parameters for undigested DNA were as follows: initial pulse, 1 s; final pulse, 25 s; voltage, 6 V/cm; time, 24 h. The following running parameters were used for DNA digested with restriction enzymes: initial pulse, 1 s; final pulse, 12 s; voltage, 6 V/cm; time, 15 h. After pulsed-field gel electrophoresis, the gel was stained with ethidium bromide, washed with distilled water, and photographed under UV light. Mid-Range or Low-Range PFG markers (New England Biolabs) were used as molecular size standards, as appropriate.
Southern hybridization.
Digoxigenin (DIG)-labeled probes were prepared using the primers listed in Table SI in the supplemental material. Southern hybridization of pulsed-field gels was performed as described previously (19). DIG labeling and detection reagents were obtained from Roche Applied Science. The CSPD substrate (Roche Applied Science) was used for detection of hybridized probes according to the manufacturer's instructions.
PCR analyses.
Template DNA for all PCRs was obtained from colony lysates, which were prepared as described previously (28). Each PCR mixture contained 5 μl of template DNA, 40 μl of TAQ Complete 1.1 Master Mix (Gene Choice), and 2.5 μl of each primer pair (final concentration, 1 μM). The primers used to investigate whether the variable region or the tcp locus of the conserved region in type A cpe plasmids pCPF4969 and pCPF5603 are also present in type D isolates were described previously by Miyamoto et al. (19). New overlapping PCR primers were designed for the remaining conserved region (∼20 kb) of pCPF5603 (see Table SII in the supplemental material), and these primers were used to investigate whether the entire conserved region of pCPF5603 is present in type D isolates. PCRs were performed with a Techne (Burkhardtsdorf, Germany) thermocycler using previously described conditions (19). PCR products were separated on 1% agarose gels and visualized with ethidium bromide staining.
The presence of the lam gene encoding lambda toxin or the rep gene encoding the Rep protein of pCW3 (1) was assessed using a primer set shown in Table SI in the supplemental material. The PCR conditions used for amplification of the lam gene were as follows: 94°C for 3 min and then 35 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. The PCR products were electrophoresed on a 1% gel and stained with ethidium bromide for visualization.
PCR analysis of the association between etx and ISs.
Template DNAs used for the PCR analysis of the association between etx and ISs were prepared as described above. Internal amplification of IS1151 was performed using the following primer set, which should have yielded a 638-bp PCR product: IS1151F (5′-CTGTACGGCTCCATTATCTC-3′) and reverse primer IS1151R (5′-CAGTAAGTTCAATTGTTTCGCC-3′). Attempts were also made to connect IS1151 to the etx gene using the following primer set: IS1151F1 (5′-GTTAAATTAGAGCGATTCATGTGC-3′) and etxR2 (5′-CCACTTACTTGTCCTACTAAC-3′). The following PCR primers were used to amplify the downstream (IS406) transposase detected previously near the etx gene of one type D isolate (GenBank accession number X60694): etx-dnR (5′-CTTCATCAGTAGGAAAAGCTG-3′) and etx-dnF (5′-GGAAATGTAAAGTTAGTAGGAC-3′). The PCR conditions used for amplification were as follows: 94°C for 3 min and then 35 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min. The PCR products were electrophoresed on a 1% gel and stained with ethidium bromide for visualization.
Sequencing of the etx gene.
DNA for sequencing was prepared as described previously (18). The following three forward primers were used for sequencing the etx ORF in selected type D isolates: extF1 (5′-GTTTTAAAATACAAGTTTTATG-3′), extF2 (5′-GAGAAAGGAAGATATAATAC-3′), and extF3 (5′-CTAGTTATAGTTTTGCAAATAC-3′). The two reverse primers used for sequencing were etxR1 (5′-GACCTAACTTTACATTTC-3′) and etxR2 (5′-CCACTTACTTGTCCTACTAAC-3′). Sequencing was performed at the University of Pittsburgh core sequencing facility (http://www.genetics.pitt.edu/services.html), and the sequences were then analyzed using BioEdit and BLAST (NCIB).
PCR identification of possible circular transposition intermediates.
Template DNAs used for PCR identification of possible circular transposition intermediates were prepared as described previously (28). Amplification of possible circular transposition intermediates containing only IS1151 and the etx ORF was performed using the following primer set, which should have yielded an ∼1.1-kb PCR product: 1R (5′-CTGTTATACTGCCTTTTCTTTG-3′) and 3F (5′-CACAAGATATACTAGTACCAGC-3′). Amplification of a second possible circular transposition intermediate containing IS406, the etx ORF, and IS1151 was performed using the following primer set, which should have yielded a ∼1.6-kb PCR product: 4F (5′-GAAAGGCATGTCTACACGAG-3′) and 2R (5′-CCATGGCCGTCAACCTAAG-3′). Finally, amplification of a third possible circular transposition intermediate containing only IS406 and the etx ORF was performed using the following primer set, which should have yielded a ∼127-bp PCR product: 4F (5′-GAAAGGCATGTCTACACGAG-3′) and 1R (5′-CTGTTATACTGCCTTTTCTTTG-3′). The PCR conditions used for these amplifications were as follows: 94°C for 3 min and then 35 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min. The PCR products were electrophoresed on a 1% gel and stained with ethidium bromide for visualization. The PCR products were cloned into the pCR2.1-TOPO vector (Invitrogen, Carlsbad, CA) and sequenced using vector-specific primers M13F and M13R.
Western immunoblotting.
Type D isolates were first grown in FTG, and then a 0.1-ml aliquot of each overnight culture was transferred into 10 ml of TGY and grown overnight. Samples were collected, and supernatants were then mixed with an equal volume of loading buffer and electrophoresed in a 10% polyacrylamide gel. The separated protein bands were transferred to a nitrocellulose membrane, and Western blotting for detection of ɛ-toxin was performed as described previously (26).
RESULTS
Characterization of type D etx plasmid diversity and toxin gene carriage.
Previous studies have shown that C. perfringens chromosomal genes do not enter pulsed-field gels unless the genes are digested with restriction endonucleases (7, 9, 10, 14). However, their smaller sizes allow even undigested plasmids to enter pulsed-field gels. Furthermore, studies with other C. perfringens plasmids have shown that on Southern blots of pulsed-field gels, probes react mainly with the nicked, linear forms of plasmids (7, 9, 10, 14, 19). Therefore, their migration on pulsed-field Southern blots provides an accurate estimate of the size of C. perfringens plasmids. This conclusion is supported by the results shown in Fig. SIB in the supplemental material for pCPF5603, which indicated that the cpe plasmid was ∼75 kb in size, which matched the sequencing-determined size of this type A cpe plasmid (19).
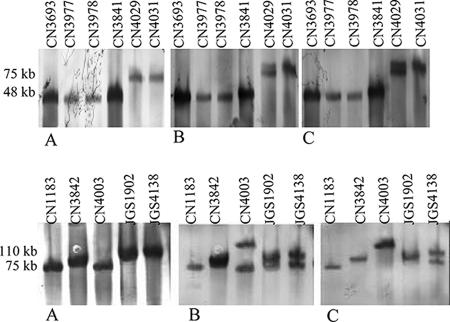
When this pulsed-field gel Southern blot approach was used to evaluate the location of the etx gene (chromosomal versus plasmid) in a collection of type D isolates, three isolates (e.g., CN1675 in Fig. SIB in the supplemental material) showed smearing, which indicated that their nuclease levels were high (7), and they could not be analyzed by this technique. All of the remaining 23 type D isolates surveyed were found to carry a plasmid-borne etx gene; i.e., their DNA hybridized with the etx probe entered pulsed-field gels without any restriction endonuclease digestion. For type D isolates with a simple plc and etx toxin genotype (i.e., neither the cpe gene nor the cpb2 gene was present), the etx gene was generally present on an ∼48-kb plasmid, although a few such type D isolates carried the etx gene on larger plasmids (∼73 to 75 kb) (see Fig. SIA in the supplemental; Table 1). Type D isolates with a more complicated toxin genotype (cpe+ and/or cpb2+) carried the etx gene on large plasmids that ranged from ∼75 to 110 kb in size (Fig. 1 and Table 2; see Fig. SIB in the supplemental material). In these type D isolates, the cpe or cpb2 toxin genes were also present on plasmids, which varied from ∼48 to 110 kb in size. For some cpe- and/or cpb2-positive type D isolates (e.g., CN1183 and CN3842), the etx, cpe, and cpb2 probes all hybridized to the same Southern blot location (Fig. 1 and Table 2). However, for other cpe- and/or cpb2-positive type D isolates, the etx probe and the cpe or cpb2 probes hybridized to different plasmids. For example, type D isolate CN4003 clearly carries three distinct toxin-encoding plasmids, including an ∼75-kb etx plasmid, an ∼110-kb cpe plasmid, and an ∼48-kb cpb2 plasmid (Fig. 1).
FIG. 1.
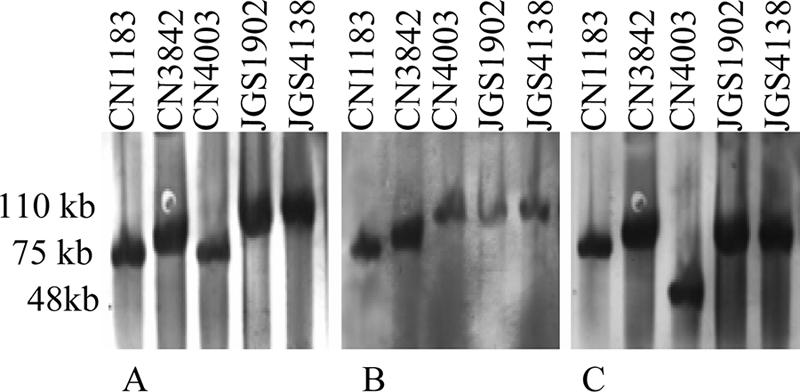
Southern blot comparison of pulsed-field gels electrophoresed with DNA from cpe+ and/or cpb2+ type D isolates. The blot was first hybridized with a DIG-labeled, etx-specific probe (A) and then stripped and reprobed with a DIG-labeled, cpe-specific probe (B). Finally, the blot was striped again and reprobed with a DIG-labeled, cpb2-specific probe (C). The positions of molecular size markers are indicated on the left, and isolate designations are indicated above the lanes.
Evaluation of whether comigrating toxin genes are present on the same or different plasmids.
As shown in Fig. 1, DNA from several type D isolates hybridized to multiple toxin gene probes at the same blot location, indicating that these isolates carry multiple toxin genes on either the same plasmid or on two distinct plasmids whose sizes are similar. To discriminate between these two possibilities, four representative type D isolates were digested with a battery of restriction endonucleases. For type D isolates CN1183 (Fig. 2) and CN3842 (not shown), migration of the etx gene, migration of the cpe gene, and migration of the cpb2 gene all exhibited similar susceptibility to restriction enzyme digestion, a result consistent with the hypothesis that these three toxin genes are located on the same plasmid (Fig. 2 and Table 2). However, for type D isolate JGS1902 (Fig. 2), there were differences in the digestion susceptibility patterns for the plasmid DNA carrying the etx gene and the plasmid DNA carrying the cpe gene, suggesting that the etx and cpe genes in this isolate may be on two distinct ∼110-kb plasmids. In this isolate the cpb2 gene is present on a smaller (75-kb) plasmid (Fig. 1). For type D isolate JGS4138 (data not shown), there was no difference between the digestion susceptibility pattern for the plasmid DNA carrying the etx gene and the digestion susceptibility pattern for the plasmid DNA carrying the cpe gene, suggesting that the etx and cpe genes of this strain are present on the same ∼110-kb plasmid. In JGS4138 the cpb2 gene is present on a smaller (75-kb) plasmid (Fig. 1).
FIG. 2.
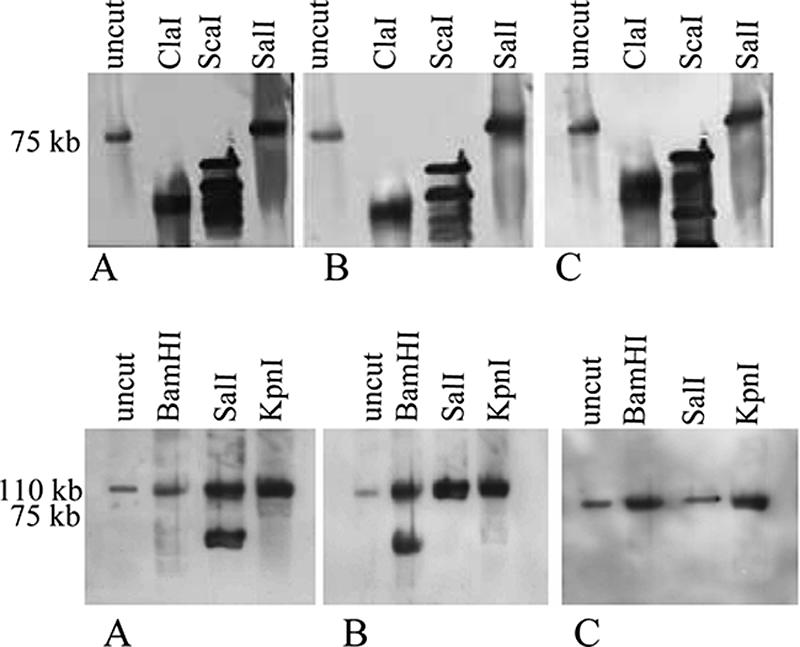
Southern blot analysis of pulsed-field gels electrophoresed with DNA from CN1183 (top panel) or JGS1902 (bottom panel) that had been digested with specified restriction enzymes. Each Southern blot was first hybridized with a DIG-labeled, etx-specific probe (A) and then stripped and reprobed with a DIG-labeled, cpe-specific probe (B) before it was stripped and probed with a DIG-labeled, cpb2-specific probe (C). The positions of molecular size markers are indicated on the left, and the restriction enzymes used are indicated above the lanes.
Presence of the tcp transfer locus in type D isolates.
In previous studies, (1, 19) workers reported that tcp genes, which mediate conjugative transfer of C. perfringens plasmid pCW3 (and probably many cpe plasmids of type A isolates [5, 19]), are present in type D isolate NCTC8346 and a second, unspecified type D isolate. To investigate whether tcp genes are commonly found in type D isolates and, if so, whether they are present on etx plasmids or other virulence plasmids in these isolates, Southern blot analyses of pulsed-field gels were performed using probes specific for two tcp genes (tcpF and tcpH) known to be required for pCW3 conjugative plasmid transfer (1). In most type D isolates surveyed, tcpF and tcpH probes hybridized to the same blot location containing the etx plasmid (Fig. 3 and Tables 1 and 2). However, in isolate CN1634, tcpF and tcpH probes did not hybridize with the etx plasmid, although they did appear to hybridize to a smaller ∼48-kb non-toxin-encoding plasmid (data not shown). These tcp Southern blot analyses also strongly suggested that type D isolates JGS1902 and JGS4138 carry a distinct cpb2 plasmid with tcpF and tcpH genes (Fig. 3).
FIG. 3.
Southern blot analysis of pulsed-field gels electrophoresed with DNA from cpe-negative cpb2-negative (top panel) or cpe+ and/or cpb2+ (bottom panel) type D isolates and hybridized with a DIG-labeled, etx-specific probe (A). The blot was then stripped and reprobed with a DIG-labeled, tcpF- and tcpH-specific probe (B) before it was stripped and probed with a DIG-labeled IS1151-specific probe (C). The positions of molecular size markers are indicated on the left, and isolate designations are indicated above the lanes.
PCR analysis also detected the presence of the gene encoding the pCW3 Rep protein (1), which is also present in pCPF4969 and pCPF5603 (19), in all 10 type D isolates surveyed. Southern blots of pulsed-field gels containing DNA from seven of these type D isolates showed that there was comigration of the hybridized rep and etx probes, suggesting that rep is commonly present on etx plasmids (data not shown).
Presence of the lambda toxin gene in type D isolates.
Lambda toxin is a C. perfringens metalloprotease that can proteolytically activate ɛ-toxin (13, 20). Since the lambda toxin gene (lam) has been detected previously (13) in two type D isolates (945P and NCTC2062), we PCR tested our 23 type D isolates and 15 other type D isolates to determine whether they carried the lam gene. This survey detected the lam gene in 9 of the 38 type D isolates. To confirm these results and to assess whether the lam gene is plasmid borne in lam+ type D isolates, we performed a Southern blot analysis of pulsed-field gels. In this analysis (Fig. 4) we detected no lam probe hybridization with DNA from two type D isolates that were PCR negative for the lam gene. In contrast, DNA from three lam PCR-positive type D isolates hybridized with the lam probe. For one of these three type D isolates, the lam gene was clearly not present on the etx plasmid but instead appeared to be present on a larger, non-toxin-encoding plasmid. For the other two type D isolates, the lam probe apparently hybridized with the etx plasmid.
FIG. 4.
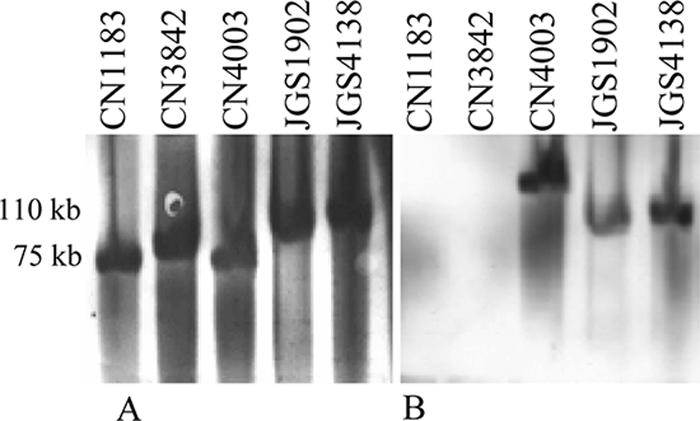
Southern blot analysis of pulsed-field gels electrophoresed with DNA from CN1183, CN3842, CN4003, JGS1902, and JGS4138. The Southern blot was first hybridized with a DIG-labeled, etx-specific probe (A) and then stripped and reprobed with a DIG-labeled, lam-specific probe (B). The positions of molecular size markers are indicated on the left.
We also used Western blotting to compare ɛ-toxin processing by lam+ and lam-negative type D isolates. Proteolytic processing of ɛ-toxin was sometimes evident (data not shown) in supernatants from one of eight lam+ isolates. Interestingly, two of four lam-negative isolates also showed intermittent ɛ-toxin processing (data not shown).
PCR studies to evaluate whether the variable or conserved regions of pCPF5603 or pCPF4969 are present in type D isolates.
As mentioned above, two major cpe plasmid families have been identified in type A isolates, and a representative of each family (pCPF4969 and pCPF5603) has now been completely sequenced (19). Since tcp genes are present in pCPF5603, pCPF4969, and most type D isolates (19) (Fig. 3), PCR analyses were performed to evaluate the possible presence of other pCPF4969 or pCPF5603 genes in type D isolates. Individual ORF PCR surveys (see Table SIII in the supplemental material) revealed that most type D isolates carry many ORFs found in the conserved region of pCPF4969 and pCPF5603. However, with the exception of type D isolates CN4003 and JGS1182, individual ORFs of either the pCPF5603 or pCPF4969 variable region were not found in the type D isolates surveyed.
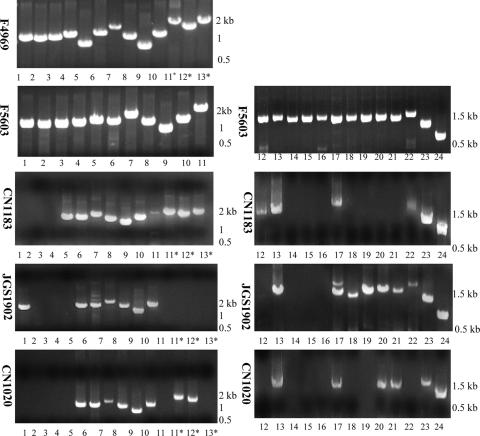
Overlapping PCR primer sets were then employed to evaluate whether the conserved region ORFs detected by individual ORF PCRs are arranged similarly in type D isolates and in pCPF4969 and pCPF5603. The tcp locus was the only product amplified from the type D isolates surveyed by these overlapping PCR assays (Fig. 5), suggesting that compared to the conserved region of pCPF5603 and pCPF4969, there are substantial ORF rearrangements or sequence differences upstream and downstream of the putative tcp transfer region in the etx plasmids of type D isolates.
FIG. 5.
Conserved region of control cpe plasmids pCPF4969 and pCPF5603 from type A isolates PCR amplified with an overlapping PCR battery (19). The same PCR primers and conditions were then used to assess the presence of this region in different type D isolates. The positions of molecular size markers are indicated on the right. Positive reactions 5 to 10 correspond to the tcp locus. Isolate designations are indicated on the left, and reaction numbers are indicated below the lanes. Asterisks indicate reactions specific for pCPF4969 sequences (19).
As expected from the individual ORF PCR survey results, overlapping PCR analyses also did not detect (data not shown) the pCPF5603 or pCPF4969 variable region in most type D isolates (although the surveys were positive for type A control plasmids pCPF5603 and pCPF4969 [data not shown]). Even the two type D isolates (JGS1182 and CN4003) that amplified most individual pCPF5603 variable ORFs did not generate positive reactions using the overlap PCR battery for the pCPF5603 variable region (data not shown), indicating that there are substantial ORF rearrangements or sequence differences between the variable region of pCPF5603 and the plasmids in type D isolates JGS1182 or CN4003.
Presence of the IS1151 sequence in type D isolates.
In previous studies, workers have demonstrated that an IS1151 sequence is located upstream of the etx gene in at least one type D isolate (8, 24). Therefore, we surveyed whether there is a similar etx locus arrangement in other type D isolates using an overlap PCR capable of linking the etx gene and IS1151 in this previously analyzed type D isolate. This PCR analysis successfully linked an upstream IS1151 to the etx gene in all cpb2-negative cpe-negative type D isolates surveyed (Fig. 6B). However, the same primer set did not amplify a product from type D isolates also carrying the cpb2 and/or cpe genes, even though these isolates were PCR positive when internal IS1151 primers were used (Fig. 6A). These results are consistent with pulsed-field gel Southern blot results showing that IS1151 is variably associated with the etx plasmid in the cpb2- and/or cpe-positive type D isolates (Fig. 3).
FIG. 6.
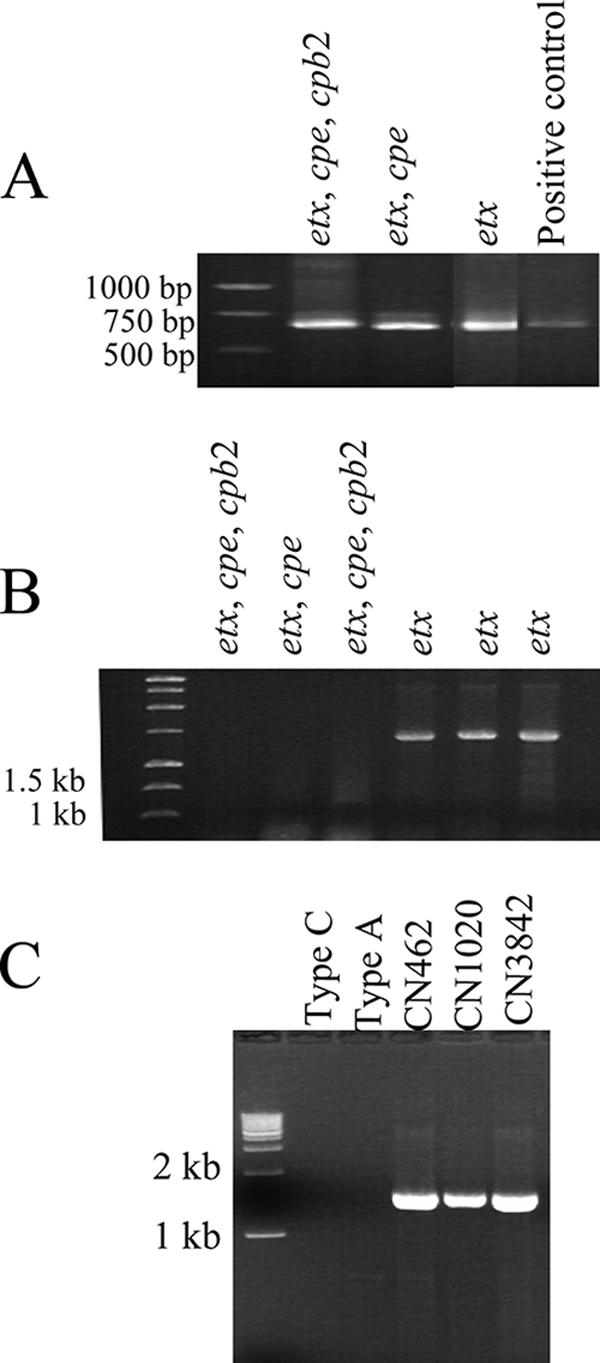
Presence of insertion sequences in type D isolates. (A) PCR amplification of internal IS1151 sequences from three different type D isolates. The toxin-borne genes present in each isolate are indicated above the lanes. (B) PCR amplification linking the upstream IS1151 sequence and the etx gene in six different type D isolates. (C) PCR amplification linking the downstream IS406 sequence and the etx gene in three different type D isolates. The positions of molecular size markers are indicated on the left. Genotypes and isolate designations are indicated above the lanes.
In another previous study workers showed that IS406 sequences (GenBank accession number X60694) were present downstream of the etx gene in one type D isolate. In the current study, PCR analysis linked the downstream transposase to the etx gene in all type D isolates surveyed irrespective of the toxin genes that they carried (Fig. 6C).
PCR identification of possible circular transposition intermediates.
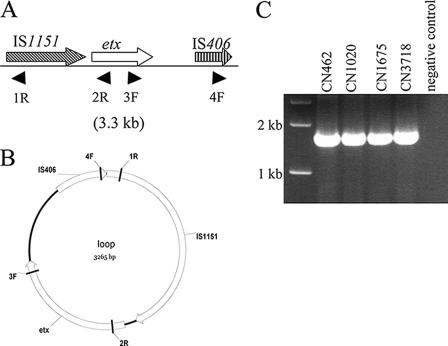
Results shown in Fig. 6 confirmed the previous finding (GenBank accession number X60694) that in cpe-negative cpb2-negative type D isolates there are two ISs flanking the etx gene, suggesting that this locus may represent an integrated genetic element. Movement of ISs and their associated genes often involves formation of small circular transposition intermediates (4). To test whether the ISs near the etx gene in cpe-negative cpb2-negative type D isolates might mobilize this toxin gene into circular intermediates, primers were designed to identify three possible circular transposition intermediates in these isolates (Fig. 7). In these PCR analyses we clearly identified a transposition intermediate including both ISs and the etx gene (∼3.3 kb). This finding was confirmed by sequencing the PCR product, which contained IS1151-etx-IS406 sequences. Primers for other possible circular intermediates also amplified products, but amplification of multiple products in these PCRs precluded sequencing attempts to confirm whether these products represent the expected circular transposition intermediates.
FIG. 7.
(A) Arrangement of the etx locus in cpe-negative cpb2-negative type D isolates based on the results shown in Fig. 6. The designations below the line indicate the primers used to evaluate circular intermediate formation. (B) Map of the circular intermediate based on PCR and sequencing (not shown). (C) PCR amplification of the circular intermediate. The positions of molecular size markers are indicated on the left, and isolate designations are indicated above the lanes.
Sequencing of the etx ORF in type D isolates.
In a previous study (11), workers sequenced the etx ORF from one type D isolate (NCTC8346) and one type B isolate (NCTC8533), and the results suggested that there might be type-specific etx ORF differences. Therefore, we sequenced the etx ORF from eight selected type D isolates, including a mixture of cpe-negative cpb2-negative type D isolates and type D isolates carrying cpe or both cpe and cpb2. These analyses showed that the etx genes in the type D isolates surveyed encode mature ɛ-toxins with the same amino acid sequence. However, some type D isolates carried the etx ORF previously associated with the etx ORF of type B isolates; i.e., their etx ORF encoded a threonine (rather than serine) at prototoxin amino acid position 321.
DISCUSSION
Type D isolates producing ɛ-toxin cause natural enterotoxemias of lambs, goats, and other animals when ɛ-toxin, an overlap CDC/U.S. Department of Agriculture class B select toxin, is absorbed through the intestinal mucosa. The absorbed toxin then spreads via the circulation to different internal organs, such as the kidney and brain, and then causes elevated blood pressure, edema, and neurological signs (16, 21, 25). The mechanism of action of ɛ-toxin has been studied intensively in the past several years, but very little is known about etx genetics.
Our current findings confirm previous suggestions, based on analysis of only a few type D isolates, that the etx gene commonly resides on large plasmids (2, 14). However, we discovered considerable variation in etx plasmid size. In cpe-negative cpb2-negative type D isolates, the etx plasmid is typically ∼48 kb long, but it can be as large as ∼73 to 75 kb. In type D isolates that also carry a cpe and/or cpb2 gene, the etx plasmid is typically very large, up to ∼110 kb in size. In these type D isolates with a more complicated toxin genotype, the etx, cpe, and cpb2 genes are sometimes present on the same plasmid; i.e., a single type D plasmid can encode three different lethal toxins. However, cpb2-positive type D isolates generally carry the cpb2 gene on a smaller plasmid distinct from the etx plasmid. In fact, results of the current study revealed that it is possible for a simple type D isolate to carry three different toxin plasmids.
The current plasmid analyses also demonstrated that tcp locus genes (1) essential for conjugative transfer of pCW3 are also apparently associated with the etx plasmid in many type D isolates. Furthermore, our results indicate these tcp genes can sometimes also be associated with non-etx-containing cpe or cpb2 plasmids in type D isolates; the latter discovery is interesting since in previous studies workers did not identify any tcp-carrying cpe-negative cpb2+ plasmids in type A isolates (19). Since the tcp locus can mediate conjugative transfer of pCW3 (1) and probably also mediates conjugative transfer of pCPF4969 and other cpe plasmids (5, 19), these findings suggest that most etx plasmids should be transferable by conjugation. However, studies are needed to confirm this hypothesis.
Recently, two cpe-carrying type A plasmids have been completely sequenced, and the sequence information was used to identify two major type A cpe plasmid families that share a conserved region (about 50% of each plasmid) but also carry quite different variable regions (19). When we evaluated (by overlapping and individual PCRs) whether the conserved or variable regions of type A cpe plasmids are also present in type D isolates, individual conserved region ORFs of type A cpe plasmids were identified in type D isolates. However, the arrangement or sequence of these conserved region ORFs in type D isolates must be substantially different from the arrangement or sequence in type A cpe plasmids. This suggests that the type A cpe plasmids and the type D etx plasmids, even when they carry the cpe gene, may not be closely related beyond their tcp sequences. Further studies are needed to evaluate whether the type A cpe plasmid conserved region ORFs detected are present on the etx plasmid in type D isolates and, if so, how they are arranged on this plasmid. While two type D isolates did carry substantial type A cpe plasmid variable region ORFs, even the two shared ORFs appeared to have substantially different sequences or were arranged differently in these two type D isolates and the type A cpe plasmids. Collectively, these findings support the conclusion that sequencing of one or more etx plasmids from type D isolates is necessary in order to understand the contributions to virulence and the evolution of these plasmids.
There is emerging evidence that there is a close association between IS sequences and many C. perfringens toxin genes (6, 18, 24). For example, IS1469, IS1151, and IS1470 sequences are located near the cpe genes of type A isolates (4, 6, 18), while IS1151 sequences are located near the ι-toxin genes in type E isolates (3, 15). It has been suggested that, at least for the chromosomal cpe gene (6) and ι-toxin gene (15), these IS sequences can mobilize the adjacent toxin gene, thereby affecting the evolution of virulence of individual C. perfringens isolates and, by extension, the entire species. In a previous study the workers identified an IS1151 sequence upstream of etx in a few type D isolates (8, 24), but our findings suggest that there may be a more complicated relationship between IS1151 and etx. While most or all type D isolates carry IS1151, this IS was found only immediately upstream of etx in cpe-negative cpb2-negative type D isolates. These findings are the first report of etx locus differences among type D isolates. At least in vitro, these etx locus variations do not affect ɛ-toxin expression levels (26; this study), but it is notable that isolates carrying etx on a ∼110-kb plasmid consistently produced low levels of ɛ-toxin (26; this study). The current study also showed that in contrast to the variations present upstream of the etx gene, most or all type D isolates carry a similar IS406-like sequence downstream of the etx gene.
Our Southern blot and overlapping PCR results clearly indicate that the etx gene occurs on several different plasmids. The association between IS sequences and the etx gene in type D strains (9; this study) suggests a possible mechanism to explain this observed etx plasmid diversity; i.e., nearby IS sequences could mobilize etx sequences from one etx plasmid, followed by subsequent insertion of the etx-carrying mobile element into a second plasmid. Consistent with this hypothesis, a circular form with a circular transposition intermediate containing IS406-etx-IS1151 was identified by PCR in this study. The suggested mobility of the etx gene and other toxin genes (9) has implications for the evolution of the virulence of C. perfringens; e.g., this could help explain why some type D isolates carry a single plasmid with three different toxin genes.
In our etx sequencing study we found that most or all type D isolates have an etx gene sequence that is highly conserved at the nucleotide level, as well as the amino acid level. Previously, it was shown that the etx gene of one type B isolate encodes an ɛ-toxin prototoxin with serine at amino acid residue 321, while the etx gene of one type D isolate encodes an ɛ-toxin with a threonine at this position (11). Our sequencing results indicated that both etx ORF variants can be found in type D isolates; i.e., these etx ORF variations are not type specific. It should be noted that this single amino acid change does not affect the activity of mature ɛ-toxin, since it occurs in a portion of the ɛ-toxin prototoxin that is removed by trypsin or chymotrypsin during the maturation process (20).
ɛ-Toxin requires proteolytic activation. Lambda toxin is capable of activating ɛ-toxin (13), but the importance of this effect is unclear since trypsin or chymotrypsin also activates ɛ-toxin (20). Our results strongly suggest that many type D isolates do not carry the lam gene. Since the type D isolates surveyed were associated with animal diseases, this suggests that lambda toxin production may not be required for virulence. This contention is consistent with our recent mouse lethality results (26) indicating that the ɛ-toxin activity of culture supernatants from most type D isolates, including several isolates surveyed by Western blotting for ɛ-toxin processing in the current study, is low or nonexistent unless it is activated with trypsin. Even supernatants from lam-positive type D isolates contained little or no ɛ-toxin activity prior to trypsin treatment (26; this study), suggesting either that the lam gene of these isolates is not expressed or that it is expressed at levels insufficient to obtain full ɛ-toxin activation under the experimental conditions tested. Furthermore, here we report Western blot evidence for proteolytic processing of ɛ-toxin by lam+ isolates, indicating that other C. perfringens proteases can also process ɛ-toxin. However, without trypsin treatment, little lethality was detected in the supernatants from the lam+ isolates proteolytically processing ɛ-toxin (26), leaving the question whether these non-lambda toxin proteases can activate ɛ-toxin unresolved.
Finally, type B isolates also produce ɛ-toxin, although the virulence genetics of type B isolates have been investigated even less than those of type D isolates. Therefore, studies are now under way to characterize the virulence plasmids of type B isolates and to compare the results with our results obtained with type D isolates. The findings should provide further insight into the contribution of C. perfringens plasmids to virulence and the evolution of virulence plasmids.
Supplementary Material
Acknowledgments
National Institute of Allergy and Infectious Diseases grants AI056177-04 and T32 AI060525-01A1 (Ruth L. Kirschstein National Service Award) supported this research.
We thank J. I. Rood and Glenn Songer for supplying type D isolates used in this study and J. I. Rood and D. J. Fisher for their suggestions. We also thank P. Hauser for supplying ɛ-toxin monoclonal antibodies.
Editor: D. L. Burns
Footnotes
Published ahead of print on 5 March 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Bannam, T. L., W. L. Teng, D. Bulach, D. Lyras, and J. I. Rood. 2006. Functional identification of conjugation and replication regions of the tetracycline resistance plasmid pCW3 from Clostridium perfringens. J. Bacteriol. 188:4942-4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentancor, A. B., M. R. Fermepín, L. D. Bentancor, and R. A. de Torres. 1999. Detection of the etx gene (epsilon-toxin inducer) in plasmids of high molecular weight in Clostridium perfringens type D. FEMS Immunol. Med. Microbiol. 24:373-377. [DOI] [PubMed] [Google Scholar]
- 3.Billington, S. J., E. U. Wieckowski, M. R. Sarker, D. Bueschel, J. G. Songer, and B. A. McClane. 1998. Clostridium perfringens type E animal enteritis isolates with highly conserved, silent enterotoxin gene sequences. Infect. Immun. 66:4531-4536. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Brynestad, S., and P. E. Granum. 1999. Evidence that Tn5565, which includes the enterotoxin gene in Clostridium perfringens, can have a circular form which may be a transposition intermediate. FEMS Microbiol. Lett. 170:281-286. [DOI] [PubMed] [Google Scholar]
- 5.Brynestad, S., M. R. Sarker, B. A. McClane, P. E. Granum, and J. I. Rood. 2001. Enterotoxin plasmid from Clostridium perfringens is conjugative. Infect. Immun. 69:3483-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brynestad, S., B. Synstad, and P. E. Granum. 1997. The Clostridium perfringens enterotoxin gene is on a transposable element in type A human food poisoning strains. Microbiology 143:2109-2115. [DOI] [PubMed] [Google Scholar]
- 7.Cornillot, E., B. Saint-Joanis, G. Daube, S. Katayama, P. E. Granum, B. Canard, and S. T. Cole. 1995. The enterotoxin gene (cpe) of Clostridium perfringens can be chromosomal or plasmid-borne. Mol. Microbiol. 15:639-647. [DOI] [PubMed] [Google Scholar]
- 8.Daube, G., P. Simon, and A. Kaeckenbeeck. 1993. IS1151, an IS-like element of Clostridium perfringens. Nucleic Acids Res. 21:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupuy, B., G. Daube, M. R. Popoff, and S. T. Cole. 1997. Clostridium perfringens urease genes are plasmid borne. Infect. Immun. 65:2313-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher, D. J., K. Miyamoto, B. Harrison, S. Akimoto, M. R. Sarker, and B. A. McClane. 2005. Association of beta2 toxin production with Clostridium perfringens type A human gastrointestinal disease isolates carrying a plasmid enterotoxin gene. Mol. Microbiol. 56:747-762. [DOI] [PubMed] [Google Scholar]
- 11.Havard, H. L., S. E. Hunter, and R. W. Titball. 1992. Comparison of the nucleotide sequence and development of a PCR test for the epsilon toxin gene of Clostridium perfringens type B and type D. FEMS Microbiol. Lett. 76:77-81. [DOI] [PubMed] [Google Scholar]
- 12.Hunter, S. E., I. N. Clarke, D. C. Kelly, and R. W. Titball. 1992. Cloning and nucleotide sequencing of the Clostridium perfringens epsilon-toxin gene and its expression in Escherichia coli. Infect. Immun. 60:102-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin, F., O. Matsushita, S. Katayama, S. Jin, C. Matsushita, J. Minami, and A. Okabe. 1996. Purification, characterization, and primary structure of Clostridium perfringens lambda-toxin, a thermolysin-like metalloprotease. Infect. Immun. 64:230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katayama, S., B. Dupuy, G. Daube, B. China, and S. T. Cole. 1996. Genome mapping of Clostridium perfringens strains with I-CeuI shows many virulence genes to be plasmid-borne. Mol. Gen. Genet. 251:720-726. [DOI] [PubMed] [Google Scholar]
- 15.Li, J., K. Miyamoto, and B. McClane. 2007. Comparison of virulence plasmids among Clostridium perfringens type E isolates. Infect. Immun. In press. [DOI] [PMC free article] [PubMed]
- 16.McClane, B. A., F. A. Uzal, M. F. Miyakawa, D. Lyerly, and T. Wilkins. 2006. The enterotoxic clostridia, p. 688-752. In M. Dworkin, S. Falkow, E. Rosenburg, K. H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes, vol. 4. Springer-Verlag, New York, NY. [Google Scholar]
- 17.Meer, R. R., and J. G. Songer. 1997. Multiplex polymerase chain reaction assay for genotyping Clostridium perfringens. Am. J. Vet. Res. 58:702-705. [PubMed] [Google Scholar]
- 18.Miyamoto, K., G. Chakrabarti, Y. Morino, and B. A. McClane. 2002. Organization of the plasmid cpe locus in Clostridium perfringens type A isolates. Infect. Immun. 70:4261-4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto, K., D. J. Fisher, J. Li, S. Sayeed, S. Akimoto, and B. A. McClane. 2006. Complete sequencing and diversity analysis of the enterotoxin-encoding plasmids in Clostridium perfringens type A non-food-borne human gastrointestinal disease isolates. J. Bacteriol. 188:1585-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyata, S., O. Matsushita, J. Minami, S. Katayama, S. Shimamoto, and A. Okabe. 2001. Cleavage of a C-terminal peptide is essential for heptamerization of Clostridium perfringens epsilon-toxin in the synaptosomal membrane. J. Biol. Chem. 276:13778-13783. [DOI] [PubMed] [Google Scholar]
- 21.Nagahama, M., H. Iida, and J. Sakurai. 1993. Effect of Clostridium perfringens epsilon toxin on rat isolated aorta. Microbiol. Immunol. 37:447-450. [DOI] [PubMed] [Google Scholar]
- 22.Petit, L., M. Gilbert, and M. R. Popoff. 1999. Clostridium perfringens: toxinotype and genotype. Trends Microbiol. 7:104-110. [DOI] [PubMed] [Google Scholar]
- 23.Rokos, E. A., J. I. Rood, and C. L. Duncan. 1978. Multiple plasmids in different toxigenic types of Clostridium perfringens. FEMS Microbiol. Lett. 4:323-326. [Google Scholar]
- 24.Rood, J. I. 1998. Virulence genes of Clostridium perfringens. Annu. Rev. Microbiol. 52:333-360. [DOI] [PubMed] [Google Scholar]
- 25.Sakurai, J., M. Nagahama, and Y. Fujii. 1983. Effect of Clostridium perfringens epsilon toxin on the cardiovascular system of rats. Infect. Immun. 42:1183-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sayeed, S., M. E. Fernandez-Miyakawa, D. J. Fisher, V. Adams, R. Poon, J. I. Rood, F. A. Uzal, and B. A. McClane. 2005. Epsilon-toxin is required for most Clostridium perfringens type D vegetative culture supernatants to cause lethality in the mouse intravenous injection model. Infect. Immun. 73:7413-7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smedley, J. G. III, D. J. Fisher, S. Sayeed, G. Chakrabarti, and B. A. McClane. 2004. The enteric toxins of Clostridium perfringens. Rev. Physiol. Biochem. Pharmacol. 152:183-204. [DOI] [PubMed] [Google Scholar]
- 28.Wen, Q., and B. A. McClane. 2004. Detection of enterotoxigenic Clostridium perfringens type A isolates in American retail foods. Appl. Environ Microbiol. 70:2685-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.