Abstract
The innate immune response of macrophages (Mφ) to spores, the environmentally acquired form of Bacillus anthracis, is poorly characterized. We therefore examined the early Mφ cytokine response to B. anthracis spores, before germination. Mφ were exposed to bacilli and spores of Sterne strain 34F2 and its congenic nongerminating mutant (ΔgerH), and cytokine expression was measured by real-time PCR and an enzyme-linked immunosorbent assay. The exosporium spore layer was retained (exo+) or removed by sonication (exo−). Spores consistently induced a strong cytokine response, with the exo− spores eliciting a two- to threefold-higher response than exo+ spores. The threshold for interleukin-1β (IL-1β) production by wild-type Mφ was significantly lower than that required for tumor necrosis factor alpha expression. Cytokine production was largely dependent on MyD88, suggesting Toll-like receptor involvement; however, the expression of beta interferon in MyD88−/− Mφ suggests involvement of a MyD88-independent pathway. We conclude that (i) the B. anthracis spore is not immunologically inert, (ii) the exosporium masks epitopes recognized by the Mφ, (iii) the Mφ cytokine response to B. anthracis involves multiple pattern recognition receptors and signaling pathways, and (iv) compared to other cytokines, IL-1β is expressed at a lower spore concentration.
Bacillus anthracis, the causative agent of anthrax, is a highly virulent gram-positive, spore-forming bacterium that is typically acquired through exposure to spores from anthrax spore-infected animals or animal products or atypically through intentional exposure as a biological weapon (7, 13). Virulent strains of B. anthracis carry two large plasmids, pXO1 and pXO2, which carry the genes encoding anthrax toxin production and capsule formation, respectively, each of which is critical to the pathogenesis of anthrax infection.
B. anthracis exists in the environment as dormant spores capable of resisting adverse conditions. Upon uptake by macrophages (Mφ), B. anthracis spores, the form initially encountered by Mφ, rapidly germinate, with the outgrowth of vegetative bacilli constituting the first stage of anthrax spore infection (14). Spore germination enables the bacteria to actively proliferate, synthesize their virulence factors (lethal and edema toxins, capsule), and disseminate within the host, leading to massive septicemia (7). Thus, the recognition and elimination of B. anthracis by Mφ (6, 7, 28, 29, 45, 46) represent critical early events in anthrax pathogenesis.
Since a successful innate immune response to B. anthracis might prevent the replication and dissemination of the vegetative form, we examined the Mφ cytokine response to B. anthracis spores. Little is known about the ability of B. anthracis spores to induce Mφ cytokine responses, which might be expected to activate macrophagicidal activity, thereby lowering the replication and dissemination of bacilli. To address this question, we used two approaches. First, we used the germination-proficient B. anthracis Sterne strain 34F2 (pXO1+ pXO2−) and examined the spore Mφ interaction at 1 h, prior to the spore germination and cytokine production, by employing quantitative PCR to study cytokine gene expression. Second, we measured cytokine protein responses by Mφ by using the congenic germination-deficient ΔgerH (gerH-null) strain spores (43, 44). The tri-cistronic gerHabc operon encodes germinant sensors in the presence of Mφ and Mφ-conditioned media and is required for endospore germination within the Mφ environment (43). Since the germination-deficient (ΔgerH) mutant does not form vegetative bacilli in Mφ cultures, it is a useful tool for dissecting the Mφ response to the B. anthracis spore in the absence of germination and allows us to distinguish the response elicited to the spore form of B. anthracis that does not express toxins or capsule from that elicited to the vegetative form.
A second goal of this study was to determine the role of the exosporium (exo) in the innate immune response to B. anthracis. The exosporium of B. anthracis is the outermost layer of the spores and contains a major structural glycoprotein, Bacillus collagen-like protein of anthracis (BclA) (35, 36, 40), as well as many enzymes, such as arginase and superoxide dismutase, whose functions in B. anthracis pathogenesis are beginning to be elucidated (3, 33). We recently reported that the presence of the exosporium may influence spore germination within the Mφ (3) and that Sterne 34F2 spores lacking an exosporium (exo−) are more easily killed than those in which the exosporium is retained (exo+) (20). We hypothesized that one explanation for the decreased B. anthracis killing that we observed with spores retaining the exosporium is that this structure may mask important structures on the spore that are normally responsible for the induction of a more robust proinflammatory cytokine response. Alternatively, proteins on the exosporium (35), such as arginase and superoxide dismutase (3, 33), may subvert the formation of oxidative radicals or other bactericidal mechanisms in Mφ.
Toll-like receptors (TLRs) are a family of pattern recognition receptors expressed on cells of the immune system whose role in innate immunity is to recognize microbial pathogens. Mφ express a molecule encoded by the myeloid differentiation primary response gene 88 (the MyD88 gene), which serves as an adaptor molecule and mediates TLR signaling for all but two TLR responses (TLR3 and TLR4) (2, 4, 11, 16, 41). A previous study found that heat-killed vegetative bacilli induced tumor necrosis factor alpha (TNF-α) expression by Mφ through TLRs in a MyD88-dependent manner (15). Previous studies have also reported the involvement of TLRs in the response to B. anthracis toxins or heat-killed bacilli or in the systemic response to B. anthracis spore challenge (15, 26), but relatively little is known about the involvement of TLRs in the Mφ response to B. anthracis spores prior to germination. Consequently, in this study we examined the early cytokine mRNA response of MyD88-dependent and -independent pathways to B. anthracis spores prior to their germination to the vegetative form.
We find that while spores are not immunologically inert, ≥105 B. anthracis spores/ml are required to elicit the expression of TNF-α and other cytokines by Mφ; however, as few as 100 spores induced a robust interleukin-1β (IL-1β) response compared to the untreated control. Further, the exosporium blunted spore-induced cytokine generation by Mφ. Finally, while we confirm the dependence on the MyD88 adaptor protein for many B. anthracis-induced cytokine responses, we also find that B. anthracis spores activated a MyD88-independent signaling pathway manifested by the induction of beta interferon (IFN-β).
MATERIALS AND METHODS
B. anthracis strains and spore preparation.
B. anthracis Sterne 34F2 and the ΔgerH (the congenic gerH-null) strain constructed from Sterne 34F2 were prepared as previously described (20). For each experiment, viable spore titer was determined by dilution plating before and after heat killing (65°C for 30 min). The vegetative forms of Sterne 34F2 and ΔgerH B. anthracis were generated by growing the spores in germinant-rich media (LB broth) that enabled the ΔgerH spores to germinate. Bacillus numbers were determined by dilution plating on LB agar plates.
Sonication of spores.
The exosporium was removed by disruption of the spores with sonication as previously described (20). This treatment did not alter the viability of the spore or its ability to grow in laboratory culture medium (20).
Preparation of murine peritoneal Mφ and culture.
Primary peritoneal Mφ were obtained from mice 4 days after intraperitoneal inoculation with 3 ml of 3% thioglycolate as previously described (20). While Mφ from C57/BL6 mice (Jackson Laboratory, Bar Harbor ME) were used for most studies, for selected experiments we used MyD88−/− mice on a C57/BL6 background (kindly provided by Stefanie Vogel, Baltimore, MD). In addition, C3H/HeJ (LPSd) and C3H/HeN (LPSn) mice were obtained from Charles River Laboratory (Wilmington, MA). Mφ were cultured according to the method of Fortier et al. with some minor modifications (12). Washed cell suspensions were adjusted to 1.5 × 106 Mφ per ml in culture medium containing RPMI 1640 with heat-inactivated fetal bovine serum (2%) and penicillin and streptomycin (100 U/ml) and incubated in a 6- or 12-well flat-bottomed tissue culture plate (Nunc A/S, Roskilde, Denmark) in 5% CO2 at 37°C overnight. Cells were washed two or three times on the day of the experiment with sterile phosphate-buffered saline (Gibco, Invitrogen, Grand Island, NY), infected with the spores at a requisite multiplicity of infection (MOI), and incubated in RPMI 1640 without fetal bovine serum or antibiotics at 37°C in 5% CO2 for 60 min. Cells were then washed and lysed to obtain mRNA. The recombinant endotoxin-neutralizing protein (rENP) from Saccharomyces cerevisiae was obtained from Seikagaku America (Falmouth, MA), and B. anthracis spores or lipopolysaccharide (LPS) was preincubated for 15 min with the rENP as per the manufacturer's instructions prior to being added to Mφ cultures.
Isolation of mRNA and real-time PCR.
Total cellular RNA was extracted from Mφ as described elsewhere (22, 41) and reverse transcribed using AMV reverse transcriptase (Promega) and poly-T priming as recommended by the manufacturer. The resulting cDNA was quantified by using real-time PCR using SYBR green PCR master mix (Applied Biosystems) and an ABI Prism 7900HT cycler. Intron-spanning primers for detection of IL-1β, TNF-α, IFN-β, IL-6, MIP1-β, and hypoxanthine phosphoribosyltransferase (HPRT) mRNAs were designed using the Primer Express 2.0 program (Applied Biosystems). Relative gene expression was calculated using the ΔCT method, where CT (threshold cycle) refers to the cycle number at which the PCR product for a particular gene is detected by the light cycler. The housekeeping (HPRT) gene was used as an internal control, and relative gene expression was normalized to HPRT gene expression by the following formula:  . The data are averages for two to five independent experiments, and the samples from each experiment were analyzed in duplicate or triplicate more than two times. Statistical analysis was performed by one-way analysis of variance with repeated measures, followed by the use of Dunnet's multiple-comparison or Tukey's multiple-paired-comparison test, with P values of ≤0.05 considered significant.
. The data are averages for two to five independent experiments, and the samples from each experiment were analyzed in duplicate or triplicate more than two times. Statistical analysis was performed by one-way analysis of variance with repeated measures, followed by the use of Dunnet's multiple-comparison or Tukey's multiple-paired-comparison test, with P values of ≤0.05 considered significant.
Measurement of cytokines.
Cell culture supernatants were quantified at the University of Maryland Cytokine Core Laboratory for cytokine levels by using a standard two-antibody enzyme-linked immunosorbent assay (ELISA) with commercial antibody pairs and recombinant standards (Pierce-Endogen, Boston, MA) as previously described (18). The data were analyzed using a computer program (SoftPro; Molecular Devices).
RESULTS
B. anthracis spores elicit a dose-dependent cytokine response in primary Mφ.
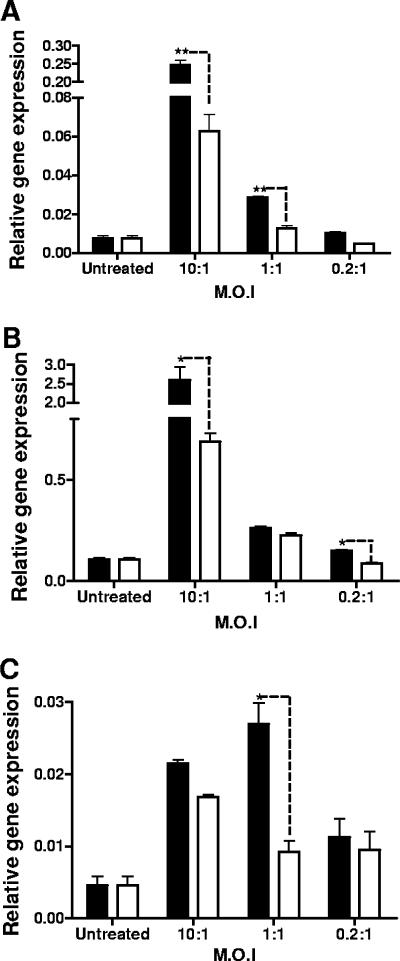
In order to determine whether B. anthracis spores induced a cytokine response in Mφ, we employed two approaches to minimize the possibility that vegetative bacilli would confound interpretation of the data. Real-time PCR allowed an evaluation of cytokine mRNA 1 h after addition of spores to Mφ. Second, we used the ΔgerH mutant of Sterne 34F2, which germinates poorly in the presence of Mφ (43, 44). We measured levels of IL-1β, TNF-α, IFN-β, RANTES, and IL-6 because of their importance in stimulating different components of the immune response, such as phagocytes (TNF-α, IL-1β), B cells, and antibody formation (IL-6). We also looked at message levels of IFN-β and RANTES as an indicator of the MyD88-independent pathway of activation of Mφ. We first measured IL-1β, TNF-α, and IFN-β mRNA levels at three different MOIs: 3 × 107 spores per 3 × 106 Mφ (MOI, 10:1), 3 × 106 spores per 3 × 106 Mφ (MOI, 1:1), and 6 × 105 spores per 3 × 106 Mφ (MOI, 0.2:1). These MOIs ranged from higher values used in other studies (15, 26, 29) to lower values (0.2:1) not reported previously, to our knowledge. We find that compared to uninfected Mφ, spores were able to induce IL-1β, TNF-α, and IFN-β mRNA in a dose-dependent fashion, with detectable TNF-α and IFN-β mRNA expression at MOIs of 0.2:1 and 1:1, respectively (Fig. 1). During the 1-hour incubation, we observed minimal germination of spores in the serum-free medium. Nevertheless, to ensure that the cytokine induction was not attributable to vegetative bacilli, we compared the ability of the spores to induce cytokine mRNA to that of heat-killed vegetative bacilli. As shown in Fig. 1, at each MOI there was greater induction of cytokine mRNA by the spores than by the bacilli. Since the bacilli have a tendency to form chains, the number of bacilli added on the basis of CFU may actually be an underestimate of the number of bacilli added to the cultures.
FIG. 1.
B. anthracis spores elicit an early cytokine mRNA response in primary Mφ. B. anthracis spores (filled bars) or heat-killed bacilli (open bars) were added at different MOIs to a culture of primary Mφ (3 × 106) that were placed in serum-free RPMI 1640 for the duration of the infection (1 h). The mRNA levels for IL-1β (A), TNF-α (B), and IFN-β (C) were quantified. **, P ≤ 0.01; *, P ≤ 0.05.
To confirm the observation that the spore form of B. anthracis induces a Mφ cytokine response, we added ΔgerH spores at a single MOI of 0.2:1 and measured cytokine protein (Fig. 2). Here, too, the spores induced significant cytokine responses. Further, these were greater than the responses to heat-killed bacilli generated from ΔgerH spores that were added to the Mφ cultures. These differences were greatest for IL-1β, TNF-α, and RANTES and less pronounced for IL-6. Thus, the results shown in Fig. 1 and 2 demonstrate that spores of the Sterne strain induce cytokine responses in Mφ and that these responses are not due to vegetative bacilli that may have emerged during germination.
FIG. 2.
The spore form of ΔgerH B. anthracis elicits a stronger cytokine protein response than the vegetative form in murine Mφ. Primary murine Mφ were exposed to the heat-killed vegetative or the exo+ form of the ΔgerH B. anthracis spores at an MOI of 0.2:1 in serum-free RPMI 1640. The Mφ were viable after 24 h, when the supernatants were harvested and analyzed by ELISA for amounts of IL-1β (A), TNF-α (B), IL-6 (C), and RANTES protein (D) made by the Mφ. **, P ≤ 0.01.
Removal of the exosporium increases spore-induced cytokine responses.
In previous studies, we demonstrated that the exosporium layer that surrounds the spore has an important role in the initial interaction of the spore with the host Mφ (20). In addition to the structural protein BclA, the exosporium contains at least 20 different enzymes which may play a role in the pathogenesis of anthrax spore infection (33, 34). We therefore studied whether the exosporium affected spore-induced cytokine responses by Mφ. Sonication of spores removes the exosporium, as monitored by electron microscopy, but does not alter its growth curve in laboratory medium (20). We noted that for each cytokine examined (Fig. 3), removal of the exosporium resulted in a more robust cytokine mRNA expression in Mφ 1 hour after infection. This was also observed when the ΔgerH spores were used in place of Sterne. The exosporium itself, however, did not induce any cytokine response (data not shown). These data suggest either that the exosporium masks structures on the spore cortex critical to cytokine induction or that enzymes on the exosporium may alter expression of the spore-induced cytokines.
FIG. 3.
Exosporium-deficient spores elicit a stronger cytokine message response than spores covered with exosporium. Primary murine Mφ were exposed to the exo+ or exo− forms of the Sterne spores at an MOI of 0.2:1, and cytokine message levels were quantified 1 h after exposure by real-time PCR. The exo− spores induced a significantly higher response for IFN-β (A), IL-1β (B), TNF-α (C), and IL-6 (D). **, P ≤ 0.01; *, P ≤ 0.05.
Low concentrations of B. anthracis spores induce IL-1β.
We observed a robust ratio of IL-1β expression (both mRNA and protein) to B. anthracis spores at an MOI ratio of 6 × 106 spores to 6 × 106 Mφ (1:1) (Fig. 1 and 2). Since initial contact with spores will most likely be at considerably lower concentrations, we determined the threshold doses of B. anthracis ΔgerH required to produce detectable levels of IL-1β and another proinflammatory cytokine, TNF-α. At every dose of spores examined, the levels of IL-1β generated were four- to sixfold greater than that of TNF-α. Moreover, at doses as low as 102 spores/106 Mφ, the IL-1β response was significantly greater than that for the medium control, whereas at the same dose, the TNF-α production was not significantly higher than that for the medium control (Fig. 4). The finding that IL-1β production was easily detectable at doses of B. anthracis spores that may closely approximate an environmental exposure suggests that this cytokine may play an early and important role in the innate immune response to B. anthracis infections. The relatively high threshold for TNF-α induction is also consistent with the hypothesis that at the levels of spores likely to be encountered via inhalation, there is relatively little cytokine generation; however, at levels of B. anthracis likely to be encountered in the later phases of anthrax spore infection, TNF-α and undoubtedly other cytokines may play an important role.
FIG. 4.
IL-1β is expressed at lower concentrations of spores than TNF-α. Primary murine Mφ plated at 1 × 106 cells/well were challenged overnight with increasing amounts of the ΔgerH exo+ spores, and the supernatants were assayed by ELISA for IL-1β (A) and TNF-α (B) production. **, P ≤ 0.01; *, P ≤ 0.05; n.s., not significant, in comparison to medium control levels.
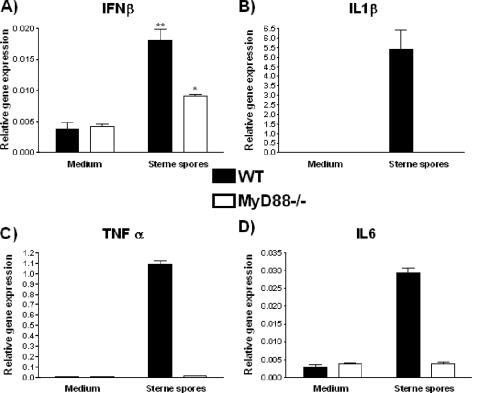
Cytokine response in Mφ is MyD88 dependent.
Since Hughes et al. reported that the TNF-α response to vegetative B. anthracis bacilli was MyD88 dependent (15), we assessed whether the Mφ response to B. anthracis spores was similarly dependent on MyD88 (Fig. 5). In the absence of MyD88, there was loss of B. anthracis spore-induced expression of all cytokines (IL-1β, TNF-α, IL-6) except for IFN-β. This was true for exo+ and exo− responses. The IFN-β mRNA expression was reduced but not entirely abrogated in MyD88−/− Mφ (Fig. 5). This observation was consistent with the known induction of IFN-β by MyD88-independent signaling through the TRIF/TRAM pathway (41).
FIG. 5.
The Mφ mRNA cytokine response is dependent on MyD88. Primary murine Mφ from MyD88−/− or wild-type (WT) control C57/BL6 mice were exposed to Sterne exo+ spores of B. anthracis at an MOI of 0.2:1 for 1 h, and their cytokine message levels for IFN-β (A), IL-1β (B), TNF-α (C), and IL-6 (D) were determined by real-time PCR. **, P ≤ 0.01; *, P ≤ 0.05, for comparison to medium control levels.
The IFN-β response is not due to contaminating LPS.
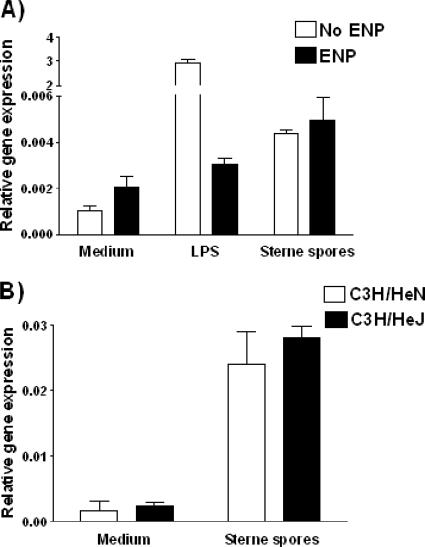
The B. anthracis spores consistently upregulated IFN-β message levels in Mφ, and this response was partially dependent on MyD88 (Fig. 5). It therefore could be suggested that the IFN-β message is being elicited in response to a TLR 4 agonist, such as LPS, that may contaminate our spore preparations. In order to rule out the possibility of endotoxin contamination, we preincubated our spore preparations with and without the ENP from the horseshoe crab (Limulus polyphemus) and quantified cytokine mRNA levels in wild-type Mφ. The up-regulation of IFN-β message levels in response to either Sterne or ΔgerH (data not shown) spores was unaltered in the presence of ENP, whereas the response to LPS was significantly abrogated (Fig. 6A), confirming that the IFN-β response to the B. anthracis spores was not due to contaminating endotoxin in the spore preparations. These results were further supported by the data from the C3H/HeJ mice, which lack functional TLR4 responses (30). Despite the lack of a functional TLR4-mediated signaling pathway, we still observed an IFN-β mRNA response to the B. anthracis spores (Fig. 6B). These data indicate that the IFN-β response to the B. anthracis spores is independent of signaling through TLR4 and not due to an endotoxin contamination. Thus, there appear to be multiple signaling pathways required for cytokine responses to B. anthracis spores.
FIG. 6.
The IFN-β response is not mediated by TLR4 or due to endotoxin contamination. (A) B. anthracis spores (MOI, 0.2:1) or LPS (100 ng/ml) was preincubated for 30 min with recombinant ENP (ENP) (3 μg/ml) or phosphate-buffered saline prior to being added to the culture of primary murine Mφ. The IFN-β message level of the Mφ 1 h poststimulation was determined by real-time PCR. (B) Mφ from TLR4-defective (C3H/HeJ) or control (C3H/HeN) mice were challenged with Sterne spores at an MOI of 0.2:1, and their IFN-β message levels 1 hour after challenge were determined by real-time PCR.
DISCUSSION
We find that upon contact with Mφ, spores, the form of B. anthracis acquired by the host from the environment, are able to initiate both an mRNA and a protein response for all proinflammatory cytokines examined; however, the concentrations of B. anthracis required for cytokine expression (≥104 B. anthracis spores) are likely to exceed environmental inhalational exposures. Significantly, however, unlike for the other cytokines, as few as 100 spores per 106 Mφ were required to induce IL-1β. This suggests a potentially important role for IL-1β in the innate immune response to B. anthracis. Further, the presence of the exosporium that surrounds the spore reduces the ability of B. anthracis spores to induce the cytokines. Finally, since B. anthracis induces the expression of IFN-β in a MyD88-independent manner, and since IL-1β and TNF-α appear to be induced differently, the cytokine response to B. anthracis spores likely requires the involvement of multiple signaling pathways.
The initial host response to inhaled B. anthracis spores may limit the replication and dissemination of the vegetative form that leads to the production of the lethal and edema toxins and ultimately host death. Consequently, it is critical to differentiate between the Mφ responses to the spore and to the later vegetative forms, particularly since the toxins expressed by vegetative forms may compromise host defenses. For example, in susceptible mice, B. anthracis infection causes the alteration in function and/or death of the Mφ, which may allow it to avoid detection by the innate immune system (13). Largely due to its inhibitory effect on mitogen-activated protein kinases, lethal toxin (LT) induces apoptosis of susceptible Mφ (1, 9, 25, 27, 32); however, lysis of Mφ may also occur as a result of the accumulation of large numbers of bacilli (6).
Most studies of the Mφ cytokine responses to B. anthracis have employed either the vegetative form (bacilli) or a mixture of spores and bacilli, since in the time required to induce cytokine protein, the spores rapidly germinate to the vegetative form within the Mφ environment. In order to circumvent this problem, we have studied the Mφ response to B. anthracis spores by real-time PCR as early as 1 hour postexposure, when few of the spores are germinating. More importantly, we also examined the cytokine response to germination-deficient (ΔgerH) B. anthracis spores to ensure that the Mφ response was to the spores alone. This congenic mutant of Sterne 34F2 has a markedly delayed germination within Mφ but otherwise has growth characteristics in laboratory medium similar to those of its parent strain (20). We find that Mφ responses to B. anthracis are stronger against the spore than the vegetative form, which indicates that the Mφ may have an early and critical role in inducing protection against anthrax spore infection prior to its germination to the vegetative form. We need to interpret the higher response to spores than to bacilli by keeping in mind that the vegetative form that we used was from the Sterne strain, which had the toxins but lacked the capsule, which is normally present in fully virulent B. anthracis. It is possible that the encapsulated bacilli may induce a cytokine response that is just as robust as or even stronger than that induced by the spores at low MOIs.
Using the ΔgerH strain, we previously reported that Mφ are unable to kill the spore form of B. anthracis but rather kill the emerging vegetative form in an NO-dependent manner (33). Since IL-1β expression is required for Mφ NO formation (10, 23, 39), the expression of this cytokine by Mφ after exposure to relatively low concentrations of spores suggests that the IL-1β response is critical to host defenses against B. anthracis. Our finding of IL-1β protein in supernatants of Mφ culture with as few as 100 spores could be attributable in part to the effect of secondary mediators generated during the 24-hour culture; however, no similar phenomenon was apparent for TNF-α. We recently observed with LPS treatment of Mφ that the expression of pro-IL-1β, the inactive precursor of IL-1β, is MyD88 dependent; however, the processing of this inactive precursor to its mature form by caspase-1 required an IFN-β-mediated signaling pathway that was MyD88 independent (17). Similar MyD88-dependent and -independent mechanisms may be required for B. anthracis-induced IL-1β as well. Thus, killing of B. anthracis by Mφ may involve the formation of an “inflammasome” complex that involves caspase-1, adaptor proteins, and IFN-β-activated STAT1 (17, 19, 21, 24). Others have reported that since LT or B. anthracis induces IL-1β, the induction of this cytokine may be involved in the lethality of anthrax infection or in apoptosis, respectively (31, 32). In that event, it is argued, targeting IL-1β may be of therapeutic benefit. If, however, IL-1β were important to host defenses against B. anthracis at a stage of the pathogenesis of anthrax spore infection that precedes germination and LT formation, as our data suggest, then neutralizing IL-1β may allow the organism to gain a foothold within the host and promote dissemination.
A second objective of this study was to determine whether the exosporium plays a role in evading the immune response. The Mφ response to the B. anthracis spores was more robust when the exosporium was not present. Immediately below the exosporium is a multilayer proteinaceous layer composed of a complex mixture of spore coat proteins, many of unknown functions (8). It is conceivable that among this array of constituents are agonists capable of engaging host cell pattern recognition receptors. Our data suggest that the exosporium either masked immunogenic epitopes on the B. anthracis spore, similar to the effect reported with some bacterial capsular polysaccharides (5), or actively down-modulated the Mφ response via the activity of some protein on the exosporium. Mφ exposed to the exosporium alone did not produce any cytokines or upregulate any cytokine genes (data not shown), indicating that for the Mφ, the exosporium is “inert.” Recent studies, including our own, have shown that in addition to the hair-like structural protein BclA, the exosporium contains over 20 proteins on its surface (34), including enzymes that either can promote germination (e.g., superoxide dismutase) or can actively interfere with the killing ability of Mφ (e.g., arginase) (33). As an example, both exo− and exo+ spores induced similar amounts of inducible nitric oxide synthase (iNOS) in Mφ; however, the exo+ B. anthracis spores generated less of the microbicidal NO, most likely because of the competition of the arginase on the exosporium for the iNOS substrate in the medium, arginine (33). Thus, the B. anthracis exosporium may be an important target for future therapeutic strategies for modulation of the immune response to B. anthracis.
Our data suggest that the signaling pathways involved in the cytokine response to B. anthracis are complex. The different levels of B. anthracis spores required to express IL-1β and TNF-α indicate that the spore utilizes distinct receptor pathways for the induction of these cytokines. Our data also support published reports that TLRs have an important role in the Mφ response to B. anthracis (15, 26). These conclusions are based in part on the loss of generation of some cytokines in Mφ obtained from MyD88−/− mice (15). Yet the identity(ies) of the TLR(s) that mediates these cytokine responses remains elusive.
Of particular interest, we find that the IFN-β response to B. anthracis spores is retained in the absence of MyD88. These results were not due to contaminating endotoxin that may signal through TLR4 via the known MyD88-independent TRIF/TRAM pathway, since the response is not abrogated in the presence of ENP and since the IFN-β response was observed in C3H/HeJ mice that lack a functional TLR4 complex (Fig. 6).
We also find that the Mφ iNOS response to the spore, but not LPS, is critically dependent on the ability of the Mφ to internalize the spore (33). This suggests that there may be intracellular receptors, such as nucleofide-binding oligomerization domain 1 or 2 or TLR3, TLR7, TLR8, or TLR9, that play a role in early responses to the B. anthracis spore. These may recognize epitopes that are usually hidden by the exosporium and may become exposed when the spore germinates within the Mφ. Significantly, peptidoglycan derived from a non-toxin-producing Bacillus strain induced IL-1β expression in RAW 264.7 cells, demonstrating that IL-1β can be induced by bacilli independently of toxins (42). Alternatively, cytosolic DNA from an invasive strain of Listeria monocytogenes as well as bacterial RNA was recently found to activate a potent IFN-I response (21, 24, 37, 38). This activation of IFN-I was TLR independent, as we observed for B. anthracis in our studies, and required IFN regulatory factor 3, which is required for transcriptional activation of IFN-β.
In conclusion, our data show that B. anthracis spores induce the expression of cytokines by Mφ. This cytokine expression occurs as early as 1 hour after exposure to B. anthracis spores and in a dose-dependent manner, with IL-1β expression occurring at significantly lower spore concentrations than are required for expression of other cytokines examined. Cytokine induction by B. anthracis spores involves multiple signaling pathways, including both MyD88-dependent and -independent (e.g., IFN-β) cascades. Further, given the difference in the number of spores required for the induction of IL-1β and TNF-α, these cytokines are likely induced by distinct pathways. Finally, while the exosporium itself does not induce the expression of cytokines, its removal enhances the cytokine response to the spores. Thus, the early interactions between B. anthracis spores and Mφ, before germination and subsequent toxin and capsule production, appear to be both complex and important in the pathogenesis of anthrax infection. A better understanding of these events will be required before optimal therapeutic strategies can be implemented.
Acknowledgments
This work was supported by NIH NIAID Mid-Atlantic Regional Center of Excellence grant U54 AI-057168 and NIH NIAID R21 AI-059093.
Editor: D. L. Burns
Footnotes
Published ahead of print on 5 March 2007.
REFERENCES
- 1.Agrawal, A., and B. Pulendran. 2004. Anthrax lethal toxin: a weapon of multisystem destruction. Cell. Mol. Life Sci. 61:2859-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira, S., K. Hoshino, and T. Kaisho. 2000. The role of Toll-like receptors and MyD88 in innate immune responses. J. Endotoxin Res. 6:383-387. [PubMed] [Google Scholar]
- 3.Baillie, L., S. Hibbs, P. Tsai, G. L. Cao, and G. M. Rosen. 2005. Role of superoxide in the germination of Bacillus anthracis endospores. FEMS Microbiol. Lett. 245:33-38. [DOI] [PubMed] [Google Scholar]
- 4.Basu, S., and M. J. Fenton. 2004. Toll-like receptors: function and roles in lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L887-L892. [DOI] [PubMed] [Google Scholar]
- 5.Cross, A. S. 1990. The biologic significance of bacterial encapsulation. Curr. Top. Microbiol. Immunol. 150:87-95. [DOI] [PubMed] [Google Scholar]
- 6.Dixon, T. C., A. A. Fadl, T. M. Koehler, J. A. Swanson, and P. C. Hanna. 2000. Early Bacillus anthracis-macrophage interactions: intracellular survival survival and escape. Cell. Microbiol. 2:453-463. [DOI] [PubMed] [Google Scholar]
- 7.Dixon, T. C., M. Meselson, J. Guillemin, and P. C. Hanna. 1999. Anthrax. N. Engl. J. Med. 341:815-826. [DOI] [PubMed] [Google Scholar]
- 8.Driks, A. 2004. From rings to layers: surprising patterns of protein deposition during bacterial spore assembly. J. Bacteriol. 186:4423-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duesbery, N. S., C. P. Webb, S. H. Leppla, V. M. Gordon, K. R. Klimpel, T. D. Copeland, N. G. Ahn, M. K. Oskarsson, K. Fukasawa, K. D. Paull, and G. F. Vande Woude. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280:734-737. [DOI] [PubMed] [Google Scholar]
- 10.Dwyer-Nield, L. D., M. C. Srebernak, B. S. Barrett, J. Ahn, P. Cosper, A. M. Meyer, L. R. Kisley, A. K. Bauer, D. C. Thompson, and A. M. Malkinson. 2005. Cytokines differentially regulate the synthesis of prostanoid and nitric oxide mediators in tumorigenic versus non-tumorigenic mouse lung epithelial cell lines. Carcinogenesis 26:1196-1206. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald, K. A., E. M. Palsson-McDermott, A. G. Bowie, C. A. Jefferies, A. S. Mansell, G. Brady, E. Brint, A. Dunne, P. Gray, M. T. Harte, D. McMurray, D. E. Smith, J. E. Sims, T. A. Bird, and L. A. O'Neill. 2001. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413:78-83. [DOI] [PubMed] [Google Scholar]
- 12.Fortier, A. H., T. Polsinelli, S. J. Green, and C. A. Nacy. 1992. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect. Immun. 60:817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukao, T. 2004. Immune system paralysis by anthrax lethal toxin: the roles of innate and adaptive immunity. Lancet Infect. Dis. 4:166-170. [DOI] [PubMed] [Google Scholar]
- 14.Guidi-Rontani, C., M. Weber-Levy, E. Labruyere, and M. Mock. 1999. Germination of Bacillus anthracis spores within alveolar macrophages. Mol. Microbiol. 31:9-17. [DOI] [PubMed] [Google Scholar]
- 15.Hughes, M. A., C. S. Green, L. Lowchyj, G. M. Lee, V. K. Grippe, M. F. Smith, Jr., L. Y. Huang, E. T. Harvill, and T. J. Merkel. 2005. MyD88-dependent signaling contributes to protection following Bacillus anthracis spore challenge of mice: implications for Toll-like receptor signaling. Infect. Immun. 73:7535-7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hultmark, D. 1994. Macrophage differentiation marker MyD88 is a member of the Toll/IL-1 receptor family. Biochem. Biophys. Res. Commun. 199:144-146. [DOI] [PubMed] [Google Scholar]
- 17.Joshi, V. D., D. V. Kalvakolanu, W. Chen, L. Zhang, T. J. Kang, K. E. Thomas, S. N. Vogel, and A. S. Cross. 2006. A role for Stat1 in the regulation of lipopolysaccharide-induced interleukin-1beta expression. J. Interferon Cytokine Res. 26:739-747. [DOI] [PubMed] [Google Scholar]
- 18.Joshi, V. D., D. V. Kalvakolanu, J. D. Hasday, R. J. Hebel, and A. S. Cross. 2002. IL-18 levels and the outcome of innate immune response to lipopolysaccharide: importance of a positive feedback loop with caspase-1 in IL-18 expression. J. Immunol. 169:2536-2544. [DOI] [PubMed] [Google Scholar]
- 19.Joshi, V. D., D. V. Kalvakolanu, J. R. Hebel, J. D. Hasday, and A. S. Cross. 2002. Role of caspase 1 in murine antibacterial host defenses and lethal endotoxemia. Infect. Immun. 70:6896-6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang, T. J., M. J. Fenton, M. A. Weiner, S. Hibbs, S. Basu, L. Baillie, and A. S. Cross. 2005. Murine macrophages kill the vegetative form of Bacillus anthracis 1. Infect. Immun. 73:7495-7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanneganti, T. D., N. Ozoren, M. Body-Malapel, A. Amer, J. H. Park, L. Franchi, J. Whitfield, W. Barchet, M. Colonna, P. Vandenabeele, J. Bertin, A. Coyle, E. P. Grant, S. Akira, and G. Nunez. 2006. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440:233-236. [DOI] [PubMed] [Google Scholar]
- 22.Manthey, C. L., M. E. Brandes, P. Y. Perera, and S. N. Vogel. 1992. Taxol increases steady-state levels of lipopolysaccharide-inducible genes and protein-tyrosine phosphorylation in murine macrophages. J. Immunol. 149:2459-2465. [PubMed] [Google Scholar]
- 23.Meroni, S. B., A. M. Suburo, and S. B. Cigorraga. 2000. Interleukin-1beta regulates nitric oxide production and gamma-glutamyl transpeptidase activity in sertoli cells. J. Androl. 21:855-861. [PubMed] [Google Scholar]
- 24.Ozoren, N., J. Masumoto, L. Franchi, T. D. Kanneganti, M. Body-Malapel, I. Erturk, R. Jagirdar, L. Zhu, N. Inohara, J. Bertin, A. Coyle, E. P. Grant, and G. Nunez. 2006. Distinct roles of TLR2 and the adaptor ASC in IL-1beta/IL-18 secretion in response to Listeria monocytogenes. J. Immunol. 176:4337-4342. [DOI] [PubMed] [Google Scholar]
- 25.Park, J. M., F. R. Greten, Z. W. Li, and M. Karin. 2002. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science 297:2048-2051. [DOI] [PubMed] [Google Scholar]
- 26.Park, J. M., V. H. Ng, S. Maeda, R. F. Rest, and M. Karin. 2004. Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists. J. Exp. Med. 200:1647-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pellizzari, R., C. Guidi-Rontani, G. Vitale, M. Mock, and C. Montecucco. 1999. Anthrax lethal factor cleaves MKK3 in macrophages and inhibits the LPS/IFNgamma-induced release of NO and TNFalpha. FEBS Lett. 462:199-204. [DOI] [PubMed] [Google Scholar]
- 28.Pickering, A. K., and T. J. Merkel. 2004. Macrophages release tumor necrosis factor alpha and interleukin-12 in response to intracellular Bacillus anthracis spores. Infect. Immun. 72:3069-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickering, A. K., M. Osorio, G. M. Lee, V. K. Grippe, M. Bray, and T. J. Merkel. 2004. Cytokine response to infection with Bacillus anthracis spores. Infect. Immun. 72:6382-6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, H. C. Van, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 31.Popov, S. G., T. G. Popova, E. Grene, F. Klotz, J. Cardwell, C. Bradburne, Y. Jama, M. Maland, J. Wells, A. Nalca, T. Voss, C. Bailey, and K. Alibek. 2004. Systemic cytokine response in murine anthrax. Cell. Microbiol. 6:225-233. [DOI] [PubMed] [Google Scholar]
- 32.Popov, S. G., R. Villasmil, J. Bernardi, E. Grene, J. Cardwell, A. Wu, D. Alibek, C. Bailey, and K. Alibek. 2002. Lethal toxin of Bacillus anthracis causes apoptosis of macrophages. Biochem. Biophys. Res. Commun. 293:349-355. [DOI] [PubMed] [Google Scholar]
- 33.Raines, K. W., T. J. Kang, S. Hibbs, G. L. Cao, J. Weaver, P. Tsai, L. Baillie, A. S. Cross, and G. M. Rosen. 2006. Importance of nitric oxide synthase in the control of infection by Bacillus anthracis. Infect. Immun. 74:2268-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redmond, C., L. W. Baillie, S. Hibbs, A. J. Moir, and A. Moir. 2004. Identification of proteins in the exosporium of Bacillus anthracis. Microbiology 150:355-363. [DOI] [PubMed] [Google Scholar]
- 35.Steichen, C., P. Chen, J. F. Kearney, and C. L. Turnbough, Jr. 2003. Identification of the immunodominant protein and other proteins of the Bacillus anthracis exosporium. J. Bacteriol. 185:1903-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steichen, C. T., J. F. Kearney, and C. L. Turnbough, Jr. 2005. Characterization of the exosporium basal layer protein BxpB of Bacillus anthracis. J. Bacteriol. 187:5868-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stetson, D. B., and R. Medzhitov. 2006. Antiviral defense: interferons and beyond. J. Exp. Med. 203:1837-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stetson, D. B., and R. Medzhitov. 2006. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24:93-103. [DOI] [PubMed] [Google Scholar]
- 39.Stoffels, K., L. Overbergh, A. Giulietti, A. Kasran, R. Bouillon, C. Gysemans, and C. Mathieu. 2004. NOD macrophages produce high levels of inflammatory cytokines upon encounter of apoptotic or necrotic cells. J. Autoimmun. 23:9-15. [DOI] [PubMed] [Google Scholar]
- 40.Sylvestre, P., E. Couture-Tosi, and M. Mock. 2002. A collagen-like surface glycoprotein is a structural component of the Bacillus anthracis exosporium. Mol. Microbiol. 45:169-178. [DOI] [PubMed] [Google Scholar]
- 41.Toshchakov, V. U., S. Basu, M. J. Fenton, and S. N. Vogel. 2005. Differential involvement of BB loops of toll-IL-1 resistance (TIR) domain-containing adapter proteins in. J. Immunol. 175:494-500. [DOI] [PubMed] [Google Scholar]
- 42.Vermeulen, M. W., and G. R. Gray. 1984. Processing of Bacillus subtilis peptidoglycan by a mouse macrophage cell line. Infect. Immun. 46:476-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiner, M. A., and P. C. Hanna. 2003. Macrophage-mediated germination of Bacillus anthracis endospores requires the gerH operon. Infect. Immun. 71:3954-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiner, M. A., T. D. Read, and P. C. Hanna. 2003. Identification and characterization of the gerH operon of Bacillus anthracis endospores: a differential role for purine nucleosides in germination. J. Bacteriol. 185:1462-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welkos, S., A. Friedlander, S. Weeks, S. Little, and I. Mendelson. 2002. In-vitro characterisation of the phagocytosis and fate of anthrax spores in macrophages and the effects of anti-PA antibody. J. Med. Microbiol. 51:821-831. [DOI] [PubMed] [Google Scholar]
- 46.Welkos, S., S. Little, A. Friedlander, D. Fritz, and P. Fellows. 2001. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology 147:1677-1685. [DOI] [PubMed] [Google Scholar]