Abstract
Transmission of leptospirosis occurs through contact of mucous membranes and abraded skin with freshwater contaminated by pathogenic Leptospira spp. Exposure to physiological osmolarity induces leptospires to express high levels of the Lig surface proteins containing imperfect immunoglobulin-like repeats that are shared or differ between LigA and LigB. We report that osmotic induction of Lig is accompanied by 1.6- to 2.5-fold increases in leptospiral adhesion to immobilized extracellular matrix and plasma proteins, including collagens I and IV, laminin, and especially fibronectin and fibrinogen. Recombinant LigA-unique and LigB-unique repeat proteins bind to these same host ligands. We found that the avidity of LigB in binding fibronectin is comparable to that of the Staphylococcus aureus FnBPA D repeats. Both LigA- and LigB-unique repeats interact with the amino-terminal fibrin- and gelatin-binding domains of fibronectin, which are also recognized by fibronectin-binding proteins mediating the adhesion of other microbial pathogens. In contrast, repeats common to both LigA and LigB do not bind these host proteins, and nonrepeat sequences in the carboxy-terminal domain of LigB show only weak interaction with fibronectin and fibrinogen. A functional role for the binding activity of LigA and LigB is suggested by the ability of the recombinants to inhibit leptospiral adhesion to fibronectin by 28% and 21%, respectively. The binding of LigA and LigB to multiple ligands present in different tissues suggests that these adhesins may be involved in the initial colonization and dissemination stages of leptospirosis. The characterization of the Lig adhesin function should aid the design of Lig-based vaccines and serodiagnostic tests.
Leptospirosis is a zoonotic disease caused by pathogenic strains of the Leptospira spirochete (35). It occurs worldwide, affecting accidental hosts, such as livestock, companion animals, and people, with mild to fatal sequelae (3). The susceptible population includes inhabitants of impoverished urban areas and flood-prone regions of developing countries, farmers, and even adventure athletes (42). The transmission of leptospirosis involves a Leptospira life cycle that has been adapted to include mammalian reservoir hosts, such as the rat, in which the spirochetes colonize the kidneys, from which infective organisms are shed in the urine (3). Susceptible hosts are infected via contact with contaminated water by dermal abrasions, mucous membranes, and conjunctivae (17, 58). Current vaccines against leptospirosis target the lipopolysaccharide coat of leptospires, which is highly variable for the >200 serovars identified, thus limiting cross-protection (35). To overcome the obstacle of serologic variability, it is of interest to identify antigenically conserved surface constituents that would serve as broader vaccine targets. In addition, the characterization of leptospiral components contributing to pathogenesis would aid in the development of improved diagnostic strategies.
LigA and LigB, encoded by separate genes, are members of the family of bacterial proteins containing immunoglobulin-like repeats that have been identified in adhesins relevant to microbial pathogenesis, such as intimin in Escherichia coli and invasin in Yersinia pseudotuberculosis (22, 24, 39, 46). The Lig proteins are expressed only on the surfaces of leptospiral pathogens isolated from infected animals and not by saprophytic Leptospira species (39). Differential Lig expression is recapitulated in culture: Lig is present in low-passage-number growth of infective organisms but is absent in highly passaged, culture-attenuated leptospires that have lost virulence in a hamster model of leptospirosis (39). Moreover, we have recently shown that Lig expression in pathogenic Leptospira is induced by a change in the osmolarity of the culture medium that mimics the transition that the bacteria might encounter upon entry into a mammalian host (40). In addition, sera from patients recovering from leptospirosis contain antibodies to Lig proteins, confirming their expression by infectious spirochetes (39, 46). Thus, the Lig proteins appear to be closely associated with infection of the mammalian host, suggesting that they may be protective immunogens. Indeed, recent studies have demonstrated that recombinant forms of Lig serve as effective vaccines in animal models (31, 47).
Their structural resemblance to known adhesins and close association with virulence suggest that the Lig proteins may be MSCRAMMs (microbial surface components recognizing adhesive matrix molecules), an important category of bacterial proteins involved in the colonization of host tissue (50). Several adhesins that bind host extracellular matrix proteins have been identified in pathogenic spirochetes. Fibronectin-binding proteins include BBK32 in Borrelia burgdorferi (52, 53), a Borrelia garinii protein (32), a 36-kDa protein in Leptospira interrogans serovar Icterohaemorrhagiae (43), and several proteins in Treponema spp., including Msp and OppA in Treponema denticola and Tp0155 and Tp0483 in Treponema pallidum (6, 10). Treponemes also have several collagen-binding proteins, including Msp in T. denticola, as well as laminin-binding proteins, such as Msp in T. denticola and Tp0751 in T. pallidum (5, 10). Lsa24 in L. interrogans serovar Copenhageni has recently been characterized as a laminin-binding protein (2). This leptospiral adhesin was previously identified as LfhA, a factor H-binding protein in pathogenic L. interrogans (66). In B. burgdorferi, there are the decorin-binding MSCRAMMs DbpA and DbpB (15). Early studies reported an association between virulence and the adhesion of Leptospira to extracellular matrix proteins (23). In the current study, we show that the physiological osmotic induction of Lig is accompanied by increased adhesion of live leptospires to fibronectin, fibrinogen, collagen, and laminin, which are some of the host proteins that might be encountered upon entry at an infection site, in transit through the bloodstream, and during invasion of tissue. Furthermore, we show that LigA and LigB are indeed adhesins binding extracellular matrix proteins and fibrinogen and are thus considered to be Leptospira MSCRAMMs involved in interaction with a host. Our results suggest that vaccines targeting the Lig adhesins common to pathogenic Leptospira species could potentially provide effective protection for humans, pets, and livestock susceptible to leptospirosis.
MATERIALS AND METHODS
Bacterial strains and culture.
Leptospira interrogans serovar Copenhageni strain Fiocruz L1-130 was isolated from a patient in Salvador, Brazil (30). Virulent leptospires isolated from infected golden Syrian hamsters were grown in Ellinghausen-McCullough-Johnson-Harris (EMJH) medium supplemented with 1% rabbit serum and 100 μg/ml 5-fluorouracil at 30°C (27). Induction by physiological osmolarity was done overnight starting at mid-log-phase growth in EMJH supplemented with 120 mM NaCl. A portion of the mid-log-phase culture was also grown overnight to obtain noninduced cells. Cells were harvested by centrifugation at 7,600 × g and 4°C for 10 min, washed, and resuspended in serum-free EMJH without added salt.
Immunoblot analysis.
About 1 × 109 leptospires were collected from 1 ml of noninduced and induced cultures by brief centrifugation and were frozen until use. Cellular protein was solubilized, resolved by reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 10% acrylamide), immunoblotted, and probed with a rabbit antiserum specific for LigA and LigB (39), which were both visualized with a horseradish peroxidase-conjugated donkey antibody against rabbit immunoglobulin G (IgG; GE Healthcare) and photographic capture of chemiluminescence.
Leptospire-binding ELISA.
High-binding microtiter wells (Costar) were coated overnight at 4°C with 1 μg of either human plasma fibronectin (Sigma-Aldrich), affinity-purified human fibrinogen (Aniara), bovine collagen type I (Sigma-Aldrich), human collagen type IV (Sigma-Aldrich), murine laminin (Sigma-Aldrich), or bovine serum albumin (BSA; Serologicals) in 0.1 ml phosphate-buffered saline (PBS), pH 7.2. Briefly, a standard enzyme-linked immunosorbent assay (ELISA) was done by blocking nonspecific binding sites with 2% BSA in PBS; incubating resuspended leptospires (1 × 108 to 3 × 108) in 0.1 ml serum-free EMJH at 30°C for 1 h; removing unattached bacteria with three serum-free EMJH washes; fixing the adherent spirochetes with methanol at 4°C for 10 min (or 4% paraformaldehyde at room temperature for 1 h for detection of Lig on cell surfaces); probing with a rabbit antiserum against LipL32, a noninduced leptospiral protein (16), or against LigA and LigB; developing with a horseradish peroxidase-conjugated monoclonal antibody against rabbit IgG (Sigma-Aldrich) and a tetramethyl benzidine substrate (Pierce); and performing spectrophotometry at 450 nm. In assays measuring the effect on leptospire adhesion of prebinding fibronectin with recombinant Lig proteins, 0.5 to 1 μM Lig in 0.1 ml PBS (found to be saturating amounts in Lig-binding ELISAs) was incubated at 37°C for 1 h before washing with PBS to remove unbound Lig, followed by the addition of cells. The prebound Lig remained stable throughout the procedure for incubation with cells and washes to remove unattached bacteria.
Lig-binding ELISA.
The Lig protein-binding ELISA was done like the leptospire-fibronectin binding assay, except that unbound Lig was removed with PBS washes and there was no fixing step before probing with an anti-His tag monoclonal antibody (Novagen) and developing with horseradish peroxidase-conjugated anti-mouse IgG (Novagen). In assays for fibronectin domain binding, the wells were coated with proteolytic fragments of human plasma fibronectin: the 30-kDa N-terminal domain (NTD) (Sigma-Aldrich), the 45-kDa gelatin-binding domain (GBD) (Sigma-Aldrich), and a 70-kDa protein (Sigma-Aldrich) comprising both domains. Equivalent results were obtained with 1 and 10 μg of each fibronectin fragment. Coating with Matrigel (BD Biosciences) was done with 100 μg in 0.1 ml PBS. The apparent Kd for saturation binding was estimated with the half-maximal binding concentration of Lig protein.
Lig subcloning and purification of recombinant proteins.
In dividing LigA and LigB for subcloning, the strategy was to minimize the potential disruption of structure and function by keeping the immunoglobulin-like repeats intact and, for fragments ending with a repeat, to also include the short sequence that links it to the next repeat (for the amino acid coordinates, see Results). In the absence of repeat sequences as a guide, the LigB carboxy-terminal domain (CTD) was inspected with Lasergene software (DNASTAR) for junctions predicted to produce turns in the molecule. Lig sequences in low-passage-number L. interrogans genomic DNA were amplified with a high-fidelity enzyme, Phusion DNA polymerase (New England Biolabs), using the PCR primers listed in Table 1. The PCR products were inserted at the NdeI site and either the HindIII or the XhoI site of pET20b(+) (Novagen), which added a six-histidine carboxy tail to the recombinant proteins. The fibronectin-binding region of FnBPA containing the first three D repeats (amino acids 743 to 862) was cloned with a His tag tail from Staphylococcus aureus strain 8325-4 genomic DNA (26) with the forward and reverse primers shown in Table 1 for insertion at the BamHI and XhoI sites in pGEX-4T-3 (GE Healthcare).
TABLE 1.
LigA and LigB proteins tested for extracellular matrix protein- and fibrinogen-binding activity
| Protein | Regiona | Primerb |
|---|---|---|
| Lig1-3 | Shared repeats 1 to 3 (aa 52-311) | GGTAACTCCCATATGACTATTACAAGAATCGAACTCAGTTAT |
| AGCAAGCTTTGCTGGAGTAACGATTAGTTTTACGGA | ||
| Lig4-6 | Shared repeats 4 to 6 (aa 312-581) | ATCGTTACTCATATGGCCTTAGTTTCTATTTCTGTTTCTCCG |
| AGCAAGCTTTGCAGCTGTAACAGATAACGTAGAAAC | ||
| LigA U | Unique repeats 7 to 13 (aa 631-1224) | AACATATCTCATATGCTTACCGTTTCCAACACAAACGCCAA |
| TTCCTCGAGTGGCTCCGTTTTAATAGAGGCTAAT | ||
| LigB U1 | Unique repeats 7 to 12 (aa 631-1118) plus CTD (aa 1119-1125) | GAAGATTCGCATATGGCTGAAATTAAAAATACCAGTGGAAG |
| AACCTCGAGAGCTATCGTGTCCGTTTTGTTTACT | ||
| LigB U2 | Unique repeats 7 to 12 (aa 631-1118) plus CTD (aa 1119-1257) | GAAGATTCGCATATGGCTGAAATTAAAAATACCAGTGGAAG |
| AGACTCGAGCACTTGGTTTAAGGAATTACAAACT | ||
| LigB CTD | CTD (aa 1613-1890) | ATAGTCCATATGGACAATTATGAAACTTATACATCTGATA |
| CAGTTCTCGAGTTGATTCTGTTGTCTGTAAATTTTGAC | ||
| S. aureus FnBPA | D repeats 1 to 3 (aa 743-862) | CGACAGGATCCGAAGGTGGCCAAAATAGC |
| TATATCTCGAGTCAATGATGATGATGATGATGATTTTGGCCGCTTAC |
See Results for details of the Lig region.
Forward (top line) and reverse (bottom) PCR primers for amplification of each Lig region are displayed 5′ to 3′; restriction endonuclease sites are underlined (see the text for details).
Overexpression of the recombinant proteins in mid-log-phase cultures of Escherichia coli BLR(DE3)pLysS transformants was induced with 1 mM isopropyl β-d-thiogalactopyranoside at 30°C for 1 to 2 h. Cellular protein was solubilized with BugBuster (Novagen), and the His-tagged recombinants were purified by nickel affinity chromatography (nickel-nitriloacetic acid agarose [QIAGEN] or nickel-iminodiacetic acid cellulose cartridges [Novagen]) and eluted with a buffer of 20 mM Tris-HCl, 100 mM NaCl, 500 mM imidazole, and 20% glycerol (pH 7.9). The recombinant proteins were checked for purity and integrity by SDS-PAGE with Coomassie staining and immunoblotting and were stored in PBS or elution buffer at 4°C. Protein levels were estimated by the bicinchoninic acid assay (Pierce) or by absorbance at 280 nm using the extinction coefficients calculated for the recombinants by Lasergene software (DNASTAR). The possible effect on the ELISA of any carryover of proprietary detergents in BugBuster during protein purification was tested, and none was found.
RESULTS
Physiological osmolarity induces leptospiral adhesion.
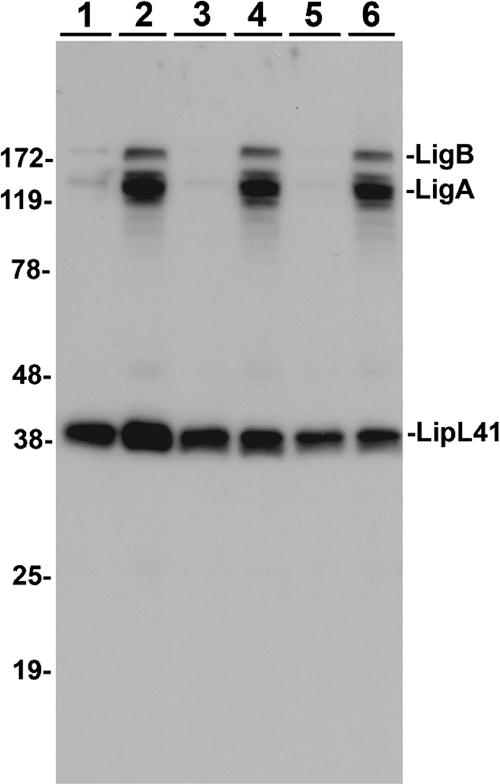
The expression of LigA and LigB by pathogenic Leptospira increases strongly when the osmotic strength of the EMJH culture medium (67 mosmol/liter) is raised severalfold by salt supplementation to a physiological level (∼300 mosmol/liter) that infectious spirochetes would encounter in a mammalian host (40). The salt-induced leptospires retained viability and motility when observed by dark-field microscopy. Figure 1 shows the immunoblot analysis of LigA and LigB in low-passage-number cultures of L. interrogans Fiocruz L1-130 used in three independent experiments reported in the current study, demonstrating the consistent elevation from virtually no expression to strong expression of Lig proteins under the conditions of osmotic induction used. Only the cell-associated Lig is shown here; a large amount of LigA is also released from leptospires upon induction (40). The increase in Lig levels on the surface of induced cells is also evident in an ELISA using a rabbit anti-Lig antiserum (data not shown).
FIG. 1.
Osmotic induction of Lig expression. Total protein of ∼1 × 108 leptospires recovered from overnight cultures of low-passage-number Leptospira interrogans Fiocruz L1-130 grown in standard EMJH medium (odd-numbered lanes) or induced with physiological osmolarity in EMJH plus 120 mM NaCl (even-numbered lanes) was resolved by SDS-10% PAGE and analyzed by immunoblotting. LigA and LigB were detected with a Lig-specific rabbit antiserum. For reference, a noninduced protein, LipL41, was also assayed. The results from three independent experiments shown here illustrate the uniformly strong induction of LigA and LigB expression in the cultures used in this study.
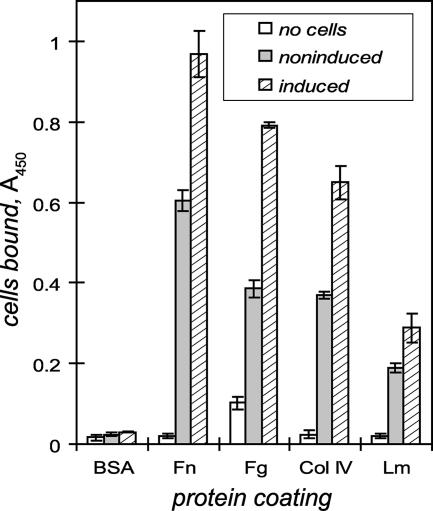
The osmotic induction of Lig expression is accompanied by enhanced adhesion of live leptospires to fibronectin and other host proteins. In the representative experiment for which results are shown in Fig. 2, noninduced Leptospira grown overnight in EMJH adhered to fibronectin, fibrinogen, collagen type IV, and laminin immobilized in microtiter wells. More leptospires attached to fibronectin than to fibrinogen (48% of the level with fibronectin minus the background without cells), collagen type IV (59%), and laminin (29%). When leptospires were grown under conditions of physiological osmolarity, they bound to fibronectin on average 1.9 times as much as bacteria cultured in EMJH without a salt supplement. The range of induction in seven different experiments was 1.6- to 2.5-fold (P < 0.01 for all two-sample comparisons). Osmotic induction also increased spirochete adhesion to fibrinogen (2.4-fold; 73% of the induced level of fibronectin binding), collagen (1.8-fold; 66%), and laminin (1.6-fold; 28%). The comparable levels of induction for binding to the four host proteins suggested that a single type of receptor may be involved, although it is possible that multiple receptors could be similarly induced.
FIG. 2.
Physiological osmolarity induces Leptospira adhesion to host proteins. Leptospiral binding was measured by an ELISA using microtiter wells coated with 1 μg of fibronectin (Fn), fibrinogen (Fg), collagen type IV (Col IV), or laminin (Lm). BSA was used to measure nonspecific adsorption of bacteria. Serum-free EMJH medium alone (no cells) or Leptospira interrogans Fiocruz L1-130 (3 × 108 cells) grown overnight in EMJH alone (noninduced) or in EMJH plus 120 mM NaCl (induced) was added for 1 h at 30°C. Leptospires bound to host proteins were detected with a LipL32-specific antiserum and horseradish peroxidase conjugated to a secondary antibody. Adhesion to all four proteins was enhanced by increased osmolarity (P < 0.01 by Student's t test). Means and standard deviations from triplicate wells are shown.
Subcloning of LigA and LigB domains.
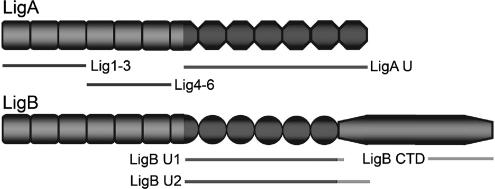
The enhanced adhesion of Leptospira accompanying Lig induction by physiological osmolarity suggested that LigA and/or LigB might be an adhesin involved in the pathogenesis of leptospirosis. We thus prepared recombinant His-tagged Lig proteins to assay for their binding to host proteins. LigA (1,224 amino acids; 128 kDa) and LigB (1,890 amino acids; 212 kDa) have 13 and 12 imperfect tandem immunoglobulin-like repeats, respectively, that are either conserved or differ between the two proteins (Fig. 3) (39, 46). LigB also has a large nonrepeat CTD. The full-length recombinant proteins have limited solubility and are susceptible to proteolysis (data not shown). Therefore, representative putative structural domains of LigA and LigB were subcloned from low-passage-number L. interrogans Fiocruz L1-130 genomic DNA, expressed in E. coli, and purified by nickel affinity chromatography. The Lig proteins used in this study remain stable, soluble, and functional for months at 4°C.
FIG. 3.
Cloning of LigA and LigB recombinant proteins. The structures of LigA and LigB are shown with the immunoglobulin-like repeats that occur in both proteins (squares), those that are specific to each (equilateral octagons for LigA, circles for LigB), and the nonrepeat CTD in LigB (elongated octagon). Short sequences linking the repeats are not shown. The Lig subclones used in this study are shown with lines delimiting their sequences within the native proteins. The primers used to amplify their coding sequences from L. interrogans Fiocruz L1-130 genomic DNA are listed in Table 1.
LigA and LigB both share amino-proximal repeats 1 to 6, which are represented by the 29-kDa Lig1-3 recombinant protein, composed of repeats 1 to 3, and the 29-kDa Lig4-6 protein, containing repeats 4 to 6 (Table 1; Fig. 3). Lig1-3 starts with the first repeat at amino acid 52 and terminates with amino acid 311; Lig4-6 begins with the fourth repeat at residue 312 and ends at residue 581 (39, 46). In LigA, the shared repeats are followed directly by the second half of the molecule, consisting of LigA-unique repeats 7 to 13. Recombinant LigA U (63 kDa) starts near the middle of repeat 7, where the sequence diverges from LigB at amino acid 631, and ends at amino acid 1224, the carboxy terminus of LigA, where a 12-residue tail follows the final repeat 13 (Table 1; Fig. 3) (39, 46).
For LigB, we examined the repeats in the middle of the protein that differ from LigA in addition to the CTD. Both LigB U1 and U2 contain repeats 7 through 12 plus a portion of the CTD (Table 1; Fig. 3). The 52-kDa LigB U1 begins in the middle of repeat 7 at amino acid 631, runs to the end of repeat 12 at amino acid 1118, and terminates with a 7-residue segment of the CTD, amino acids 1119 to 1125 (39, 46). LigB U2 (67 kDa) replicates LigB U1 and contains a larger, 139-amino-acid portion of the CTD, residues 1119 to 1257 (Table 1; Fig. 3). In addition, we tested a separate region of the CTD, comprising the last 278 amino acids of LigB from residue 1613 to 1890, with the 32-kDa LigB CTD protein (Table 1; Fig. 3).
Lig binds extracellular matrix proteins and fibrinogen.
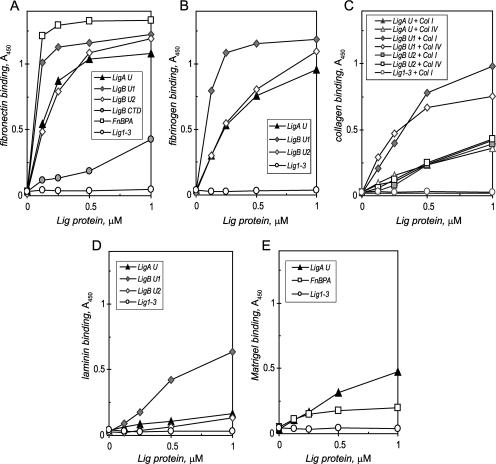
In an ELISA using immobilized extracellular matrix proteins and fibrinogen as test ligands, the unique repeats of LigA bound strongly to the matrix/plasma protein fibronectin (Fig. 4A). The apparent Kd for LigA U-fibronectin binding is 126 ± 41 nM (mean and standard deviation from 17 independent experiments). For comparison, the activity of the fibronectin-binding domain cloned from the well-characterized FnBPA of Staphylococcus aureus is also shown; its estimated Kd in this assay is 69.0 ± 7.7 nM, suggesting that FnBPA binds fibronectin twice as strongly as LigA U. In addition to saturation binding to another plasma protein, fibrinogen (Kd, 221 ± 63 nM) (Fig. 4B), LigA U showed weaker interactions with collagen types I and IV, binding almost equally to each (Fig. 4C) and binding to laminin with even lower avidity (Fig. 4D). LigA U also displayed concentration-dependent binding to Matrigel, a biologically active meshwork of extracellular matrix proteins, including fibronectin, as suggested by the binding of FnBPA (Fig. 4E). In contrast, the repeats shared by LigA and B in Lig1-3 showed little interaction with the host proteins tested (Fig. 4A to E).
FIG. 4.
LigA and LigB bind to extracellular matrix and plasma proteins. For each ligand, an ELISA was used to measure binding. After a 1-h incubation at 37°C, the bound recombinant protein was washed and detected with a monoclonal antibody to its histidine tag along with horseradish peroxidase conjugated to a goat antibody to mouse IgG. (A) Measurement of the abilities of the unique repeats of LigA U and LigB U, the shared repeats in Lig1-3, and LigB CTD to bind to 1 μg immobilized fibronectin. The S. aureus FnBPA D repeats served as a positive reference. (B) LigA and LigB bind to fibrinogen. The abilities of the unique repeats of LigA U and LigB U and the shared repeats in Lig1-3 to bind to 1 μg immobilized fibrinogen were measured. (C) LigA and LigB bind to collagen. The abilities of the unique repeats of LigA U and LigB U and the shared repeats in Lig1-3 to bind to 1 μg immobilized collagen type I or IV were measured. Lig1-3 also did not bind collagen type IV. (D) LigA and LigB bind to laminin. The abilities of the unique repeats of LigA U and LigB U and the shared repeats in Lig1-3 to bind to 1 μg immobilized laminin were measured. (E) LigA binds to biologically active matrix. The abilities of the unique repeats of LigA U and the shared repeats in Lig1-3 to bind to 100 μg of Matrigel were measured. The S. aureus FnBPA D repeats were also tested.
The LigB-specific repeats have a binding profile similar to that of LigA U, indicating that both LigA and LigB are Leptospira MSCRAMMs induced by physiological osmolarity. LigB U1 and U2, containing repeats 7 to 12, bound fibronectin with similar or higher avidity than LigA U; the binding by LigB U1 was comparable to that by FnBPA (Fig. 4A). The apparent Kds with LigB U1 and U2 are 72.6 ± 11.7 nM and 132 ± 18 nM, respectively. Whereas the unique repeats in LigB U1 also bound strongly to fibrinogen, with a Kd of 87.1 ± 2.4 nM, the additional 139-amino-acid nonrepeat CTD sequence in LigB U2 appeared to weaken the interaction with the plasma protein (Kd, 269 ± 24 nM) (Fig. 4B). Table 2 compares the apparent Kds for saturation binding to fibronectin and fibrinogen by the Lig proteins. In terms of collagen and laminin binding, the LigB-specific repeats in LigB U1 displayed much stronger interaction than LigB U2 and LigA U (Fig. 4C and D). As with LigA U, both LigB proteins bound collagen types I and IV similarly, with LigB U1 showing high avidity. LigB U1 also bound laminin substantially, in contrast to the weak interaction of LigB U2 and LigA U with laminin. In summary, LigB U1 binds host proteins significantly more strongly than LigB U2 and LigA U, which have similar binding activities.
TABLE 2.
Fibronectin and fibrinogen binding by LigA and LigB variable repeats
| Binding protein |
Kda
|
||||
|---|---|---|---|---|---|
| Fn | NTD | GBD | 70 kDa | Fg | |
| LigA U | 126 | 141 | 114 | 149 | 221 |
| LigB U1 | 72.6 | 77.0 | 72.8 | 68.0 | 87.1 |
| LigB U2 | 132 | 199 | 222 | 173 | 269 |
| FnBPA | 69.0 | 63 | 177 | 126 | ND |
| LigB CTD | NS | ND | ND | ND | NS |
Estimated in nM binding protein; see the text for details. ND, not done. NS, no saturating activity.
The LigB CTD protein, representing the C-terminal end of LigB, which contains neither shared nor unique repeats, bound weakly to fibronectin in comparison to LigA U and LigB U1 and 2 (Fig. 4A). It displayed similarly low level binding with fibrinogen and virtually no activity with collagen and laminin (data not shown). Thus, the matrix and plasma protein-binding activities of LigA and LigB are attributed mainly to their unique repeats rather than to their shared repeats or the CTD of LigB.
LigA and LigB bind the F1 module-containing domains of fibronectin.
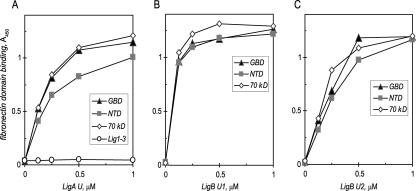
The interaction of the unique repeats in LigA and LigB with fibronectin was examined further by identifying the specific domains involved in the host ligand. In an ELISA using immobilized proteolytic fragments of fibronectin, LigA U bound about 20 to 40% more strongly to the isolated 45-kDa GBD of fibronectin than to the 30-kDa fibrin/heparin-binding NTD (e.g., the Kd was 114 ± 27 nM with the GBD versus 141 nM [range, 85 nM] with the NTD) (Fig. 5A; Table 2). These estimated binding constants are comparable to that obtained with intact fibronectin (126 ± 41 nM), indicating that binding to either domain can account for the interaction of LigA U with this host protein. The moderate preference for the GBD shown by LigA U differs from the known pronounced affinity of FnBPA for the NTD of fibronectin (in our assay, the Kd was 63 nM with the NTD versus 177 nM with the GBD) (20, 21, 25, 26, 62). LigA U interaction with the 70-kDa domain from which the F1 module-containing GBD and NTD are derived is also shown (Kd, 149 nM; range, 23 nM). In contrast, there was weak interaction of LigA U with the RGD sequence from the cell-binding domain of fibronectin that is recognized by integrins of host cells (data not shown) (57). Lig1-3 did not bind any fibronectin fragment (GBD results are shown in Fig. 5A).
FIG. 5.
LigA and LigB interact with the amino-terminal domains of fibronectin. An ELISA was used to identify the fibronectin domains involved in binding by the unique repeats in LigA U (A), LigB U1 (B), or LigB U2 (C). After a 1-h incubation at 37°C with 1 μg immobilized proteolytic fragments of fibronectin that included either the GBD, the NTD, or the 70-kDa fragment containing both the GBD and the NTD, bound recombinant Lig protein was measured as for Fig. 4. Lig1-3 did not bind any fibronectin fragments (shown for GBD in panel A).
LigB U1 and U2 bind both the GBD and the NTD of fibronectin with similar avidity. LigB U1 binds the two domains with apparent Kds of 72.8 ± 16.4 nM and 77.0 ± 8.3 nM, respectively, compared to 72.6 ± 11.7 nM with intact fibronectin and 68.0 ± 9.5 nM with the 70-kDa fragment, suggesting that interaction with either domain can also account for LigB U1-fibronectin binding (Fig. 5B; Table 2). LigB U2 binds both isolated domains with less avidity than LigB U1 (Fig. 5C; Table 2). The estimated Kds, 222 nM (range, 16 nM) with the GBD and 199 ± 52 nM with the NTD, are substantially greater than that obtained with intact fibronectin, 132 ± 18 nM, suggesting that neither domain alone could account for LigB U2-fibronectin binding. However, the Kd estimated for interaction with the 70-kDa fragment of fibronectin, 173 nM (range, 7 nM), is comparable to that obtained with intact fibronectin, suggesting that LigB U2 binding involves the F1 modules in both the NTD and the GBD.
LigA and LigB partially inhibit leptospiral adhesion to fibronectin.
The results described above suggested that the osmotically inducible Lig proteins are adhesins that contribute to the increase in fibronectin binding observed with induced Leptospira. In a modification of the ELISA used for Fig. 2 to show spirochete adhesion to host proteins, fibronectin binding by Leptospira was measured after the immobilized fibronectin had been incubated with saturating amounts of recombinant Lig proteins (0.5 to 1 μM) for 1 h at 37°C. Preincubation with the unique repeats of LigA partially inhibited leptospiral binding to fibronectin that was induced two- to threefold by increased osmolarity. In contrast, there was no significant effect on fibronectin binding by noninduced spirochetes (absorbance, 0.285 ± 0.048 without Lig preincubation and 0.232 ± 0.030 with Lig [n = 3; P > 0.08]), suggesting that their adhesion is mediated primarily by a non-Lig MSCRAMM(s). On average, LigA U reduced the level of increased adhesion of induced cells by 28%, with a range of 17 to 39% for seven independent experiments, in which individual P values ranged from < 0.05 to < 0.01 by a Student t test comparing the means from triplicate or quadruplicate measurements. Figure 6 shows a representative example of the effect on Lig-mediated adhesion. In contrast, the shared repeats in Lig1-3 did not affect cell adhesion to fibronectin (data not shown). We examined whether the release of large amounts of LigA from osmotically induced cells might account for the incomplete inhibition obtained with prebound LigA U. Preincubation of fibronectin with either LigB U1 or U2 failed to produce greater interference with adhesion than preincubation with LigA U. LigB U1, but not LigB U2, blocked leptospiral binding 20 to 21% (P < 0.05). The incomplete inhibition of fibronectin binding indicates that LigA and LigB are not the only fibronectin-binding adhesins involved in the enhanced adhesion of osmotically induced Leptospira.
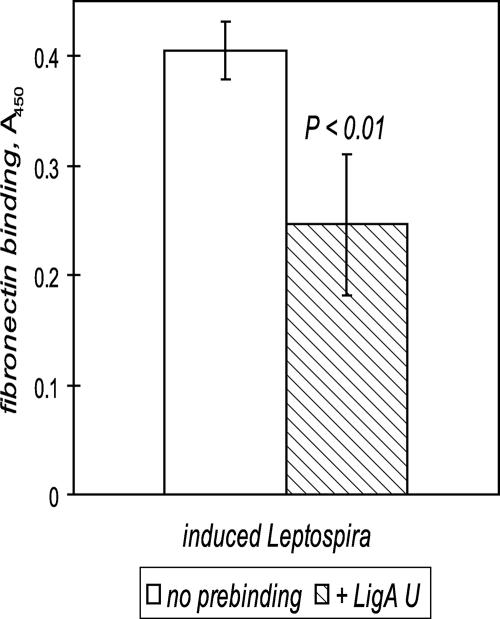
FIG. 6.
Recombinant LigA U inhibits osmotically induced fibronectin binding. Fibronectin adhesion by osmotically induced L. interrogans was measured by an ELISA with a LipL32-specific antiserum after the immobilized fibronectin had been incubated with PBS or LigA U. Fibronectin binding by noninduced leptospires was subtracted to reflect the level of adhesion due to exposure to physiological osmolarity, which induced binding 2.4-fold in this representative experiment, increasing the absorbance from 0.285 ± 0.048 to 0.690 ± 0.026 (mean ± standard deviation for triplicates; P < 0.001). Prebound LigA U (1 μM) reduced the level of induced adhesion by 39% (from an absorbance of 0.405 ± 0.026 without Lig to an absorbance of 0.246 ± 0.065 with Lig [P < 0.01]).
DISCUSSION
Adhesion is an essential first step in bacterial pathogenesis. Its management by both microorganism and host during infection is critical for successful bacterial colonization or clearance by the host. The bacterial surface proteins that mediate binding to the extracellular matrix in the host are known as MSCRAMMs (50). Their surface exposure renders MSCRAMMs vulnerable to the host immune system and makes them important vaccine candidates. Among the best-studied adhesins in spirochetes are B. burgdorferi DbpA, DbpB, and BBK32, whose mechanisms for binding decorin or fibronectin, in vivo function in Lyme disease, and use in vaccines are being examined (4, 15, 29, 36, 37, 53-55, 61).
In leptospirosis, the spirochetes initially migrate from contaminated water in the environment into the tissues of a mammal. We recently discovered that the change in osmolarity encountered during this transition causes a dramatic increase in expression of the two surface-exposed Lig proteins, LigA and LigB, in cultures of pathogenic strains of Leptospira (40). An association of virulence with Lig expression is also suggested by diminished levels of Lig in nonpathogenic strains and pathogenic leptospires that have lost virulence after extensive subculturing in an artificial medium (39). In Lyme disease, there is an analogous response by B. burgdorferi entering the environment of a mammalian host, where BBK32 expression is induced from a low level inside the gut of the tick vector (12, 36, 37, 45, 55, 64).
Pathogenic leptospires are known to adhere to extracellular matrix proteins, such as fibronectin, collagen, and laminin (23). We now show that the osmotic induction of Lig expression is accompanied by the enhanced binding of virulent spirochetes to fibronectin, collagen, laminin, and fibrinogen (Fig. 2). Fibrinogen binding by Leptospira has not been reported previously. We anticipate that our findings here with the important human pathogen L. interrogans also apply to other pathogenic strains such as Leptospira kirschneri serovar Grippotyphosa strain RM52, where we have shown the osmotic induction of both Lig expression (40) and leptospiral adhesion to fibronectin (unpublished data).
The increased adhesion of leptospires in which Lig expression has been induced by physiological osmolarity and the structural similarity of LigA and LigB proteins to other adhesins suggested that the Lig proteins may function as MSCRAMMs. In testing recombinant proteins derived from Lig domains, we identified the immunoglobulin-like repeats that differ between LigA and LigB as the primary structures with activity in binding to fibronectin, collagen, laminin, and fibrinogen. We have thus established that the Lig proteins are osmotically inducible multifunctional Leptospira SCRAMMs (Fig. 4A to E). The demonstration of LigA and LigB as fibrinogen-binding MSCRAMMs also provides an explanation for our finding that leptospires adhere to this plasma protein (Fig. 2). LigA and LigB bind fibronectin and fibrinogen with higher avidity than collagen and laminin (Fig. 4A and B), which is consistent with the adhesion of osmotically induced leptospires being strongest with fibronectin and fibrinogen (Fig. 2). The fibronectin and fibrinogen interactions are also about two to three times stronger with LigB U1 than with LigA U or LigB U2. LigB U2 differs from LigB U1 in that the former contains a larger portion of the nonrepetitive CTD. The role of the CTD in LigB remains unclear, but the data suggest a role for negative modulation of binding to host proteins (Fig. 4A to D).
Fibrinogen binding has been reported for several MSCRAMMs with immunoglobulin-like repeats (9, 68). However, the direct involvement of the LigA and LigB immunoglobulin-like repeats in fibronectin binding differs from the non-immunoglobulin-like binding domains utilized in the tandem β-zipper mechanism described for other fibronectin-binding proteins, including borrelial BBK32 (29, 54, 59, 60). Thus, although LigA and LigB have structural similarities with other MSCRAMMs that bind the F1 repeat modules of fibronectin, the difference in the type of structural motif involved may reflect a divergence from previously described interactions of fibronectin-binding proteins with the five F1 modules in the NTD of fibronectin (20, 21, 25, 26, 54, 60, 62). Another indication that fibronectin binding by the Lig proteins is unique comes from their binding to both the NTD and the GBD; the latter also contains F1 modules plus two F2 modules that are not present elsewhere in fibronectin (Fig. 5) (51). By comparison, the D repeats from FnBPA bind the NTD about three times more strongly than they bind the adjacent GBD in fibronectin. Thus, the immunoglobulin-like repeats in the Lig proteins differ from other MSCRAMMs in their interaction with fibronectin. Additional examples of differences in fibronectin binding by spirochetes come from the 36-kDa protein in L. interrogans serovar Icterohaemorraghiae and Msp in T. denticola. The former binds the GBD and the latter the NTD (10, 43).
The functional significance of the binding of the Lig proteins to specific domains of fibronectin and to a biologically active meshwork containing fibronectin is supported by the correlation between strong binding by recombinant Lig proteins and strong adherence by osmotically induced spirochetes (Fig. 2 and 4). We confirmed a role for LigA and LigB in leptospiral binding by demonstrating inhibition of induced leptospiral adhesion to fibronectin by exogenous LigA U (Fig. 6) and LigB U1. The partial inhibition of leptospiral adhesion by recombinant Lig proteins suggests the presence of additional adhesins in Leptospira, which is consistent with the binding to host proteins by noninduced cells that express relatively small amounts of Lig (Fig. 1 and 2) and the absence of an effect of exogenous Lig proteins on fibronectin binding by noninduced leptospires. The genomic sequencing of L. interrogans also reveals the presence of other possible MSCRAMMs (44). It is possible that additional fibronectin-binding proteins also interact with the N-terminal region of fibronectin but with higher avidity than Lig. At least one other fibronectin-binding protein has been found in L. interrogans serovar Icterohaemorrhagiae, and it binds the GBD (43). The deletion of the bbk32 gene leaves intact as much as half of the level of fibronectin binding observed with the parental isogenic wild-type strain, suggesting the presence of multiple fibronectin-binding proteins in B. burgdorferi (61). Other examples of incomplete blocking of fibronectin binding in spirochetes are provided by recombinant Msp, which inhibits T. denticola adhesion by 40% at most (10), and Tp0155 and Tp0483, each of which inhibits about 70% of T. pallidum binding (6).
The multiple binding activities of the Lig proteins suggest that they may be involved during different stages of infection. Multifunctional MSCRAMMs have been reported for other spirochetes, including B. burgdorferi BBK32, which binds glycosaminoglycans, including heparin, in addition to fibronectin (14); T. denticola Msp, which has a broad spectrum of ligands like those of Lig, including fibronectin, fibrinogen, collagen, laminin, and also keratin and heparin (10); T. denticola OppA, which binds fibronectin and plasminogen (11); and L. interrogans LfhA/Lsa24, which binds factor H and laminin (2, 66). Multifunctional MSCRAMMs in gram-positive bacteria include S. aureus FnBPA and FnBPB, which bind fibronectin, fibrinogen, and elastin (56, 68); Streptococcus pyogenes protein F, which binds both fibronectin and fibrinogen (28); and S. aureus Efb, which binds fibrinogen and the C3b component of complement (34, 49).
The regulation by osmotic change is consistent with an early role for the Lig proteins during infection. Exposure of leptospires to the osmolarity of bodily fluids in damaged epidermis or on mucous membranes would induce Lig expression, thereby promoting adhesion to fibronectin, fibrinogen, collagen, and laminin at the site of entry. A functional role for the Lig proteins early in infection by L. interrogans is also suggested by our recent microarray studies showing that Lig induction by a shift in culture temperature from 30°C to 37°C is followed by repression with prolonged incubation at 37°C (38).
A substantial portion of LigA is released from the cell surface as a result of the induction of expression by physiological osmolarity (40). The effect of LigA release on leptospiral adhesion and the fate of extracellular LigA are unknown. The release of LigA may promote leptospiral dissemination following attachment at the initial site of infection. This hypothesis is consistent with the lack of skin lesions, which distinguishes leptospirosis from Lyme disease and syphilis (19). Similar roles in promoting colonization by Bordetella pertussis and Haemophilus influenzae, which also release the filamentous hemagglutinin and Hap adhesins, respectively, are proposed (8, 13). However, extracellular LigA may have an entirely nonadhesin function, like that of another extracellular MSCRAMM in S. aureus, Efb, which binds fibrinogen, thereby inhibiting platelet aggregation (48), which could otherwise interfere with bacterial dissemination. Efb also inhibits complement activation by binding C3b, thus promoting the survival of S. aureus during infection (33, 34). These potential functions for extracellular LigA may also be in the repertoire of cellular LigB.
Leptospires are known to become decorated with fibronectin (7). In the bloodstream, Lig-mediated binding to plasma fibronectin may help the spirochetes to avoid the host immune system. However, neutrophils have been shown to attach to fibronectin-decorated L. interrogans via CR3, a neutrophil fibronectin receptor (7). Thus, a likely function for fibronectin bound on the surface of leptospires is in adhesion to host cells during tissue colonization. The osmotic induction of both Lig expression and leptospiral adhesion to fibronectin and other host proteins suggests a basis for earlier reports of virulent Leptospira attachment to mammalian cells (1, 7, 63, 65, 67). In addition to fibronectin, fibrinogen, collagen, laminin, and another extracellular matrix protein, vitronectin, all share the RGD recognition sequence for integrins, suggesting a potential mechanism for leptospiral adhesion to host cells (57). Leptospire-host cell bridges could be constructed with matrix-protein or fibrinogen spans anchored by Lig and integrins on their respective cell surfaces.
The colonization of distal organs could be promoted by Lig binding to collagen type I in connective tissue and collagen type IV and laminin in basement membranes. For example, Leptospira in the circulation may gain access to the proximal tubule in the kidney via the glomerulus, where the basal lamina is exposed to the blood. Consistent with this notion is our recent finding of a high density of leptospires in the glomeruli of infected hamsters (41).
In summary, the multifunctional Lig proteins not only confer advantages on Leptospira in initiating and establishing an infection but also make the spirochetes potentially more vulnerable to vaccine-mediated protection due to the possible involvement of Lig in multiple steps of pathogenesis that include the critical process of adhesion. If it can be confirmed that Lig expression is common among pathogenic leptospires, this would provide an additional rationale for studies on the efficacy of Lig-based vaccines and diagnostic tests. Antigens based on conserved proteins, such as OmpL1 and LipL41, protect hamsters from leptospirosis and could elicit broader protection against heterologous strains (18). Effective protection by Lig vaccines has been demonstrated in two other animal models (31, 47). Further identification of the Lig sequences required for interaction with the host will aid in vaccine development.
In concert with the protection by Lig vaccines demonstrated in animal models of leptospirosis, our findings here are consistent with the hypothesis that osmotic regulation of leptospiral attachment mediated by Lig binding to host matrix and plasma proteins is a vital part of pathogenesis. Additional studies with animal models will determine Lig expression at different stages of infection and Lig localization relative to extracellular matrix and host cells.
Acknowledgments
We thank Jane T. Babbitt for helpful comments on the manuscript and Magnus Höök and Albert I. Ko for helpful discussions.
This work was supported by Public Health Service grant AI-34431 (to D.A.H.) from the National Institute of Allergy and Infectious Diseases and by VA Medical Research funds (to J.M. and D.A.H.).
Editor: V. J. DiRita
Footnotes
Published ahead of print on 12 February 2007.
REFERENCES
- 1.Ballard, S. A., M. Williamson, B. Adler, T. Vinh, and S. Faine. 1986. Interactions of virulent and avirulent leptospires with primary cultures of renal epithelial cells. J. Med. Microbiol. 21:59-67. [DOI] [PubMed] [Google Scholar]
- 2.Barbosa, A. S., P. A. Abreu, F. O. Neves, M. V. Atzingen, M. M. Watanabe, M. L. Vieira, Z. M. Morais, S. A. Vasconcellos, and A. L. Nascimento. 2006. A newly identified leptospiral adhesin mediates attachment to laminin. Infect. Immun. 74:6356-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharti, A. R., J. E. Nally, J. N. Ricaldi, M. A. Matthias, M. M. Diaz, M. A. Lovett, P. N. Levett, R. H. Gilman, M. R. Willig, E. Gotuzzo, and J. M. Vinetz. 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 3:757-771. [DOI] [PubMed] [Google Scholar]
- 4.Brown, E. L., J. H. Kim, E. S. Reisenbichler, and M. Höök. 2005. Multicomponent Lyme vaccine: three is not a crowd. Vaccine 23:3687-3696. [DOI] [PubMed] [Google Scholar]
- 5.Cameron, C. E., N. L. Brouwer, L. M. Tisch, and J. M. Kuroiwa. 2005. Defining the interaction of the Treponema pallidum adhesin Tp0751 with laminin. Infect. Immun. 73:7485-7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron, C. E., E. L. Brown, J. M. Kuroiwa, L. M. Schnapp, and N. L. Brouwer. 2004. Treponema pallidum fibronectin-binding proteins. J. Bacteriol. 186:7019-7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cinco, M., B. Cini, S. Perticarari, and G. Presani. 2002. Leptospira interrogans binds to the CR3 receptor on mammalian cells. Microb. Pathog. 33:299-305. [DOI] [PubMed] [Google Scholar]
- 8.Coutte, L., S. Alonso, N. Reveneau, E. Willery, B. Quatannens, C. Locht, and F. Jacob-Dubuisson. 2003. Role of adhesin release for mucosal colonization by a bacterial pathogen. J. Exp. Med. 197:735-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deivanayagam, C. C., E. R. Wann, W. Chen, M. Carson, K. R. Rajashankar, M. Höök, and S. V. Narayana. 2002. A novel variant of the immunoglobulin fold in surface adhesins of Staphylococcus aureus: crystal structure of the fibrinogen-binding MSCRAMM, clumping factor A. EMBO J. 21:6660-6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards, A. M., H. F. Jenkinson, M. J. Woodward, and D. Dymock. 2005. Binding properties and adhesion-mediating regions of the major sheath protein of Treponema denticola ATCC 35405. Infect. Immun. 73:2891-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenno, J. C., M. Tamura, P. M. Hannam, G. W. Wong, R. A. Chan, and B. C. McBride. 2000. Identification of a Treponema denticola OppA homologue that binds host proteins present in the subgingival environment. Infect. Immun. 68:1884-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fikrig, E., W. Feng, S. W. Barthold, S. R. Telford III, and R. A. Flavell. 2000. Arthropod- and host-specific Borrelia burgdorferi bbk32 expression and the inhibition of spirochete transmission. J. Immunol. 164:5344-5351. [DOI] [PubMed] [Google Scholar]
- 13.Fink, D. L., L. D. Cope, E. J. Hansen, and J. W. Geme III. 2001. The Haemophilus influenzae Hap autotransporter is a chymotrypsin clan serine protease and undergoes autoproteolysis via an intermolecular mechanism. J. Biol. Chem. 276:39492-39500. [DOI] [PubMed] [Google Scholar]
- 14.Fischer, J. R., K. T. LeBlanc, and J. M. Leong. 2006. Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans. Infect. Immun. 74:435-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo, B. P., E. L. Brown, D. W. Dorward, L. C. Rosenberg, and M. Höök. 1998. Decorin-binding adhesins from Borrelia burgdorferi. Mol. Microbiol. 30:711-723. [DOI] [PubMed] [Google Scholar]
- 16.Haake, D. A., G. Chao, R. L. Zuerner, J. K. Barnett, D. Barnett, M. Mazel, J. Matsunaga, P. N. Levett, and C. A. Bolin. 2000. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect. Immun. 68:2276-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haake, D. A., M. Dundoo, R. Cader, B. M. Kubak, R. A. Hartskeerl, J. J. Sejvar, and D. A. Ashford. 2002. Leptospirosis, water sports, and chemoprophylaxis. Clin. Infect. Dis. 34:e40-e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haake, D. A., M. K. Mazel, A. M. McCoy, F. Milward, G. Chao, J. Matsunaga, and E. A. Wagar. 1999. Leptospiral outer membrane proteins OmpL1 and LipL41 exhibit synergistic immunoprotection. Infect. Immun. 67:6572-6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heath, C. W., Jr., A. D. Alexander, and M. M. Galton. 1965. Leptospirosis in the United States. Analysis of 483 cases in man, 1949, 1961. N. Engl. J. Med. 273:857-864, 915-922. [DOI] [PubMed] [Google Scholar]
- 20.Huff, S., Y. V. Matsuka, M. J. McGavin, and K. C. Ingham. 1994. Interaction of N-terminal fragments of fibronectin with synthetic and recombinant D motifs from its binding protein on Staphylococcus aureus studied using fluorescence anisotropy. J. Biol. Chem. 269:15563-15570. [PubMed] [Google Scholar]
- 21.Ingham, K. C., S. Brew, D. Vaz, D. N. Sauder, and M. J. McGavin. 2004. Interaction of Staphylococcus aureus fibronectin-binding protein with fibronectin: affinity, stoichiometry, and modular requirements. J. Biol. Chem. 279:42945-42953. [DOI] [PubMed] [Google Scholar]
- 22.Isberg, R. R., D. L. Voorhis, and S. Falkow. 1987. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell 50:769-778. [DOI] [PubMed] [Google Scholar]
- 23.Ito, T., and R. Yanagawa. 1987. Leptospiral attachment to four structural components of extracellular matrix. Nippon Juigaku Zasshi 49:875-882. [DOI] [PubMed] [Google Scholar]
- 24.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joh, D., P. Speziale, S. Gurusiddappa, J. Manor, and M. Höök. 1998. Multiple specificities of the staphylococcal and streptococcal fibronectin-binding microbial surface components recognizing adhesive matrix molecules. Eur. J. Biochem. 258:897-905. [DOI] [PubMed] [Google Scholar]
- 26.Joh, H. J., K. House-Pompeo, J. M. Patti, S. Gurusiddappa, and M. Höök. 1994. Fibronectin receptors from gram-positive bacteria: comparison of active sites. Biochemistry 33:6086-6092. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, R. C., and V. G. Harris. 1967. Differentiation of pathogenic and saprophytic leptospires. I. Growth at low temperatures. J. Bacteriol. 94:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katerov, V., A. Andreev, C. Schalen, and A. A. Totolian. 1998. Protein F, a fibronectin-binding protein of Streptococcus pyogenes, also binds human fibrinogen: isolation of the protein and mapping of the binding region. Microbiology 144:119-126. [DOI] [PubMed] [Google Scholar]
- 29.Kim, J. H., J. Singvall, U. Schwarz-Linek, B. J. Johnson, J. R. Potts, and M. Höök. 2004. BBK32, a fibronectin binding MSCRAMM from Borrelia burgdorferi, contains a disordered region that undergoes a conformational change on ligand binding. J. Biol. Chem. 279:41706-41714. [DOI] [PubMed] [Google Scholar]
- 30.Ko, A. I., M. Galvao Reis, C. M. Ribeiro Dourado, W. D. Johnson, Jr., L. W. Riley, et al. 1999. Urban epidemic of severe leptospirosis in Brazil. Lancet 354:820-825. [DOI] [PubMed] [Google Scholar]
- 31.Koizumi, N., and H. Watanabe. 2004. Leptospiral immunoglobulin-like proteins elicit protective immunity. Vaccine 22:1545-1552. [DOI] [PubMed] [Google Scholar]
- 32.Kopp, P. A., M. Schmitt, H. J. Wellensiek, and H. Blobel. 1995. Isolation and characterization of fibronectin-binding sites of Borrelia garinii N34. Infect. Immun. 63:3804-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, L. Y., M. Höök, D. Haviland, R. A. Wetsel, E. O. Yonter, P. Syribeys, J. Vernachio, and E. L. Brown. 2004. Inhibition of complement activation by a secreted Staphylococcus aureus protein. J. Infect. Dis. 190:571-579. [DOI] [PubMed] [Google Scholar]
- 34.Lee, L. Y., X. Liang, M. Höök, and E. L. Brown. 2004. Identification and characterization of the C3 binding domain of the Staphylococcus aureus extracellular fibrinogen-binding protein (Efb). J. Biol. Chem. 279:50710-50716. [DOI] [PubMed] [Google Scholar]
- 35.Levett, P. N. 2001. Leptospirosis. Clin. Microbiol. Rev. 14:296-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li, X., X. Liu, D. S. Beck, F. S. Kantor, and E. Fikrig. 2006. Borrelia burgdorferi lacking BBK32, a fibronectin-binding protein, retains full pathogenicity. Infect. Immun. 74:3305-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo, M., D. M. Bulach, D. R. Powell, D. A. Haake, J. Matsunaga, M. L. Paustian, R. L. Zuerner, and B. Adler. 2006. Effects of temperature on gene expression patterns in Leptospira interrogans serovar Lai as assessed by whole-genome microarrays. Infect. Immun. 74:5848-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsunaga, J., M. A. Barocchi, J. Croda, T. A. Young, Y. Sanchez, I. Siqueira, C. A. Bolin, M. G. Reis, L. W. Riley, D. A. Haake, and A. I. Ko. 2003. Pathogenic Leptospira species express surface-exposed proteins belonging to the bacterial immunoglobulin superfamily. Mol. Microbiol. 49:929-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsunaga, J., Y. Sanchez, X. Xu, and D. A. Haake. 2005. Osmolarity, a key environmental signal controlling expression of leptospiral proteins LigA and LigB and the extracellular release of LigA. Infect. Immun. 73:70-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsunaga, J., K. Werneid, R. L. Zuerner, A. Frank, and D. A. Haake. 2006. LipL46 is a novel, surface-exposed lipoprotein expressed during leptospiral dissemination in the mammalian host. Microbiology 152:3777-3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McBride, A. J., D. A. Athanazio, M. G. Reis, and A. I. Ko. 2005. Leptospirosis. Curr. Opin. Infect. Dis. 18:376-386. [DOI] [PubMed] [Google Scholar]
- 43.Merien, F., J. Truccolo, G. Baranton, and P. Perolat. 2000. Identification of a 36-kDa fibronectin-binding protein expressed by a virulent variant of Leptospira interrogans serovar icterohaemorrhagiae. FEMS Microbiol. Lett. 185:17-22. [DOI] [PubMed] [Google Scholar]
- 44.Nascimento, A. L., A. I. Ko, E. A. Martins, C. B. Monteiro-Vitorello, P. L. Ho, D. A. Haake, S. Verjovski-Almeida, R. A. Hartskeerl, M. V. Marques, M. C. Oliveira, C. F. Menck, L. C. Leite, H. Carrer, L. L. Coutinho, W. M. Degrave, O. A. Dellagostin, H. El-Dorry, E. S. Ferro, M. I. Ferro, L. R. Furlan, M. Gamberini, E. A. Giglioti, A. Goes-Neto, G. H. Goldman, M. H. Goldman, R. Harakava, S. M. Jeronimo, I. L. Junqueira-de-Azevedo, E. T. Kimura, E. E. Kuramae, E. G. Lemos, M. V. Lemos, C. L. Marino, L. R. Nunes, R. C. de Oliveira, G. G. Pereira, M. S. Reis, A. Schriefer, W. J. Siqueira, P. Sommer, S. M. Tsai, A. J. Simpson, J. A. Ferro, L. E. Camargo, J. P. Kitajima, J. C. Setubal, and M. A. Van Sluys. 2004. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J. Bacteriol. 186:2164-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palaniappan, R. U., Y. F. Chang, S. S. Jusuf, S. Artiushin, J. F. Timoney, S. P. McDonough, S. C. Barr, T. J. Divers, K. W. Simpson, P. L. McDonough, and H. O. Mohammed. 2002. Cloning and molecular characterization of an immunogenic LigA protein of Leptospira interrogans. Infect. Immun. 70:5924-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palaniappan, R. U., S. P. McDonough, T. J. Divers, C. S. Chen, M. J. Pan, M. Matsumoto, and Y. F. Chang. 2006. Immunoprotection of recombinant leptospiral immunoglobulin-like protein A against Leptospira interrogans serovar Pomona infection. Infect. Immun. 74:1745-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palma, M., O. Shannon, H. C. Quezada, A. Berg, and J. I. Flock. 2001. Extracellular fibrinogen-binding protein, Efb, from Staphylococcus aureus blocks platelet aggregation due to its binding to the alpha-chain. J. Biol. Chem. 276:31691-31697. [DOI] [PubMed] [Google Scholar]
- 49.Palma, M., D. Wade, M. Flock, and J. I. Flock. 1998. Multiple binding sites in the interaction between an extracellular fibrinogen-binding protein from Staphylococcus aureus and fibrinogen. J. Biol. Chem. 273:13177-13181. [DOI] [PubMed] [Google Scholar]
- 50.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Höök. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 51.Potts, J. R., and I. D. Campbell. 1996. Structure and function of fibronectin modules. Matrix Biol. 15:313-321. [DOI] [PubMed] [Google Scholar]
- 52.Probert, W. S., and B. J. Johnson. 1998. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol. Microbiol. 30:1003-1015. [DOI] [PubMed] [Google Scholar]
- 53.Probert, W. S., J. H. Kim, M. Höök, and B. J. Johnson. 2001. Mapping the ligand-binding region of Borrelia burgdorferi fibronectin-binding protein BBK32. Infect. Immun. 69:4129-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raibaud, S., U. Schwarz-Linek, J. H. Kim, H. T. Jenkins, E. R. Baines, S. Gurusiddappa, M. Höök, and J. R. Potts. 2005. Borrelia burgdorferi binds fibronectin through a tandem beta-zipper, a common mechanism of fibronectin binding in staphylococci, streptococci, and spirochetes. J. Biol. Chem. 280:18803-18809. [DOI] [PubMed] [Google Scholar]
- 55.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roche, F. M., R. Downer, F. Keane, P. Speziale, P. W. Park, and T. J. Foster. 2004. The N-terminal A domain of fibronectin-binding proteins A and B promotes adhesion of Staphylococcus aureus to elastin. J. Biol. Chem. 279:38433-38440. [DOI] [PubMed] [Google Scholar]
- 57.Ruoslahti, E., and M. D. Pierschbacher. 1987. New perspectives in cell adhesion: RGD and integrins. Science 238:491-497. [DOI] [PubMed] [Google Scholar]
- 58.Sasaki, D. M., L. Pang, H. P. Minette, C. K. Wakida, W. J. Fujimoto, S. J. Manea, R. Kunioka, and C. R. Middleton. 1993. Active surveillance and risk factors for leptospirosis in Hawaii. Am. J. Trop. Med. Hyg. 48:35-43. [DOI] [PubMed] [Google Scholar]
- 59.Schwarz-Linek, U., M. Höök, and J. R. Potts. 2004. The molecular basis of fibronectin-mediated bacterial adherence to host cells. Mol. Microbiol. 52:631-641. [DOI] [PubMed] [Google Scholar]
- 60.Schwarz-Linek, U., J. M. Werner, A. R. Pickford, S. Gurusiddappa, J. H. Kim, E. S. Pilka, J. A. Briggs, T. S. Gough, M. Höök, I. D. Campbell, and J. R. Potts. 2003. Pathogenic bacteria attach to human fibronectin through a tandem beta-zipper. Nature 423:177-181. [DOI] [PubMed] [Google Scholar]
- 61.Seshu, J., M. D. Esteve-Gassent, M. Labandeira-Rey, J. H. Kim, J. P. Trzeciakowski, M. Höök, and J. T. Skare. 2006. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol. Microbiol. 59:1591-1601. [DOI] [PubMed] [Google Scholar]
- 62.Signas, C., G. Raucci, K. Jonsson, P. E. Lindgren, G. M. Anantharamaiah, M. Höök, and M. Lindberg. 1989. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc. Natl. Acad. Sci. USA 86:699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas, D. D., and L. M. Higbie. 1990. In vitro association of leptospires with host cells. Infect. Immun. 58:581-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tokarz, R., J. M. Anderton, L. I. Katona, and J. L. Benach. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 72:5419-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsuchimoto, M., M. Niikura, E. Ono, H. Kida, and R. Yanagawa. 1984. Leptospiral attachment to cultured cells. Zentbl. Bakteriol. Mikrobiol. Hyg. A 258:268-274. [DOI] [PubMed] [Google Scholar]
- 66.Verma, A., J. Hellwage, S. Artiushin, P. F. Zipfel, P. Kraiczy, J. F. Timoney, and B. Stevenson. 2006. LfhA, a novel factor H-binding protein of Leptospira interrogans. Infect. Immun. 74:2659-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vinh, T., S. Faine, and B. Adler. 1984. Adhesion of leptospires to mouse fibroblasts (L929) and its enhancement by specific antibody. J. Med. Microbiol. 18:73-85. [DOI] [PubMed] [Google Scholar]
- 68.Wann, E. R., S. Gurusiddappa, and M. Höök. 2000. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J. Biol. Chem. 275:13863-13871. [DOI] [PubMed] [Google Scholar]