Abstract
Leishmania donovani is an intracellular protozoan parasite that impairs the host macrophage immune response to render it suitable for its survival and establishment. L. donovani-induced immunosuppression and alteration of host cell signaling is mediated by ceramide, a pleiotropic second messenger playing an important role in regulation of several kinases, including mitogen-activated protein kinase and phosphatases. We observed that the endogenous ceramide generated during leishmanial infection led to the dephosphorylation of protein kinase B (PKB) (Akt) in infected cells. The study of ceramide-mediated Akt phosphorylation revealed that Akt was dephosphorylated at both Thr308 and Ser473 sites in infected cells. Further investigation demonstrated that ceramide was also responsible for the induction of PKCζ, an atypical Ca-independent stress kinase, as well as the ceramide-activated protein phosphatases (e.g., protein phosphatase 2A [PP2A]). We found that Akt dephosphorylation was mediated by ceramide-induced PKCζ-Akt association and PP2A activation. In addition, treatment of L. donovani-infected macrophages with PKCζ-specific inhibitor peptide could restore the translocation of phosphorylated Akt to the cell membrane. This study also revealed that ceramide is involved in the inhibition of proinflammatory cytokine tumor necrosis factor alpha release by infected macrophages. These observations strongly suggest the importance of ceramide in the alteration of normal cellular functions, impairment of the kinase/phosphatase balance, and thereby establishment of leishmaniasis in the hostile macrophage environment.
Leishmania donovani is an obligate intracellular protozoan that resides in the mononuclear phagocytes of infected mammalian hosts (4, 10). Since macrophages are specialized for killing invading pathogens and priming the host immune response, Leishmania has evolved a range of sophisticated mechanisms to subvert normal macrophage function (1, 34). It is well known that L. donovani infection induces macrophage dysfunction, resulting in dampening of the respiratory burst, impairment of protein kinase C (PKC) activity, and suppression of other proinflammatory molecules (5, 6, 32). Recent studies suggest that ceramide is a pleiotropic lipid second messenger which plays an important role in a plethora of cellular functions, such as immunomodulation and apoptosis (21, 22, 27, 35). It exerts a diverse array of cellular functions by means of a subtle regulation of downstream kinases and phosphatases. In our previous studies we observed that L. donovani infection induced the endogenous ceramide synthesis pathway (18, 19). The resulting increase in ceramide generation not only is responsible for the impaired PKC activity and extracellular signal-regulated kinase phosphorylation and activity but also inhibits the AP-1 and NF-κB activities (18, 19). Ceramide-induced tyrosine phosphatase plays a major role in the deactivation of the mitogen-activated protein kinases in Leishmania-infected macrophages (18, 19).
PKB (Akt) is another important cellular factor which plays crucial roles in normal cellular signaling, including normal cellular proliferation and the respiratory burst mechanism (2, 11, 13). Earlier studies established that disruption of Akt kinase activation is important for immunosuppression induced by measles virus (3). Recently it was found that Akt plays a major role in the establishment of Neisseria gonorrhoeae in human cervical epithelial cells (16). In another study it was found that in cases of visceral leishmaniasis, sodium antimony gluconate, the established antileishmanial drug, induces the phosphorylation of Akt, which subsequently stimulates generation of reactive oxygen species in infected cells (29). However, the role of endogenous ceramide in Akt kinase activity in visceral leishmaniasis is yet to be explored. Ceramide is also capable of activating protein phosphatases such as protein phosphatase 1 (PP1) and PP2A (21, 22, 27). It is through these protein phosphatases that ceramide inhibits kinases which are key components of progrowth signaling processes, such as the classical as well as novel PKC isoforms and Akt (21, 22, 27, 35).
PKC is a family of structurally related serine/threonine protein kinases. The mammalian PKC isotypes have been grouped into three subfamilies, namely, classical PKCs, novel PKCs, and atypical PKCs, on the basis of their structural and regulatory properties (28, 30, 31, 41). The atypical isotypes have been shown to be activated by ceramide in different cell types (8, 40).
It was reported earlier that ceramide dephosphorylates Akt by a PKCζ-dependent mechanism in different cell types (8, 15, 26, 36). In this study we also found that the L. donovani-induced elevation of endogenous ceramide level leads to dephosphorylation of Akt through a PKC-dependent mechanism. An elevated level of endogenous ceramide induces the expression of PKCζ in L. donovani-infected host macrophages. Direct binding of PKCζ to Akt serves to negatively regulate Akt activation (8, 15, 36). Apart from PKCζ, ceramide also activates the PP2A, which in turn dephosphorylates the Akt kinase. These results suggest that the elevated endogenous ceramide level helps the induction of PKCζ and PP2A activities in Leishmania-infected macrophages, which ultimately dephosphorylate the Akt kinase and inhibit its translocation to the membrane.
MATERIALS AND METHODS
Materials.
Anti-PKCζ antibody, anti-Akt(1/2/3) antibody, p-Akt(1/2/3) (Ser473) antibody (a gift from Arun Bandyopadhyay, IICB, Kolkata, India), p-Akt(1/2/3) (Thr308) antibody, and anti-PP2A (catalytic subunit) primary antibody (rabbit polyclonal; reacts with mouse origin) were obtained from Santa Cruz Biotechnology. Anti-β-actin primary antibody (mouse monoclonal) and fluorescein isothiocyanate (FITC)-conjugated anti-mouse secondary antibodies were obtained from Sigma. Cell-permeative PKCζ inhibitor peptide (includes amino acids 113 to 125 of the pseudosubstrate region, Myr-Ser-Ile-Tyr-Arg-Arg-Gly-Ala-Arg-Arg-Trp-Arg-Lys-Leu-OH) was purchased from CalBiochem, Germany. All other chemicals were purchased from either Sigma or Merck.
Animals and parasites.
BALB/c mice were purchased from the National Center for Laboratory Animal Sciences, India. For each experiment 8 to 10 mice (4 to 6 weeks old) were used, regardless of sex. L. donovani strain AG-83 (MHOM/IN/1983/AG-83) was maintained in vitro in medium 199 (Sigma) containing 10% fetal calf serum. Amastigotes were prepared as described elsewhere (23). Promastigotes were obtained by suitable transformation. Experiments were done with promastigotes of stationary phase.
Peritoneal macrophage preparation.
Mouse macrophages were isolated by peritoneal lavage with ice-cold phosphate-buffered saline (PBS) at 48 h after intraperitoneal injection of 1.0 ml of sterile 4% thioglycolate broth (Difco). Cells were cultured as described by Fahey et al. (17). The adherent cell population was cultured for 48 h prior to any treatment, to achieve the resting state.
Preparation of cell lysate.
We checked the viability of infected macrophages as well as uninfected macrophages, and equal numbers of viable cells were taken for each experiment. The adherent cell population was scraped and centrifuged at 400 × g for 15 min at 4°C. The cells were then resuspended in ice-cold extraction buffer containing 50 mM Tris-HCl (pH 7.5), 50 mM EGTA, antiprotease mixture, and 50 mM β-mercaptoethanol. The antiprotease mixture consisted of 0.33 mM leupeptin, 0.2 mM phenylmethylsulfonyl fluoride, 0.35 mM antipain, 0.24 mg/ml of chymostatin, 0.35 mM pepstatin, and 4.8 trypsin inhibitor units of aprotinin/ml (14, 25). The macrophage-containing suspension was sonicated at 4°C and centrifuged at 4,250 × g for 10 min at 4°C, and then the supernatant was used for experiments.
Coimmunoprecipitation of PKCζ with Akt.
The adherent cell population was scraped and centrifuged at 400 × g for 15 min at 4°C. The cells were then resuspended in ice-cold extraction buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 1 mM NaF, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 0.2% Nonidet P-40, and 10 μg/ml aprotinin. The cell lysates were centrifuged at 14,000 × g for 10 min at 4°C. The supernatants (200 μg of protein) from the cell lysates were subjected to immunoprecipitation with anti-Akt antibody overnight at 4°C. The immunocomplexes were captured by incubation with anti-rabbit immunoglobulin G (IgG) agarose (Gibco-BRL) secondary antibody for 1 h at 4°C. The immunocomplexes then washed twice with PBS and once with lysis buffer and then assessed for coimmunoprecipitation with PKCζ by probing the blot with anti-PKCζ primary antibody (8). Equal loading of samples was verified by reprobing the blots with anti-Akt antibody.
Electrophoresis and immunoblotting.
Proteins were quantified with the Bio-Rad protein assay reagent, using bovine serum albumin as a standard. Equal amount of protein in each lane were subjected to sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The membrane was blocked overnight with 3% bovine serum albumin in Tris-saline buffer (pH 7.5), and immunoblotting was done as described by Majumdar and coworkers (14, 25).
Confocal microscopy.
Cells were grown on coverslips and treated with different inhibitors, lipopolysaccharide (LPS), and L. donovani parasites for the indicated times. Cells were washed in PBS and fixed in 1% paraformaldehyde in PBS at room temperature for at least 10 min. Cells were washed twice in PBS and permeabilized by a 15-min incubation in PBS supplemented with 0.1% saponin. Cells were washed again in PBS and stained for 45 min at room temperature with rabbit anti-mouse polyclonal anti-Akt antibody (Santa Cruz Biotechnology; 1:250 dilution) (31). An FITC-labeled goat anti-rabbit secondary antibody (Sigma; 1:100 dilution) was then added and incubated for another 45 min. The coverslips were mounted on a glass slide, and the images were observed under a confocal laser scanning microscope (LSM 510; Zeiss) (9).
PP2A activity.
Phosphatase activity was measured with para-nitrophenyl phosphate as the substrate, as described previously (39). Infected cells were lysed in lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 1 mM EGTA, 10% glycerol, 1.5 mM magnesium chloride, 1% Triton X-100, 1 μg of leupeptin/ml, 50 U of aprotinin/ml, 1 mM phenylmethylsulfonyl fluoride). Clarified supernatants were incubated with anti-PP2A antibody and protein G-agarose for 2 h at 4°C. The washed immunoprecipitates were resuspended in assay buffer (50 mM Tris [pH 7.0], 0.1 mM calcium chloride) containing 2.5 mM nickel chloride and 900 μg of para-nitrophenyl phosphate/ml and incubated for 30 min at 30°C. The amount of para-nitrophenol produced was determined by measuring the absorbance at 405 nm.
Cytokine analysis by sandwich ELISA.
The level of mouse tumor necrosis factor alpha (TNF-α) in the conditioned medium of the macrophage culture was measured by use of a mouse TNF-α enzyme-linked immunosorbent assay (ELISA) kit (Factor-Test-X; R & D Systems) in accordance with the manufacturer's instruction. The detection limit of this assay is 15.0 pg/ml.
Densitometric analysis.
Autoradiographs of endogenous protein phosphorylation and immunoblots were analyzed using a model GS-700 imaging densitometer and Molecular Analyst (version 1.5; Bio-Rad Laboratories, Hercules, CA).
Statistical analysis.
The in vitro cultures were in triplicate, and a minimum of four mice were used per group for in vivo experiments. The data, represented as means ± standard deviations (SD), are from one experiment which was performed at least three times. Student's t test was employed to assess the significance of the differences between the mean values of control and experimental sets. A P value of less than 0.05 was considered significant, a P value of less than 0.003 was considered highly significant, and a P value of less than 0.001 was considered very highly significant.
RESULTS
Dephosphorylation of Akt mediated by ceramide in L. donovani-infected macrophages.
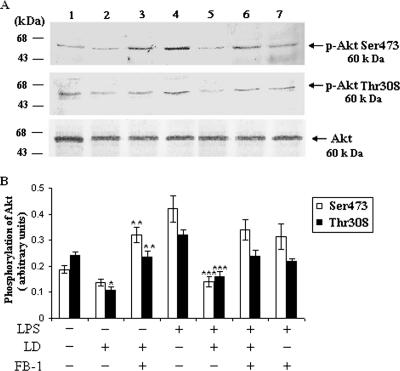
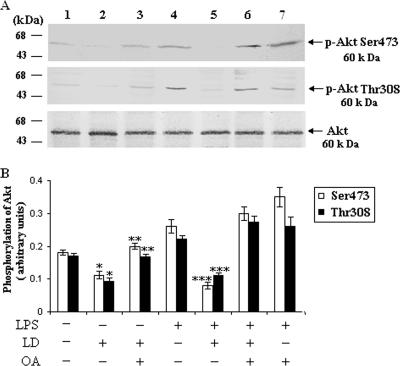
There are two key regulatory sites of Akt, i.e., Thr308 and Ser473. Phosphorylation of these two sites leads to the activation of Akt (13). LPS (1 μg/ml), a well-documented activator of Akt (20, 37, 43), induced the phosphorylation of Akt in the uninfected BALB/c macrophages. To explore the ceramide-mediated downstream signaling, we studied the effect of L. donovani infection on the phosphorylation of Akt in infected cells. From the immunoprecipitation study we found that in L. donovani-infected macrophages there was a significant (P < 0.05) inhibition of Akt phosphorylation at both Thr308 and Ser473 phosphorylation sites compared to uninfected macrophages treated with or without LPS (Fig. 1A, lanes 3 and 4). To investigate the role of ceramide in Akt phosphorylation, we pretreated the cells with fumonisin B-1 (FB-1) (a specific inhibitor of de novo synthesis of ceramide) (18, 19, 42) and infected the cells with L. donovani. From the Western blot analysis it was found that there was a significant (P < 0.003) restoration of Akt phosphorylation at both Thr308 and Ser473 phosphorylation sites in FB-1-treated infected cells (Fig. 1A). These results clearly indicate that the elevated level of ceramide in infected cells (19) was responsible for the deactivation of Akt.
FIG. 1.
L. donovani infection dephosphorylates Akt via ceramide. Macrophages were pretreated with or without FB-1 (10 μM) for 1 h and then challenged with L. donovani promastigotes (LD) (cell/parasite ratio, 1:10) for 4 h, noningested promastigotes were removed, and macrophages were cultured for another 20 h. LPS (1 μg/ml) was added where indicated for 30 min prior to the lysis of cells. Cell lysates were prepared, immunoprecipitated with anti-Akt antibody, and subjected to immunoblotting with p-Akt Ser473 and p-Akt Thr308 antibodies. The blots were reprobed with anti-Akt antibody to confirm equal protein loading. The blots shown in panel A are representative of a single experiment. The data shown in panel B are the means ± SD from four separate experiments. The lane numbers above the Western blots correspond to the x axis of the densitometric analysis below. The arbitrary values shown were obtained by densitometric analyses of the protein bands. Asterisks indicate statistically significant inhibition (*, P < 0.05) compared to normal macrophages, restoration (**, P < 0.003) compared to infected macrophages, and inhibition (***, P < 0.001) of phosphorylation of Akt compared to LPS-stimulated macrophages.
Induction of PKCζ by ceramide in L. donovani-infected macrophages.
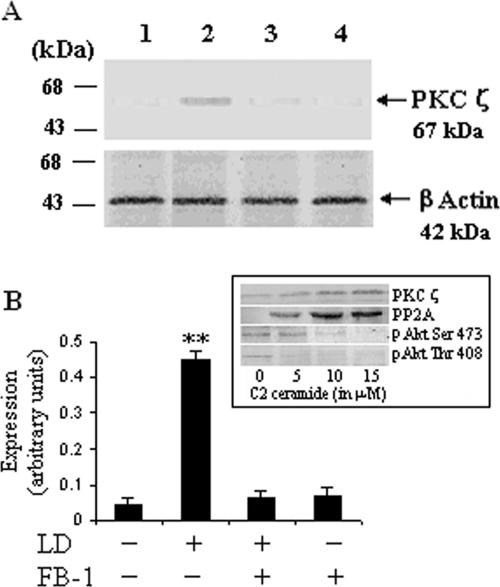
We next investigated the possible mechanism by which ceramide could inhibit Akt phosphorylation. It was reported earlier that ceramide might function as the endogenous signal regulating PKCζ-dependent inhibition of Akt (15, 33, 36). PKCζ is an atypical PKC having important implications in stress-induced altered signaling pathways (27). It has been reported that PKCζ is a potent downstream effector molecule of ceramide (15, 27, 33). The Western blot analysis clearly showed that L. donovani infection of macrophages for 24 h led to a significant (P < 0.003) enhancement in PKCζ expression (Fig. 2A, lane 2). To explore the role of ceramide in the induction of PKCζ, we pretreated the cells with FB-1 and then infected them with L. donovani promastigotes. We observed that in FB-1-pretreated cells infected with L. donovani there was a significant inhibition of PKCζ expression compared to that of infected macrophages (Fig. 2A, lane 3). To further confirm the effect of ceramide on the dephosphorylation of Akt, we studied the role of exogenously added cell-permeative C2 ceramide in uninfected macrophages. The immunoblot analysis (Fig. 2A, inset) clearly revealed that C2 ceramide enhanced the PKCζ and PP2A expression but decreased Akt phosphorylation in a dose-dependent manner.
FIG. 2.
Induction of PKC-ζ by ceramide in L. donovani-infected macrophages. Macrophages were cultured and treated with FB-1 and L. donovani promastigotes (LD) as described in the legend to Fig. 1. After 24 h of culture, cell lysates were prepared and subjected to immunoblot analysis with anti-PKCζ antibody. The blots were reprobed with anti-β-actin antibody to confirm equal protein loading. The blots shown in panel A are representative of a single experiment. The inset shows the effect of exogenously added cell-permeative C2 ceramide on the expression of PKCζ, phosphorylation of Akt, and expression of PP2A catalytic subunit. C2 ceramide was added in the culture medium of uninfected macrophages and cultured for 24 h. The data shown in pane B are the means ± SD from four separate experiments. The lane numbers above the Western blots correspond to the x axis of the densitometric analysis below. The arbitrary values shown were obtained by densitometric analyses of the protein bands. Asterisks indicate statistically significant induction (**, P < 0.003) of expression of PKCζ compared to control cells.
Enhanced intracellular ceramide induces the association of Akt and PKCζ in L. donovani-infected macrophages.
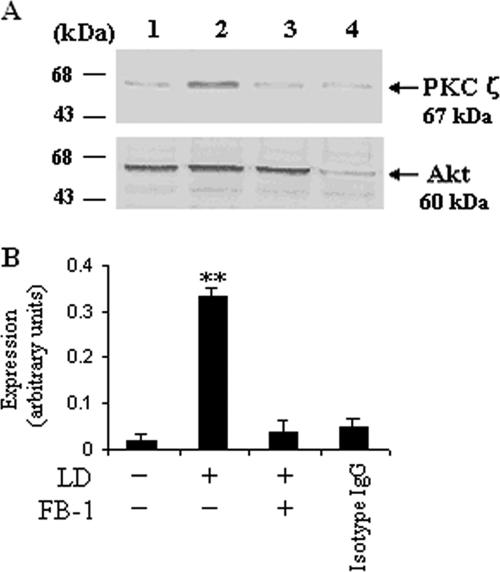
As we have clearly shown that ceramide activates PKCζ while inhibiting Akt phosphorylation, we investigated whether this elevated ceramide orchestrates a direct interaction between Akt and PKCζ in L. donovani-infected cells. The cell lysates were subjected to immunoprecipitation with anti-Akt antibody or control mouse IgG, followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Western blotting was done with anti-PKCζ antibody. A strong selective association of PKCζ with Akt was observed in L. donovani-infected macrophages (Fig. 3A, lane 2), whereas in the case of FB-1-pretreated cells following infection, there was a significant (P < 0.003) inhibition of PKCζ-Akt interaction (Fig. 3A, lane 3).
FIG. 3.
Enhanced intracellular ceramide enhances the association between Akt and PKCζ in L. donovani-infected macrophages. Macrophages were cultured and treated with FB-1 and L. donovani promastigotes (LD) as described in the legend to Fig. 1. After 24 h of culture, cell lysates were prepared and subjected to immunoprecipitation with anti-Akt antibody or mouse IgG (as a control), and the blots were probed with anti-PKCζ antibody as described in Materials and Methods. To verify equal protein loading of samples, the blots were reprobed with anti-Αkt antibody; this is representative of four individual experiments. The blots shown in panel A are representative of a single experiment. The data shown in panel B are the means ± SD from four separate experiments. The lane numbers above Western blots correspond to the x axis of the densitometric analysis below. The arbitrary values shown were obtained by densitometric analyses of the protein bands. Asterisks indicate statistically significant induction (*, P < 0.003) of expression of PKCζ compared to control cells.
PKCζ is necessary for ceramide-induced inhibition of Akt phosphorylation in L. donovani-infected cells.
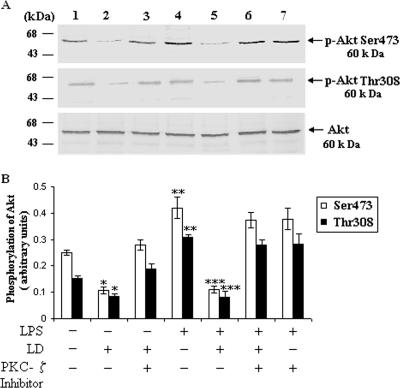
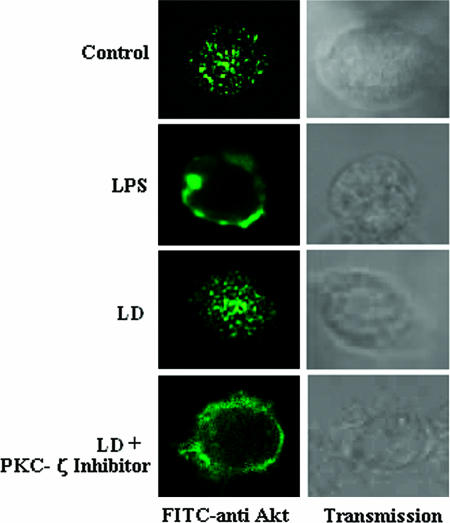
To further confirm the inhibition of PKCζ-mediated Akt phosphorylation, we used the cell-permeative PKCζ inhibitor peptide and analysis by Western blotting as well as confocal microscopy. From the Western blot analysis it was observed that in the presence of PKCζ inhibitor peptide there was significant restoration of phosphorylation of Akt in L. donovani-infected macrophages (Fig. 4A). As previously reported, the phosphorylation of Akt led to its translocation to the cell membrane (2). When the cells were stimulated with LPS, there was translocation of Akt from cytosol to membrane in normal macrophages (Fig. 5). In the case of L. donovani-infected cells, Akt was localized in the cytoplasm. When we treated the cells with cell-permeative PKCζ inhibitor, there was a significant translocation of Akt to the plasma membrane in infected cells (Fig. 5).
FIG. 4.
PKCζ is necessary for ceramide-induced inhibition of Akt phosphorylation in L. donovani-infected cells. (A) PKCζ inhibits the phosphorylation of Akt. Macrophages were pretreated with or without cell-permeative PKCζ inhibitor peptide (100 nM) for 1 h and then challenged with L. donovani promastigotes (LD) (cell/parasite ratio, 1:10) for 4 h, noningested promastigotes were removed, and macrophages were cultured for another 20 h. LPS (1 μg/ml) was added where indicated at 30 min prior to the lysis of cells. The lysates were immunoprecipitated with anti-Akt antibody and subjected to immunoblotting with p-Akt Ser473 and p-Akt Thr308 antibodies. The blots were reprobed with anti-Akt antibody to confirm equal protein loading. The lane numbers above Western blots correspond to the x axis of the densitometric analysis below. The data shown in panel B are the means ± SD from four separate experiments. The arbitrary values shown were obtained by densitometric analyses of the protein bands. Asterisks indicate statistically significant inhibition (*, P < 0.05) compared to normal macrophages, restoration (**, P < 0.003) compared to infected macrophages, and inhibition (***, P < 0.001) of phosphorylation of Akt compared to LPS-stimulated macrophages.
FIG. 5.
PKCζ inhibits the translocation of Akt to the membrane. Macrophages were cultured in coverslips, treated with or without LPS, and also pretreated with or without cell-permeative PKCζ inhibitor peptide (100 nM) for 1 h, followed by infection with L. donovani promastigotes (LD), as described in the legend to Fig. 1. After 24 h of culture, cells were stained with FITC-conjugated anti-Akt antibody and observed by confocal microscopy.
Ceramide-induced activation of PP2A in L. donovani-infected macrophages.
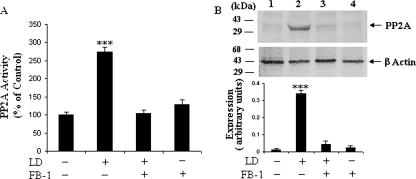
In addition to the activation of PKCζ, ceramide has been suggested to activate protein phosphatases, particularly serine/threonine phosphatases, that also dephosphorylate Akt (21, 22, 27). To investigate the activation of phosphatases in L. donovani-infected cells, we assessed the PP2A activity by a spectrophotometric method and also by Western blot analysis using anti-PP2A antibody (specific for the catalytic subunit). We found that there was very highly significant (P < 0.001) enzymatic activity of PP2A in L. donovani-infected macrophages (Fig. 6A); however, when we pretreated the cells with FB-1, there was significant inhibition of this activity. The Western blot analysis of the PP2A catalytic subunit also corroborated with the enzyme analysis data. In the case of infection, there was a significant induction of the catalytic subunit of PP2A (Fig. 6B, lane 2), and when we treated the infected cells with FB-1 to inhibit the endogenous ceramide synthesis, PP2A was also inhibited to a great extent (Fig. 6B, lane 3).
FIG. 6.
Ceramide activates PP2A, which in turn dephosphorylates Akt in L. donovani-infected macrophages. (A) Ceramide induces PP2A activity. Macrophages were incubated with or without FB-1 (10 μM) for 1 h and challenged with L. donovani promastigotes (LD) (cell/parasite ratio, 1:10) for 4 h. Cell lysates were prepared, and the PP2A activity was measured as described in Materials and Methods. Asterisks indicate statistically significant induction (***, P < 0.001) of PP2A activity compared to control cells. (B) Western blot analysis of PP2A (upper panel). Cell lysates were subjected to immunoblot analysis with anti-PP2A antibody. The data shown in the lower panel are the means ± SD from four separate experiments. The lane numbers above Western blots correspond to the x axis of the densitometric analysis below. The arbitrary values shown were obtained by densitometric analyses of the protein bands. Asterisks indicate statistically significant induction (***, P < 0.001) of phosphorylation of Akt.
Ceramide-induced PP2A dephosphorylates Akt in L. donovani-infected macrophages.
To further investigate whether PP2A had any role in the dephosphorylation of Akt, we treated the cells with okadaic acid, a specific inhibitor of PP2A, prior to L. donovani infection. We found that okadaic acid treatment significantly restored the Akt phosphorylation in infected cells (Fig. 7A), suggesting that PP2A was also responsible for the dephosphorylation of Akt in L. donovani-infected cells.
FIG. 7.
PP2A dephosphorylates the Akt in L. donovani-infected cells. Macrophages were pretreated with or without okadaic acid (OA) (10 μM) for 1 h and then challenged with L. donovani promastigotes (LD) (cell/parasite ratio, 1:10) for 4 h, noningested promastigotes were removed, and macrophages were cultured for another 20 h. The lysates were immunoprecipitated with anti-Akt antibody and subjected to immunoblotting with p-Akt Ser473 and p-Akt Thr308 antibodies. The blots were reprobed with anti-Akt antibody to confirm equal protein loading. The blots shown in panel A are representative of a single experiment. The data shown in panel B are the means ± SD from four separate experiments. The lane numbers above the Western blots correspond to the x axis of the densitometric analysis below. The arbitrary values shown were obtained by densitometric analyses of the protein bands. Asterisks indicate statistically significant inhibition (*, P < 0.05) compared to normal macrophages, restoration (**, P < 0.003) compared to infected macrophages, and inhibition (***, P < 0.001) of phosphorylation of Akt compared to LPS-stimulated macrophages.
Role of ceramide in the release of TNF-α in L. donovani-infected macrophages.
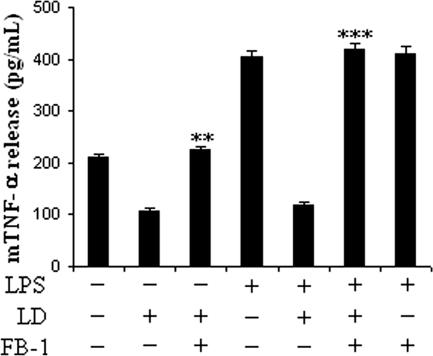
The generation of nitric oxide (NO) is an important host defense mechanism which is impaired in the susceptible host, and enhanced ceramide plays a major role in this process (18). As TNF-α, a potent NO-stimulatory molecule, is also impaired in the infected macrophages, here we tried to investigate whether enhanced ceramide plays any role in the impairment process. We cultured the macrophages with or without LPS and then treated them with FB-1, followed by infection and culture of the cells for another 20 h. We then studied the level of release of TNF-α in the supernatant. In infected cells there was inhibition of TNF-α release; however, in FB-1-treated infected cells the TNF-α release was restored (Fig. 8). Interestingly, in LPS-treated infected cells there was inhibition of TNF-α release, though FB-1 completely abrogated this inhibition in L. donovani-infected cells (Fig. 8). These results clearly suggest that enhanced ceramide in infected cells not only dephosphorylated the Akt but also affected the other antimicrobial machinery of the macrophages.
FIG. 8.
Effect of a ceramide inhibitor on TNF-α release by L. donovani-infected macrophages. Macrophages were cultured in a 96-well plate (106 cells/ml) with or without LPS (1 μg/ml) and treated with FB-1, followed by infection with L. donovani promastigotes as described in the Fig. 1 legend. The mouse TNF-α (mTNF-α) level in the cell culture supernatant was measured by sandwich ELISA (see Materials and Methods). Results are expressed as means ± SD of data from replicate experiments. **, P < 0.003 (restoration of mTNF-α release with respect to infected control macrophages); ***, P < 0.001 (restoration of mTNF-α release with respect to LPS-stimulated infected macrophages).
DISCUSSION
Ceramide plays a pivotal role in the signaling of different stress responses such as ischemia and insulin-resistant diabetes, as well as parasitized conditions (21, 22, 27). From our previous studies we found that ceramide plays an active part in the signal transduction of macrophages infected with L. donovani and is perhaps the most important regulatory switch operated by the parasite in the deactivation of macrophages (18, 19). Since ceramide has a wide range of effects on the intracellular signaling parameters, we were interested in investigating the effect of this molecule on the activation of Akt.
Akt has been shown to exert diverse cellular effects depending upon the cell type and stimulus (11, 13). In addition to its well-established role as a cell survival kinase, Akt is implicated in superoxide anion generation and activation of the immune response in different kinds of immune cells (11, 24). Activation of Akt leads to conformational changes in Akt, with subsequent phosphorylation at Thr308 and Ser473 (2). After being phosphorylated, Akt is translocated to the nucleus, where it regulates the synthesis of numerous proteins. From Fig. 1A, it was evident that Akt phosphorylation was significantly (P < 0.05) inhibited in L. donovani infection, and the elevated level of endogenous ceramide was one of the important factors, since FB-1 pretreatment restored (P < 0.003) the inhibition of Akt phosphorylation on both sites.
PKCζ has been implicated as a putative mediator of Akt inactivation (7). The mechanism by which PKCζ regulates the phosphorylation and activation of Akt also seem to be complex. Earlier we reported that ceramide activates the PKCζ in human neutrophils as well as murine macrophages (12, 19), and some other studies also reported that ceramide induces the activity of atypical PKCζ (7, 8, 40). Here we observed that, in L. donovani-infected macrophages, the elevated endogenous ceramide level facilitated PKCζ expression significantly (P < 0.003) compared to that in uninfected macrophages (Fig. 2). The ability of PKCζ to negatively regulate Akt implied that an interaction between PKCζ and Akt might play significant role in inhibiting Akt phosphorylation (8, 15, 40). Our studies further supported the biological significance of PKCζ as one of the potential targets for ceramide. It was reported earlier that PKCζ binds to the serine/threonine kinase (Akt) to inhibit its phosphorylation (8, 15, 36). From our coimmunoprecipitation study we found that PKCζ interacts with Akt in L. donovani-infected macrophages (Fig. 3). Interestingly, when we inhibited the endogenous ceramide level by pretreatment with FB-1, the PKCζ-Akt interaction was also inhibited. These studies suggest that in L. donovani-infected cells, an elevated level of endogenous ceramide induces PKCζ, which subsequently interacts with Akt, leading to its dephosphorylation. C2 ceramide has also been shown to inhibit Akt phosphorylation and upregulate PKCζ expression (Fig. 2B, inset).
However, a simple interaction between two proteins does not imply direct inhibition of Akt. Therefore, we assessed the critical role of PKCζ in the inhibition of Akt phosphorylation in L. donovani-infected cells. We used the cell-permeative PKCζ inhibitor in infected cells and then studied Akt phosphorylation. Figure 4 shows that there was a significant enhancement of Akt phosphorylation in PKCζ-inhibited cells infected with L. donovani. As translocation from cytosol to membrane is a very crucial parameter following the activation of Akt, we also studied the localization of Akt in treated cells. From the confocal microscopic studies (Fig. 5), it was clearly observed that in L. donovani-infected cells, Akt resided in the cytoplasm, but when the cells were treated with PKCζ inhibitor prior to infection, there was a significant translocation of Akt to the membrane. These studies clearly suggest that PKCζ interacts with Akt to inhibit its phosphorylation.
In addition to activation of PKCζ, ceramide has been suggested to activate protein phosphatases that have been known to dephosphorylate Akt (38). It was reported earlier that ceramide induces the serine/threonine phosphatase PP2A in different cell types (21, 22, 27). Here we observed that in L. donovani-infected cells there was a significant (P < 0.001) induction of PP2A activity (Fig. 6A). To assess the role of PP2A in Akt dephosphorylation, we inhibited the phosphatases by using different phosphatase inhibitors and then studied the phosphorylation status of Akt. Figure 7 shows that treatment with okadaic acid, a specific inhibitor of PP2A, abrogated the inhibition of Akt phosphorylation in infected cells, though calyculin, an inhibitor of PP1, had no effect on the phosphorylation of Akt. These studies implied that in L. donovani-infected cells endogenous ceramide induces the PP2A that in turn is responsible for the dephosphorylation of Akt. Earlier we established that enhanced ceramide impairs the nitric oxide generation in L. donovani-infected macrophages (18), and here we observed that ceramide was also involved in the process of inhibition of TNF-α release in L. donovani-infected macrophages (Fig. 8).
In summary, the observations presented here clearly demonstrate that in leishmaniasis endogenous ceramide is one of the most crucial factors for the dephosphorylation of Akt. To understand the mechanisms for Akt deactivation, we observed that ceramide induced the PKCζ-Akt interaction along with the serine/threonine phosphatase PP2A. Both mechanisms are operative in L. donovani-infected macrophages. These observations strongly suggest that endogenous ceramide generation induced in host cells by the parasite was responsible for the impairment of the normal macrophage antimicrobial machinery, thus pointing to the possibility of the utilization of ceramide as a possible therapeutic target against visceral leishmaniasis.
Acknowledgments
This work was financially supported by the Council of Scientific and Industrial Research (CSIR) and the Department of Atomic Energy (DAE), Government of India.
We are grateful to the Director, Bose Institute, Kolkata, for his encouragement. We thank Prabal Gupta for his technical assistance.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Alexander, J., R. A. Satoskar, and D. G. Russel. 1999. Leishmania species: models of intracellular parasitism. J. Cell Sci. 112:2993-3002. [DOI] [PubMed] [Google Scholar]
- 2.Andjelkovic, M., R. D. Alessi, R. Meier, A. Fernandez, J. C. Lamb, J. Ned, M. Frech, P. Cron, P. Cohen, M. J. Lucocq, and B. A. Hemmings. 1997. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272:31515-31524. [DOI] [PubMed] [Google Scholar]
- 3.Avota, E., A. Avots, S. Niewiesk, P. L. Kane, U. Bommhardt, V. Meulen, and S. Schneider-Schaulies. 2001. Disruption of Akt kinase activation is important for immunosuppression induced by measles virus. Nat. Med. 7:725-731. [DOI] [PubMed] [Google Scholar]
- 4.Bates, P. A. 1994. The developmental biology of Leishmania promastigotes. Exp. Parasitol. 79:215-218. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharyya, S., S. Ghosh, P. L. Jhonson, S. Bhattacharya, and S. Majumdar. 2001. Immunomodulatory role of interleukin-10 in visceral leishmaniasis: defective activation of protein kinase C-mediated signal transduction events. Infect. Immun. 69:1499-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharyya, S., S. Ghosh, P. Sen, S. Roy, and S. Majumdar. 2001. Selective impairment of protein kinase C isotypes in murine macrophage by Leishmania donovani. Mol. Cell. Biochem. 216:47-57. [DOI] [PubMed] [Google Scholar]
- 7.Bourbon, A. N., J. Yun, and M. Kester. 2000. Ceramide directly activates protein kinase Cζ to regulate a stress-activated protein kinase signaling complex. J. Biol. Chem. 275:35617-35623. [DOI] [PubMed] [Google Scholar]
- 8.Bourbon, A. N., L. Sandirasegarane, and M. Kester. 2002. Ceramide-induced inhibition of Akt is mediated through protein kinase Cζ. J. Biol. Chem. 277:3286-3292. [DOI] [PubMed] [Google Scholar]
- 9.Chakraborty, D., S. Banerjee, A. Sen, K. K. Banerjee, P. Das, and S. Roy. 2005. Leishmania donovani affects antigen presentation of macrophage by disrupting lipid rafts. J. Immunol. 175:3214-3224. [DOI] [PubMed] [Google Scholar]
- 10.Chang, K. P., and G. Choudhuri. 1990. Molecular determinates of Leishmania virulence. Annu. Rev. Microbiol. 44:499-529. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Q., D. W. Powell, M. J. Rane, S. Singh, W. Butt, J. B. Klein, and K. R. McLeish. 2003. Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. J. Immunol. 170:5302-5308. [DOI] [PubMed] [Google Scholar]
- 12.Das, S., S. Bhattacharyya, S. Ghosh, and S. Majumdar. 2000. Signal transduction mechanism in human neutrophil: comparatative study between the z and b-protein kinase isotypes. Mol. Cell. Biochem. 203:143-151. [DOI] [PubMed] [Google Scholar]
- 13.Datta, R. S., A. Brunet, and E. M. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 14.Dey, R., A. Sarkar, N. Majumder, S. M. Bhattacharyya, K. Roychoudhury, S. Bhttacharyya, S. Roy, and S. Majumdar. 2005. Regulation of impaired protein kinase C signaling by chemokines in murine macrophages during visceral leishmaniasis. Infect. Immun. 73:8334-8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doornbos, R. P., M. Thelen, P. C. van der Hoeven, W. J. van Blitterswijk, A. J. Verkleij, and P. M. van Bergen en Henegouwen. 1999. Protein kinase Cζ is a negative regulator of Protein Kinase B activity. J. Biol. Chem. 274:8589-8596. [DOI] [PubMed] [Google Scholar]
- 16.Edwards, J. L., and M. A. Apicella. 2006. Neisseria gonorrhoeae PLD directly interacts with Akt kinase upon infection of primary, human cervical epithelial cells. Cell. Microbiol. 8:1253-1271. [DOI] [PubMed] [Google Scholar]
- 17.Fahey, J. T., K. J. Tracey, P. T. Olson, L. S. Cousens, W. G. Jones, G. T. Shires, and B. Sherry. 1992. Macrophage inflammatory protein 1 modulates macrophage function. J. Immunol. 148:2764-2769. [PubMed] [Google Scholar]
- 18.Ghosh, S., S. Bhattacharyya, M. Sirkar, G. S. Sa, T. Das, D. Majumdar, S. Roy, and S. Majumdar. 2002. Leishmania donovani suppresses activated protein 1 and NF-κB in host macrophages via ceramide generation: involvement of extracellular signal-regulated kinase. Infect. Immun. 70:6828-6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh, S., S. Bhattacharyya, S. Das, S. Raha, N. Maulik, K. D. Das, S. Roy, and S. Majumdar. 2001. Generation of ceramide in murine macrophages infected with Leishmania donovani alters macrophage signaling events and aids intracellular parasitic survival. Mol. Cell. Biochem. 223:47-60. [DOI] [PubMed] [Google Scholar]
- 20.Guha, M., and N. Mackman. 2001. LPS induction of gene expression in human monocytes. Cell Signal. 13:85-94. [DOI] [PubMed] [Google Scholar]
- 21.Hannun, Y. A. 1996. Functions of ceramide in coordinating cellular response to stress. Science 274:1855-1859. [DOI] [PubMed] [Google Scholar]
- 22.Hannun, Y. A., and L. M. Obeid. 1995. Ceramide: an intracellular signal for apoptosis. Trends Biochem. Sci. 20:73-77. [DOI] [PubMed] [Google Scholar]
- 23.Hart, T. D., K. Vickerman, G. H. Coombs, and A. Quick. 1981. Simple method for purifying L. mexicana amastigotes in large numbers. Parasitology 82:345-355. [DOI] [PubMed] [Google Scholar]
- 24.Kane, L. P., P. G. Andres, K. C. Howland, A. K. Abbas, and A. Weiss. 2001. Akt provides the CD28 co-stimulatory signal for up-regulation of IL-2 and IFN-γ but not Th2 cytokines. Nat. Immunol. 2:37-44. [DOI] [PubMed] [Google Scholar]
- 25.Majumdar, S., M. W. Rossi, T. Fujiki, W. A. Phillips, S. Disn, C. F. Queen, R. B. Jhonston, O. M. Rosen, B. E. Corkey, and H. M. Korchak. 1991. Protein kinase C isotypes and signaling in neutrophils. J. Biol. Chem. 266:9285-9294. [PubMed] [Google Scholar]
- 26.Mao, M., X. Fang, U. L. Yiling., R. LaPushin, C. R. Bast, and G. B. Mills. 2000. Inhibition of growth factor induced phosphorylation and activation of protein kinase B/Akt by atypical protein kinase C in breast cancer cells. Biochem. J. 352:475-482. [PMC free article] [PubMed] [Google Scholar]
- 27.Mathias, S., A. L. Pena, and R. N. Kolesnick. 1998. Signal transduction of stress via ceramide. Biochem. J. 335:465-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mellor, H., and P. J. Parker. 1998. The extended protein kinase C super-family. Biochem. J. 332:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mookerjee Basu, J., A. Mookerjee, P. Sen, S. Bhaumik, P. Sen, S. Banerjee, K. Naskar, S. K. Choudhury, B. Saha, S. Raha, and S. Roy. 2006. Sodium antimony gluconate induces the generation of reactive oxygen species and nitric oxide via phosphoinositide-3-kinase and mitogen-activated protein kinase activation in Leishmania donovani-infected macrophages. Antimicrob Agents Chemother. 50:1788-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newton, A. C. 1995. Protein kinase C: structure, function and regulation. J. Biol. Chem. 270:28495-28498. [DOI] [PubMed] [Google Scholar]
- 31.Nishizuka, Y. 1995. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 9:484-496. [PubMed] [Google Scholar]
- 32.Olivier, M., D. J. Gregory, and G. Forget. 2005. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin. Microbiol. Rev. 18:293-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powell, J. D., E. Hajduch, G. Kular, and H. S. Hundal. 2003. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCζ-dependent mechanism. Mol. Cell. Biol. 23:7794-7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell, D. G., S. Xu, and P. Chakraborty. 1992. Intracellular trafficking and the parasitophorous vacuole of Leishmania mexicana infected macrophages. J. Cell Sci. 103:1193-1210. [DOI] [PubMed] [Google Scholar]
- 35.Saba, J. D., L. M. Obeid, and Y. A. Hannun. 1996. Ceramide: an intracellular mediator of apoptosis and growth suppression. Philos. Trans. R. Soc. London B 351:233-240. [DOI] [PubMed] [Google Scholar]
- 36.Schubert, M. K., P. M. Scheid, and V. Duronio. 2000. Ceramide inhibits protein kinase B/Akt by promoting dephosphorylation of serine 473. J. Biol. Chem. 275:13330-13335. [DOI] [PubMed] [Google Scholar]
- 37.Sengupta, S., P. M. Chilton, and T. C. Mitchell. 2005. Adjuvant-induced survival signaling in clonally expanded T cells is associated with transient increases in pAkt levels and sustained uptake of glucose. Immunobiology 210:647-659. [DOI] [PubMed] [Google Scholar]
- 38.Stratford, S., L. K. Hoehn, F. Liu, and S. A. Summers. 2004. Regulation of insulin action by ceramide, dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 279:36608-366615. [DOI] [PubMed] [Google Scholar]
- 39.Ugi, S., T. Imamura, H. Maegawa, K. Egawa, T. Yoshizaki, K. Shi, T. Obata, Y. Ebina, A. Kashiwagi, and J. M. Olefsky. 2004. Protein phosphatase 2A negatively regulates insulin's metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes. Mol. Cell. Biol. 24:8778-8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Blitterswijk, W. J., A. H. van der Luit, R. J. Veldman, M. Verhelj, and J. Borst. 2003. Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem. J. 369:199-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Violin, J. D., and A. C. Newton. 2003. Pathway illuminated: visualising protein kinase C signaling. IUBMB Life 55:653-660. [DOI] [PubMed] [Google Scholar]
- 42.Wang, E., W. P. Norred, C. W. Bacon, R. T. Riley, and A. H. Merril. 1991. Inhibition of sphingolipid biosynthesis by fumonisins: implication for disease associated with Fusarium moniliforme. J. Biol. Chem. 266:14486-14490. [PubMed] [Google Scholar]
- 43.Xagorari, A., A. Papapetropoulos, A. Mauromatis, M. Economou, T. Fotsis, and C. Roussos. 2001. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J. Pharmacol. Exp. Ther. 296:181-187. [PubMed] [Google Scholar]