Abstract
Anthrax toxin is made up of three separate protein components: the receptor-binding protective antigen (PA), the adenylyl cyclase edema factor (EF), and the metalloproteinase lethal factor (LF). EF and PA constitute edema toxin (ET), which causes edema when injected subcutaneously. At higher doses, ET causes severe pathologies and death in BALB/cJ mice (A. M. Firoved et al., Am. J. Pathol. 167:1309-1320, 2005). A striking effect of ET at lethal doses is adrenal necrosis. Here we show that low doses of ET (10 μg) that produce no overt signs of illness in mice still cause substantial adrenal lesions. These lesions are not associated with reduced corticosterone production; instead, ET-treated mice have increased corticosterone production. Because the resistance of mice to the other component of anthrax toxin, lethal toxin (LT; LF plus PA), has been shown to be overcome by the perturbation of the endocrine system, we hypothesized that sublethal doses of ET might sensitize LT-resistant DBA/2J mice to LT-mediated lethality. We report that a low dose of ET (5 μg) is sufficient to sensitize DBA/2J mice when given concurrently with LT. Higher doses of ET (e.g., 15 μg) given to male and female DBA/2J mice 18 h prior to LT challenge also sensitize them to LT. This study using highly purified ET and LT demonstrates how the components of anthrax toxin can work together to increase lethality.
Bacillus anthracis, the causative agent of anthrax, relies on a polyglutamic capsule and anthrax toxin to establish infection and kill its host. Anthrax toxin is a tripartite toxin composed of the receptor binding protective antigen (PA), the zinc metalloprotease lethal factor (LF), and adenylyl cyclase edema factor (EF) (for toxin reviews, see references 3, 9, and 15). PA and EF together constitute edema toxin (ET), while PA and LF constitute lethal toxin (LT). PA binds the cellular receptors tumor endothelial marker 8 (TEM8) and capillary morphogenesis gene 2 (CMG2) (2, 16). Once bound to the receptor and proteolytically activated, PA forms a heptamer that delivers EF and/or LF to the cytoplasm following receptor-mediated endocytosis.
Upon entry into cells, LF cleaves members of the mitogen-activated protein kinase kinase family (4, 22). When injected into mice, LT induces pleural edema and a limited amount of tissue damage, primarily in the bone marrow, spleen, and liver, due to vascular collapse and subsequent hypoxia (12). Mice do, however, differ in their susceptibilities to death mediated by LT. Resistance or susceptibility is influenced by at least three genetic loci, of which only one (Nalp1b) has been identified (1, 11). The DBA/2J strain of mice is almost completely resistant to a bolus injection of LT, while the BALB/cJ murine strain is highly sensitive to the injection. Both strains show increased sensitivity to LT following the perturbation of the hypothalamic-pituitary-adrenal (HPA) axis, the neuroendocrine system which controls a variety of body processes from the immune response to the vascular system through the release of hormones including glucocorticoids (GC). Interference with GC production via adrenalectomy, the inhibition of GC function by the GC receptor antagonist RU-486, or the administration of excess GCs, such as the synthetic GC dexamethasone, all resulted in altered sensitivity to LT (14).
EF is dependent on host calmodulin and calcium to catalyze the conversion of ATP to cyclic AMP (8). In tissue culture, ET induces the production of anti-inflammatory cytokines and inhibits the production of lipopolysaccharide-stimulated inflammatory cytokines (7, 17). ET acts cooperatively with LT to shut down dendritic cell cytokine secretion (20). The murine adrenal gland-derived cell line Y1 and Chinese hamster ovary (CHO) cells exhibit shape changes (rounding or elongation) upon exposure to ET (6, 8). ET causes a wide range of tissue lesions in mice, including the gross observations of gastrointestinal tract effusion and hemorrhage of lymph nodes and adrenal glands (5). Histopathological examination of ET-treated mice reveals pathological lesions in heart, kidney, gastrointestinal tract, lymphoid organs, bone marrow, and adrenal glands. In the adrenal glands specifically, there is multifocal necrosis, hemorrhage, and granulocytic infiltrates in the zona fasciculata of the adrenal cortex, the region responsible for the production of GCs (5).
The increased sensitivity of mice to LT following the perturbation of the endocrine system as well as the targeting of the adrenal gland by ET led us to investigate whether low doses of ET can potentiate LT activity in DBA/2J mice in a manner similar to the sensitization induced by other mechanisms of endocrine disruption. The only previous study utilizing LT and ET together in animal models reported on synergism for LT and ET in inducing lethality in mice (of unknown origin) but a decrease in edema formation in rabbit skin (19). However, highly purified toxin was not available for those studies and the role of PA as a limiting component for LF or EF cell entry was unknown. Recently, an in vitro study demonstrated the cooperation between the two toxins in impairing dendritic cell function (20). Here, we describe the effects of highly purified ET and LT given together to mice and show that nonlethal doses of ET act to overcome DBA/2J resistance to LT.
MATERIALS AND METHODS
Toxin.
PA and LF were made in a B. anthracis expression system and purified from culture supernatants (21). N-terminal His-tagged EF was purified from Escherichia coli (18) and contained ≤10 pg of lipopolysaccharide per microgram of EF, as assayed with the limulus amebocyte lysate 120 test kit (Cambrex, East Rutherford, NJ). All toxin preparations were made in sterile 1× phosphate-buffered saline (PBS) from frozen stocks that were thawed only once before use. Toxin dosage refers to the amount of LF or EF given with an equal amount of PA (i.e., 100 μg of LT is 100 μg of LF plus 100 μg of PA).
Animals.
Female and male 12- to 16-week-old BALB/cJ and DBA/2J mice (The Jackson Laboratory, Bar Harbor, ME) were age matched for each experiment. DBA/2J mice were acclimated to the National Institute of Allergy and Infectious Diseases (NIAID) animal facility for 4 to 8 weeks prior to experimentation. Intravenous (200 μl) or intraperitoneal (1 ml) injections were carried out with sterile 1× PBS. All protocols were approved by the NIAID Animal Care and Use Committee.
Survival experiments.
For determining the sensitivity to ET, male and female BALB/cJ and DBA/2J mice were intravenously injected with various doses of ET and followed until death or terminal illness (at which time animals were euthanized by CO2 inhalation). In sensitization experiments, nonlethal doses of ET were intravenously injected into male or female DBA/2J mice (n = 5) 18 h prior to intraperitoneal injection of 100 μg of LT. Control animals received a single toxin component, either PA, LF, or EF. Concurrent delivery of ET by the intravenous and LT by the intraperitoneal routes was carried out in female DBA/2J mice. Delayed or concurrent ET and LT injection experiments were performed in duplicate, and data were pooled. Control DBA/2J mice were tested for susceptibility to 100 μg LT to ensure they showed typical resistance to LT. All survival curves were compared by the log rank test.
Histopathology.
Two DBA/2J or BALB/cJ mice of each sex were left untreated or intravenously injected with EF or ET (1, 5, 10, 20, or 37.5 μg). After 18 h, animals were euthanized by CO2 inhalation and necropsied. Adrenal glands were fixed in 10% buffered formalin phosphate (Fisher Scientific, Inc., Fairlawn, NJ). Tissues were paraffin embedded, sectioned, and stained with hematoxylin and eosin (Histoserv, Inc., Germantown, MD). Images were acquired using an Olympus BX41 compound binocular microscope (Olympus America, Inc., Melville, NY).
Corticosterone measurements.
All mice were housed on a 12-h light cycle and redistributed to clean cages (three mice per cage) 48 h prior to each experiment to minimize stress. On the day of the experiment, only one mouse per cage was injected and it was then housed with cage mates. Groups of DBA/2J and BALB/cJ mice of both sexes were intravenously injected with 20 μg ET (n = 5). Control mice were injected with 20 μg EF alone (n = 5) or PBS (n = 3). Mice were assayed for serum corticosterone levels at 6 and 24 h after injection. All mice, including controls, were euthanized at the same time of day by CO2 inhalation prior to trunk blood collection. Serum samples were analyzed by Linco Diagnostic Services, Inc. (St. Charles, MO) by using radioimmunoassay methods. The difference of means between the ET treatment group and the corresponding EF control group was evaluated using the two-tailed unpaired t test.
Statistics.
Data were processed using GraphPad PRISM 4.0 software (GraphPad Software, Inc., San Diego, CA) to generate graphs and statistical analyses.
RESULTS
ET is lethal in DBA/2J mice.
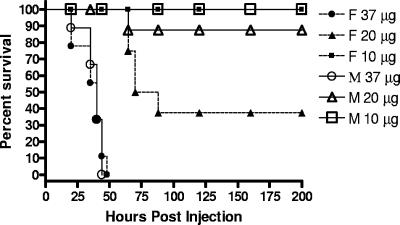
The DBA/2J mouse strain is strongly resistant to a bolus delivery of up to 200 μg of LT, with at most 10% of the mice succumbing (13, 14). We had previously shown that intravenous injection of 37.5 μg ET results in complete lethality for female BALB/cJ mice (5). This dose was lethal to both female and male DBA/2J mice (Fig. 1). Female DBA/2J mice were also sensitive to a dose of 20 μg (Fig. 1), but as observed previously (5), no BALB/cJ (male or female) mice given 20 μg of ET died (data not shown). Interestingly, we also found that the 37.5-μg dose, which was fully lethal to female BALB/cJ mice, was not lethal to male BALB/cJ mice, but both sexes fully succumbed to 50 μg ET with similar kinetics (data not shown). No DBA/2J mice died from a dose of 10 μg ET (Fig. 1) or lower (data not shown). Controls treated with 37.5 μg of EF given in the absence of PA or with PA alone (at a range of doses) also survived with no signs of illness (data not shown).
FIG. 1.
ET is lethal in DBA/2J mice. Female (F; dashed lines, closed symbols) or male (M; solid lines, open symbols) DBA/2J mice were treated with 37.5 μg (n = 9), 20 μg (n = 9), or 10 μg (n = 9) ET. All injections were delivered intravenously (200-μl volume).
ET causes adrenal lesions in BALB/cJ and DBA/2J mice.
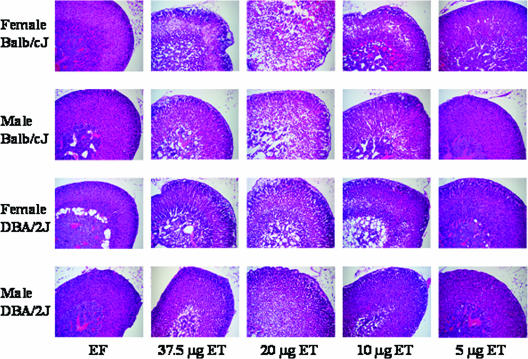
An early and consistent pathology following ET intoxication in female BALB/cJ mice is necrosis of the adrenal cortex accompanied by hemorrhage and granulocytic infiltrates (5). To examine whether this is a consistent finding across strains and sexes, we compared effects of various doses of ET on adrenals in female and male DBA/2J and BALB/cJ mice 18 h after challenge (Fig. 2). As seen previously at a dose of 37.5 μg ET (5) and here also at 20 μg ET, female BALB/cJ mice displayed multifocal necrosis and significant numbers of neutrophil infiltrates in the zona fasciculata of the adrenal cortex. Male and female DBA/2J mice as well as male BALB/cJ mice at the highest dosage showed less severe pathologies than those observed for female BALB/cJ mice, with only occasional necrotic cells and few neutrophil infiltrates. All animals given doses of 37.5 μg or 20 μg ET exhibited the disruption of the zona fasciculata cords associated with increased spacing of the sinusoidal capillary lumina and congestion. Cells within the cords were degenerate and displayed cell rounding with losses of microvesicular lipid droplets and the formation of macrovacuoles. These effects were present but less severe at the 10-μg dose and minimal at the 5-μg dose. Effects in animals given 1 μg ET were indistinguishable from those of untreated animals (data not shown) or EF-only controls. Animals that received 5 or 10 μg ET had pathological lesions in the adrenals but displayed no outward signs of illness. By comparison, at a dose of 20 μg ET, mice displayed slightly reduced activity levels and a scruffy coat, while animals that received 37.5 μg were dehydrated and lethargic at the time of necropsy.
FIG. 2.
Adrenal gland lesions in ET-treated mice. Two male or female DBA/2J or BALB/cJ mice were left untreated or were injected intravenously with 37.5 μg EF or 1, 5, 10, 20, or 37.5 μg ET. Animals were euthanized after 18 h, and adrenal glands were processed for histopathology.
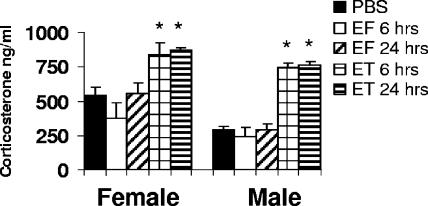
ET induces corticosterone production.
Glucocorticoids are produced in the cortex of the adrenal gland and are important for regulating immune responses as well as playing a role in glucose metabolism and cardiac homeostasis. To examine the effects of ET on corticosterone production by the adrenal glands, we administered ET, EF, or PBS to male and female DBA/2J mice. A dose of 20 μg ET was chosen due to the severe adrenal phenotypic changes induced by ET at this dose. We chose an early (6-h) time point prior to any illness and a 24-h time point to follow the verified histological changes observed at 18 h. The mice were assessed for serum corticosterone levels. Corticosterone levels in both male and female mice were increased at 6 h and remained elevated at 24 h (Fig. 3). EF alone did not increase corticosterone levels beyond those observed in PBS-injected control animals. An identical experiment performed with BALB/cJ mice of both sexes yielded similar levels for all conditions (data not shown). Therefore, the pathological lesions in the adrenal cortex following the injection of ET do not prevent corticosterone production and instead actually stimulate its production.
FIG. 3.
ET induces serum corticosterone production. DBA/2J mice were injected intravenously with 20 μg ET (n = 5), EF (n = 5), or PBS (n = 3), and corticosterone levels were assessed at 6 or 24 h. Asterisks indicate the statistically significant difference of means between ET treatment and corresponding timed EF control by two-tailed unpaired t test (P < 0.05). Error bars indicate standard deviations.
DBA lethality resulting from concurrent delivery of ET and LT.
DBA/2J mice are normally resistant to a single bolus of LT at doses that are 100% lethal in many other mouse strains (13). GC injection or other modulation of adrenal gland function sensitizes DBA/2J mice to otherwise nonlethal doses of LT (14). We hypothesized that the adrenal phenotypic changes and corticosterone production induced by ET might sensitize DBA/2J to LT. ET and LT were injected simultaneously into mice, with various doses of ET delivered intravenously, followed immediately by an intraperitoneal injection of 100 μg LT (Fig. 4). Doses of 5, 10, and 15 μg ET delivered in conjunction with LT resulted in 90, 87, and 60% lethality, respectively. Surprisingly, the lower doses of ET appeared to have stronger effects on lethality than did the higher doses, although the difference was not statistically significant (P = 0.16 for 5 μg versus 15 μg ET treatments). A minimum level of EF appears to be required, however, since 1 μg of ET resulted in only 1 of 10 animals dying (typical of DBA susceptibility to LT). Controls in which EF, LF, or PA was absent from one of the injections resulted in 0 to 20% mortality.
FIG. 4.
Concurrent activity of ET and LT on DBA/2J lethality. Animals received an intravenous injection of 1 μg (n = 10), 5 μg (n = 10), 10 μg (n = 15), or 15 μg ET (n = 10), immediately followed by an intraperitoneal injection of 100 μg LT. Control animals (n = 10) received one injection in which individual components were omitted: 15 μg PA plus 100 μg LT, 15 μg EF plus 100 μg LT, or 15 μg ET plus 100 μg PA. By the log rank test, the groups given 10 μg ET plus 100 μg LT (P = 0.0027) and 5 μg ET plus 100 μg LT (P = 0.00315) were significantly different from the 15 μg PA plus 100 μg LT group. The groups given 1 μg ET plus 100 μg LT (P = 0.5842) and 15 μg ET plus 100 μg LT (P = 0.0699) were not significantly different from the group given 15 μg PA plus 100 μg LT, although the latter group (15 μg ET) was statistically significant from the other two control groups (P < 0.05).
Pretreatment with sublethal dose of ET sensitizes DBA/2J mice to LT-mediated lethality.
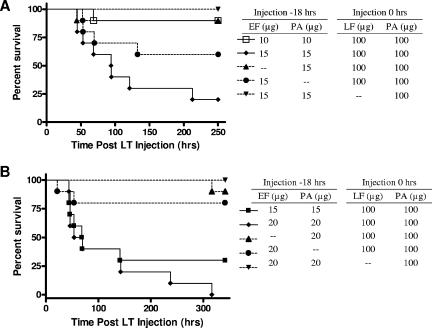
We tested whether sublethal ET doses administrated with a delay prior to LT administration would also sensitize DBA/2J mice to LT. We treated female mice with nonlethal doses (10 or 15 μg) of ET, EF, or PA 18 h prior to the injection of 100 μg LT (Fig. 5A). To ensure that dosing conditions remained consistent with previous experiments (5, 13, 14), ET was delivered intravenously, while LT was given intraperitoneally. A dose of 15 μg ET, followed by LT, resulted in 80% lethality, while 15 μg of ET, followed by PA, did not kill any animals. Similarly, pretreatment by PA alone prior to the delivery of LT killed only one animal. A dose of 10 μg ET, however, did not sensitize the DBA/2J to LT-mediated lethality. Surprisingly, in the groups that received 15 μg EF alone prior to LT treatment, 40% of the animals died (a stronger effect than that observed for 10 μg of ET).
FIG. 5.
(A) ET sensitizes female DBA/2J mice to LT lethality. Animals (n = 10 except for one control noted below) received an intravenous injection of 10 μg or 15 μg ET, followed 18 h later by an intraperitoneal injection of 100 μg LT. Control animals received 15 μg PA plus 100 μg LT, 15 μg EF plus 100 μg LT, or 15 μg ET plus 100 μg PA (n = 9). By the log rank test, the group given 15 μg ET plus 100 μg LT was significantly different from the group given 15 μg PA plus 100 μg LT control (P = 0.0034), while the groups given 10 μg ET plus 100 μg LT or 15 μg EF plus 100 μg LT were not significantly different from this control group (P = 0.9703 or 0.1605, respectively). (B) ET sensitizes male DBA/2J mice to LT lethality. Animals (n = 10) received intravenous injections of 15 or 20 μg ET, followed 18 h later by an intraperitoneal injections of 100 μg LT. Control animals received 20 μg PA plus 100 μg LT, 20 μg EF plus 100 μg LT, or 20 μg ET plus 100 μg PA. By the log rank test, the groups given 15 μg ET plus 100 μg LT (P = 0.0035) and the 20 μg ET plus 100 μg LT (P < 0.0001) were significantly different from the 20 μg PA plus 100 μg LT group.
Male DBA/2J mice showed similar sensitizations to LT with ET pretreatment (Fig. 5B). Male DBA/2J mice received ET 18 h prior to 100 μg LT. Doses of 20 or 15 μg of ET led to the sensitization of DBA/2J to LT-mediated killing (100 or 70% lethality, respectively). PA controls replacing either ET or LT did not result in significant mortality. EF alone prior to LT treatment resulted in 20% lethality in each of two replicate experiments, a greater effect than that observed when PA preceded LT.
DISCUSSION
In this report, we extend our previous work (5, 13) to show that ET is lethal for both sexes of the LT-resistant DBA/2J mouse strain. We also report that nonlethal doses of ET sensitize DBA/2J mice in a manner suggestive of synergism when delivered prior to or concurrent with LT. This sensitization may be similar to that induced by the disruption of HPA equilibrium or could result from the action of ET on other tissues or organs.
In contrast to the almost complete resistance of DBA/2J mice to LT (13, 14), DBA/2J mice are susceptible to ET-mediated lethality and may be slightly more sensitive than BALB/cJ mice are. Although no differences in percent lethality and time to death between male and female DBA/2J mice were evident at high ET doses, we did note a suggestion of a sex bias toward greater female susceptibility at the lower ET doses tested. More extensive studies specifically addressing this issue are required to determine whether ET might be more potent in female than in male mice.
The adrenal glands of ET-treated mice showed the degeneration of the cells that make up the cords in the zona fasciculata, accompanied by the congestion of the sinusoidal capillaries and occasional neutrophil infiltrates. These effects were seen in female and male BALB/cJ and DBA/2J mice. Consistent with our previous report (5), female BALB/cJ mice also showed moderate to severe multifocal necrosis of the cortex accompanied by greater numbers of granulocytic infiltrates. It may be that lesions in male BALB/cJ mice and DBA/2J mice would progress to the more severe phenotype exhibited by female BALB/cJ mice if given more time to develop. We sought to determine whether the ET-mediated adrenal morphological changes would result in the stimulation or repression of corticosteroid production. We found that ET induces corticosterone production to similar levels in both BALB/cJ and DBA/2J mice. In response to LT, however, the adrenal cortex produces corticosteroids in BALB/cJ mice but not DBA mice (14). The hyporesponsiveness of DBA/2J mice could potentially contribute to their greater resistance to LT. Indeed, any manipulation of the HPA axis in DBA/2J mice, including the surgical removal of the adrenal gland, chemical repression of glucocorticoid receptor function by RU-486, or the delivery of synthetic glucocorticoid, increased their susceptibilities to LT-mediated lethality (14).
Given that ET perturbs the adrenal gland, resulting in corticosterone release, and that the manipulation of the HPA axis sensitizes DBA/2J mice to ET-mediated lethality, we hypothesized that ET might sensitize DBA/2J mice to LT. Concurrent exposure to ET and LT required doses as low as 5 μg ET to cause full sensitization to LT, while ET delivered 18 h prior to LT exposure required a higher dose of 15 μg to sensitize the mice. There also appeared to be an inversion of the dose response in the experiments where LT and ET were given simultaneously, with 5 μg ET being slightly more potent than 10 μg or 15 μg ET, suggesting possible competition for PA or receptor depletion by ET. A greater influence on LT-mediated lethality by lower amounts of ET could indicate greater competition for LF in binding to PA when ET is at higher doses. Alternatively, rapid clearance of PA receptors by higher doses of intravenously delivered ET may make fewer cell surface receptors available to LT that may be temporally delayed due to intraperitoneal delivery. The lower dose of ET required for lethality when given in the presence of LT may explain why we saw higher-than-predicted lethality when EF was given alone prior to LT treatment. EF administered in the absence of PA may persist in the circulation, and although reduced somewhat by normal clearance mechanisms, the amounts may still be sufficient to act once PA is supplied with the LT injection. A dose of 10 μg ET, however, is insufficient to sensitize DBA/2J mice to LT and apparently is taken up by cells and cleared from circulation before PA is available from the LT injection. LF alone is able to persist in circulation and can kill mice hours later when PA is supplied (unpublished data), suggesting a possible similar scenario for EF persistence. Alternatively, it is possible that small amounts of EF can enter tissues in the absence of PA. A final possibility is that the low levels of sensitization with EF alone could be due to the small amounts of endotoxin present in EF samples, independent of EF function.
The modulation of the HPA axis may be just one mechanism by which ET and LT interact to contribute to the lethality brought about by the combined anthrax toxins. ET has been shown to upregulate the anthrax toxin receptor in macrophages (10), an effect expected to cause more rapid and extensive uptake of LT in tissues that respond in this way. This may play a role in our 18-h presensitization experiments but seems unlikely to explain the role of ET in sensitizing DBA/2J mice to LT when the toxins are given concurrently. Furthermore, receptor availability does not appear to be a limiting factor since DBA/2J mice are as sensitive, if not more so, to ET-mediated effects than the LT-susceptible BALB/cJ mice are. ET also affects a broad range of tissues, including the cardiac, renal, and lymphoid systems (5), potentially offering additional pathways on which ET and LT can act to cause murine lethality. In the DBA/2J model, ET creates a physiologic state in which LT can bring about lethality. Synergism between LT and ET has been previously suggested (19). The studies presented here demonstrate that B. anthracis ET enhances the activity of LT to overcome the natural resistance of DBA/2J mice. It is crucial, however, that similar studies be performed in other mouse models. The DBA/2J mouse exhibits the highest degree of resistance to LT and harbors macrophages resistant to this toxin. However, because different strains show such a wide range of susceptibilities to this toxin with differing cytokine responses (13), it is possible that ET effects on LT function would be different in other mouse strains. Thus, it is also important that a wider range of dosing schemes be tested in other mouse strains and animal species, especially as the relative amounts of LF and EF simultaneously present at each stage of infection are currently unknown. Studies of the activity of both toxins individually and in combination are crucial to our understanding of their greater role in anthrax pathogenesis.
Acknowledgments
We acknowledge the expertise provided by Jerold Ward and Georgina Miller in evaluating the adrenal histopathology.
W.-J.T. is supported by NIH GM62548 and Great Lakes Regional Center for Excellence pilot grants, and Y.S. is supported by an AHA postdoctoral fellowship. This research was supported by the Intramural Research Program of the NIAID, NIH.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 5 March 2007.
REFERENCES
- 1.Boyden, E. D., and W. F. Dietrich. 2006. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38:240-244. [DOI] [PubMed] [Google Scholar]
- 2.Bradley, K. A., J. Mogridge, M. Mourez, R. J. Collier, and J. A. Young. 2001. Identification of the cellular receptor for anthrax toxin. Nature 414:225-229. [DOI] [PubMed] [Google Scholar]
- 3.Collier, R. J., and J. A. T. Young. 2003. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 19:45-70. [DOI] [PubMed] [Google Scholar]
- 4.Duesbery, N. S., C. P. Webb, S. H. Leppla, V. M. Gordon, K. R. Klimpel, T. D. Copeland, N. G. Ahn, M. K. Oskarsson, K. Fukasawa, K. D. Paull, and G. F. Vande Woude. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280:734-737. [DOI] [PubMed] [Google Scholar]
- 5.Firoved, A. M., G. F. Miller, M. Moayeri, R. Kakkar, Y. Shen, J. F. Wiggins, E. M. McNally, W. J. Tang, and S. H. Leppla. 2005. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am. J. Pathol. 167:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong, J., J. Beeler, N. L. Zhukovskaya, W. He, W. J. Tang, and M. R. Rosner. 2005. Anthrax edema factor potency depends on mode of cell entry. Biochem. Biophys. Res. Commun. 335:850-857. [DOI] [PubMed] [Google Scholar]
- 7.Hoover, D. L., A. M. Friedlander, L. C. Rogers, I. K. Yoon, R. L. Warren, and A. S. Cross. 1994. Anthrax edema toxin differentially regulates lipopolysaccharide-induced monocyte production of tumor necrosis factor alpha and interleukin-6 by increasing intracellular cyclic AMP. Infect. Immun. 62:4432-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leppla, S. H. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 79:3162-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leppla, S. H. 2006. Bacillus anthracis toxins, p. 323-347. In J. E. Alouf and M. R. Popoff (ed.), The comprehensive sourcebook of bacterial protein toxins. Academic Press, Burlington, MA.
- 10.Maldonado-Arocho, F. J., J. A. Fulcher, B. Lee, and K. A. Bradley. 2006. Anthrax oedema toxin induces anthrax toxin receptor expression in monocyte-derived cells. Mol. Microbiol. 61:324-337. [DOI] [PubMed] [Google Scholar]
- 11.McAllister, R. D., Y. Singh, W. D. Du Bois, M. Potter, T. Boehm, N. D. Meeker, P. D. Fillmore, L. M. Anderson, M. E. Poynter, and C. Teuscher. 2003. Susceptibility to anthrax lethal toxin is controlled by three linked quantitative trait loci. Am. J. Pathol. 163:1735-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moayeri, M., D. Haines, H. A. Young, and S. H. Leppla. 2003. Bacillus anthracis lethal toxin induces TNF-α-independent hypoxia-mediated toxicity in mice. J. Clin. Investig. 112:670-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moayeri, M., N. W. Martinez, J. Wiggins, H. A. Young, and S. H. Leppla. 2004. Mouse susceptibility to anthrax lethal toxin is influenced by genetic factors in addition to those controlling macrophage sensitivity. Infect. Immun. 72:4439-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moayeri, M., J. I. Webster, J. F. Wiggins, S. H. Leppla, and E. M. Sternberg. 2005. Endocrine perturbation increases susceptibility of mice to anthrax lethal toxin. Infect. Immun. 73:4238-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mourez, M. 2004. Anthrax toxins. Rev. Physiol. Biochem. Pharmacol. 152:135-164. [DOI] [PubMed] [Google Scholar]
- 16.Scobie, H. M., G. J. Rainey, K. A. Bradley, and J. A. Young. 2003. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl. Acad. Sci. USA 100:5170-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen, Y., N. L. Zhukovskaya, M. I. Zimmer, S. Soelaiman, P. Bergson, C. R. Wang, C. S. Gibbs, and W. J. Tang. 2004. Selective inhibition of anthrax edema factor by adefovir, a drug for chronic hepatitis B virus infection. Proc. Natl. Acad. Sci. USA 101:3242-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soelaiman, S., B. Q. Wei, P. Bergson, Y. S. Lee, Y. Shen, M. Mrksich, B. K. Shoichet, and W. J. Tang. 2003. Structure-based inhibitor discovery against adenylyl cyclase toxins from pathogenic bacteria that cause anthrax and whooping cough. J. Biol. Chem. 278:25990-25997. [DOI] [PubMed] [Google Scholar]
- 19.Stanley, J. L., and H. Smith. 1961. Purification of factor I and recognition of a third factor of anthrax toxin. J. Gen. Microbiol. 26:49-66. [DOI] [PubMed] [Google Scholar]
- 20.Tournier, J. N., A. Quesnel-Hellmann, J. Mathieu, C. Montecucco, W. J. Tang, M. Mock, D. R. Vidal, and P. L. Goossens. 2005. Anthrax edema toxin cooperates with lethal toxin to impair cytokine secretion during infection of dendritic cells. J. Immunol. 174:4934-4941. [DOI] [PubMed] [Google Scholar]
- 21.Varughese, M., A. Chi, A. V. Teixeira, P. J. Nicholls, J. M. Keith, and S. H. Leppla. 1998. Internalization of a Bacillus anthracis protective antigen-c-Myc fusion protein mediated by cell surface anti-c-Myc antibodies. Mol. Med. 4:87-95. [PMC free article] [PubMed] [Google Scholar]
- 22.Vitale, G., L. Bernardi, G. Napolitani, M. Mock, and C. Montecucco. 2000. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem. J. 352:739-745. [PMC free article] [PubMed] [Google Scholar]