Abstract
Pseudomonas aeruginosa keratitis is an acute sight-threatening infection. We previously reported that human tear fluid could protect individual human corneal epithelial cells in vitro against invasion by and cytotoxicity due to clinical and laboratory isolates of P. aeruginosa and that the protective mechanism was independent of bacteriostatic activity. In the present study, we examined the effects of human tear fluid in vivo. Tears were collected from healthy human volunteers and were studied in vivo in mice. The effects on the virulence of both invasive and cytotoxic clinical isolates of P. aeruginosa were examined. Tear fluid was found to reduce the severity of disease when corneas were challenged with cytotoxic bacteria immediately after scratch injury, and it completely protected against susceptibility to infection by a cytotoxic strain in a model in which corneas were infected during the healing process 6 h after scratching. Visible protection correlated with the inhibition of bacterial colonization 1, 4, and 48 h postinoculation. Tear fluid also significantly reduced the severity of infections caused by invasive P. aeruginosa in the 6-h-healing model. This result also coincided with significantly reduced bacterial colonization at 48 h. In vitro, human tear fluid significantly reduced the ability of invasive and cytotoxic bacteria to translocate across corneal epithelia and increased transepithelial resistance with or without bacterial inoculation. These data show that human tear fluid can protect against P. aeruginosa corneal infection in vivo and that the mechanism likely involves enhanced epithelial barrier function in addition to protection of individual epithelial cells against bacterial internalization and cytotoxicity.
Pseudomonas aeruginosa can cause microbial keratitis associated with soft contact lens wear or corneal injury (4, 14). Clinical and laboratory isolates of this opportunistic bacterial pathogen are capable of using multiple virulence strategies to cause disease, including adherence to and invasion of epithelial cells, intoxication of epithelial cells and other cell types using type III secretion mechanisms, and extracellular secretion of potentially damaging toxins, enzymes, and proteases (1, 8, 9, 12, 15, 23, 24). Despite the presence of these traits and a plethora of genes devoted to virulence regulation, resistance to antimicrobial agents, and environmental adaptation, P. aeruginosa cannot infect the healthy cornea in vivo (22). Our long-term research goals are to understand the mechanisms involved in corneal resistance to infection and the circumstances that compromise resistance, with a view toward developing preventive and therapeutic measures for infections of the cornea and other sites.
We have shown previously that corneal epithelial cells in vitro are vulnerable to invasion by P. aeruginosa and to the type III secretion-dependent cytotoxicity of P. aeruginosa, irrespective of whether they are in cell culture or on the surface of whole eyeballs (5, 7, 10). Thus, we have been working toward understanding differences between in vivo and in vitro environments that determine resistance in the former environment and susceptibility in the latter environment. Through these efforts we found that human tear fluid could protect cells in vitro against both invasion by P. aeruginosa and cytotoxicity of P. aeruginosa in vitro and that the mechanism was independent of tear bacteriostatic activity (6). While these findings suggested that tear fluid is a critical in vivo defense against ocular surface infection, in vivo studies to show this directly have not been done previously.
To study the role of tear fluid in vivo, we examined whether human tear fluid could protect against P. aeruginosa corneal infection in mice. Two in vivo models were used; in the murine scarification model the epithelial surface is scratched to allow bacteria direct access into the underlying stromal tissue (21), and in the 6-h-healing model scratch-injured corneas are allowed to re-epithelialize for 6 h before inoculation with bacteria (13). To better understand the results obtained with the 6-h-healing model, in which bacteria must penetrate through the epithelium to cause disease, we used an in vitro model system to determine tear fluid effects on the epithelial barrier function and on the efficiency with which bacteria translocate across the corneal epithelium.
MATERIALS AND METHODS
Tear collection.
Reflex tear fluid was collected from the lower conjunctival sac of healthy human volunteers using microcapillary tubes (19). One hundred microliters of tears was collected over approximately 15 min on each occasion and was used for experiments on the same day. The tear collection protocol was approved by the Committee for the Protection of Human Subjects, University of California, Berkeley.
Bacterial strains and preparation of inocula.
Experiments were performed with two corneal isolates of P. aeruginosa; strain 6206 (serogroup O11) expresses the toxin ExoU and is thus acutely cytotoxic for mammalian cells (5, 10), and strain 6294 (serogroup O6) efficiently invades mammalian cells (5, 10). The other strains used included strain 19660 (serogroup O2) and strain PAK (serogroup O1), which are cytotoxic and invasive, respectively (10). Bacterial inocula were stored in 20% glycerol stocks at −80°C. Bacteria were inoculated onto Trypticase soy agar plates and incubated overnight at 37°C. Cultures were then suspended in Eagle's minimal essential medium with Hanks’ salts and l-glutamine (Sigma, St Louis, MO) buffered with 1 M HEPES-NaOH (pH 7.6), 0.35 g of NaHCO3 per liter, and 6 g of bovine serum albumin (Sigma) per liter (MEM). Bacteria were initially prepared at a concentration of ∼1 × 108 CFU/ml MEM as determined by spectrophotometry (optical density at 650 nm, 0.1). Bacterial suspensions were then diluted with MEM or human tear fluid to obtain the concentrations required. Bacterial numbers were confirmed by viable counting. For translocation assays, Hanks’ balanced salt solution (HBSS) was used instead of MEM.
Murine corneal infection models and bacterial colonization.
The scarification (12, 21) and 6-h-healing (13) murine models of infection described previously were used. Briefly, C57BL/6 female mice (5 to 7 weeks old) were anesthetized by intraperitoneal inoculation of an anesthetic cocktail (21 mg/ml ketamine, 2.4 mg/ml xylazine, 0.3 mg/ml acepromazine). The eyes were checked for corneal clarity using a stereomicroscope prior to initiation of the experiments. Three parallel scratches (length, ∼1 mm) were made on the left cornea of each animal using a sterile 25 5/8-gauge needle. One group of mice was inoculated immediately after the corneas were scratched, while another group was inoculated after the corneas were allowed to heal for 6 h. Scratched eyes or eyes that had healed for 6 h were topically inoculated with bacterial suspensions containing 103, 104, 105, or 106 CFU either in 5 μl of MEM (control) or in 5 μl of whole human tear fluid diluted 1:2 with MEM. Five mice were assigned to each treatment group. The animals were observed daily, and the severity of disease was graded in a masked fashion by the same observer after 24 and 48 h or in some instances after 4 and 7 days, using the following grading system (3): 0, eye macroscopically identical to the uninfected contralateral control eye; 1, faint opacity partially covering the pupil; 2, dense opacity covering the pupil; 3, dense opacity covering the entire anterior segment; and 4, perforation of cornea and/or phthisis bulbi (shrinkage of the eyeball following inflammatory disease). In some instances, the disease was assessed as being halfway between defined grades and therefore was assigned a “half-grade” (e.g., 2.5). To assess bacterial colonization, mice were euthanized, and infected eyes then immediately enucleated and homogenized in phosphate-buffered saline (PBS) (1 ml). Viable counts were the determined for the homogenates. In other experiments, mice were sacrificed 1 or 4 h after infection to determine the effect of tear fluid on early bacterial colonization. In this case, the corneas were not graded. All procedures were conducted in accordance with the policies established by the Association for the Research in Vision and Ophthalmology and were approved by the University of California, Berkeley Animal Care and Use Committee.
Bacterial translocation assay.
Rabbit corneal epithelial cells were cultured on Transwell filter membranes (pore size, 3 μm; diameter, 12 mm; Corning Life Sciences, Acton, MA) as previously described (5). Confluence of epithelial monolayers was confirmed by measuring the transepithelial resistance (TER) using an epithelial voltohmmeter (EVOM; World Precision Instruments, Sarasota, FL). Prior to infection, cells were washed once with HBSS (1 ml in the upper and lower chambers). One well was treated with EGTA (5 mM) for 15 min prior to washing as a negative control for TER. Cells were infected on the apical surface (upper chamber) with 0.5 ml of a bacterial suspension containing 106 CFU/ml in HBSS or whole tear fluid. Sterile HBSS (2 ml) was added to the lower chamber, and the preparation was incubated at 37°C for 6 h. Each hour, the TER was measured, and viable counts were determined for the upper and lower chambers.
Bacterial association assay.
Rabbit corneal epithelial cells were cultured on filters as described above for the translocation assay. After washing with PBS (0.5 ml), the apical surface of cells was inoculated with 200 μl of a bacterial suspension containing ∼106 CFU bacteria/ml of MEM or whole tear fluid. MEM (1 ml) was added to the lower (basal) chamber and incubated at 37°C for 30 min to 3 h depending on the strain. The filters were then washed three times with PBS before careful excision with sterile scissors. The filters were then homogenized in PBS (1 ml) containing Triton X-100 (0.25%, vol/vol), and the numbers of viable (associated) bacteria were determined by viable counting.
Statistical analysis.
Data were expressed as means and standard deviations, and the statistical significance of differences between groups was determined by using Student's t test and analysis of variance (ANOVA). Differences in disease severity scores or other nonparametric data were compared using the Mann-Whitney test. P values of <0.05 were considered significant.
RESULTS
Tear fluid protects against corneal disease and tissue colonization by both cytotoxic and invasive P. aeruginosa.
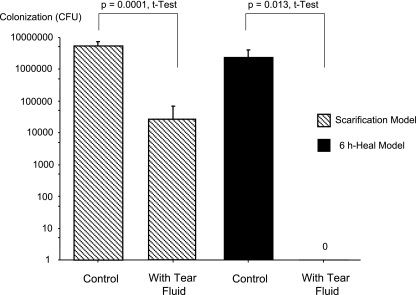
Cytotoxic P. aeruginosa strain 6206 reliably infected murine corneas, as determined by visible pathology 24 h postchallenge, in both the scarification and 6-h-healing models with an inoculum of only 103 CFU in 5 μl (Table 1). Under these circumstances, tear fluid reduced the visible pathology in both the scarification model and the 6-h-healing model (Table 1 and Fig. 1). In the scarification model, the reduced disease severity in the tear fluid-treated eyes coincided with significant reductions in the bacterial colonization rates 48 h postinoculation (Fig. 2). In the 6-h-healing model, tears were found to completely protect corneas against pathology, and this protection was associated with a total absence of colonizing bacteria (Fig. 2). When the inoculum was increased 10-fold (to 104 CFU) tear fluid was found to protect corneas only in the 6-h-healing model (Fig. 1).
TABLE 1.
Ocular disease severity scores for the scarification and 6-h-healing murine models of P. aeruginosa keratitis after inoculation with ∼1 × 103 CFU strain 6206 in 5 μl of MEM (control) or human tear fluid (diluted 1:2 in MEM)a
| Time (h) | Disease severity scores
|
|||
|---|---|---|---|---|
| Scarification model
|
6-h-healing model
|
|||
| Control | With tear fluid | Control | With tear fluid | |
| 24 | 3, 3, 3, 3, 3 | 1, 2, 0, 0, 0 | 2.5, 2, 2.5, 3, 3 | 0, 0, 0, 0, 0 |
| 48 | 3, 3, 3, 3, 3 | 2.5, 2.5, 0, 0, 0 | 2.5, 2.5, 2.5, 3, 3 | 0, 0, 0, 0, 0 |
The disease scores (as defined in Materials and Methods) for eyes treated with tear fluid were significantly lower than the disease scores for controls at each time in each model (P = 0.005, as determined by the Mann-Whitney test).
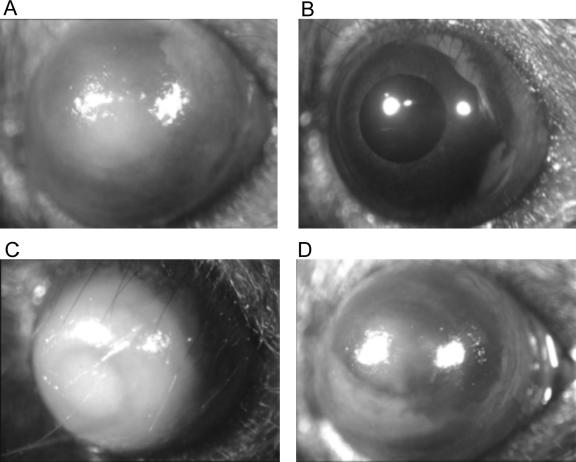
FIG. 1.
P. aeruginosa keratitis at 48 h postinoculation with cytotoxic strain 6206 using the 6-h-healing murine model of infection. (A and C) Controls. Bacteria were added in MEM. (B and D) Effect of human tear fluid. Bacteria were added in tears (diluted 1:2 in MEM). (A and B) Inoculum, ∼1 × 103 CFU in 5 μl. (C and D) Inoculum, ∼1 × 104 CFU in 5 μl.
FIG. 2.
Effect of human tear fluid on 48-h corneal colonization rates (means and standard deviations). The scarification infection model and the 6-h-healing infection model were both used, and cytotoxic P. aeruginosa strain 6206 was added to the cornea at a concentration of ∼1 × 103 CFU in 5 μl of MEM (control) or human tear fluid (diluted 1:2 in MEM).
In the healing model, tear fluid added with the bacterial inoculum (either 103 or 104 CFU) protected corneas throughout the 4 days of observation. A typical experiment (inoculum, 103 CFU bacteria) yielded 4-day (96-h) disease scores of 0 in each of three mice that were treated with tear fluid, compared to three untreated corneas of control animals whose scores were 3, 2, and 2 (P = 0.034, as determined by the Mann-Whitney test). Again, tear protection coincided with significant reductions in bacterial colonization (mean ± standard deviation for controls, 3.075 × 106 ± 2.214 × 106 CFU; mean ± standard deviation for tear fluid-treated eyes, 5.00 × 102 ± 6.93 × 102 CFU [P = 0.037, as determined by a t test]).
Tear fluid was also found to protect corneas against invasive P. aeruginosa. Table 2 shows the results of an experiment in which we used the healing model with invasive strain 6294 and inocula containing 103 and 104 CFU in 5 μl MEM. Similar to the protection seen with the cytotoxic clinical isolate, protection against the invasive clinical isolate was accompanied by significant reductions in bacterial colonization. For example, with an inoculum of 104 CFU, the median level of colonization of control eyes at 48 h was 42,500 CFU (lower quartile, 590 CFU; upper quartile, 440,000 CFU), compared to tear fluid-treated eyes, for which four of five mice showed no colonization (one mouse cornea yielded 10 CFU) (P = 0.007, as determined by the Mann-Whitney test).
TABLE 2.
Ocular disease severity scores for the 6-h-healing murine model of P. aeruginosa keratitis after inoculation with ∼1 × 103 or 1 × 104 CFU of strain 6294 in 5 μl of MEM (control) or human tear fluid (diluted 1:2 in MEM)a
| Time (h) | Disease severity scores
|
|||
|---|---|---|---|---|
| 103 inoculum
|
104 inoculum
|
|||
| Control | With tear fluid | Control | With tear fluid | |
| 24 | 2, 2, 2, 0, 2 | 0, 1, 0, 0, 1 | 1, 3, 3, 3, 2 | 1, 0, 2, 1, 0 |
| 48 | 3, 2, 1, 0, 1 | 0, 1, 0, 0, 0 | 2, 2, 3, 2, 1 | 1, 0, 1, 1, 0 |
The disease scores for eyes treated with tear fluid were significantly lower than the disease scores for the corresponding controls after 24 h with both inocula (P = 0.044 for 103 CFU and P = 0.031 for 104 CFU, as determined by the Mann-Whitney test) and after 48 h with the 104-CFU inoculum (P = 0.016, as determined by the Mann-Whitney test).
Tear fluid reduced early colonization of the cornea in vivo.
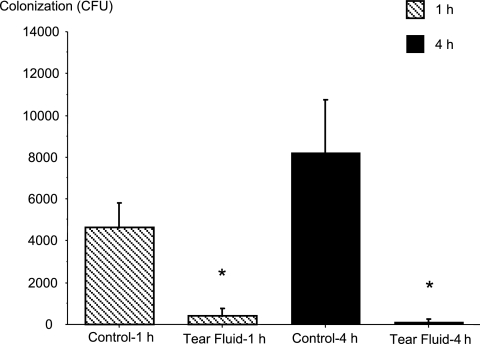
Reduced colonization at 48 h could result from a reduced ability to colonize at earlier times, or it could occur if bacteria were less able to penetrate into tissue or to survive within the tissue after penetration. To begin to explore the mechanism of the protective effects of tears, levels of early colonization with and without tear fluid were examined by viable counting 1 and 4 h postinoculation. For these experiments the cytotoxic strain P. aeruginosa 6206 and the 6-h-healing model were used. The results showed that tear fluid significantly reduced bacterial colonization of the cornea at both of these early times (Fig. 3). At 1 h, tear fluid had already reduced colonization by ∼10-fold compared to the colonization of control untreated corneas. By 4 h, there were even fewer viable bacteria colonizing treated eyes. In contrast, the colonization had increased substantially (∼2-fold) in control untreated eyes by 4 h (P < 0.05, as determined by ANOVA).
FIG. 3.
Effect of human tear fluid on 1- and 4-h corneal colonization rates (means and standard deviations). The 6-h-healing infection model was used, and cytotoxic P. aeruginosa strain 6206 was added at a concentration of ∼7 × 104 CFU in 5 μl in either MEM (control) or human tear fluid (diluted 1:2 in MEM). There was a significant difference between the groups (P = 0.0001, as determined by ANOVA). The asterisks indicate that Fisher PLSD and Scheffe F-test posthoc analysis showed that there was a significant difference between tear fluid-treated eyes and controls at each time (P < 0.05).
Tear fluid reduced P. aeruginosa adherence to corneal epithelial cells in vitro.
A possible mechanism for the reduced colonization observed as early as 1 h postinfection in the 6-h-healing model was decreased bacterial adhesion to corneal epithelial cells. Since the in vivo environment contains factors that complicate measurement of adhesion, including in vivo antimicrobial compounds and clearance mechanisms such as blinking and tear flow, etc., adhesion to corneal epithelial cells was studied in the absence of these complicating factors in an in vitro assay system.
Tear fluid was found to reduce adherence of P. aeruginosa to cultured corneal epithelial cells in vitro. Since cytotoxic strains kill cells and since P. aeruginosa is known to adhere avidly to cells that are already dead or dying, we expected that tear fluid would inhibit adhesion at later times because it protects against cytotoxic activity. Accordingly, a shorter 30-min assay was used to study tear effects on the adhesion of cytotoxic strain 6206. Even at this very early time, the adhesion of cytotoxic strain 6206 was reduced by tear fluid from 2,400 ± 819 CFU (control) to 983 ± 115 CFU (tear fluid) (P = 0.046, as determined by a t test).
At 1 h, tear fluid had no significant effect on the adhesion of invasive strain 6294 (association control value, 43,700 ± 9,460 CFU; value with tear fluid, 36,000 ± 19,500 CFU; P = 0.558, as determined by a t test). However, by 3 h the level of adhesion was reduced from 632,000 ± 112,000 CFU to 57,300 ± 21,800 CFU (P = 0.0009, as determined by a t test).
The mechanism for reduced adherence of the strains to corneal epithelial cells is not directly related to the bacteriostatic activity of tears; invasive strain 6294 is not susceptible (6), and although the susceptibility of cytotoxic strain 6206 to tear fluid bacteriostatic activity is evident at 3 h (6), it is not observed after 2 h of exposure. For example, in the present study, the viable counts for strain 6206 from the upper chamber (above the cells) after 2 h in a translocation assay were not significantly different (mean ± standard deviation) for control samples (1.19 × 106 ± 2.55 × 105 CFU/ml) and tear fluid-treated samples (8.38 × 105 ± 5.30 × 104 CFU/ml) (P = 0.198, as determined by a t test). After 3 h, however, there was a significant difference (bacteriostatic activity) between control samples (3.45 × 106 ± 1.41 × 105 CFU/ml) and tear fluid-treated samples (7.7 × 105 ± 1.56 × 105 CFU/ml) (P = 0.003, as determined by a t test).
Tear fluid reduces P. aeruginosa translocation across corneal epithelia in vitro.
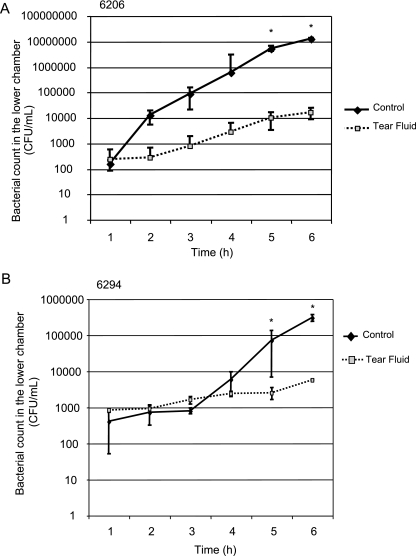
Our previous research showed that the 6-h-healing model allows re-epithelialization after scratching. One possible explanation for why better protection was observed in the healing infection model than in the scratch infection model is that tears inhibited the ability of bacteria to penetrate the corneal epithelium. To examine this possibility, an assay system was used to study tear effects on bacterial translocation across the corneal epithelium in vitro. P. aeruginosa strain 6206 was found to readily translocate across corneal epithelial cells in this assay over a 6-h period when it was added without tear fluid in MEM. With tear fluid, the bacterial translocation rates were significantly reduced. By 2 h ∼45-fold fewer bacteria had translocated across epithelial cells when they were added with tear fluid, and the protective effect increased to ∼650-fold by 5 and 6 h (Fig. 4A).
FIG. 4.
Translocation of P. aeruginosa strains 6206 (A) and 6294 (B) across cultured rabbit corneal epithelial cells grown in vitro on Transwell filter membranes. The viable bacterial counts (means ± standard deviations) in the lower (basal) compartment were determined each hour after bacteria were added to the apical surface in HBSS (control) or with undiluted human tear fluid. The asterisks indicate that for strain 6206 significantly less translocation was observed with tear-treated samples than with controls at 5 h (P = 0.027, as determined by a t test) and at 6 h (P = 0.0006, as determined by a t test) and that similar results were obtained for strain 6294 at 5 h (P = 0.049, as determined by a t test) and at 6 h (P = 0.0009, as determined by a t test).
Since 6206 is a cytotoxic strain, tear fluid protection against translocation of this strain could involve protection against cytotoxic activity. However, tear fluid also reduced translocation of invasive strain 6294, which is not cytotoxic (Fig. 4B). Inhibition of translocation of this invasive strain also verified that the mechanism is independent of bacteriostatic activity since we showed that tear fluid does not inhibit the growth of this strain (6). This was reconfirmed with the translocation assay system, since the viable counts for the upper chamber (inoculum) at each hour during the assay indicated that the bacteria grew in both tear fluid and control medium. Human tear fluid inhibition of epithelial translocation was confirmed using another invasive strain, strain PAK (data not shown).
Tear fluid increases TER with and without bacterial exposure.
Our previous studies showed than tear fluid inhibited epithelial cell invasion by bacteria, and this could contribute to the mechanism by which tears prevent invasive strain translocation across corneal epithelial cells. To determine whether effects on the epithelial barrier function also contributed, we examined the effects of tear fluid on the TER of corneal epithelial cells in the translocation assay system.
Tear fluid increased the TER of both infected and uninfected cells (Fig. 5A). This increase in barrier function upon addition of tears was observed at early times and throughout the 6-h assay. When invasive strain 6294 was used to infect cells, the TER was even greater than the TER observed when tear fluid alone was added, showing that there was synergy between the effects of tears and the effects of bacteria on TER. Interestingly, the same bacteria added alone (i.e., without tears) did not increase the TER above baseline level for uninfected cells. In the presence of 6294, the tear fluid resulted in a significant increase in the TER compared to the TER for cells infected with bacteria in HBSS (Fig. 5A).
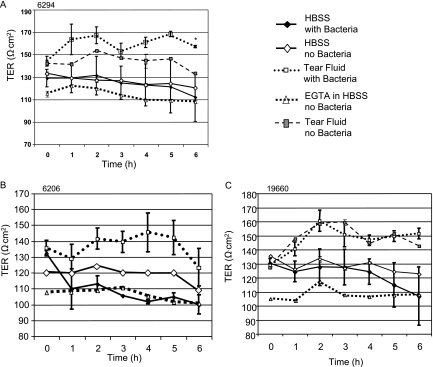
FIG. 5.
TER (means ± standard deviations) across cultured rabbit corneal epithelial cells in vitro in the presence of the invasive P. aeruginosa strain 6294 (A) or the cytotoxic P. aeruginosa strains 6206 (B) and 19660 (C) with and without undiluted human tear fluid. The asterisk indicates that in cells infected with 6294, tear fluid significantly increased the TER compared to the TER observed with cells infected with bacteria in HBSS alone (P = 0.0006 at inoculation and P = 0.0001 at each time from 1 to 6 h, as determined by a t test).
As expected, the highly cytotoxic strain 6206, which kills corneal epithelial cells, brought the TER down to the level of EGTA-treated cells (epithelial tight junctions disrupted) within the first hour of infection (Fig. 5B). Tear fluid protected cells completely against this bacterially induced loss of barrier function; indeed, the TER of cells infected in the presence of tear fluid was even higher than the TER of uninfected control cells. Similar results were obtained in separate experiments using a different cytotoxic strain of P. aeruginosa (19660), although this strain reduced TER less rapidly than the more cytotoxic strain 6206 reduced TER (Fig. 5C). In this case, the decrease in TER began at 3 h and was not complete until 6 h. Interestingly, the bacterial translocation rates reflected this time difference, with translocation beginning at 5 h, and a significant difference between control bacteria and tear fluid-treated bacteria was observed only at 6 h (the lower chamber viable count for the control at 6 h was 20,500 ± 5,090 CFU/ml, compared with the tear fluid value of 883 ± 247 [P = 0.0026, as determined by a t test]). Strain 19660 is not susceptible to tear bacteriostatic activity (6). Unlike invasive bacteria, cytotoxic bacteria did not act synergistically with tear fluid to increase the TER beyond the effects observed when tear fluid was added alone.
DISCUSSION
The data obtained in this study show that human tear fluid can protect mouse corneas against disease caused by both cytotoxic and invasive isolates of P. aeruginosa, and the effect coincided with reduced tissue colonization as soon as 1 h postinoculation. The data also show that human tear fluid can reduce bacterial translocation across corneal epithelial cells, while it also mediates an increase in TER.
The greater protection against P. aeruginosa keratitis by tear fluid in the 6-h-healing model than in the scarification model may be related to the presence of a regenerating corneal epithelium in the 6-h-healing model and its potential to prevent bacterial access to the underlying stroma (13). This hypothesis is supported by in vitro data obtained in the present study showing that tear fluid could inhibit both invasive and cytotoxic P. aeruginosa strains from crossing a corneal epithelial layer.
Several mechanisms could contribute to tear fluid inhibition of bacterial epithelial translocation in vitro. First, the ability of tear fluid to reduce P. aeruginosa invasion and cytotoxicity in vitro (6) reduces translocation via intracellular pathways and due to epithelial breakdown, respectively (2, 11). Second, inhibition of translocation by tear fluid was accompanied by an increase in TER, suggesting that increased barrier function is part of the mechanism for protection. Since TER reflects, in part, the integrity of the epithelial tight junctions, tear fluid-mediated increases in TER could reduce bacterial access to epithelial basolateral surfaces, thereby reducing translocation via a paracellular route. Reduced access to basolateral surfaces may also contribute to tear-mediated reductions in P. aeruginosa invasion and cytotoxicity that we have reported previously (5). Third, tear fluid-mediated loss of swimming motility and/or bacterial chain formation, which we also reported previously (6), might affect the epithelial translocation of P. aeruginosa irrespective of whether intracellular or paracellular pathways are taken by the bacteria. Fourth, tear fluid-mediated reductions in bacterial association (adherence) with cells may also contribute to reductions in translocation.
The data obtained in this study and in our previous studies show that the efficiency with which tear fluid protects against disease in vivo and against adherence, invasion, cytotoxicity, and translocation in vitro does not correlate with the level of bacteriostatic activity against specific strains when in vitro tests are performed. However, antimicrobial activity could still contribute to tear fluid protection against P. aeruginosa keratitis in vivo; i.e., both tear fluid-derived antimicrobial factors and endogenous antimicrobial factors triggered or activated by exposure to exogenous tear fluid could contribute to protection against bacterium-induced pathology by tears. The factors known to be in tears and/or known to be made by the ocular surface that have been shown to modify the behavior of P. aeruginosa include immunoglobulin A, defensins, mucins, and surfactant protein D (16-18, 20).
Why would tears prevent infection in mice that can make their own tear fluid? In previous in vitro studies we showed that tear fluid protection against bacterially induced invasion or cytotoxicity is highly susceptible to inactivation upon dilution of the tear fluid (6). Indeed, when there is threefold dilution, the protective activity of human tear fluid is completely abolished. In mouse corneal infection models, a large drop of medium relative to the volume of endogenous tears is added to the ocular surface during inoculation. It is quite possible that the mouse corneal infection models used by us and other workers to study P. aeruginosa require dilution of mouse tear fluid and not just corneal scarification to create susceptibility. When we use human tear fluid for suspending the inoculum, we might restore critical defenses that are otherwise diluted away during inoculation.
Larger inocula were found to overwhelm the protective activity of tear fluid. Our previous in vitro studies showed that when tears were exposed to bacteria for extended periods, protective activity was lost (6). Crossover studies of spent bacteria and spent tear fluid showed that changes in the tear fluid and not in the bacteria had occurred. P. aeruginosa produces a large array of virulence factors, including proteases and phospholipases, with the potential to break down molecular factors found in tear fluid. Whether endogenous tear fluid is overwhelmed by a large inoculum in the same way that tears mixed with bacteria prior to addition to the eye are overwhelmed has not been determined.
Our interest in exploring tear fluid as a defense was originally ignited when we found that cytotoxic P. aeruginosa could damage intact corneas on eyeballs that had been removed from the in vivo environment (7). Data obtained in this study support our hypothesis that tear fluid plays a critical role in defense against infection and that tear interactions with the epithelium are involved. Dilution of tear fluid or long-term exposure of tear fluid to bacteria reduces its ability to protect cells in vitro against bacteria, and in vivo we observed less protection with larger inocula of bacteria. These data suggest mechanisms by which contact lens wear, the most common predisposing factor for P. aeruginosa infection of the cornea, might compromise tear fluid defense mechanisms. These mechanisms include microenvironmentally induced changes in tear biochemistry under the lens as a result of compromising tear flow or exchange, alterations in the production or postproduction modification of ocular surface-derived tear components in that microenvironment, growth of bacterial biofilms on or under a lens, and the dilution of tears by addition of contact lens care packaging and care solutions to the eye.
It is interesting that tear fluid is more effective in protecting the cornea against disease if the epithelium is given time to cover the exposed stroma after scratching (6-h-healing model) (13). Together with the results of in vitro experiments described here and elsewhere (6), this suggests that the mechanism for in vivo protection by tear fluid involves the epithelium. This might be related to why corneal injury can predispose eyes to infection in the absence of factors with the potential to alter ocular surface biochemistry (e.g., contact lens wear or dry eye).
In addition to clarifying our understanding of factors that predispose eyes to infection, research in this area might provide new strategies for preventing infection. These strategies could include modifications to lens materials, designs, or modes of wear aimed at reducing disruption of critical tear factors that have been identified, or they could involve restoring the factors found to be compromised.
The corneal surface is arguably the human mucosal surface that is most exposed to a daily barrage of potential pathogens and other types of insult. It is also the least able to tolerate infection, since even a small scar at the center of the cornea can cause loss of vision. Yet the cornea lacks various features used by other tissues to defend against infection because it must be transparent; e.g., it lacks blood vessels and is immune privileged. The mechanism(s) by which its exquisite resistance is maintained in the face of these limitations is not well understood. The results of this study and previous data showing that ∼50% of P. aeruginosa isolates can grow effectively in human tear fluid (6) suggest that there is much more to this story than tear bacteriostatic activity, but that it does involve tear fluid. Research in this area has the potential to lead to new biocompatible approaches for preventing infection of the eye and of other sites.
Acknowledgments
This work was supported by NIH research grant R01-EY11221 and by a Vistakon research grant from the American Optometric Foundation.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 26 February 2007.
REFERENCES
- 1.Aristoteli, L. P., and M. D. Willcox. 2003. Mucin degradation mechanisms by distinct Pseudomonas aeruginosa isolates in vitro. Infect. Immun. 71:5565-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azghani, A. O. 1996. Pseudomonas aeruginosa and epithelial permeability: role of virulence factors elastase and exotoxin A. Am. J. Respir. Cell Mol. Biol. 15:132-140. [DOI] [PubMed] [Google Scholar]
- 3.Beisel, K. W., L. D. Hazlett, and R. S. Berk. 1983. Dominant susceptibility effect on the murine corneal response to Pseudomonas aeruginosa. Proc. Soc. Exp. Biol. Med. 172:488-491. [DOI] [PubMed] [Google Scholar]
- 4.Cheng, K. H., S. L. Leung, H. W. Hoekman, W. H. Beekhuis, P. G. Mulder, A. J. Geerards, and A. Kijlstra. 1999. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet 354:181-185. [DOI] [PubMed] [Google Scholar]
- 5.Fleiszig, S. M., D. J. Evans, N. Do, V. Vallas, S. Shin, and K. E. Mostov. 1997. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect. Immun. 65:2861-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleiszig, S. M., M. S. Kwong, and D. J. Evans. 2003. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect. Immun. 71:3866-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleiszig, S. M., E. J. Lee, C. Wu, R. C. Andika, V. Vallas, M. Portoles, and D. W. Frank. 1998. Cytotoxic strains of Pseudomonas aeruginosa can damage the intact corneal surface in vitro. CLAO J. 24:41-47. [PubMed] [Google Scholar]
- 8.Fleiszig, S. M., J. P. Wiener-Kronish, H. Miyazaki, V. Vallas, K. E. Mostov, D. Kanada, T. Sawa, T. S. Yen, and D. W. Frank. 1997. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect. Immun. 65:579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleiszig, S. M., T. S. Zaidi, E. L. Fletcher, M. J. Preston, and G. B. Pier. 1994. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect. Immun. 62:3485-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleiszig, S. M., T. S. Zaidi, M. J. Preston, M. Grout, D. J. Evans, and G. B. Pier. 1996. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect. Immun. 64:2288-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirakata, Y., R. Srikumar, K. Poole, N. Gotoh, T. Suematsu, S. Kohno, S. Kamihira, R. E. Hancock, and D. P. Speert. 2002. Multidrug efflux systems play an important role in the invasiveness of Pseudomonas aeruginosa. J. Exp. Med. 196:109-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, E. J., B. A. Cowell, D. J. Evans, and S. M. Fleiszig. 2003. Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Investig. Ophthalmol. Vis. Sci. 44:3892-3898. [DOI] [PubMed] [Google Scholar]
- 13.Lee, E. J., D. J. Evans, and S. M. Fleiszig. 2003. Role of Pseudomonas aeruginosa ExsA in penetration through corneal epithelium in a novel in vivo model. Investig. Ophthalmol. Vis. Sci. 44:5220-5227. [DOI] [PubMed] [Google Scholar]
- 14.Mah-Sadorra, J. H., S. G. Yavuz, D. M. Najjar, P. R. Laibson, C. J. Rapuano, and E. J. Cohen. 2005. Trends in contact lens-related corneal ulcers. Cornea 24:51-58. [DOI] [PubMed] [Google Scholar]
- 15.Marquart, M. E., A. R. Caballero, M. Chomnawang, B. A. Thibodeaux, S. S. Twining, and R. J. O'Callaghan. 2005. Identification of a novel secreted protease from Pseudomonas aeruginosa that causes corneal erosions. Investig. Ophthalmol. Vis. Sci. 46:3761-3768. [DOI] [PubMed] [Google Scholar]
- 16.Masinick, S. A., C. P. Montgomery, P. C. Montgomery, and L. D. Hazlett. 1997. Secretory IgA inhibits Pseudomonas aeruginosa binding to cornea and protects against keratitis. Investig. Ophthalmol. Vis. Sci. 38:910-918. [PubMed] [Google Scholar]
- 17.McNamara, N. A., R. Van, O. S. Tuchin, and S. M. Fleiszig. 1999. Ocular surface epithelia express mRNA for human beta defensin-2. Exp. Eye Res. 69:483-490. [DOI] [PubMed] [Google Scholar]
- 18.McNamara, N. A., R. Andika, M. Kwong, R. A. Sack, and S. M. Fleiszig. 2005. Interaction of Pseudomonas aeruginosa with human tear fluid components. Curr. Eye Res. 30:517-525. [DOI] [PubMed] [Google Scholar]
- 19.Nagyova, B., and J. M. Tiffany. 1999. Components responsible for the surface tension of human tears. Curr. Eye Res. 19:4-11. [DOI] [PubMed] [Google Scholar]
- 20.Ni, M., D. J. Evans, S. Hawgood, E. M. Anders, R. A. Sack, and S. M. Fleiszig. 2005. Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa. Infect. Immun. 73:2147-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preston, M. J., S. M. Fleiszig, T. S. Zaidi, J. B. Goldberg, V. D. Shortridge, M. L. Vasil, and G. B. Pier. 1995. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect. Immun. 63:3497-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramphal, R., M. T. McNiece, and F. M. Polack. 1981. Adherence of Pseudomonas aeruginosa to the injured cornea: a step in the pathogenesis of corneal infections. Ann. Ophthalmol. 13:421-425. [PubMed] [Google Scholar]
- 23.Zaidi, T., M. Mowrey-McKee, and G. B. Pier. 2004. Hypoxia increases corneal cell expression of CFTR leading to increased Pseudomonas aeruginosa binding, internalization, and initiation of inflammation. Investig. Ophthalmol. Vis. Sci. 45:4066-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaidi, T. S., S. M. Fleiszig, M. J. Preston, J. B. Goldberg, and G. B. Pier. 1996. Lipopolysaccharide outer core is a ligand for corneal cell binding and ingestion of Pseudomonas aeruginosa. Investig. Ophthalmol. Vis. Sci. 37:976-986. [PubMed] [Google Scholar]