Abstract
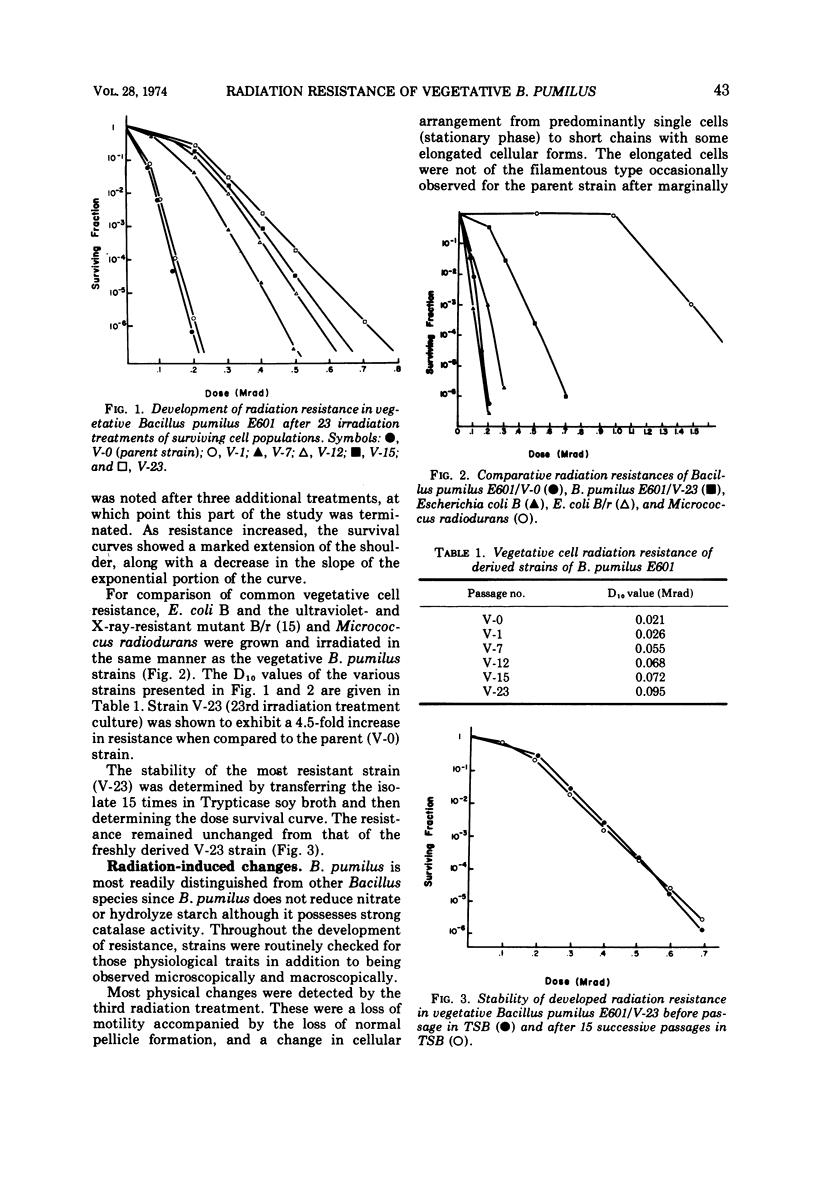
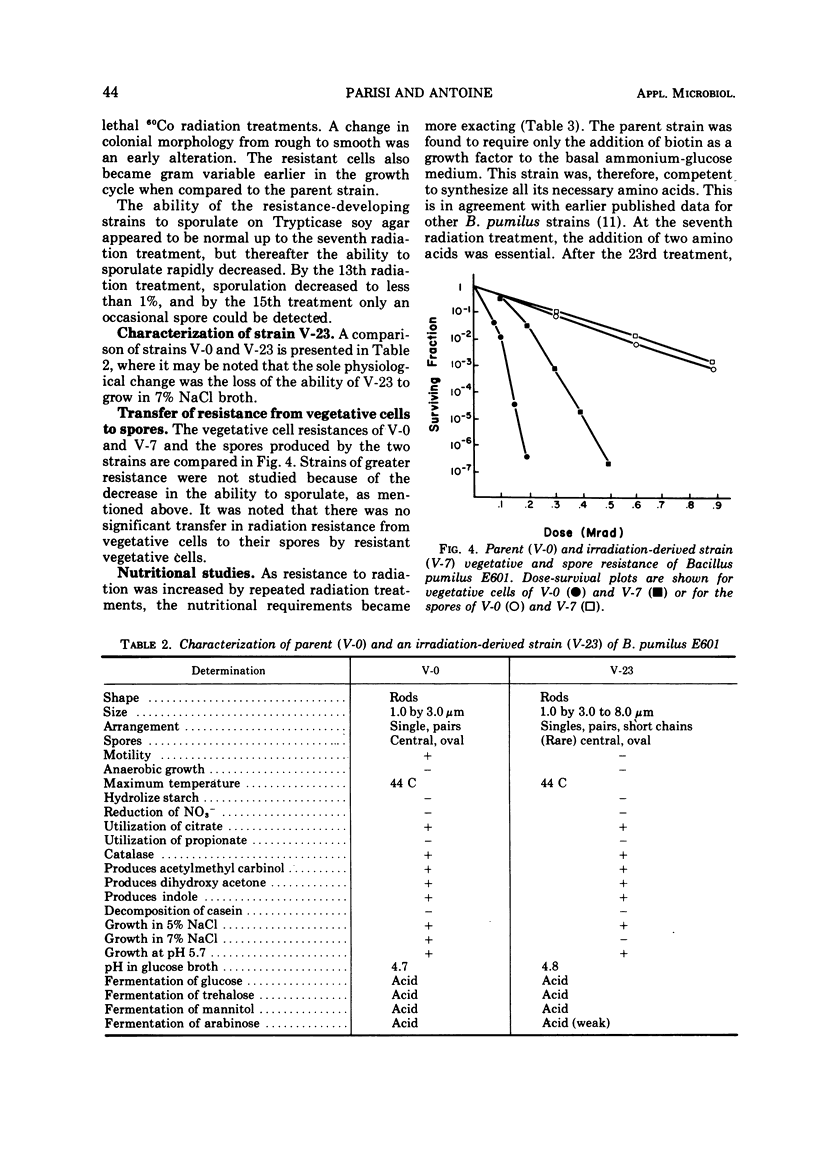
A 4.5-fold increase in vegetative cell radiation resistance of Bacillus pumilus E601, the internationally recognized biological standard for irradiation sterilization, was obtained by the repeated passage of resistant survivors through successive sublethal doses of 60Co irradiation. This increase in resistance was accompanied by a corresponding increase in spore resistance through the seventh irradiation passage. By the fifteenth passage, the ability for spore formation was lost. Other effects noted by the successive irradiation dosages included loss of motility and pellicle formation, and changes in the Gram reaction, cell morphology, and colonial morphology. Increased resistance was also accompanied by an increased nutritional requirement for specific amino acids. Radiation resistance was not transferred from vegetative cells to spores.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borick P. M., Fogarty M. G. Effects of continuous and interrupted radiation on microorganisms. Appl Microbiol. 1967 Jul;15(4):785–789. doi: 10.1128/am.15.4.785-789.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL L. L., Jr, WILLIAMS O. B. The effect of temperature on the nutritional requirements of facultative and obligate thermophilic bacteria. J Bacteriol. 1953 Feb;65(2):141–145. doi: 10.1128/jb.65.2.141-145.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTENSEN E. A., SEHESTED K. RADIATION RESISTANCE OF STREPTOCOCCUS FAECIUM AND SPORES OF BACILLUS SUBTILIS DRIED IN VARIOUS MEDIA. Acta Pathol Microbiol Scand. 1964;62:448–458. doi: 10.1111/apm.1964.62.3.448. [DOI] [PubMed] [Google Scholar]
- Davies R., Sinskey A. J. Radiation-resistant mutants of Salmonella typhimurium LT2: development and characterization. J Bacteriol. 1973 Jan;113(1):133–144. doi: 10.1128/jb.113.1.133-144.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERDMAN I. E., THATCHER F. S., MACQUEEN K. F. Studies on the irradiation of microorganisms in relation to food preservation. II. Irradiation resistant mutants. Can J Microbiol. 1961 Apr;7:207–215. doi: 10.1139/m61-027. [DOI] [PubMed] [Google Scholar]
- GADEN E. L., Jr, HENLEY E. J. Induced resistance to gamma irradiation in Escherichia coli. J Bacteriol. 1953 Jun;65(6):727–732. doi: 10.1128/jb.65.6.727-732.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNIGHT B. C. J. G., PROOM H. A comparative survey of the nutrition and physiology of mesophilic species in the genus Bacillus. J Gen Microbiol. 1950 Sep;4(3):508–538. doi: 10.1099/00221287-4-3-508. [DOI] [PubMed] [Google Scholar]
- Luckiesh M., Knowles T. Resistivity of Escherichia coli to Ultraviolet Energy (lambda 2537) as Affected by Irradiation of Preceding Cultures. J Bacteriol. 1948 Mar;55(3):369–372. doi: 10.1128/jb.55.3.369-372.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEPPER R. E., BUFFA N. T., CHANDLER V. L. Relative resistances of micro-organisms to cathode rays. III. Bacterial spores. Appl Microbiol. 1956 May;4(3):149–152. doi: 10.1128/am.4.3.149-152.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. J. The nutrition of Bacillus megaterium and Bacillus cereus. J Gen Microbiol. 1972 Aug;71(3):505–514. doi: 10.1099/00221287-71-3-505. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Inherited Differences in Sensitivity to Radiation in Escherichia Coli. Proc Natl Acad Sci U S A. 1946 Mar;32(3):59–68. doi: 10.1073/pnas.32.3.59. [DOI] [PMC free article] [PubMed] [Google Scholar]



