Abstract
While hemoplasma infections in domestic cats are well studied, almost no information is available on their occurrence in wild felids. The aims of the present study were to investigate wild felid species as possible reservoirs of feline hemoplasmas and the molecular characterization of the hemoplasma isolates. Blood samples from the following 257 wild felids were analyzed: 35 Iberian lynxes from Spain, 36 Eurasian lynxes from Switzerland, 31 European wildcats from France, 45 lions from Tanzania, and 110 Brazilian wild felids, including 12 wild felid species kept in zoos and one free-ranging ocelot. Using real-time PCR, feline hemoplasmas were detected in samples of the following species: Iberian lynx, Eurasian lynx, European wildcat, lion, puma, oncilla, Geoffroy's cat, margay, and ocelot. “Candidatus Mycoplasma haemominutum” was the most common feline hemoplasma in Iberian lynxes, Eurasian lynxes, Serengeti lions, and Brazilian wild felids, whereas “Candidatus Mycoplasma turicensis” was the most prevalent in European wildcats; hemoplasma coinfections were frequently observed. Hemoplasma infection was associated with species and free-ranging status of the felids in all animals and with feline leukemia virus provirus-positive status in European wildcats. Phylogenetic analyses of the 16S rRNA and the partial RNase P gene revealed that most hemoplasma isolates exhibit high sequence identities to domestic cat-derived isolates, although some isolates form different subclusters within the phylogenetic tree. In conclusion, 9 out of 15 wild felid species from three different continents were found to be infected with feline hemoplasmas. The effect of feline hemoplasma infections on wild felid populations needs to be further investigated.
Hemotropic mycoplasmas (also known as the hemoplasmas), the causative agents of infectious anemia, are cell-wall-free bacteria that attach to red blood cells of several mammalian species. Infections can induce acute hemolysis, and the disease is characterized by anorexia, lethargy, dehydration, weight loss, and sudden death. In domestic cats, three different hemoplasma species have been recognized: Mycoplasma haemofelis, “Candidatus Mycoplasma haemominutum” (8, 9, 20, 21, 32), and “Candidatus Mycoplasma turicensis” (35, 36). In the last few years, PCR assays have been developed for a sensitive and specific diagnosis of these agents (1, 5, 14), and recently published real-time PCR assays allow the quantification of the three feline hemoplasmas in the blood of infected cats (31, 35). Hemoplasma infections in domestic cats have been diagnosed worldwide (5, 14, 18, 28, 29, 34, 35, 37). However, the epidemiology and transmission of these agents are still poorly understood. Bloodsucking arthropods, such as ticks and fleas, are suspected to be involved in the transmission of feline hemoplasmas between domestic cats (16, 25), but an attempted experimental transmission between cats via fleas has not been conclusive (38). Based on several studies showing that feline hemoplasmas are more frequently found in male cats (19, 28, 35, 37), a direct transmission between domestic cats has also been discussed. Indeed, “Ca. Mycoplasma haemominutum” and “Ca. Mycoplasma turicensis” have been detected in the saliva of infected cats (6, 35). On the other hand, the recent discovery of “Ca. Mycoplasma turicensis” as a third feline hemoplasma species that is most closely related to rodent hemoplasmas (36) brought up the hypothesis of an interspecies transmission of hemoplasmas between mice and cats.
The occurrence of feline hemoplasma infections in wild felids has only marginally been addressed to date (11), although wild felid species could represent an important reservoir for these agents due to their common exposure to bloodsucking arthropods and their hunting activity upon rodents. The aims of the current study were to analyze 15 different wild felid species from three different continents for feline hemoplasma infections using real-time PCR assays and to molecularly characterize hemoplasma isolates from different geographical origins.
(These studies were conducted by B. Willi as partial fulfillment of the requirements for a Ph.D. degree at the Vetsuisse Faculty, University of Zurich).
MATERIALS AND METHODS
Sample collection.
Samples were available from 257 wild felids (Table 1), including blood or serosanguinous fluid from 35 Iberian lynxes (Lynx pardinus) from Spain, 36 Eurasian lynxes (Lynx lynx) from Switzerland, 31 European wildcats (Felis silvestris silvestris) from France (17), and 45 lions (Panthera leo) from Tanzania (23). In addition, one sample from a free-ranging ocelot from Brazil and 109 blood samples from 12 different captive wild felid species kept at the Fundacão Parque Zoológico de São Paulo, São Paulo, Brazil, were available (Table 2). The latter samples derived from two cheetahs (Acinonyx jubatus), four leopards (Panthera pardus), five lions, 11 Siberian tigers (Panthera tigris altaica), two snow leopards (Uncia uncia), and seven Brazilian Neotropic felid species, including seven Geoffroy's cats (Oncifelis geoffroyi), 23 jaguarundis (Herpailurus yaguaroundi), nine margays (Leopardus wiedii), six ocelots (Leopardus pardalis), 33 oncillas (Leopardus tigrinus), five Pampas cats (Oncifelis colocolo), and two pumas (Puma concolor). From the European wildcats, samples were collected from dead animals that had been hit by cars (17). Based on the findings at necropsy, the animals had not been dead for more than 48 h before retrieval. The samples from African lions were collected in the Serengeti National Park during a canine distemper virus (CDV) outbreak (23). Samples from Iberian lynxes were collected from the vena cephalica or vena saphena, the thoracic cavity, or the heart from animals that were necropsied, as well as from wild-caught and captive lynxes that were anesthetized for health screening and/or radiocollaring. Samples from Eurasian lynxes were collected from the vena cephalica, the thoracic cavity, or the heart from animals that were found dead or caught and anesthetized during biological studies and management programs. Samples from captive Brazilian wild felids were collected by venipuncture under chemical restraint. Blood from the free-ranging ocelot was obtained from the Genome Resource Bank from the National Research Center for Carnivore Conservation, a unit of the National Environmental Agency. All Brazilian samples were transported to Switzerland on dry ice in full compliance with the Convention on International Trade in Endangered Species and the Genetic Heritage Management Council. The samples were stored at −20°C or −80°C or in liquid nitrogen until use.
TABLE 1.
Gender and modus vivendi of Iberian lynxes, Eurasian lynxes, European wildcats, Serengeti lions, and Brazilian wild felids
| Variable | No. of animals with characteristic in group (n):
|
||||
|---|---|---|---|---|---|
| Iberian lynxes (35) | Eurasian lynxes (36) | European wildcats (31) | Serengeti lions (45) | Brazilian wild felids (110) | |
| Gender | |||||
| Female | 21 | 14 | 15 | 30 | 50 |
| Male | 14 | 22 | 16 | 15 | 60 |
| Modus vivendi | |||||
| Captive | |||||
| Wild caught | 17 | 0 | 0 | 0 | 44 |
| Zoo born | 2 | 0 | 0 | 0 | 65 |
| Free ranging | 16 | 36 | 31 | 45 | 1 |
TABLE 2.
Gender; modus vivendi; and numbers of M. haemofelis, “Ca. Mycoplasma haemominutum,” and “Ca. Mycoplasma turicensis” PCR-positive animals in the group of Brazilian wild felids
| Variable | No. of animals with characteristic (n)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cheetah (2) | Leopard (4) | Lion (5) | Siberian tiger (11) | Snow leopard (2) | Geoffroy's cat (7) | Jaguarundi (23) | Margay (9) | Ocelot (7) | Oncilla (33) | Pampas cat (5) | Puma (2) | |
| Gender | ||||||||||||
| Female | 1 | 3 | 3 | 6 | 1 | 2 | 9 | 4 | 4 | 12 | 4 | 1 |
| Male | 1 | 1 | 2 | 5 | 1 | 5 | 14 | 5 | 3 | 21 | 1 | 1 |
| Modus vivendi | ||||||||||||
| Captive | ||||||||||||
| Wild caught | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 8 | 3 | 24 | 3 | 2 |
| Zoo born | 2 | 4 | 5 | 11 | 2 | 6 | 20 | 1 | 3 | 9 | 2 | 0 |
| Free ranging | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Status for organism | ||||||||||||
| M. haemofelis | ||||||||||||
| Positive | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Negative | 2 | 4 | 5 | 11 | 2 | 7 | 23 | 8 | 6 | 33 | 5 | 2 |
| “Ca. Mycoplasma haemominutum” | ||||||||||||
| Positive | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 4 | 3 | 0 | 1 |
| Negative | 2 | 4 | 4 | 11 | 2 | 6 | 23 | 8 | 3 | 30 | 5 | 1 |
| “Ca. Mycoplasma turicensis” | ||||||||||||
| Positive | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Negative | 2 | 4 | 5 | 11 | 2 | 7 | 23 | 9 | 6 | 33 | 5 | 2 |
Hematology.
For the Iberian lynxes, hematological parameters were evaluated in EDTA-blood with the ADVIA 120 autoanalyzer (Bayer, Zurich, Switzerland). For the Serengeti lions and Brazilian wild felids, packed cell volume (PCV) was analyzed using a microcentrifuge.
TNA extraction.
Total nucleic acid (TNA) was extracted from 100 μl of EDTA-anticoagulated blood or serosanguinous fluid using the MagNaPure LC TNA isolation kit (Roche Diagnostics, Rotkreuz, Switzerland). To monitor for cross-contamination, negative controls consisting of 100 μl phosphate-buffered saline were concurrently prepared with each batch of 15 samples.
PCR assays.
Real-time PCR assays for the detection of M. haemofelis, “Ca. Mycoplasma haemominutum,” and “Ca. Mycoplams turicensis” were performed as previously described (35, 36). Due to the high sequence similarity, Mycoplasma haemocanis is also amplified in the M. haemofelis assay (35). To diagnose feline leukemia virus (FeLV) infections, a PCR assay was performed as described previously (27). Furthermore, all samples from dead European wildcats were subjected to a quantitative real-time PCR assay for feline glyceraldehyde-3-phosphate dehydrogenase amplification (3) to confirm the presence of amplifiable TNA. For all PCR assays, the amplification buffer contained dUTP for the use with uracil-N-glycosylase to prevent carryover of PCR amplicons and water was used as a negative control. All negative extraction and pipetting controls tested PCR negative.
Sequencing of 16S rRNA and partial RNase P genes.
The near-complete 16S rRNA genes of 21 hemoplasma isolates (six of M. haemofelis, 11 of “Ca. Mycoplasma haemominutum,” and four of “Ca. Mycoplasma turicensis”) were cloned and sequenced using previously described methods (35, 36). The RNA subunit of the RNase P gene of seven M. haemofelis-like isolates was amplified using the primers RNasePFor1 and RnasePRev1 as published elsewhere (32). All sequences obtained were compared to those of the GenBank database and aligned using CLUSTAL W (33). Alignment was manually adjusted, and percentage identity was calculated using Jalview 2.07 (4). Only positions where the nucleotide composition was known in all sequences being compared were used in the phylogenetic analyses. Phylogenetic trees were constructed with the neighbor-joining method (24) from a distance matrix corrected for nucleotide substitutions by the Kimura two-parameter model (15). The data set was resampled 1,000 times to generate bootstrap values.
Statistical evaluation.
Data were compiled and analyzed with Excel (Microsoft, Wallisellen, Switzerland), Analyze-it Clinical Laboratory (Analyze-it Software, Leeds, United Kingdom), Prism software (GraphPad, San Diego, CA), and NCSS 2004 (J. Hintze, Kaysville, UT). The following variables were assessed: wild felid species; modus vivendi (free ranging, wild caught, or zoo born); sex; infection status for M. haemofelis, “Ca. Mycoplasma haemominutum,” “Ca. Mycoplasma turicensis,” and FeLV (available for European wildcats); and PCV values (available for Iberian lynxes, Serengeti lions, and Brazilian wild felids). For 2 × 2 contingency tables, the Fisher exact test (PF, expected cell frequencies of ≤5) or χ2 test (Pchi, expected cell frequencies of >5) was used. Contingency tables with more than 2 × 2 categories were analyzed with the χ2 test. PCV values were tested for statistical differences among animals with different hemoplasma infection status by the Kruskal-Wallis one-way analysis of variance by ranks (PKW) and the Dunn post test for multiple comparisons. Differences were considered significant with P < 0.05.
Nucleotide sequence accession numbers.
The 16S rRNA and RNase P nucleotide sequences generated from feline hemoplasma isolates have been submitted to GenBank and given the accession numbers DQ825438, DQ825441, DQ825447, DQ825451, DQ825453, and DQ825458 (16S rRNA genes of M. haemofelis); DQ825439, DQ825440, DQ825442, DQ825443, DQ825444, DQ825445, DQ825446, DQ825452, DQ825455, DQ825456, and DQ825457 (16S rRNA genes of “Ca. Mycoplasma haemominutum”); DQ825448, DQ825449, DQ825450, and DQ825454 (16S rRNA genes of “Ca. Mycoplasma turicensis”); and DQ859006, DQ859007, DQ859008, DQ859009, DQ859010, DQ859011, and DQ859012 (partial RNase P genes of M. haemofelis).
RESULTS
Sample prevalence.
Among the 257 wild felids analyzed with real-time PCR assays, 96 (37%) tested positive for feline hemoplasma infections (Table 3). The frequency of feline hemoplasma infection was significantly different between the five sample groups (Iberian lynxes, Eurasian lynxes, European wildcats, Serengeti lions, and Brazilian wild felids; Pchi < 0.0001). Feline hemoplasma infections were highly prevalent in the Serengeti lions (98%) but less common in Eurasian lynxes (44%), European wildcats (39%), and Iberian lynxes (37%). The lowest feline hemoplasma prevalence was found in the group of Brazilian wild felids (10%).
TABLE 3.
Numbers and percentages of animals that tested real-time PCR positive for M. haemofelis, “Ca. Mycoplasma haemominutum,” and “Ca. Mycoplasma turicensis” in the groups of Iberian lynxes, Eurasian lynxes, European wildcats, Serengeti lions, and Brazilian wild felids
| Sample group, geographical origin (n) | No. (%) of animals PCR positive for organism:
|
||
|---|---|---|---|
| M. haemofelis | “Ca. Mycoplasma haemominutum” | “Ca. Mycoplasma turicensis” | |
| Iberian lynxes, Spain (35) | 7 (20) | 9 (26) | 3 (9) |
| Eurasian lynxes, Switzerland (36) | 4 (11) | 14 (39) | 2 (6) |
| European wildcats, France (31) | 1 (3) | 6 (19) | 11 (36) |
| Serengeti lions, Tanzania (45) | 31 (69) | 43 (96) | 34 (76) |
| Brazilian wild felids, Brazil (110) | 2 (2) | 11 (10) | 1 (0.9) |
| Total (257) | 45 (18) | 83 (32) | 51 (20) |
Fifty-five wild felids were concurrently infected with several feline hemoplasmas (Table 4). No animal was coinfected with M. haemofelis and “Ca. Mycoplasma turicensis” in the absence of “Ca. Mycoplasma haemominutum” infection. The frequency of concurrent infections with several feline hemoplasmas was significantly different between the sample groups (Pchi < 0.0001): 89% of the hemoplasma-infected Serengeti lions were concurrently infected with several species. This finding was less common in European wildcats (42%), Iberian lynxes (39%), Eurasian lynxes (25%), and Brazilian wild felids (18%). Furthermore, free-ranging animals were significantly more often coinfected with several hemoplasmas than were captive wild felids (wild caught or zoo born; Pchi = 0.0005). However, when Serengeti lions were excluded from these calculations to address a bias due to the frequent coinfections in this population, values no longer reached significance (PF = 0.1533).
TABLE 4.
Numbers and percentages of animals that were concurrently infected with several feline hemoplasmas as determined by real-time PCR in the groups of Iberian lynxes, Eurasian lynxes, European wildcats, Serengeti lions, and Brazilian wild felids
| Sample group (n) | No. (%) of animals PCR positive for coinfection with:
|
||
|---|---|---|---|
| CMhm-Mhfa | CMhm-CMtb | CMhm-Mhf-CMtc | |
| Iberian lynxes (35) | 3 (9) | 1 (3) | 1 (3) |
| Eurasian lynxes (36) | 3 (8) | 1 (3) | 0 |
| European wildcats (31) | 0 | 4 (13) | 1 (3) |
| Serengeti lions (45) | 5 (11) | 9 (20) | 25 (56) |
| Brazilian wild felids (110) | 1 (0.9)d | 0 | 1 (0.9)e |
| Total (257) | 12 (5) | 15 (6) | 28 (11) |
“Ca. Mycoplasma haemominutum” and M. haemofelis.
“Ca. Mycoplasma haemominutum” and “Ca. Mycoplasma turicensis”.
“Ca. Mycoplasma haemominutum,” M. haemofelis, and “Ca. Mycoplasma turicensis”.
Margay.
Ocelot.
Phylogenetic analyses.
All six M. haemofelis 16S rRNA gene sequences that had been analyzed showed >99% identity when aligned with an M. haemofelis 16S rRNA sequence from a domestic cat (DQ157160). Two “Ca. Mycoplasma haemominutum” 16S rRNA sequences originating from European wildcats were also >99% identical to a published sequence from a domestic cat (U88564), whereas nine sequences from other wild felid species exhibited less identity (97 to 99%). Three out of four “Ca. Mycoplasma turicensis” sequences were >99% identical to a domestic cat-derived sequence (DQ157150); the three isolates derived from one Brazilian ocelot and two European wildcats. The fourth sequence, originating from an African lion, showed only 97% identity.
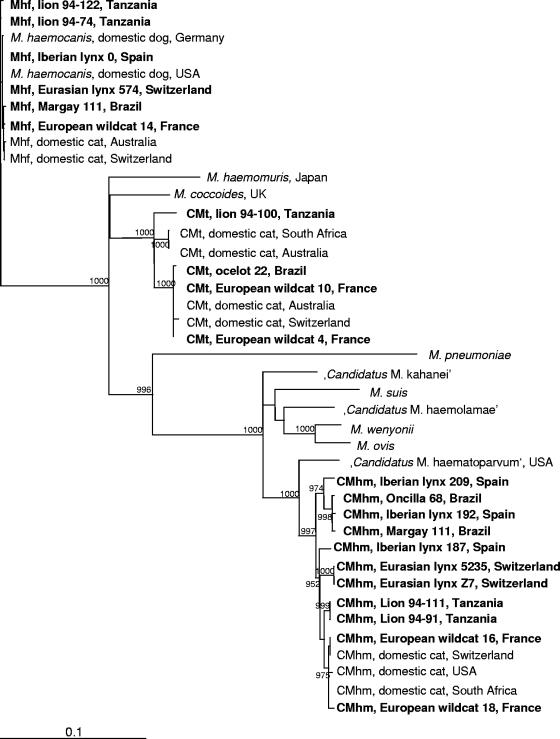
Phylogenetic analyses based on the 16S rRNA gene of M. haemofelis isolates from wild felids revealed no major subclustering in the phylogenetic tree (Fig. 1). In contrast, the “Ca. Mycoplasma haemominutum” isolates from wild felids formed different subclusters: isolates from African lions, Eurasian lynxes, and Brazilian wild felids formed three separate groups, whereas isolates from European wildcats colocalized with domestic cat-derived isolates. “Ca. Mycoplasma haemominutum” isolates from Iberian lynxes, however, were found throughout different subclusters. Finally, among the “Ca. Mycoplasma turicensis” 16S rRNA gene sequences, only the one isolate that derived from an African lion (94-100) branched away from the remaining isolates.
FIG. 1.
Phylogenetic analysis of nearly complete 16S rRNA gene sequences from M. haemofelis (Mhf), “Ca. Mycoplasma haemominutum” (CMhm), and “Ca. Mycoplasma turicensis” (CMt) isolates from different wild felid species. Bootstrap values are given at the nodes of the tree; only values of ≥900 are shown. The following sequences are shown: M. haemofelis (lion 94-122, DQ825453; lion 94-74, DQ825451; Iberian lynx 0, DQ825447; Eurasian lynx 574, DQ825458; margay 111, DQ825438; European wildcat 14, DQ825441; Australia, AY150976; Switzerland, DQ157160), M. haemocanis (Germany, AY150973; United States, AF197337), Mycoplasma haemomuris (U82963), Mycoplasma coccoides (AY171918), “Ca. Mycoplasma turicensis” (lion 94-100, DQ825454; South Africa, DQ464424; Australia, DQ464425; ocelot 22, DQ825448; European wildcat 10, DQ825450; Australia, DQ464417; Switzerland, DQ157150; European wildcat 4, DQ825449), Mycoplasma pneumoniae (M29061), “Candidatus Mycoplasma kahanei” (AF338269), Mycoplasma suis (U88565), “Candidatus Mycoplasma haemolamae” (AF306346), Mycoplasma wenyonii (AF016546), Mycoplasma ovis (AF338268), “Candidatus Mycoplasma haematoparvum” (AY532390), and “Ca. Mycoplasma haemominutum” isolates (Iberian lynx 209, DQ825446; oncilla 68, DQ825439; Iberian lynx 192, DQ825445; margay 111, DQ825440; Iberian lynx 187, DQ825444; Eurasian lynx 5235, DQ825456; Eurasian lynx Z7, DQ825457; lion 94-111, DQ825452; lion 94-91, DQ825455; European wildcat 16, DQ825442; Switzerland, DQ157149; United States, U88564; South Africa, AY150979; European wildcat 18, DQ825443). UK, United Kingdom; USA, United States.
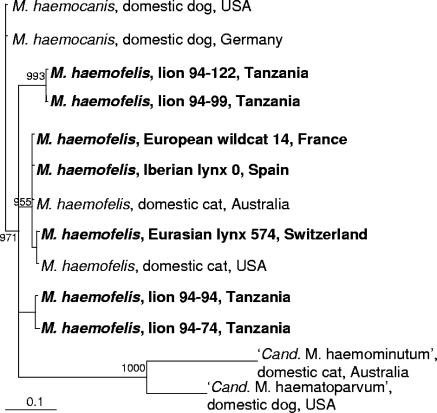
To differentiate M. haemofelis from M. haemocanis infection (2), the RNA subunit of the RNase P gene from seven isolates was sequenced. The sequences from three isolates (from one European wildcat, one Iberian lynx, and one Eurasian lynx) exhibited >98% identity to an M. haemofelis RNase P gene sequence (AY150991) but only 94% identity to an M. haemocanis RNase P gene sequence (AF407213). Different results were obtained with isolates originating from four Serengeti lions from four different prides (Barafu, Serengeti Nomads, Sangere, and Naabi). The RNase P gene sequences from two isolates (from lions 94-94 and 94-74) exhibited only 88% and 93% identity, respectively, to the M. haemofelis sequence. However, they were even less similar to the M. haemocanis RNase P gene sequence (85% and 90% identity, respectively). The RNase P gene sequences from the remaining two isolates (from lions 94-122 and 94-99) exhibited equally low identity (92%) to the RNase P gene sequences from M. haemofelis and M. haemocanis.
Phylogenetic analyses based on the RNA subunit of the RNase P gene confirmed the close evolutionary homology of three M. haemofelis isolates (from one European wildcat, one Iberian lynx, and one Eurasian lynx) to isolates from domestic cats (Fig. 2). In contrast, but in accordance with the sequencing results, the M. haemofelis isolates from the four Serengeti lions branched away from the remaining isolates and formed two different subclusters. Nevertheless, all M. haemofelis isolates from lions were localized within the M. haemofelis and not within the M. haemocanis subcluster.
FIG. 2.
Phylogenetic analysis of partial RNase P gene sequences from M. haemofelis isolates from seven wild felids. Bootstrap values are given at the nodes of the tree; only values of ≥900 are shown. The following sequences are given: M. haemocanis (United States, AF407213; Germany, AY150989), M. haemofelis (lion 94-122, DQ859010; lion 94-99, DQ859009; European wildcat 14, DQ859006; Iberian lynx 0, DQ859008; Australia, AY150991; Eurasian lynx 574, DQ859011; United States, AF407212; lion 94-94, DQ859012; lion 94-74, DQ859007), “Ca. Mycoplasma haemominutum” (AY150990), and “Candidatus Mycoplasma haematoparvum” (AY380803). USA, United States.
Case characteristics.
Taking all samples together, the frequency of feline hemoplasma infection was significantly different among the wild felid species under investigation (Pchi < 0.0001). This was also found when M. haemofelis (Pchi < 0.0001), “Ca. Mycoplasma haemominutum” (Pchi < 0.0001), and “Ca. Mycoplasma turicensis” (Pchi < 0.0001) infections were tested individually. To exclude bias due to the high hemoplasma prevalence in the Serengeti lions, calculations were repeated excluding these samples. Still the frequency of feline hemoplasma (Pchi = 0.0003), “Ca. Mycoplasma haemominutum” (Pchi = 0.0041), and “Ca. Mycoplasma turicensis” (Pchi = 0.0002) infection was significantly different among the wild felid species. The frequency of feline hemoplasma infection (Pchi = 0.0049) and “Ca. Mycoplasma haemominutum” infection (Pchi = 0.0049) was also significantly different among different species when only Brazilian wild felids were included in the calculations. Remarkably, four out of seven ocelots tested PCR positive for “Ca. Mycoplasma haemominutum” in this sample group, although the three captive ocelots were not housed together.
Free-ranging animals were significantly more often infected with feline hemoplasmas than captive wild felids (wild caught or zoo born; Pchi < 0.0001) (Table 5). Association with free-ranging status was also found for M. haemofelis (PF < 0.0001), “Ca. Mycoplasma haemominutum” (Pchi < 0.0001), and “Ca. Mycoplasma turicensis” (PF < 0.0001) infections when analyzed individually. To address a bias due to the high hemoplasma prevalence in free-ranging Serengeti lions, calculations were again repeated by excluding the latter sample group. Hemoplasma infection (Pchi < 0.0001), as well as M. haemofelis (PF = 0.0004), “Ca. Mycoplasma haemominutum” (Pchi < 0.0001), and “Ca. Mycoplasma turicensis” (PF < 0.0001) infection, was still significantly associated with free-ranging status of the felids. Association with free-ranging status was also found for M. haemofelis (PF = 0.0182) and “Ca. Mycoplasma turicensis” infection (PF = 0.0091) within the group of Brazilian wild felids and for M. haemofelis infection within the group of Iberian lynxes (PF = 0.0273). In addition, European wildcats that tested FeLV provirus positive were significantly more often infected with feline hemoplasmas than FeLV PCR-negative wildcats (PF = 0.0156).
TABLE 5.
Numbers and percentages of animals that tested real-time PCR positive for M. haemofelis, “Ca. Mycoplasma haemominutum,” and “Ca. Mycoplasma turicensis” categorized by modus vivendi
| Modus vivendi (n) | No. (%) of animals PCR positive for organism:
|
||
|---|---|---|---|
| M. haemofelis | “Ca. Mycoplasma haemominutum” | “Ca. Mycoplasma turicensis” | |
| Captive | |||
| Wild caught (61) | 2 (3) | 10 (16) | 2 (3) |
| Zoo born (67) | 0 | 3 (5) | 0 |
| Free ranging (129) | 43 (33) | 70 (54) | 49 (38) |
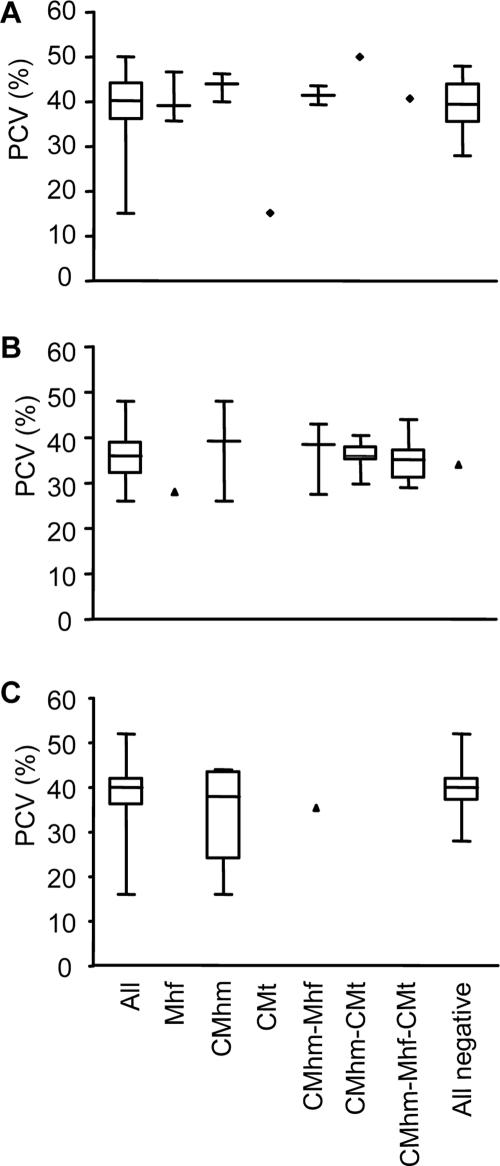
PCV values were not significantly different between hemoplasma-uninfected animals and animals singly infected or coinfected with feline hemoplasmas as evaluated for Iberian lynxes (PKW = 0.2814; Fig. 3A), Serengeti lions (PKW = 0.3752; Fig. 3B), and Brazilian wild felids (PKW = 0.4635; Fig. 3C). There was no association of feline hemoplasma infection with gender of the wild felids under investigation.
FIG. 3.
PCV values of Iberian lynxes (A), Serengeti lions (B), and Brazilian wild felids (C) grouped by hemoplasma infection status. Boxes represent the 25th, 50th (median), and 75th quartiles with whiskers extending to the greatest and smallest values. All, all animals analyzed; Mhf, animals PCR positive for M. haemofelis alone; CMhm, animals PCR positive for “Ca. Mycoplasma haemominutum” alone; CMt, animals PCR positive for “Ca. Mycoplasma turicensis” alone; CMhm-Mhf, animals coinfected with “Ca. Mycoplasma haemominutum” and M. haemofelis; CMhm-CMt, animals coinfected with “Ca. Mycoplasma haemominutum” and “Ca. Mycoplasma turicensis”; CMhm-Mhf-CMt, animals concurrently infected with all three feline hemoplasmas; All negative, animals not infected with any feline hemoplasma.
Clinical examination findings and follow-up.
Clinical data for 9 out of 13 hemoplasma PCR-positive Iberian lynxes were available: while eight animals were found to be healthy, a 1-year-old feline Iberian lynx that tested PCR positive for “Ca. Mycoplasma turicensis” showed pale mucous membranes and results from hematology revealed a nonregenerative anemia (PCV, 15%; reticulocyte count of 12,900/μl). The latter lynx tested also PCR positive for Cytauxoon felis but was PCR negative for other infectious agents (details will be published elsewhere). The lynx was again examined 6 months later and showed full recovery from anemia.
Hemoplasma infections were followed in four Iberian lynxes between 2 months and 2 years. For three animals, the hemoplasma PCR-positive status changed during the follow-up period: one “Ca. Mycoplasma haemominutum” PCR-positive animal turned PCR negative in the last of five blood samples analyzed over the duration of 2 years. One lynx coinfected with “Ca. Mycoplasma haemominutum” and M. haemofelis turned PCR negative for both hemoplasmas in a follow-up sample collected after 11 months. Finally, one animal coinfected with “Ca. Mycoplasma haemominutum” and M. haemofelis turned PCR negative for M. haemofelis in a sample collected 2 months after the first evaluation.
DISCUSSION
This is the first study to report feline hemoplasma infections in nine captive and free-ranging wild felid species from three different continents. An especially high prevalence was found in free-ranging animals, and concurrent infections with different hemoplasmas were frequently observed. Studies of hemoplasma infections in domestic cats reveal that these agents are more commonly detected in regions with warmer climates (5, 18, 19, 29), suggesting that distinct bloodsucking arthropods may play a role in the transmission of hemoplasmas in different countries. Accordingly, we found a high sample prevalence in lions from Tanzania. However, hemoplasma infections were also rather common in Eurasian lynxes from Switzerland, although we recently found a very low prevalence for these agents in Swiss pet cats (35). As the present work was carried out using convenience-sampled populations, the limitations of which have been discussed previously (26), conclusions concerning the general hemoplasma prevalence in the wild felids under investigation cannot be drawn from this study.
Hemoplasma infections were significantly associated with wild felid species, and concurrent infections with several hemoplasmas were most frequently detected in the Serengeti lions. Different susceptibilities of animals of different wild felid species to hemoplasma infections cannot be excluded, but the dissimilar living environment and health status of the wild felids under investigation seem a more likely explanation for this association. Correspondingly, we also found a lower prevalence of feline hemoplasma infections in captive than in free-ranging animals. The hemoplasma prevalence in wild felids kept at São Paulo Zoo in Brazil was comparable to the prevalence reported in a study investigating 54 captive nondomestic cats in the United States (11). In contrast, free-ranging animals may be more exposed to bloodsucking arthropods and exhibit higher fighting activity than nondomestic cats in a zoo environment; thus, they might experience a higher infection risk with agents such as hemoparasites or retroviruses. It has been shown that free-ranging felids often exhibit multiple infections with different pathogens (7, 13, 17); in particular all except two African lions included in the present study had CDV and feline immunodeficiency virus infections, and many of the animals showed distinct lymphopenia (22, 23). Additionally, Hepatozoon as well as Babesia and Theileria/Cytauxoon-like organisms were identified by microscopic evaluation on blood smears of the Serengeti lions (data not shown). Concurrent infections, especially those with immunosuppressive agents, such as CDV, retroviruses, and Theileria spp., could have influenced the frequency of hemoplasma infections. This has also been suspected for domestic cats (10, 12) and would be in agreement with the finding that hemotropic mycoplasmas were more common in FeLV-infected than FeLV-uninfected European wildcats in the present study.
The pathogenic potential of M. haemofelis and to a lesser extent of “Ca. Mycoplasma turicensis” in domestic cats has been demonstrated by experimental infection studies (8, 36). Phylogenetic analyses revealed that most hemoplasma isolates from wild felids were indeed very closely related to hemotropic mycoplasmas from domestic cats. However, hemoplasma PCR-positive wild felids did not exhibit significantly lower PCV values than did hemoplasma PCR-negative animals. The lack of clear clinical signs in most of the infected animals could be explained by the possibility that the cats were sampled during a chronic carrier status and not during acute infection; domestic cats that recover from acute infection usually lack signs of anemia although they test PCR positive and occasionally show high hemoplasma loads (8, 30, 35). Indeed, a healthy Iberian lynx in the present study remained PCR positive for “Ca. Mycoplasma haemominutum” over a 22-month follow-up period.
Interestingly, two out of four “Ca. Mycoplasma haemominutum”-infected Iberian lynxes that were followed during infection turned PCR negative for this agent. This is in agreement with our recent results where we reported clearance of this agent from the blood in 3 out of 15 “Ca. Mycoplasma haemominutum”-infected domestic cats, with or without antibiotic treatment (35). Spontaneous or treatment-induced elimination from the blood was also reported for “Ca. Mycoplasma turicensis”-infected domestic cats (35, 36). In contrast, in M. haemofelis infection, an occasional PCR-negative result might not necessarily indicate clearance of the agent but could be due to marked fluctuations of M. haemofelis blood loads (31, 35). This might also have been the case in the two M. haemofelis-infected Iberian lynxes followed in this study.
Phylogenetic analyses based on the 16S rRNA or partial RNase P genes revealed that hemoplasma isolates from European wildcats were very closely related to domestic cat-derived isolates. In contrast, the hemoplasma isolates from Serengeti lions branched away from domestic cat isolates and formed different subclusters within the phylogenetic trees. These findings could imply that in European wildcats, but to a lesser extent in Serengeti lions, an interchange of hemoplasma isolates between wild felids and domestic cats might occur, e.g., via bloodsucking arthropods. Furthermore, analyses of the RNase P gene sequences showed that the M. haemofelis-like isolates analyzed from Serengeti lions were more closely related to M. haemofelis than to M. haemocanis. A recent study reporting M. haemofelis infection in two tigers (11), another species of the genus Panthera, did not include RNase P gene analyses and therefore could not distinguish between M. haemofelis and M. haemocanis infection.
In conclusion, hemoplasma infections were highly prevalent in numerous wild felid species from three different continents. Free-ranging animals were frequently infected, and concurrent infections with several hemoplasmas were often observed. A conclusion about the pathogenic potential of these agents in wild felids cannot yet be drawn. Future studies should therefore address the significance of feline hemoplasma infections in wild felid populations.
Acknowledgments
We thank B. Weibel, T. Meili Prodan, N. Tschopp, E. Gönczi, B. Pineroli, A. Pepin, R. Tandon, G. Dasen, B. Riond, and N. Wengi for excellent laboratory assistance and helpful support. We are indebted to the Environmental Council of the Government of Andalusia, southern Spain, for providing the Iberian lynx samples, and to J. Pastor and E. Bach from the Ecopathology Service, University of Bologna, who performed the hematology on these samples. We also express our appreciation to CENAP-IBAMA, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundo Nacional de Meio Ambiente (FNMA), Pró-Reitoria de Pós-Graduação from the University of São Paulo, and the International Relations Office from the University of Zurich. Laboratory work was done using the facilities of the Center for Clinical Studies at the Vetsuisse Faculty of the University of Zurich.
The collection of the African lion samples was funded by the Messerli Foundation, Zurich, Switzerland, in collaboration with Tanzania National Parks and the Serengeti Research Institute. This work was supported by a research grant (Forschungskredit 2002) of the University of Zurich; by the Janggen-Poehn Foundation, St. Gallen; by the Roche Research Foundation, Basel; and by Merial GmbH, Germany. R.H.-L. is the recipient of a professorship from the Swiss National Science Foundation (PP00B-102866).
Footnotes
Published ahead of print on 14 February 2007.
REFERENCES
- 1.Berent, L. M., J. B. Messick, and S. K. Cooper. 1998. Detection of Haemobartonella felis in cats with experimentally induced acute and chronic infections, using a polymerase chain reaction assay. Am. J. Vet. Res. 59:1215-1220. [PubMed] [Google Scholar]
- 2.Birkenheuer, A. J., E. B. Breitschwerdt, A. R. Alleman, and C. Pitulle. 2002. Differentiation of Haemobartonella canis and Mycoplasma haemofelis on the basis of comparative analysis of gene sequences. Am. J. Vet. Res. 63:1385-1388. [DOI] [PubMed] [Google Scholar]
- 3.Cattori, V., R. Tandon, A. Pepin, H. Lutz, and R. Hofmann-Lehmann. 2006. Rapid detection of feline leukemia virus provirus integration into feline genomic DNA. Mol. Cell. Probes 20:172-181. [DOI] [PubMed] [Google Scholar]
- 4.Clamp, M., J. Cuff, S. M. Searle, and G. J. Barton. 2004. The Jalview Java alignment editor. Bioinformatics 20:426-427. [DOI] [PubMed] [Google Scholar]
- 5.Criado-Fornelio, A., A. Martinez-Marcos, A. Buling-Sarana, and J. C. Barba-Carretero. 2003. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: a molecular study. Vet. Microbiol. 93:307-317. [DOI] [PubMed] [Google Scholar]
- 6.Dean, R., C. R. Helps, T. J. Gruffydd-Jones, and S. Tasker. 2005. Use of real-time PCR to detect M. haemofelis and ‘Candidatus Mycoplasma haemominutum’ in the saliva and salivary glands of haemoplasma-infected cats, p. 554. Proc. BSAVA Congr. British Small Animal Veterinary Association, Gloucester, United Kingdom.
- 7.Filoni, C., J. L. Catão-Dias, G. Bay, E. L. Durigon, R. S. Jorge, H. Lutz, and R. Hofmann-Lehmann. 2006. First evidence of feline herpesvirus, calicivirus, parvovirus, and Ehrlichia exposure in Brazilian free-ranging felids. J. Wildl. Dis. 42:470-477. [DOI] [PubMed] [Google Scholar]
- 8.Foley, J. E., S. Harrus, A. Poland, B. Chomel, and N. C. Pedersen. 1998. Molecular, clinical, and pathologic comparison of two distinct strains of Haemobartonella felis in domestic cats. Am. J. Vet. Res. 59:1581-1588. [PubMed] [Google Scholar]
- 9.Foley, J. E., and N. C. Pedersen. 2001. ‘Candidatus Mycoplasma haemominutum’, a low-virulence epierythrocytic parasite of cats. Int. J. Syst. Evol. Microbiol. 51:815-817. [DOI] [PubMed] [Google Scholar]
- 10.George, J. W., B. A. Rideout, S. M. Griffey, and N. C. Pedersen. 2002. Effect of preexisting FeLV infection or FeLV and feline immunodeficiency virus coinfection on pathogenicity of the small variant of Haemobartonella felis in cats. Am. J. Vet. Res. 63:1172-1178. [DOI] [PubMed] [Google Scholar]
- 11.Haefner, M., T. J. Burke, B. E. Kitchell, L. A. Lamont, D. J. Schaeffer, M. Behr, and J. B. Messick. 2003. Identification of Haemobartonella felis (Mycoplasma haemofelis) in captive nondomestic cats. J. Zoo Wildl. Med. 34:139-143. [DOI] [PubMed] [Google Scholar]
- 12.Harrus, S., E. Klement, I. Aroch, T. Stein, H. Bark, E. Lavy, M. Mazaki-Tovi, and G. Baneth. 2002. Retrospective study of 46 cases of feline haemobartonellosis in Israel and their relationships with FeLV and FIV infections. Vet. Rec. 151:82-85. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann-Lehmann, R., D. Fehr, M. Grob, M. Elgizoli, C. Packer, J. S. Martenson, S. J. O'Brien, and H. Lutz. 1996. Prevalence of antibodies to feline parvovirus, calicivirus, herpesvirus, coronavirus, and immunodeficiency virus and of feline leukemia virus antigen and the interrelationship of these viral infections in free-ranging lions in east Africa. Clin. Diagn. Lab. Immunol. 3:554-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen, W. A., M. R. Lappin, S. Kamkar, and W. J. Reagan. 2001. Use of a polymerase chain reaction assay to detect and differentiate two strains of Haemobartonella felis in naturally infected cats. Am. J. Vet. Res. 62:604-608. [DOI] [PubMed] [Google Scholar]
- 15.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 16.Lappin, M. R., B. Griffin, J. Brunt, A. Riley, D. Burney, J. Hawley, M. M. Brewer, and W. A. Jensen. 2006. Prevalence of Bartonella species, haemoplasma species, Ehrlichia species, Anaplasma phagocytophilum, and Neorickettsia risticii DNA in the blood of cats and their fleas in the United States. J. Feline Med. Surg. 8:85-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leutenegger, C. M., R. Hofmann-Lehmann, C. Riols, M. Liberek, G. Worel, P. Lups, D. Fehr, M. Hartmann, P. Weilenmann, and H. Lutz. 1999. Viral infections in free-living populations of the European wildcat. J. Wildl. Dis. 35:678-686. [DOI] [PubMed] [Google Scholar]
- 18.Lobetti, R. G., and S. Tasker. 2004. Diagnosis of feline haemoplasma infection using a real-time PCR assay. J. S. Afr. Vet. Assoc. 75:94-99. [DOI] [PubMed] [Google Scholar]
- 19.Luria, B. J., J. K. Levy, M. R. Lappin, E. B. Breitschwerdt, A. M. Legendre, J. A. Hernandez, S. P. Gorman, and I. T. Lee. 2004. Prevalence of infectious diseases in feral cats in Northern Florida. J. Feline Med. Surg. 6:287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messick, J. B., L. M. Berent, and S. K. Cooper. 1998. Development and evaluation of a PCR-based assay for detection of Haemobartonella felis in cats and differentiation of H. felis from related bacteria by restriction fragment length polymorphism analysis. J. Clin. Microbiol. 36:462-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neimark, H., K. E. Johansson, Y. Rikihisa, and J. G. Tully. 2001. Proposal to transfer some members of the genera Haemobartonella and Eperythrozoon to the genus Mycoplasma with descriptions of ‘Candidatus Mycoplasma haemofelis', ‘Candidatus Mycoplasma haemomuris’, ‘Candidatus Mycoplasma haemosuis’ and ′Candidatus Mycoplasma wenyonii’. Int. J. Syst. Evol Microbiol. 51:891-899. [DOI] [PubMed] [Google Scholar]
- 22.Roelke, M. E., J. Pecon-Slattery, S. Taylor, S. Citino, E. Brown, C. Packer, S. Vandewoude, and S. J. O'Brien. 2006. T-lymphocyte profiles in FIV-infected wild lions and pumas reveal CD4 depletion. J. Wildl. Dis. 42:234-248. [DOI] [PubMed] [Google Scholar]
- 23.Roelke-Parker, M. E., L. Munson, C. Packer, R. Kock, S. Cleaveland, M. Carpenter, S. J. O'Brien, A. Pospischil, R. Hofmann-Lehmann, H. Lutz, et al. 1996. A canine distemper virus epidemic in Serengeti lions (Panthera leo). Nature 379:441-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 25.Shaw, S. E., M. J. Kenny, S. Tasker, and R. J. Birtles. 2004. Pathogen carriage by the cat flea Ctenocephalides felis (Bouche) in the United Kingdom. Vet. Microbiol. 102:183-188. [DOI] [PubMed] [Google Scholar]
- 26.Sukura, A., Y. T. Grohn, J. Junttila, and T. Palolahti. 1992. Association between feline immunodeficiency virus antibodies and host characteristics in Finnish cats. Acta Vet. Scand. 33:325-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tandon, R., V. Cattori, M. A. Gomes-Keller, M. L. Meli, M. C. Golder, H. Lutz, and R. Hofmann-Lehmann. 2005. Quantitation of feline leukaemia virus viral and proviral loads by TaqMan real-time polymerase chain reaction. J. Virol. Methods 130:124-132. [DOI] [PubMed] [Google Scholar]
- 28.Tasker, S., S. H. Binns, M. J. Day, T. J. Gruffydd-Jones, D. A. Harbour, C. R. Helps, W. A. Jensen, C. S. Olver, and M. R. Lappin. 2003. Use of a PCR assay to assess the prevalence and risk factors for Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’ in cats in the United Kingdom. Vet. Rec. 152:193-198. [DOI] [PubMed] [Google Scholar]
- 29.Tasker, S., J. A. Braddock, R. Baral, C. R. Helps, M. J. Day, T. J. Gruffydd-Jones, and R. Malik. 2004. Diagnosis of feline haemoplasma infection in Australian cats using a real-time PCR assay. J. Feline Med. Surg. 6:345-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tasker, S., S. M. Caney, M. J. Day, R. S. Dean, C. R. Helps, T. G. Knowles, P. J. Lait, M. D. Pinches, and T. J. Gruffydd-Jones. 2006. Effect of chronic FIV infection, and efficacy of marbofloxacin treatment, on Mycoplasma haemofelis infection. Vet. Microbiol. 117:169-179. [DOI] [PubMed] [Google Scholar]
- 31.Tasker, S., C. R. Helps, M. J. Day, T. J. Gruffydd-Jones, and D. A. Harbour. 2003. Use of real-time PCR to detect and quantify Mycoplasma haemofelis and “Candidatus Mycoplasma haemominutum” DNA. J. Clin. Microbiol. 41:439-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tasker, S., C. R. Helps, M. J. Day, D. A. Harbour, S. E. Shaw, S. Harrus, G. Baneth, R. G. Lobetti, R. Malik, J. P. Beaufils, C. R. Belford, and T. J. Gruffydd-Jones. 2003. Phylogenetic analysis of hemoplasma species: an international study. J. Clin. Microbiol. 41:3877-3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe, M., M. Hisasue, K. Hashizaki, M. Furuichi, M. Ogata, S. Hisamatsu, E. Ogi, M. Hasegawa, R. Tsuchiya, and T. Yamada. 2003. Molecular detection and characterization of Haemobartonella felis in domestic cats in Japan employing sequence-specific polymerase chain reaction (SS-PCR). J. Vet. Med. Sci. 65:1111-1114. [DOI] [PubMed] [Google Scholar]
- 35.Willi, B., F. S. Boretti, C. Baumgartner, S. Tasker, B. Wenger, V. Cattori, M. L. Meli, C. E. Reusch, H. Lutz, and R. Hofmann-Lehmann. 2006. Prevalence, risk factor analysis, and follow-up of infections caused by three feline hemoplasma species in cats in Switzerland. J. Clin. Microbiol. 44:961-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willi, B., F. S. Boretti, V. Cattori, S. Tasker, M. L. Meli, C. Reusch, H. Lutz, and R. Hofmann-Lehmann. 2005. Identification, molecular characterization, and experimental transmission of a new hemoplasma isolate from a cat with hemolytic anemia in Switzerland. J. Clin. Microbiol. 43:2581-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willi, B., S. Tasker, F. S. Boretti, M. G. Doherr, V. Cattori, M. L. Meli, R. G. Lobetti, R. Malik, C. E. Reusch, H. Lutz, and R. Hofmann-Lehmann. 2006. Phylogenetic analysis of “Candidatus Mycoplasma turicensis” isolates from pet cats in the United Kingdom, Australia, and South Africa, with analysis of risk factors for infection. J. Clin. Microbiol. 44:4430-4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woods, J. E., M. M. Brewer, J. R. Hawley, N. Wisnewski, and M. R. Lappin. 2005. Evaluation of experimental transmission of Candidatus Mycoplasma haemominutum and Mycoplasma haemofelis by Ctenocephalides felis to cats. Am. J. Vet. Res. 66:1008-1012. [DOI] [PubMed] [Google Scholar]