Abstract
Early detection of multidrug-resistant Mycobacterium tuberculosis (MDR-TB) is of primary importance for both patient management and infection control. Optimal methods for identifying drug-resistant Mycobacterium tuberculosis in a timely and affordable way in resource-limited settings are not yet available. This study prospectively evaluated a low-technology but rapid drug susceptibility testing method, the microscopic observation drug susceptibility assay (MODS), in the concurrent detection of M. tuberculosis and its susceptibilities to isoniazid and rifampin (two drugs defining multidrug-resistant M. tuberculosis) directly from sputum specimens. Sputum samples were collected from 262 smear-positive TB patients in Addis Ababa, Ethiopia. To undertake MODS, 100 μl of decontaminated samples was inoculated into a 24-well plate containing 1 ml of 7H9 broth with and without appropriate drugs. The assay uses an inverted-light microscope to detect characteristic mycobacterial growth in liquid culture. Of 262 smear-positive patients, MODS detected 254 (96.9%) and culture in Löwenstein-Jensen medium detected 247 (94.3%) (P = 0.016). For the 247 cultures, the sensitivity, specificity, and accuracy of MODS for detecting MDR-TB were 92.0, 99.5, and 98.8%, respectively, using the method of proportion as a reference (concordance, 98.8%; kappa value, 0.932). Results for MODS were obtained in a median time of 9 days. MODS is an optimal alternative method for identifying MDR-TB in a timely and affordable way in resource-limited settings.
Tuberculosis (TB), once in decline, is now experiencing a resurgence fueled in part by the human immunodeficiency virus (HIV)/AIDS pandemic. Multidrug-resistant Mycobacterium tuberculosis (MDR-TB), defined as resistant to at least isoniazid (INH) and rifampin (RIF), is complicating TB control efforts in several low- and middle-income countries. A critical mass of resistance seen in high-burden countries has great potential not only to halt the progress of TB control but also to reverse it (34). Implementation of additional strategies, such as reducing transmission by detecting cases earlier and improving infection control in settings with shared-air spaces, is urgently needed (29). Optimal methods for identifying drug-resistant M. tuberculosis in a timely and affordable way in resource-limited settings are not yet available. The currently available drug susceptibility testing (DST) methods with solid media are inexpensive but slow and laborious and cannot meet such a demand. Liquid automated commercial systems (13, 14, 20, 28), such as the BACTEC MGIT 960 (Becton Dickinson, Sparks, MD), are rapid but require heavy, expensive equipment, have high running costs, and are technically complex. Molecular genetic methods (16, 17, 22, 29) are fast but too expensive and require well-trained manpower in order to be used in resource-poor settings. In addition, not all mutations conferring resistance to anti-TB drugs are known. Several low-technology methods (1, 2, 8, 10, 26, 30, 32) are also available. Most are indirect DST methods requiring more time and considerable laboratory expertise. The microscopic observation drug susceptibility assay (MODS) is a low-technology, direct DST method which detects resistance to INH and RIF directly from sputa, with positive results available within 2 weeks in most cases. The assay was developed and evaluated in Peru (9, 24, 25). Introducing such inexpensive but rapid tests in resource-poor settings, like Ethiopia, after evaluation is timely for improving both patient management and infection control. This study, using 262 smear-positive sputum samples, had three specific objectives: (i) to compare the sensitivity of detection of mycobacterial growth by MODS with that by standard culture using Löwenstein-Jensen (LJ) medium, (ii) to compare INH and RIF DST using MODS directly on sputum specimens with the indirect method of proportion (MOP) on agar media, and (iii) to determine the time elapsed between the processing of the sputum specimen and the availability of culture detection and susceptibility results by MODS.
MATERIALS AND METHODS
Study design.
This was a cross-sectional, hospital-based test validation study. Samples were collected from 262 TB patients (one sample per patient) visiting St. Peter's TB-specialized hospital in Addis Ababa, Ethiopia. A patient was eligible for inclusion in the study if registered as a sputum smear-positive patient (newly or previously treated but not on TB treatment) according to the WHO/IUATLD definition. Both male and female patients belonging to all age groups were included. After collection, samples were transported and stored in a cold chain. The study was reviewed and approved by the Faculty Research and Publication Committee of the Faculty of Medicine, Addis Ababa University, and the Research and Ethical Clearance Committee of the Ethiopian Health and Nutrition Research Institute.
Sputum digestion and decontamination.
All sputum specimens were digested and decontaminated using the modified Petroff method, which is used routinely in the Ethiopian national TB reference laboratory. An equal volume of sputum was added to 2 ml of 4% sodium hydroxide. The sodium hydroxide-sputum mixture was vortexed for 1 min until homogenized and then left to stand for 15 min, after which 20 ml of phosphate buffer solution (pH 6.8) was added. After centrifugation for 15 min at 3,000 × g, the supernatant was decanted and the pellet was resuspended into 2 ml of 7H9 broth containing OADC (oleic acid, albumin, dextrose, and catalase) (Becton, Dickinson and Company, Sparks, MD).
M. tuberculosis growth detection and direct antimicrobial susceptibility test using MODS.
Middlebrook 7H9 broth was prepared by using a Middlebrook 7H9 broth base (Difco, Detroit, MI), 0.31% of glycerol (Sigma Chemical Co., St. Louis, MO), 0.125% of Bacto casitone, and 10% of OADC containing PANTA antibiotic (Becton, Dickinson and Company, Sparks, MD). Antibiotic stock solutions were added to give final critical concentrations of 0.1 and 0.4 μg/ml for INH and 2 μg/ml for RIF. In a sterile 24-well plate, 1 ml of drug-free broth was distributed in the first row. In the other three rows, 1 ml of broth with isoniazid at 0.1 and 0.4 μg/ml and rifampin at 2 μg/ml, respectively, was distributed. Each column of the plate was used to test one sample (at least one sample was left uninoculated as a broth control), and four to five samples were run in a single plate. The reference strain, H37Rv MTB (ATCC 27294), was included in each test batch as a quality control for both culture and susceptibility testing. One hundred microliters of decontaminated sputum samples was inoculated into control wells containing drug-free media and also into wells of drug-containing media. The plate was then covered with its lid and securely sealed throughout its edge using polyethylene tape. The date of inoculation and plate number were recorded on the plate. The dates, plate numbers, and corresponding isolate numbers were noted on a plate layout laboratory worksheet prepared for the recording of results. Each plate was placed in a polyethylene bag to prevent evaporation and incubated at 37°C. A convenience sample of 19 specimens selected randomly (every 13th sample) was processed in duplicate to check for reproducibility.
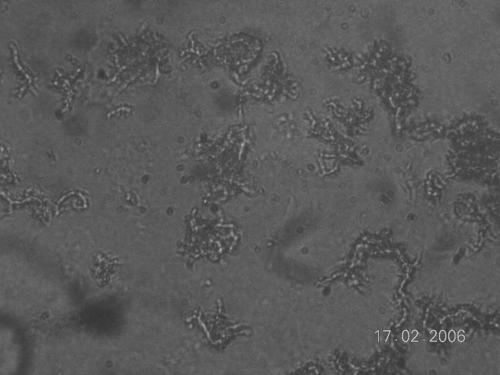
Observation of drug-free control wells was commenced on day 3 of incubation and performed each day, using an inverted-light microscope at ×20 magnification, except on holidays. After 15 days of incubation, observation was limited to once or twice a week. Growth was defined as the emergence of visually detectable serpentine clusters of bacteria (Fig. 1). Drug susceptibility results were interpreted on the same day that distinct growth was visualized in control wells. A sample was considered susceptible if growth was visible in the drug-free well but not in the drug-containing well. A sample was considered resistant if both the drug-free and the drug-containing wells showed visible growth (7, 9, 27).
FIG. 1.
Characteristic serpentine structure of young M. tuberculosis colonies grown in Middlebrook 7H9 broth for MODS, as seen under an inverted-light microscope (original magnification, ×20).
Growth detection using LJ medium.
The resuspended sediment (100 μl) was inoculated on two tubes of LJ medium. Each slant was properly labeled with the sample number and date of inoculation. The cultures were incubated at 37°C for 8 weeks or until growth of colonies was observed; they were inspected first after 48 h and then weekly. Each isolate was examined for morphology and pigmentation; the week of the appearance of colonies was noted. If there was no growth by 8 weeks or if contamination was present, the cultures were discarded and the laboratory forms completed accordingly. All positive cultures were kept at −20°C as a backup for further antimicrobial susceptibility testing using MOP. In addition to cultural characteristics, nitrate reductase and 68°C catalase tests were used for identification of M. tuberculosis (18, 33).
Agar-based MOP for DST.
Indirect MOP was undertaken for all isolates (in LJ media) using Middlebrook 7H10 (Difco, Detroit, MI) supplemented with OADC (Becton, Dickinson and Company, Sparks, MD) and glycerol (Sigma Chemical Co., St. Louis, MO) containing critical drug concentrations of 0.2 and 1 μg/ml for INH and 1 μg/ml for RIF. Interpretation was made as described by Kent and Kubica (18).
Statistical analysis.
All data were entered and managed using SPSS version 11 (SPSS, Chicago, IL) for Windows (Microsoft Corp., Redmond, WA). Descriptive statistics were analyzed using this package, and 2-by-2 contingency tables were used for calculation of diagnostic parameters, such as sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and test efficiency. McNemar's chi-square test was used to test the null hypothesis of equality between results for MODS and the reference methods.
RESULTS
M. tuberculosis culture detection by MODS and Löwenstein-Jensen media.
MODS detected 254 (96.9%) of the 262 smear-positive sputum cultures, whereas the LJ method detected 247 (94.3%) (P = 0.016). Contamination was observed in one (0.4%) and four (1.5%) cultures in MODS and the LJ method, respectively. Seven (2.7%) samples for MODS and 11 (4.2%) for the LJ method were negative (Table 1). In sample-free control wells containing only broth with or without drugs, no growth was observed, indicating no MODS cross-contamination. In MODS, 100% concordance was found for samples run in duplicate. Based on growth characteristics in both broth and solid media and biochemical test results, all MODS and LJ isolates were classified as M. tuberculosis complex.
TABLE 1.
Culture detection by MODS and LJ of sputa from smear-positive patients
| Test outcomea | Frequency (%) |
|---|---|
| Both positive | 247 (94.3) |
| MODS+/LJ− | 4 (1.5) |
| MODS+/LJ contaminated | 3 (1.1) |
| Both contaminated | 1 (0.4) |
| Both negative | 7 (2.7) |
| Total | 262 (100) |
+, Positive; −, negative.
TAT for MODS.
The time elapsed from the date of inoculation (sample processing) to the date of positive-result availability (concurrent detection and susceptibility results) for each sample was registered as a turnaround time (TAT) for MODS (Table 2). TAT for MODS ranged from 5 to 29 days, with a median of 9 days.
TABLE 2.
Frequency table of MODS TAT for culture-positive sputa
| MODS TAT (no. of days) | Frequency | % | Cumulative % |
|---|---|---|---|
| 5 | 5 | 2.0 | 2.0 |
| 6 | 25 | 9.8 | 11.8 |
| 7 | 37 | 14.6 | 26.4 |
| 8 | 39 | 15.4 | 41.7 |
| 9 | 27 | 10.6 | 52.4 |
| 10 | 36 | 14.2 | 66.5 |
| 11 | 42 | 16.5 | 83.1 |
| 12 | 13 | 5.1 | 88.2 |
| 13 | 8 | 3.1 | 91.3 |
| 14 | 11 | 4.3 | 95.7 |
| 15 | 2 | 0.8 | 96.5 |
| 17 | 3 | 1.2 | 97.6 |
| 18 | 3 | 1.2 | 98.8 |
| 19 | 1 | 0.4 | 99.2 |
| 21 | 1 | 0.4 | 99.6 |
| 29 | 1 | 0.4 | 100.0 |
| Total | 254 | 100.0 |
Comparison of MODS with the reference MOP using agar media.
Of 246 isolates tested for susceptibility to INH at the low concentration (0.1 μg/ml), results obtained by MODS and MOP using agar media showed agreement for 237 (96.3%) isolates (187 susceptible and 50 resistant) (Table 3). Results for INH at the high concentration (0.4 μg/ml) showed agreement for 238 (96.7%) isolates (195 susceptible and 43 resistant). Of 247 isolates tested for susceptibility to RIF, complete agreement between MODS and MOP results was found for 244 (98.8%) strains (220 susceptible and 24 resistant). For the detection of MDR-TB, results for the two methods agreed for 244 (98.8%) strains (23 MDR and 221 not MDR) (Table 3).
TABLE 3.
Susceptibilities of M. tuberculosis isolates to INH and RIF as determined by MODS and MOP using Middlebrook 7H10 media
| Druga | Total no. of strains tested | No. (%) of isolates with indicated resultb
|
Agreement data
|
|||||
|---|---|---|---|---|---|---|---|---|
| S by both methods | MODS S, 7H10 R | MODS R, 7H10 S | R by both methods | No. (%) of isolates with:
|
Kappa valuec (P < 0.001) | |||
| Concordance | Discordance | |||||||
| INHL | 246 | 187 (76.0) | 3 (1.2) | 6 (2.4) | 50 (20.3) | 237 (96.3) | 9 (3.7) | 0.894 |
| INHH | 246 | 195 (79.3) | 5 (2.0) | 3 (1.2) | 43 (17.5) | 238 (96.7) | 8 (3.3) | 0.895 |
| RIF | 247 | 220 (89.1) | 2 (0.8) | 1 (0.4) | 24 (9.7) | 244 (98.8) | 3 (1.2) | 0.934 |
| MDR | 247 | 221 (89.5) | 2 (0.8) | 1 (0.4) | 23 (9.3) | 244 (98.8) | 3 (1.2) | 0.932 |
Drugs were used at the following concentrations (in micrograms per milliliter): INH at low concentrations, 0.1 (MODS) and 0.2 (7H10); INH at high concentrations, 0.4 (MODS) and 1.0 (7H10); RIF, 2.0 (MODS) and 1.0 (7H10).
S, susceptible; R, resistant.
The kappa value is a measure of test reliability, with the values interpreted as follows: <0.4, poor; 0.4 to 0.75, fair to good; >0.75, strong.
Performance characteristics, using the MOP result as the reference standard, are shown in Table 4. Specificities, i.e., abilities to detect true susceptibility, were 96.9 and 98.5% for INH at the low and high concentrations, respectively, and 99.5% for both RIF and MDR-TB detection. Sensitivities, i.e., abilities to detect true resistance, were 94.3 and 89.6% for INH at the low and high concentrations, respectively, and 92.3 and 92% for RIF and MDR-TB detection, respectively.
TABLE 4.
Accuracy and reliability data for MODS compared with those for MOP using agar media (Middlebrook 7H10)
| Drug | Patient group | Specificity (%) | Sensitivity (%) | NPV (%) | PPV (%) | Efficiency |
|---|---|---|---|---|---|---|
| INHL | New | 97.3 | 92.3 | 98.9 | 82.8 | 0.967 |
| Previously treated | 90.0 | 96.3 | 90.0 | 96.3 | 0.946 | |
| Combined | 96.9 | 94.3 | 98.4 | 89.3 | 0.964 | |
| INHH | New | 99.5 | 95.7 | 99.5 | 95.7 | 0.990 |
| Previously treated | 83.3 | 84.0 | 71.4 | 91.3 | 0.838 | |
| Combined | 98.5 | 89.6 | 97.5 | 93.5 | 0.967 | |
| RIF | New | 100.0 | 100.0 | 100.0 | 100.0 | 1.000 |
| Previously treated | 94.7 | 89.5 | 89.5 | 94.4 | 0.919 | |
| Combined | 99.5 | 92.3 | 99.1 | 96 | 0.988 | |
| MDR | New | 100.0 | 100.0 | 100.0 | 100.0 | 1.000 |
| Previously treated | 94.4 | 89.5 | 89.5 | 94.4 | 0.919 | |
| Combined | 99.5 | 92 | 99.1 | 95.8 | 0.988 |
There was no statistically significant difference between the results for the two DST methods for either drug (for INH at the low concentration [P = 0.453 for new patients and P = 1 for previously treated patients], for INH at the high concentration [P = 1 for new patients and P = 0.687 for previously treated patients], and for RIF and MDR-TB detection [P = 1 for both new and previously treated patients]).
DISCUSSION
In African settings with high HIV prevalences, not only MDR-TB but also extensively drug-resistant (XDR) M. tuberculosis (defined as resistant to at least isoniazid, rifampin, any fluoroquinolone, and either aminoglycosides [amikacin, kanamycin] or capreomycin or both) is emerging (5, 11, 12, 35). The recent emergence of a cluster of XDR-TB cases in KwaZulu Natal, South Africa, has demonstrated devastating effects on patients and health care workers, alarmingly high mortality rates in those coinfected with HIV, and a rapid nosocomial spread (11, 12). An essential component of the response to drug-resistant M. tuberculosis is a wide-scale application of rapid diagnostic tests capable of identifying drug resistance in order to initiate appropriate treatment earlier and halt further transmission.
MODS is a rapid but relatively simple test which was developed recently in Peru. This novel test permits inexpensive, rapid, and effective detection of the organism responsible for TB and MDR-TB directly from sputa (9, 25). Cost is an important factor for any health intervention, particularly in resource-limited settings. An analysis by Caviedes et al. (9) indicated that the cost of MODS is lower than those of other available DST methods. In this study, MODS was evaluated for its performance in concurrent culture detection of M. tuberculosis and identification of MDR-TB among 262 smear-positive tuberculosis patients. This study is the first evaluation of MODS in a high-TB-burden African country, where such a test is needed the most.
Culture detection rate was significantly higher in MODS (96.9%) than the LJ method (94.3%) (P = 0.016). Similar results were obtained in four independent studies undertaken in Peru. In a large study using a broad group of patients, Moore et al. (24) reported culture detection sensitivities of 97.8, 89.0, and 84.0% for the MODS, automated, and LJ mycobacterial methods, respectively. In another study of selected patients, Moore et al. (25) also reported culture detection rates of 94% for MODS and 86.9% for the LJ method. Caviedes et al. (9) observed comparable culture detection rates for MODS (92%) and the most sensitive comparator, MGIT (93%). In the same study, methods using solid media were less sensitive than MODS, with detection rates of 76% for LJ and 78% for microagar 7H11 (9). Mayta et al. (22) also reported culture detection rates of 94.9% for MODS and 90.2% for the LJ method. The culture detection rate in the present study is high in part because our study was based solely on known smear-positive patients. Theoretically, the percentage of specimens positive by smear but negative by culture should be less than 1%, and most smear-positive, culture-negative specimens are seen in patients who are taking antimicrobial therapy (21). Laboratory errors, prolonged specimen decontamination, shortened incubation times of culture, cross-contamination of smears, and use of water and stains contaminated with acid-fast organisms can result in smear-positive, culture-negative specimens (21). A decontamination method with sodium hydroxide (a modified Petroff method) routinely used in this laboratory and applied for this study could be partly associated with smear-positive, culture-negative results. This is supported by our observed contamination rate of 1.5% for the LJ method: a contamination rate below 3% may indicate an overharsh decontamination lethal to mycobacteria (23). Liquid systems, such as BACTEC-MGIT, allow rapid growth of mycobacteria (within 1 to 3 weeks), compared to solid media, where growth takes 3 to 8 weeks, but provide an opportunity to examine colony morphology and detect mixed cultures (3). However, in addition, MODS allows detection of contaminants and examination of colonies under the microscope.
Rapid detection of MDR-TB was possible using MODS. In this study, the median time required to detect M. tuberculosis and its pattern of resistance to INH and RIF directly from sputa was 9 days, with a range of 5 to 29 days. Results for 96.5% of 254 culture-positive sputa were available within 15 days. Using studies with a large data set, Moore et al. (24) reported a median time of 7 days in a MODS-experienced laboratory. Besides providing early drug susceptibility results, MODS requires minimal workload, cost, and space and presents the least possible biohazard risk (four to five samples are handled in a single plate, results are read rapidly, and subsequently, materials are discarded in less time than in the conventional methods). An alternative direct agar dilution susceptibility test is a recognized inexpensive alternative that can provide DST results within 3 to 4 weeks (18, 20). However, direct agar DST can be confounded by bacterial contamination; under- or overgrowth in controls, which invalidates about 15% of tests; and potential inactivation of the test drug during prolonged incubation. Libonati et al. (20) reported that direct agar DST provided reportable results in only 41% of smear-positive and 62% of culture-positive cases (20). Other low-technology techniques, such as colorimetric methods (microplate alamar blue and tetrazolium microplate assays) and nitrate reductase assays using both solid and liquid media, have been described previously (2, 4, 10, 26, 30). Most are indirect tests requiring pure M. tuberculosis isolates (which take at least 3 weeks to obtain and involve the potential laboratory biohazard of culture manipulation); this method requires more time than MODS and considerable laboratory expertise. Promising results have been reported for a direct colorimetric assay, with a sensitivity above 90.5% and a specificity of 100% (1, 32). However, available performance evaluations are based on small sample sizes and a lack of field testing and are more costly than MODS (8). Direct assessment of cultural growth characteristics of M. tuberculosis in broth media in the direct colorimetric assay is impossible, and ruling out growth of organisms other than M. tuberculosis is unreliable.
For 247 culture-positive sputa, DST agreement between MODS and the reference susceptibility method was excellent (kappa value of 0.9 and concordance above 95%). For testing of INH resistance, two critical concentrations (low [0.1 μg/ml] and high [0.4 μg/ml]) were applied. This has clinical importance, since for patients whose isolates are resistant to the low concentration only, therapeutic effects may be achieved with an adjustment in dosage (15). Compared with those for conventional methods, the agreement rates for both the low and the high concentrations were excellent. However, for INH at the low concentration, MODS had a lower positive (82.8%) than negative (98.9%) predictive value and generally overdiagnosed resistance more than the conventional methods of proportion, particularly in new cases.
For RIF, a critical concentration of 2 μg/ml was used in this study. A 100% agreement was obtained for newly diagnosed patients. Agreement was still high for retreatment patients; however, two (0.8%) resistant patients were misclassified as susceptible by MODS. Caviedes et al. (9) reported that MODS misclassifies more isolates as resistant at 0.5-μg/ml critical concentrations than at 1 μg/ml. At 2 μg/ml, using indirect MODS, Park et al. (27) reported an agreement of 100% with MOP, using the 7H10 agar method as the reference.
The performance results for MODS in the detection of MDR-TB are similar to those obtained in other studies (9, 24, 25, 27). The combined results for INH and RIF (in MDR-TB detection) had excellent agreement rates (kappa value, 0.932; concordance, 98.8%) compared with those for the conventional methods of proportion. A somewhat high PPV (94.4%), compared with a lower NPV (89.5%), for retreatment patients in our study suggests that MODS very slightly underdiagnosed MDR-TB. In contrast, Moore et al. (25) report an overall lower PPV (81.8%) than NPV (97.7%), suggesting an overdiagnosis of disease caused by MDR organisms. This discrepancy may be explained by the use of differing critical concentrations for RIF: 2 μg/ml in the present study and 1 μg/ml in the previous one (25).
Even between well-standardized drug susceptibility tests, agreement is not 100%. There is no “gold standard” by which to judge a typing method or to consistently define true-positive or true-negative results (6). One advantage of MODS is that it is flexible enough to be adjusted for the local context (by changing the critical concentration). For example, by lowering the critical cutoff points for RIF to 1 μg/ml, MODS can be used as a screening test to permit much earlier isolation of infectious patients who have a high likelihood of MDR-TB infection (thereby reducing MDR-TB transmission) and could facilitate more-focused DST practices for first- and second-line TB drugs and more-efficient use of resources.
This work evaluated MODS performance only in the detection of INH and RIF resistance. This approach was adopted because these two drugs are the key elements in short-course TB chemotherapy and provide the most-robust drug susceptibility results (13). Moreover, the only susceptibility data that are likely to be usefully translated into clinical action (in terms of a change in drug regimen) in a programmatic setting would be identification of MDR-TB (25). Another consideration was that previous studies using MODS for streptomycin and ethambutol indicated less favorable outcomes, in part due to a lack of clearly defined cutoff points for these two drugs (25). This is also seen with other well-standardized methods, as resistance results for ethambutol and streptomycin are less reliable than those for INH and RIF (19). Yet, discrimination between probably resistant and probably susceptible strains is more reliable for INH and RIF than for other drugs (19).
This study has a limitation, as it was based on smear-positive cases only. However, in operational studies which included patients with suspected TB, prescreened patients at high risk, and patients with HIV infection, MODS has a significantly higher sensitivity than automated or LJ culture (24, 31). In countries with high prevalences of smear-negative TB, the high sensitivity of MODS and the rapid and concurrent availability of drug susceptibility results may help to increase the proportion of microbiologically confirmed patients and thereby reduce the number of empirically treated cases (24, 31). To further assess the feasibility, impact, and cost-effectiveness of MODS, it is necessary to establish large-scale demonstration projects in high-burden-TB and high-prevalence-HIV settings to provide further evidence for policy change and further adoption in resource-limited settings, like Ethiopia.
Footnotes
Published ahead of print on 24 January 2007.
REFERENCES
- 1.Abate, G., A. Aseffa, A. Selassie, S. Goshu, B. Fekade, D. WoldeMeskel, and H. Miörner. 2004. Direct colorimetric assay for rapid detection of rifampin-resistant Mycobacterium tuberculosis. J. Clin. Microbiol. 42:871-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abate, G., R. N. Mshana, and H. Miörner. 1998. Evaluation of a colorimetric assay based on 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) for rapid detection of rifampicin resistance in Mycobacterium tuberculosis. Int. J. Tuber. Lung Dis. 2:580-584. [PubMed] [Google Scholar]
- 3.American Thoracic Society. 2000. Diagnostic standards and classification of tuberculosis in adults and childern. Am. J. Respir. Crit. Care Med. 161:1376-1395. [DOI] [PubMed] [Google Scholar]
- 4.Ángeby, K. A. K., L. Klintz, and S. E. Hoffner. 2002. Rapid and inexpensive drug susceptibility testing of Mycobacterium tuberculosis with a nitrate reductase assay. J. Clin. Microbiol. 40:553-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anonymous. 2006. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs—worldwide, 2000-2004. Morb. Mortal. Wkly. Rep. 55:301-305. [PubMed] [Google Scholar]
- 6.Arbeit, R. D. 1999. Laboratory procedures for epidemiologic analysis of microorganisms, p. 116-137. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, DC.
- 7.Caviedes, L., J. Coronel, C. Evans, B. Gilman, and D. Moore. 2004. MODS—a user guide. [Online.] http://www.upch.edu.pe/facien/dbmbqf/mods/mods.htm.
- 8.Caviedes, L., J. Delgado, and R. H. Gilman. 2002. Tetrazolium microplate assay as a rapid and inexpensive colorimetric method for determination of antibiotic susceptibility of Mycobacterium tuberculosis. J. Clin. Microbiol. 40:1873-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caviedes, L., T.-S. Lee, R. H. Gilman, P. Sheen, E. Spellman, E. H. Lee, D. E. Berg, S. Montenegro-James, and the Tuberculosis Working Group in Peru. 2000. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. J. Clin. Microbiol. 38:1203-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins, L., and S. Franzblau. 1997. Microplate alamar blue assay versus BCTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. J. Clin. Microbiol. 41:1004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donald, E., and A. V. Rie. 2006. XDR tuberculosis: an indicator of public-health negligence. Lancet 368:1554-1556. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi, N. R., A. Moll, A. W. Sturm, R. Pawinski, T. Govender, U. Lalloo, K. Zeller, J. Andrews, and G. Friedland. 2006. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368:1575-1580. [DOI] [PubMed] [Google Scholar]
- 13.Goloubeva, V., M. Lecocq, P. Lassowsky, F. Matthys, F. Portaels, and I. Bastian. 2001. Evaluation of Mycobacteria Growth Indicator Tube for direct and indirect susceptibility testing of Mycobacterium tuberculosis from respiratory specimens in a Siberian prison hospital. J. Clin. Microbiol. 39:1501-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heifets, L. B., and G. A. Cangelosi. 1999. Drug susceptibility testing of Mycobacterium tuberculosis: a neglected problem at the turn of the century. Int. J. Tuber. Lung Dis. 3:541-581. [PubMed] [Google Scholar]
- 15.Inderlied, C. B., and M. Salfinger. 1999. Antimicrobial agents and susceptibility tests, p. 1601-1623. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, DC.
- 16.Johansen, I. S., B. Lundgren, A. Sosnovskaja, and V. Ø. Thomsen. 2003. Direct detection of multidrug-resistant Mycobacterium tuberculosis in clinical specimens in low- and high-incidence countries by line probe assay. J. Clin. Microbiol. 41:4454-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juréen, P., J. Werngren, and S. E. Hoffner. 2004. Evaluation of the line probe assay (LiPA) for rapid detection of rifampicin resistance in Mycobacterium tuberculosis. Tuberculosis 84:311-316. [DOI] [PubMed] [Google Scholar]
- 18.Kent, P. T., and G. P. Kubica. 1985. Public health mycobacteriology: a guide for the level III laboratory. Centers for Disease Control, Atlanta, GA.
- 19.Kim, S. J. 2005. Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur. Respir. J. 25:564-569. [DOI] [PubMed] [Google Scholar]
- 20.Libonati, J. P., C. E. Stager, J. R. Davis, and S. H. Siddiqi. 1988. Direct antimicrobial drug susceptibility testing of Mycobacterium tuberculosis by the radiometric method. Diagn. Microbiol. Infect. Dis. 10:41-48. [DOI] [PubMed] [Google Scholar]
- 21.Lipsky, G. J., J. Gates, F. C. Tenover, and J. Plorde. 1984. Factors affecting the clinical value for acid-fast bacilli. Rev. Infect. Dis. 6:214-222. [DOI] [PubMed] [Google Scholar]
- 22.Mayta, H., R. H. Gilman, F. Arenas, T. Valencia, L. Caviedes, S. H. Montenegro, E. Ticona, J. Ortiz, R. Chumpitaz, C. A. Evans, D. L. Williams, and the Tuberculosis Working Group in Peru. 2003. Evaluation of PCR-based universal heteroduplex generator assay as a tool for rapid detection of multidrug-resistant Mycobacterium tuberculosis in Peru. J. Clin. Microbiol. 41:5774-5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metchock, B. G., F. S. Nolte, and R. J. Wallace. 1999. Mycobacterium, p. 399-437. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, DC.
- 24.Moore, D. A. J., C. A. W. Evans, R. H. Gilman, L. Caviedes, J. Coronel, A. Vivar, E. Sanchez, Y. Pinedo, J. C. Saravia, C. Salazar, R. Oberhelman, M. G. Hollm-Delgado, D. LaChira, R. Escombe, and J. S. Friedland. 2006. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N. Engl. J. Med. 355:1539-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore, D. A. J., D. Mendoza, R. H. Gilman, C. A. W. Evans, M. G. Hollm Delgado, J. Guerra, L. Caviedes, D. Vargas, E. Ticona, J. Ortiz, G. Soto, J. Serpa, and the Tuberculosis Working Group in Peru. 2004. Microscopic observation drug susceptibility assay, a rapid, reliable diagnostic test for multidrug-resistant tuberculosis suitable for use in resource-poor settings. J. Clin. Microbiol. 42:4432-4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mshana, R. N., G. Tadess, G. Abate, and H. Miörner. 1998. Use of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide for rapid detection of rifampin-resistant Mycobacterium tuberculosis. J. Clin. Microbiol. 36:1214-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park, G. W., W. R. Bishai, R. E. Chaisson, and S. E. Dorman. 2002. Performance of microscopic observation drug susceptibility assay in drug susceptibility testing for Mycobacterium tuberculosis. J. Clin. Microbiol. 40:4750-4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rüsch-Gerdes, S., C. Domehl, G. Nardi, M. R. Gismondo, H. M. Welscher, and G. E. Pfyffer. 1999. Multicenter evaluation of the mycobacterium growth indicator tube for testing susceptibility of Mycobacterium tuberculosis to first-line drugs. J. Clin. Microbiol. 37:45-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skendersn, G., A. M. Fry, I. Prokopovica, S. Greckoseja, L. Broka, B. Metchock, T. H. Holtz, C. D. Wells, and V. Leimane. 2005. Multidrug-resistant tuberculosis detection, Latvia. Emerg. Infect. Dis. 11:1461-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syre, H., S. Phyu, P. Sandven, B. Bjorvatn, and H. M. S. Grewal. 2003. Rapid colorimetric method for testing susceptibility of Mycobacterium tuberculosis to isoniazid and rifampin in liquid cultures. J. Clin. Microbiol. 41:5173-5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vargas, D., L. Garcia, R. H. Gilman, C. Evans, E. Ticona, M. Navincopa, R. F. Luo, L. Caviedes, C. Hong, R. Escombe, and D. A. J. Moore. 2005. Diagnosis of sputum-scarce HIV-associated pulmonary tuberculosis in Lima, Peru. Lancet 365:150-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WoldeMeskel, D., G. Abate, M. Lakew, S. Gosh, S. Selassie, H. Miorner, and A. Aseffa. 2005. Evaluation of a direct colorimetric assay for rapid detection of rifampicin resistant Mycobacterium tuberculosis. Ethiop. J. Health Dev. 19:51-54. [Google Scholar]
- 33.World Health Organization. 1998. Laboratory service in tuberculosis control: part III, culture. WHO/TB/98.258. World Health Organization, Geneva, Switzerland.
- 34.World Health Organization. 2004. Anti-tuberculosis drug resistance in the world. Third global report. The WHO/IUATLD global project on anti-tuberculosis drug resistance surveillance 1999-2002. WHO/HTM/TB/2004.343. World Health Organization, Geneva, Switzerland.
- 35.World Health Organization. 2006. Laboratory XDR-TB definitions. Meeting of the Global XDR-TB Task Force. World Health Organization, Geneva, Switzerland.