Abstract
We report the molecular epidemiology of astrovirus infection in 335 infants with diarrhea in Wuhan City, China. Astrovirus RNA was detected in the stool specimens of 33 children (9.87%). Genotyping analysis indicated that 23 out of 24 astroviruses identified were classified as belonging to genotype 1, with highest identity (>98%) to a Mongolian strain.
Diarrhea is a major cause of childhood morbidity and mortality, especially in developing countries (2). Human astrovirus may be the second most common cause of viral gastroenteritis in young children, with rotavirus being the first (6-8, 14). Human astrovirus infections occur worldwide and have been detected in people at different ages, but mostly in children and the elderly (7). The incidence of astrovirus infection ranges from about 2 to 9% in children with gastroenteritis in both developing and developed countries (3-5, 11, 13, 15), although some studies have reported that the infection rate may be as high as 26% (10). In order to investigate the role of astrovirus infection in childhood diarrhea in Wuhan City, China, we examined astrovirus RNA in the fecal specimens of 335 infants (under the age of 3 years) from the outpatient department of Wuhan Children's Hospital between June 2004 and May 2005.
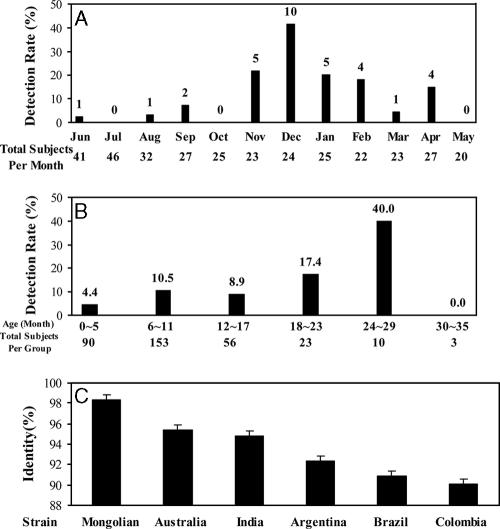
Among 335 stool specimens examined, 33 were positive for astrovirus RNA as determined by reverse transcription-PCR with the primer set Mon340/Mon348 (Invitrogen, Shanghai, China) that permits detection of all eight serotypes of human astroviruses (1). The infection rate (9.87%) of our study is in agreement with that reported in other parts of China (9) and other developing countries (5). There was no significant difference (P = 0.23) in the infection rates between boys (11.44%, 23/201) and girls (7.46%, 10/134). As demonstrated in Fig. 1A, the incidence in the winter and spring months (November 2004 to April 2005) was significantly higher (P < 0.05) than that in the summer and autumn months (June to October 2004 and May 2005). December was the month with the highest incidence of astrovirus infection (41.67%, 10/24). This seasonal pattern of astrovirus infection in Wuhan City is consistent with other epidemiological studies of temperate regions (7) and is similar to that seen in patients with rotavirus infection. The children of 24 to 29 months of age appeared to have the highest infection rate (40%, 4/10) (Fig. 1 B). However, since there were only 10 specimens collected from this age group, the significance of this finding remains to be determined.
FIG. 1.
Molecular epidemiology of astrovirus infection among children under the age of 3 years in Wuhan City, China. (A) Monthly distribution of astroviruses detected in stool specimens between June 2004 and May 2005. The numbers above the bars indicate the number of samples positive for astrovirus in each month. (B) Detection rates of astrovirus infection in different age groups. The numbers above the bars indicate the percentage of samples positive for astrovirus in each age group. (C) Comparison (percent identity) of Wuhan astrovirus strains (genotype 1, 21 out of 23 strains) with astrovirus strains from other regions in the world.
Twenty-four specimens positive for astrovirus RNA were genotyped by sequencing a 449-bp region of ORF2 of the astroviruses as previously described by Noel et al. (12). Reverse transcription-PCR was performed with the primer set Mon269/Mon270, which was considered to be more universal than the Mon244/Mon245 set (12). The purified DNA was sequenced by Shanghai Sangon Biological Engineering & Technology and Service Co., Ltd., with an ABI3730 automatic sequencer. Each nucleotide sequence of the 348-bp region was compared to that of each reference strain by using the BLAST program (National Center for Biotechnology Information); the comparison showed that 23 out of 24 viruses were classified as genotype 1 viruses, while only 1 was identified as genotype 3. Genotype 1 has been considered the most frequent and dominant type in most parts of the world, with a few exceptions (16).
Because the analysis of astrovirus genes from different regions of the world is useful for developing detection kits and a vaccine for astrovirus infection, we used MegAlign in the DNAStar program to determine the sequence identities of the Wuhan astroviruses to those from different areas in the world. The majority (87.5%, 21/24) of the astroviruses identified in this study showed very little or almost no divergence among themselves. Twenty-one of these genotype 1 viruses showed the highest identity (96.8 to 98.6%) to a Mongolian strain (GenBank accession number AY590261) and lesser identities to strains from Australia (AY175258; ca. 94.0 to 95.7%), India (AB241064; ca. 93.4 to 95.1%), Argentina (AY324859; ca. 90.8 to 92.6%), Brazil (DQ381480; ca. 89.4 to 91.1%), and Colombia (DQ157438; ca. 88.5 to 90.3%) (Fig. 1C). The remaining two strains (accession numbers DQ788596 and DQ788612) of the 23 genotype 1 astroviruses had high identity (97.7%) to a Brazilian strain (DQ381480).
Our data clearly indicate that astrovirus as an etiological agent has a significant role in infant gastroenteritis in Wuhan City. Since the majority of the astroviruses identified in the infants in Wuhan are classified as belonging to one genotype (type 1), it would be feasible to develop an effective vaccine against type 1 astrovirus infection in the Wuhan area. Further studies are needed to understand the nature of astrovirus infection and to provide strategies for the prevention and control of astrovirus infection in children in China.
Nucleotide sequence accession numbers.
The nucleotide sequences of the 348-bp regions of the 24 astroviruses have been submitted to GenBank under the following accession numbers: DQ788590 to DQ788612 and DQ630763.
Footnotes
Published ahead of print on 14 February 2007.
REFERENCES
- 1.Belliot, G., H. Laveran, and S. S. Monroe. 1997. Detection and genetic differentiation of human astroviruses: phylogenetic grouping varies by coding region. Arch. Virol. 142:1323-1334. [DOI] [PubMed] [Google Scholar]
- 2.Bern, C., J. Martines, I. de Zoysa, and R. I. Glass. 1992. The magnitude of the global problem of diarrhoeal disease: a ten-year update. Bull. W. H. O. 70:705-714. [PMC free article] [PubMed] [Google Scholar]
- 3.Bon, F., P. Fascia, M. Dauvergne, D. Tenenbaum, H. Planson, A. M. Petion, P. Pothier, and E. Kohli. 1999. Prevalence of group A rotavirus, human calicivirus, astrovirus, and adenovirus type 40 and 41 infections among children with acute gastroenteritis in Dijon, France. J. Clin. Microbiol. 37:3055-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley, B., J. O'Mahony, S. M. Morgan, C. Hill, and J. G. Morgan. 2000. Detection of sporadic cases of Norwalk-like virus (NLV) and astrovirus infection in a single Irish hospital from 1996 to 1998. J. Clin. Virol. 17:109-117. [DOI] [PubMed] [Google Scholar]
- 5.Gaggero, A., M. O'Ryan, J. S. Noel, R. I. Glass, S. S. Monroe, N. Mamani, V. Prado, and L. F. Avendano. 1998. Prevalence of astrovirus infection among Chilean children with acute gastroenteritis. J. Clin. Microbiol. 36:3691-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass, R. I., J. Noel, D. Mitchell, J. E. Herrmann, N. R. Blacklow, L. K. Pickering, P. Dennehy, G. Ruiz-Palacios, M. L. de Guerrero, and S. S. Monroe. 1996. The changing epidemiology of astrovirus-associated gastroenteritis: a review. Arch. Virol. Suppl. 12:287-300. [DOI] [PubMed] [Google Scholar]
- 7.Guix, S., S. Caballero, C. Villena, R. Bartolome, C. Latorre, N. Rabella, M. Simo, A. Bosch, and R. M. Pinto. 2002. Molecular epidemiology of astrovirus infection in Barcelona, Spain. J. Clin. Microbiol. 40:133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrmann, J. E., D. N. Taylor, P. Echeverria, and N. R. Blacklow. 1991. Astroviruses as a cause of gastroenteritis in children. N. Engl. J. Med. 324:1757-1760. [DOI] [PubMed] [Google Scholar]
- 9.Liu, C. Y., K. L. Shen, S. X. Wang, Y. Y. Liu, and G. T. Zhaori. 2004. Astrovirus infection in young children with diarrhea hospitalized at Beijing Children's Hospital. Chin. Med. J. (Engl. Ed.). 117:353-356. [PubMed] [Google Scholar]
- 10.Maldonado, Y., M. Cantwell, M. Old, D. Hill, M. L. Sanchez, L. Logan, F. Millan-Velasco, J. L. Valdespino, J. Sepulveda, and S. Matsui. 1998. Population-based prevalence of symptomatic and asymptomatic astrovirus infection in rural Mayan infants. J. Infect. Dis. 178:334-339. [DOI] [PubMed] [Google Scholar]
- 11.Mustafa, H., E. A. Palombo, and R. F. Bishop. 2000. Epidemiology of astrovirus infection in young children hospitalized with acute gastroenteritis in Melbourne, Australia, over a period of four consecutive years, 1995 to 1998. J. Clin. Microbiol. 38:1058-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noel, J. S., T. W. Lee, J. B. Kurtz, R. I. Glass, and S. S. Monroe. 1995. Typing of human astroviruses from clinical isolates by enzyme immunoassay and nucleotide sequencing. J. Clin. Microbiol. 33:797-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svenungsson, B., A. Lagergren, E. Ekwall, B. Evengard, K. O. Hedlund, A. Karnell, S. Lofdahl, L. Svensson, and A. Weintraub. 2000. Enteropathogens in adult patients with diarrhea and healthy control subjects: a 1-year prospective study in a Swedish clinic for infectious diseases. Clin. Infect. Dis. 30:770-778. [DOI] [PubMed] [Google Scholar]
- 14.Ulloa, J. C., A. Matiz, L. Lareo, and M. F. Gutierrez. 2005. Molecular analysis of a 348 base-pair segment of open reading frame 2 of human astrovirus. A characterization of Colombian isolates. In Silico Biol. 5:537-546. [PubMed] [Google Scholar]
- 15.Walter, J. E., and D. K. Mitchell. 2000. Role of astroviruses in childhood diarrhea. Curr. Opin. Pediatr. 12:275-279. [DOI] [PubMed] [Google Scholar]
- 16.Walter, J. E., D. K. Mitchell, M. L. Guerrero, T. Berke, D. O. Matson, S. S. Monroe, L. K. Pickering, and G. Ruiz-Palacios. 2001. Molecular epidemiology of human astrovirus diarrhea among children from a periurban community of Mexico City. J. Infect. Dis. 183:681-686. [DOI] [PubMed] [Google Scholar]