Abstract
We performed Etest, disk diffusion, and broth microdilution susceptibility testing of posaconazole against 146 clinical isolates of filamentous fungi. By using provisional breakpoints for comparison purposes only, categorical agreement between the results of the agar-based methods and those of broth microdilution were 96 to 98%, with no very major errors. These agar-based methods hold promise as simple and reliable methods for determining the posaconazole susceptibilities of filamentous fungi.
Infections from filamentous fungi are the causes of high rates of morbidity and mortality and result in prolonged hospitalizations and increased health care costs (2). Technological advances in health care and the increased use of immunosuppressive regimens have resulted in an increase in the incidence of invasive infections due to common and emerging filamentous fungi (5, 6, 7). Appropriate diagnosis and early therapeutic intervention are critical components in the management of these infections (11). The Clinical Laboratory and Standards Institute (CLSI; formerly NCCLS) broth microdilution M38-A method (8) is currently the standard protocol for determining the susceptibilities of filamentous fungi to antifungal agents. Accurate, alternative agar-based methods offering decreased time to reporting and relative ease of use are of great interest.
Based on the previous good correlation between the results of the broth microdilution (BMD) and the agar-based methods of susceptibility testing of posaconazole (POS) against the yeasts (10, 12) and between the results of Etest, disk diffusion (DD), and BMD for molds (3, 4), we extended the comparative analysis of three different methods—DD, Etest, and BMD—to encompass the filamentous fungi using a collection of 140 clinical isolates of Aspergillus spp. and 6 clinical isolates of zygomycetes.
A total of 146 clinical isolates of filamentous fungi, including 68 Aspergillus fumigatus isolates, 45 A. flavus isolates, 15 A. niger isolates, 9 A. terreus isolates, 3 A. niveus isolates, 2 A. nidulans isolates, 2 Mucor spp., and 4 Rhizopus spp., were collected from 2000 to 2005 and tested in parallel by DD, Etest, and BMD. Isolates were sent to a central reference laboratory on a charcoal swab with Amies medium and subcultured onto potato dextrose agar (PDA) slants (Remel, Inc.), and the identification was confirmed by conventional methods and microscopic examination. To ensure viability and purity before storage, each isolate was subcultured onto a PDA slant and incubated for 7 days to ensure maximum conidial or sporangiospore formation. The isolates were stored in vials with water at ambient room temperature until they were tested.
In preparation for testing, isolates were subcultured on PDA slants and incubated at 35°C for 7 days. Stock suspensions were made by covering the colonies with 1 ml 0.85% saline and harvesting the conidia or sporangia with gentle probing by using the tip of a transfer pipette. The stock suspensions were processed by transferring them to a sterile 15-ml glass tube containing additional saline, vortexing for 15 s, and allowing hyphal fragments to settle for 3 to 5 min. To reduce the hydophobicity of the conidia and to aid with the formation of uniform conidial suspensions, Tween 80 (diluted to a 0.2% final concentration) was added to the saline. The transmittances of the conidial suspensions were adjusted according to the CLSI M38-A protocol (8) by using a Sequoia-Turner model 340 spectrophotometer set at a wavelength of 540 nm. Suspensions were applied to the surfaces of two different agar media by using swab applicators: Mueller-Hinton (MH) agar (Difco) supplemented with 2% glucose and 0.5 μg/ml methylene blue (1) and RPMI buffered with morpholinepropanesulfonic acid and supplemented with 2% glucose (Remel, Inc.). The plates were allowed to dry for 20 min. A 5-μg POS paper disk was applied to the center of the MH agar plates, and the plates were incubated at 35°C for 24 h. POS was provided by Schering Plough, and the disks were manufactured by Becton Dickinson (Sparks, MD). Zone diameters were measured at the point where the growth significantly decreased and were recorded to the nearest millimeter by using digital calipers. POS Etest strips were applied to the RPMI agar, and the plates were incubated at 35°C for 48 h. Etest MICs were recorded at the gradient concentration where the inhibition ellipse intersected the plastic strip. From the adjusted suspensions, conidia or sporangia were further diluted to a final concentration of 0.4 × 104 to 5 × 104 CFU/ml and then transferred in 100-μl increments to a BMD panel containing 2× concentrations of POS (final concentration, 0.007 to 8.0 μg/ml). For purposes of comparison only, MIC and disk interpretive criteria for POS were the same as those recently adopted by the CLSI for voriconazole (9): susceptible, ≤1 μg/ml and ≥17 mm, respectively; susceptible dose dependent, 2 μg/ml and 14 to 16 mm, respectively; and resistant, ≥4 μg/ml and ≤13 mm, respectively.
Following incubation, the DD zone diameters and Etest MICs were plotted against the respective POS BMD MIC. The method of least squares was used to calculate a regression line for each comparison. Categorical agreement between DD and BMD and between Etest and BMD were based on the interpretive breakpoints listed above. Major errors (MEs) were defined as instances in which DD or Etest results revealed resistance and BMD revealed susceptibility. Very major errors (VMEs) were defined as instances in which the DD or the Etest results revealed susceptibility and the BMD results revealed resistance. Minor errors were defined as instances in which one test result was susceptible or resistant and the comparator test was susceptible dose dependent.
Quality control was performed by using the methods described in the CLSI M38-A document. American Type Culture Collection (ATCC) isolates Candida krusei (ATCC 6258) and Candida parapsilosis (ATCC 22019) were used to monitor the testing conditions and performance.
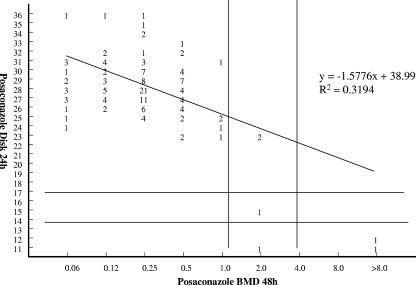
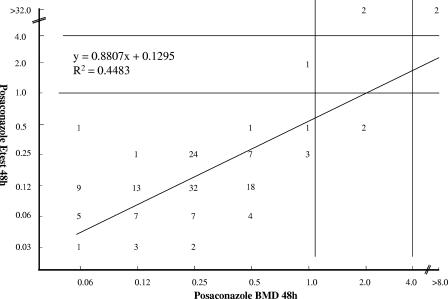
By BMD testing, 95.9% of the 146 filamentous fungi were susceptible to POS, 2.7% were susceptible dose dependent, and 1.4% were resistant. The overall categorical agreement between DD and the reference BMD method for all molds tested was 97.9%, with 0% VMEs, 0% MEs, and 2.1% minor errors (Fig. 1). The categorical agreement between DD and BMD for Aspergillus spp. only was 100% (data not shown). The categorical agreement between Etest and the reference BMD method was 96.6%, with 0% VMEs, 0% MEs, and 3.4% minor errors (Fig. 2). The agreement between Etest and BMD for Aspergillus spp. was 99.3%, with 0% VMEs, 0% MEs, and 0.7% minor errors (data not shown). DD and Etest were both 100% sensitive for the detection of isolates that were POS resistant (≥4 μg/ml) by the BMD method.
FIG. 1.
Comparison of DD zone diameter (24 h) and the results of the BMD reference method reveals categorical agreement for all molds tested of 97%, with 0% VMEs, 0% MEs, and 2.1% minor errors.
FIG. 2.
Comparison of agar-based Etest MICs (48 h) and the results of the BMD reference method reveals categorical agreement for all molds tested of 96.6%, with 0% VMEs, 0% MEs, and 3.4% minor errors.
The regression statistics showed statistically significant correlations between both the DD zone diameters and the Etest MICs with the BMD MICs (Fig. 1 and 2).
The POS disk diffusion and Etest antifungal susceptibility test results demonstrated excellent agreement with the results of the CLSI M38-A BMD method (categorical agreement, 96 to 98%). While this study was limited by the small numbers of isolates that were “resistant” to POS, the correlation between the DD and Etest results and the reference BMD results suggests that both agar-based methods demonstrate promise as reliable alternatives for determining the susceptibilities of filamentous fungi to POS. These results are consistent with previous data from Espinel-Ingroff and Rezusta (3) and Espinel-Ingroff (4), which have demonstrated essential agreement (within 3 dilutions) between POS Etest and BMD of 94 to 97% for Aspergillus and other filamentous fungi (3, 4) and a significant correlation between the POS disk diameters and the BMD MICs for filamentous fungi (4). Our data confirm and extend these findings by including isolates from a multicenter, international surveillance network and by generating an estimate of categorical agreement rates by using sample (provisional) breakpoints. Future multicenter, collaborative studies should include more isolates of filamentous fungi that demonstrate elevated POS MICs.
Acknowledgments
This study was supported in part by research and educational grants from Schering Plough Research Institute.
Footnotes
Published ahead of print on 31 January 2007.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2004. Method for antifungal disk diffusion susceptibility testing of yeasts: approved guidance M44-A. Clinical and Laboratory Standards Institute, Wayne, PA.
- 2.Dasbach, E., G. Davies, and S. Teutsch. 2000. Burden of aspergillus-related hospitalizations in the United States. Clin. Infect. Dis. 31:1524-1528. [DOI] [PubMed] [Google Scholar]
- 3.Espinel-Ingroff, A., and A. Rezusta. 2002. Etest method for testing susceptibilities of Aspergillus spp. to the new triazoles voriconazole and posaconazole and to established antifungal agents: comparison with NCCLS broth microdilution method. J. Clin. Microbiol. 40:2101-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espinel-Ingroff, A. 2006. Comparison of three commercial assays and a modified disk diffusion assay with two broth microdilution reference assays for testing zygomycetes, Aspergillus spp., Candida spp., and Cryptococcus neoformans with posaconazole and amphotericin B. J. Clin. Microbiol. 44:3616-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwen, P. C., M. E. Rupp, and S. H. Hinrichs. 1997. Invasive mold sinusitis: 17 cases in immunocompromised patients and review of the literature. Clin. Infect. Dis. 24:1178-1184. [DOI] [PubMed] [Google Scholar]
- 6.Lortholary, O., M.-C. Meyohas, B. Dupont, J. Cadranel, D. Salmon-Ceron, D. Peyramond, D. Simonin, et al. 1993. Invasive aspergillosis in patients with acquired immunodeficiency syndrome: report of 33 cases. Am. J. Med. 95:177-187. [DOI] [PubMed] [Google Scholar]
- 7.Marr, K., R. Carter, F. Crippa, A. Wald, and L. Corey. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34:909-917. [DOI] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 9.Pfaller, M. A., D. J. Diekema, J. H. Rex, A. Espinel-Ingroff, E. M. Johnson, D. Andes, V. Chaturvedi, M. A. Ghannoum, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, P. Troke, T. J. Walsh, and D. W. Warnock. 2006. Correlation of MIC with outcome for Candida species tested against voriconazole: analysis and proposal for interpretive breakpoints. J. Clin. Microbiol. 44:819-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfaller, M. A., S. A. Messer, K. Mills, et al. 2001. Evaluation of Etest method for determining posaconazole MICs for 314 clinical isolates of Candida species. J. Clin. Microbiol. 39:3952-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rapp, R. P. 2004. Changing strategies for the management of invasive fungal infections. Pharmacotherapy 24(2 pt 2):4S-28S. [PubMed] [Google Scholar]
- 12.Sims, C. R., V. L. Paetznick, J. R. Rodriguez, et al. 2006. Correlation between microdilution, Etest, and disk diffusion methods for antifungal susceptibility testing of posaconazole against Candida spp. J. Clin. Microbiol. 44:2105-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]