Abstract
Bovine vaccinia virus outbreaks have been occurring in different regions of Brazil. We report here the time course of natural human infection by vaccinia virus and describe important clinical and epidemiological aspects of this zoonotic infection. The diagnosis of vaccinia virus infection was based on clinical, serological, and molecular procedures.
CASE REPORT
We examined the clinical and epidemiological aspects of one case of zoonotic human infection during a bovine vaccinia virus outbreak and characterized the causative agent as a vaccinia virus (VACV) strain.
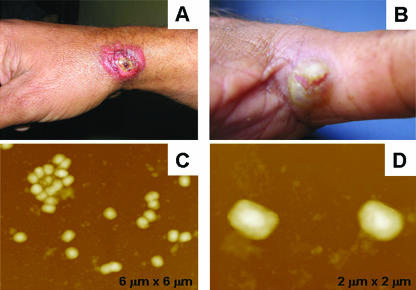
In August 2000, vesicular and exanthemous disease outbreaks affecting dairy cattle and milkers were observed on several farms in Minas Gerais, the most important milk-producing State in Brazil. At one farm, a smallpox unvaccinated 19-year-old male dairy farm milker (Patient one) became sick and developed many lesions after being in contact with cows presenting lesions on their udders. These lesions started as itchy points on the hands, followed by the appearance of local edema and, after 3 days, the patient reported fever, headache, exhaustion, and vesicles on his hands. After a period of 3 or 4 days, these vesicles evolved to ulcerated lesions mainly characterized as umbilicated pustules surrounded by inflammatory tissue and painful ulcers on the hands and fingers (Fig. 1A and B). At this time, the milker reported the presence of peripheral erythema and lymphadenitis, with enlarged lymph nodes and a secondary bacterial infection on the lesions. Despite having the clinical symptoms of systemic illness, the patient did not cease working. Only on day 10 was the patient admitted at the local public hospital and received treatment based on fever and pain relievers and topical antibiotics due to the secondary bacterial infection. A serum sample and a biopsy from the necrotic black scab were collected and sent to the laboratory in order to establish the etiology of the lesions. Antibodies to orthopoxvirus were detected in the patient's serum sample by a serum neutralization assay. The biopsy sample was then ground, homogenized, and inoculated onto a chorioallantoic membrane of embryonated chicken eggs where, after 72 h, white and opaque classical pock formations were observed, suggesting a VACV infection (3, 6, 9). The “pocks” were used for virus propagation in Vero cells, where a typical cytopathic effect was also observed. The isolated virus showed typical poxvirus brick-shaped particles when visualized by atomic force microscopy (AFM) (5) (Fig. 1C and D).
FIG. 1.
(A and B) Typical ulcerative lesions on a milker's fingers and hands. (C and D) AFM height images ranging from 6 to 2 μm. Viral particles were imaged in air with multimode equipment and a NanoScope IIIa controller (Digital Instruments, Santa Barbara, CA). Tapping mode imaging was performed in air by using silicon cantilevers from Nanosensors.
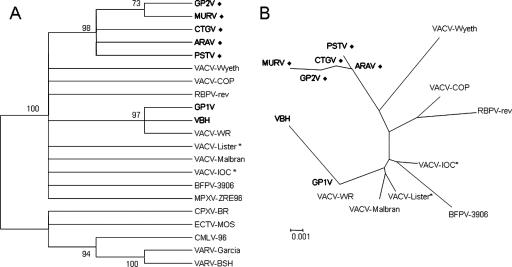
In addition, for the molecular characterization of the isolated poxvirus, the thymidine kinase (tk), vaccinia virus growth factor (vgf), and hemagglutinin (ha) genes were amplified, the amplicons were cloned into the pGEM-T vector (pGEM-T Easy Vector Systems; Promega Corp., Madison, WI), and three clones for each gene were sequenced (3, 6, 9). tk and vgf are conserved genes in the Orthopoxvirus genus and have previously been used as taxonomic tools to study ungrouped poxvirus (3, 9). When the tk and vgf nucleotide sequences from the isolated virus were compared to orthopoxvirus sequences, higher similarity values were found among the VACV sequences (greater than 99 and 97%, respectively), confirming that the isolated virus was, indeed, a VACV strain. On the other hand, ha is a less conserved gene among VACV strains that has been used as molecular tool to differentiate Brazilian VACV samples (1, 3, 6, 9). The ha nucleotide sequence presented the same signature deletion comprising 18 nucleotides as also observed for other Brazilian VACV strains isolated from cattle during vaccinia virus outbreaks in the southeast Brazil: Passatempo (PSTV) (3), Araçatuba virus (ARAV) (9), Guarani P2 (GP2V) (10), and Cantagalo virus (CTGV) (1). Despite these similarities, the ha sequence of the isolated virus also presented unique differences that allow for its differentiation from the other Brazilian VACV strains. We compared the deduced amino acid sequence from the isolated virus to those belonging to PSTV, ARAV, GP2V, CTGV, and VACV-Lister; identity values ranging from 94 to 98% were obtained. Orthopoxvirus ha nucleotide sequences were aligned by CLUSTAL W (8) and used to construct phylogenetic trees by the neighbor-joining method using the Tamura-Nei model of nucleotide substitution (7) implemented in the software MEGA3 (2). The newly isolated virus was clustered with VACV strains (Fig. 2A) and presented a closer relationship with the Brazilian VACV strains PSTV (3), ARAV (9), GP2V (10), and CTGV (1) (Fig. 2). Molecular characterization and phylogenetic analysis suggest that Muriae virus (MURV) might represent yet another Brazilian VACV strain obtained from bovine VACV infection outbreaks and the first one isolated from a human infection accompanied by a time course.
FIG. 2.
Orthopoxvirus phylogenetic analysis (A) and VACV phylogenetic analysis (B) based on the ha gene. The midpoint-rooted condensed trees (cutoff value of 70% from 1,000 bootstrap replicates) were constructed based on ha gene sequences by the neighbor-joining method using the Tamura-Nei model of nucleotide substitution implemented in MEGA3. Brazilian strains of VACV are shown in boldface, and a “⧫” symbol indicates the strains associated with bovine vaccinia virus outbreaks. An asterisk indicates vaccine strains used in Brazil during the smallpox vaccination campaign. Nucleotide sequences were obtained from GenBank with the following accession numbers: MURV (DQ247770); ARAV (AY523994); PSTV (DQ070848); GP1V, Guarani P1 virus (DQ206436); GP2V (DQ206437); VBH, Belo Horizonte virus (DQ206435); CTGV (AF229247); VACV-WR, VACV-Western Reserve (AY243312); VACV-Lister (AY678276); VACV-Malbran (AY146624); VACV-IOC, Instituto Oswaldo Cruz (AF229248); VACV-COP, VACV-Copenhagen (M35027); VACV-Wyeth (VVZ99051); BFLVbfl-3906, buffalopox virus (AF375077); RPXV-rev, rabbitpox virus rev (AF375118); ECTV-MOS, ectromelia virus Moscow (AF012825); CPXV-BR, cowpox virus Brighton Red (AF482758); CMLV-M96, camelpox virus M96 (AF438165); VARV-BSH, variola virus Bangladesh (L22579); VARV-Garcia, variola virus minor Garcia-1966 (Y16780); MPXV-ZRE, monkeypox virus Zaire-96-I-16 (AF380138).
In addition, it is important to mention that at least 3 other patients, all male milkers from the same rural area as the patient, presented the same type of painful lesions with fever and lymphadenitis, characterizing a systemic illness. Consistent with other outbreaks investigated in Brazil, most cases in this outbreak share the same epidemiological aspects (1, 3, 4, 6, 9). All four patients were male and milkers or farmers that had direct contact with dairy herds, characterizing this disease as an occupational zoonosis. In addition, in almost all reported cases, infected milkers were unable to work, causing economic losses to their families. Besides being in contact with affected cows, the milkers also related contact with other domestic animals, such as pigs and dogs that were apparently not affected.
It is clear that this case is part of an ongoing epidemic zoonosis caused by different strains of VACV that has continually affected rural areas of southeast Brazil (1, 3, 6, 9, 10). The emergence of this zoonosis 30 years after the end of smallpox vaccination certainly points to a decline in cohort immunity against orthopoxviruses, resulting in an important public health problem. Also of great importance is the fact that clinicians and other health professionals had problems with diagnosing and managing these infections. Many of the patients presenting lesions caused by poxviruses were treated with topical corticoids and often had the lesions excised. Both factors favor the spread of the virus to different parts of the body and the delay in the healing process. Another serious risk is represented by the nosocomial spread of the virus during a patient's hospitalization, which emphasizes the necessity of patient isolation and/or strengthening of disinfection measures.
Upon participation in this study signed informed consents from all patients investigated during this outbreak were obtained before biological samples were collected and the questionnaires were completed. The documents presented to the patients the objectives of study, as well as possible risks and benefits involved on their participation.
The nucleotide sequences from MURV were deposited at GenBank under the following accession numbers: ha, DQ247770; tk, DQ247771; and vgf, DQ247772.
Zoonotic orthopoxvirus infections are increasingly being recognized in unusual geographical areas, such as the detection of monkeypox virus infections in the United States and VACV infections in Brazil; therefore, some aspects of the outbreak described here could provide insights toward understanding the epidemiological and clinical features of zoonotic poxvirus diseases (3, 4, 9). The objective of the present study was to describe the clinical aspects of this emergent disease in humans in order to alert and help physicians and other health authorities as to how to recognize the lesions and the procedures that should be avoided by clinicians and health staff. Also, our findings confirm the circulation of a novel VACV strain, raising questions about the establishment of these viruses in Brazil. In this context, more studies should be conducted that focus on this zoonosis and the viruses that cause it in order to characterize the epidemiology of the disease, such as the identification of potential reservoirs of the viruses and the chain or transmission and also to have a better understanding of the immune response after a natural infection with an orthopoxvirus.
Acknowledgments
We thank Sandro Alvim and Flávia Medeiros for help with the collection of human samples. We are grateful for the assistance of the Instituto Mineiro de Agropecuária, Escola de Medicina Veterinária/UFMG, and we also thank the Laboratório de Vírus, ICB, UFMG, for excellent technical assistance. We thank Margareth Spangler Andrade and José Mário Vilela from the Laboratório de Nanoscopia/CETEC, where the AFM images were made. Fabrício R. dos Santos and the Laboratório de Biodiversidade e Evolução Molecular, where the sequences were made, are also gratefully acknowledged.
We thank CAPES, CNPq, and FAPEMIG for financial support. G.D.S.T. received a fellowship from the NIH. E.G.K., B.P.D., J.A.L., M.A.C., F.G.D.F., and Z.I.P.L. received fellowships from CNPq. M.I.M.C.G. received a fellowship from FAPEMIG. F.G.D.F. is supported by an NIH grant, and M.L.N.'s laboratory is supported by FAPESP and CNPq grants.
Footnotes
Published ahead of print on 7 February 2007.
REFERENCES
- 1.Damaso, C. R. A., J. J. Esposito, R. Condit, and N. Moussatché. 2000. An emergent poxvirus from humans and cattle in Rio de Janeiro state: Cantagalo virus may derive from Brazilian smallpox vaccine. Virology 277:439-449. [DOI] [PubMed] [Google Scholar]
- 2.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 3.Leite, J. A., B. P. Drumond, G. S. Trindade, Z. I. P. Lobato, F. G. da Fonseca, J. R. dos Santos, M. C. Madureira, M. I. M. C. Guedes, J. M. S. Ferreira, C. A. Bonjardim, P. C. Ferreira, and E. G. Kroon. 2005. Passatempo virus: a novel vaccinia virus isolated during a zoonotic outbreak in Brazil. Emerg. Infect. Dis. 11:1935-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis-Jones, S. 2004. Zoonotic poxvirus infections in humans. Curr. Opin. Infect. Dis. 17:81-89. [DOI] [PubMed] [Google Scholar]
- 5.Malkin, A. J., A. McPherson, and P. D. Gershon. 2003. Structure of intracellular mature vaccinia virus visualized by in situ atomic force microscopy. J. Virol. 77:6332-6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagasse-Sugahara, T. K., J. J. Kisielius, M. Ueda-Ito, S. P. Curti, C. A. Figueiredo, A. S. Cruz, M. M. Silva, C. H. Ramos, M. C. Silva, T. Sakurai, and L. F. Salles-Gomes. 2004. Human vaccinia-like virus outbreaks in Sao Paulo and Goias States, Brazil: virus detection, isolation, and identification. Rev. Inst. Med. Trop. Sao Paulo 46:315-322. [DOI] [PubMed] [Google Scholar]
- 7.Tamura, K., and M. Nei. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10:512-526. [DOI] [PubMed] [Google Scholar]
- 8.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trindade, G. S., F. G. Fonseca, J. T. Marques, M. L. Nogueira, L. C. Mendes, A. S. Borges, E. M. Pituco, C. A. Bonjardim, P. C. Ferreira, and E. G. Kroon. 2003. Araçatuba virus: a vaccinia-like virus associated with cattle and human infection. Emerg. Infect. Dis. 9:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trindade, G. S., Z. I. P. Lobato, B. P. Drumond, J. A. Leite, R. C. Trigueiro, M. I. M. C. Guedes, et al. 2006. Isolation of two vaccinia virus strains from a single bovine vaccinia outbreak in rural area from Brazil: implications on the emergence of zoonotic orthopoxviruses. Am. J. Trop. Med. Hyg. 75:486-490. [PubMed] [Google Scholar]