Abstract
An outbreak of gastroenteritis occurred at a kindergarten in Yokote City, Japan, between February 2006 and March 2006. Sapovirus was identified in 19 of 26 stool specimens by reverse transcription-PCR. A high viral shedding pattern was found for this strain, which was shown to be antigenically distinct from other genogroups.
Sapoviruses (SaV) and noroviruses (NoV) are etiological agents of human gastroenteritis. Human NoVs are the most important cause of outbreaks of gastroenteritis worldwide, whereas SaV infections are mostly associated with sporadic gastroenteritis in young children, though only a limited number of studies have been conducted. The most widely used method of detection is reverse transcription-PCR (RT-PCR), which has a high sensitivity and can also be used for genetic analysis (7). Real-time RT-PCR is also a useful method and can be practical for molecular epidemiological studies (11, 13). SaV strains can be divided into five genogroups (GI to GV), among which GI, GII, GIV, and GV are known to infect humans, whereas SaV GIII infects porcine species. Human NoV and SaV strains are noncultivable, but expression of the recombinant capsid protein (rVP1) in insect cells results in the self-assembly of virus-like particles (VLPs) that are antigenically similar to native viruses (5, 9). The purpose of this study was to describe a recent SaV outbreak of gastroenteritis at a kindergarten in Japan.
Between 20 February 2006 and 3 March 2006, an outbreak of gastroenteritis occurred at a kindergarten in Yokote City, Akita prefecture, Japan. In total, 66 of 107 (61%) children and 1 of 11 staff members reported symptoms associated with gastroenteritis at the kindergarten. Of the 107 children attending the kindergarten, 44 became absent due to diarrhea and vomiting on 1 March 2006. Stool specimens were collected within 48 h of symptoms from 26 children aged between 4 and 6 years and examined for the presence of viral agents as mentioned previously (4).
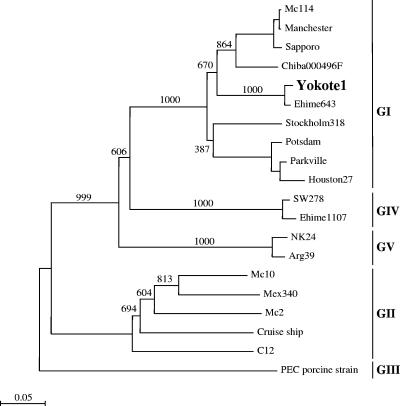
SaVs were detected in 19 of 26 (73%) stool specimens by nested RT-PCR, which included 14 of 18 females and 5 of 8 males. Other viral pathogens were not detected in the stool specimens, namely, norovirus and rotavirus. Nucleotide sequences were analyzed as described previously (3). Sequence analysis of several positive RT-PCR specimens showed identical sequences, which indicated the presence of a single SaV strain causing the outbreak at the kindergarten (strain Yokote1: accession number AB253740). Phylogenetic analysis of capsid (nucleotide) sequences showed the different genogroups and clusters (Figure 1). The closest matching sequence to the Yokote1 sequence was the Ehime643 strain (accession number DQ366345), having 96.1% and 95.4% nucleotide identity for the complete VP1 and VP2 genes, respectively. Interestingly, both the Yokote1 and Ehime643 VP1 and VP2 sequences were exactly 1,701 and 495 nucleotides in length, respectively, suggesting they were closely related. The Ehime643 strain was isolated from a sporadic case of gastroenteritis in a 2-year-old male in March 2000 from a different island in Japan (8). The Yokote1 and Ehime643 sequences belonged to a distinct GI cluster; however, similar sequences were also found in the database for isolates from other countries, including England, France, Hong Kong, and Russia.
FIG. 1.
Phylogenetic analysis of SaV capsid (nucleotide) sequences showing the different genogroups and clusters. The numbers on each branch indicate the bootstrap values for the genotype. Bootstrap values of 950 or higher were considered statistically significant for the grouping. The scale represents nucleotide substitutions per site. GenBank accession numbers for the reference strains are as follows: Arg39, AY289803; C12, AY603425; Chiba000496F, AJ412800; Cruise ship, AY289804; Ehime643, DQ366345; Ehime1107, DQ058829; Houston27, U95644; Manchester, X86560; Mc2, AY237419; Mc10, AY237420; Mex340, AF435812; Parkville, U73124; PEC, AF182760; Potsdam, AF294739; Sapporo, U65427; Stockholm318, AF194182; and SW278, DQ125333.
Real-time RT-PCR was used to further confirm the presence of SaV and to determine the viral loads (13). Sixteen of 26 stool specimens were positive by real-time RT-PCR (Table 1). Three positive RT-PCR specimens were found to be negative by real-time RT-PCR for an unknown reason, but this could be related to the sensitivity of the assay, i.e., nested RT-PCR was more sensitive than the real-time RT-PCR used in this study. Nevertheless, we found that the number of SaV cDNA copies ranged from 5.8 × 106 to 2.2 × 109 copies per gram of stool specimen with a median of 2.9 × 108 copies per gram of stool specimen. In a recent report, the number of NoV cDNA copies per gram of stool specimen was analyzed and a discrepancy was found between the different NoV genogroups (1). Chan et al. found that NoV GI and GII had a median of 8.4 × 105 and 3.0 × 108 copies per gram of stool specimen, respectively, and speculated that the increased viral loads were due to the higher transmissibility of NoV GII strains. The median viral load of the SaV Yokote1 strain isolated in this study was as high as that of NoV GII strains. These novel results suggested that the high viral shedding pattern of the SaV Yokote1 strain was a possible reason for the high transmissibility at the kindergarten, i.e., 57% (the number presenting illness was 67 of 118, children and staff included). However, further studies are clearly needed with other SaV strains.
TABLE 1.
RT-PCR and real-time RT-PCR results
| Straina | Positivity of sample by nested RT-PCR | Viral load (no. of copies/ g stool) determined by real-time RT-PCR | Age of patient (yr) | Sex of patientb | Date of sample collectionc |
|---|---|---|---|---|---|
| Yokote1 | + | 1.43 × 106 | 5 | F | 3/1/06 |
| Yokote2 | + | 8.51 × 106 | 5 | F | 3/1/06 |
| Yokote3 | + | − | 4 | M | 3/1/06 |
| Yokote4 | + | 2.85 × 107 | 4 | F | 3/1/06 |
| Yokote5 | + | 1.07 × 108 | 4 | M | 3/1/06 |
| Yokote6 | − | − | 5 | M | 3/1/06 |
| Yokote7 | − | − | 5 | M | 3/1/06 |
| Yokote8 | + | 6.55 × 108 | 4 | F | 3/1/06 |
| Yokote9 | + | 1.59 × 108 | 5 | M | 3/1/06 |
| Yokote10 | + | 1.67 × 108 | 5 | M | 3/1/06 |
| Yokote11 | + | 3.71 × 108 | 4 | M | 3/1/06 |
| Yokote12 | + | − | 4 | F | 3/1/06 |
| Yokote13 | − | − | 4 | F | 3/1/06 |
| Yokote14 | + | 5.77 × 106 | Adult | F | 3/1/06 |
| Yokote15 | + | 3.37 × 108 | 5 | F | 3/1/06 |
| Yokote16 | + | 2.25 × 108 | 4 | F | 3/1/06 |
| Yokote17 | + | 4.74 × 107 | 4 | F | 3/1/06 |
| Yokote18 | + | − | 6 | F | 3/2/06 |
| Yokote19 | − | − | 5 | F | 3/2/06 |
| Yokote20 | − | − | 4 | F | 3/2/06 |
| Yokote21 | + | 8.09 × 107 | 4 | F | 3/2/06 |
| Yokote22 | − | − | 5 | M | 3/2/06 |
| Yokote23 | − | − | 6 | F | 3/2/06 |
| Yokote24 | + | 2.19 × 109 | 6 | F | 3/2/06 |
| Yokote25 | + | 1.77 × 107 | 4 | F | 3/2/06 |
| Yokote26 | + | 1.82 × 108 | 4 | F | 3/2/06 |
Sequences of Yokote1, Yokote5, Yokote14, and Yokote18 were analyzed and were found to be identical. VLPs were expressed using Yokote5.
M, male; F, female.
Month/day/year.
We expressed Yokote1 rVP1 in insect cells in order to antigenically classify the Yokote1 strain. Yokote1 VP1 successfully formed VLPs morphologically similar to native SaV as determined by electron microscopy and had a molecular weight of approximately 60 kDa (data not shown). Hyperimmune rabbit and guinea pig antisera raised against purified Yokote1 VLPs were developed as described earlier, and an antigen enzyme-linked immunosorbent assay was used to examine the cross-reactivities (5). The purified Yokote1 VLPs and antiserum were tested against SaV Mc114 (accession number AY237422) and NK24 (accession number AY646856), which belonged to GI and GV, respectively. Based on our previous study, a specimen with an A492 (P − N) of >0.1 and a P/N A492 ratio of >1.34 (where P = hyperimmune antiserum and N = preimmune antiserum) was considered significantly positive (2). The VLPs reacted strongly against the homologous antiserum, i.e., a Mc114 A492 (P − N) of 0.4095 and a P/N value of 9.19; for Yokote1, the A492 (P − N) was 0.9295 and the value for P/N was 19.59; for NK24, the A492 (P − N) was 1.0290 and the value for P/N was 21.58 (Table 2). There was no obvious cross-reactivity among the heterogeneous antiserum, i.e., all values for P − N were less than 0.1 and those for P/N were less than 1.34 (Table 2). As a result, the Yokote1 VLPs were considered to have no cross-reactivity against either the Mc114 or NK24 antiserum, even though the Mc114 and Yokote1 strains both belonged to the same SaV genogroup (GI). Mc114 VP1 had 76.5% and 79.0% nucleotide and amino acid identity to Yokote1 VP1, respectively. These novel findings have shown SaV intragenogroup cross-reactivity for the first time and that the Mc114 GI genetic cluster was indeed genetically and antigenically distinct from the Yokote1 GI cluster. However, as we have shown with a recent NoV cross-reactivity study, genotyping and cross-reactivity may not directly correlate to the complete capsid amino acid sequence, since secondary structures and/or specific amino acid residues may also have an important influence (6).
TABLE 2.
Antigen enzyme-linked immunosorbent assay showing cross-reactivities among different VLPs
| Antiserum specificity | OD492a of VLPs
|
||
|---|---|---|---|
| Mc114 | Yokote1 | NK24 | |
| Mc114 | 0.4095 (9.19) | 0 (1.00) | 0 (1.00) |
| Yokote1 | 0.0065 (1.13) | 0.9295 (19.59) | 0 (1.00) |
| NK24 | 0 (1.00) | 0 (1.00) | 1.0290 (21.58) |
Optical density at 492 nm, expressed as the value for P minus the value for N (P/N ratio).
Little is known about SaV infections except that they are considered to be only a minor cause of sporadic gastroenteritis in children. Outbreaks of SaV are not as common as those of NoV; however, in a recent study, SaV was found to be the cause of an adult outbreak of gastroenteritis in Sweden (10). In addition, we recently identified several novel recombinant SaV strains (8, 12) and found an increase in SaV infections in Australia (7). None of the food handlers associated with the kindergarten in this study reported symptoms of gastroenteritis, and to the best of our knowledge SaV has not yet been detected in food destined for human consumption. However, in a recent study we detected SaV in 7 of 69 water samples, which included untreated wastewater, treated wastewater, and a river in Japan (8a). Further environmental surveillance studies of these viruses are clearly needed.
Acknowledgments
This work was supported in part by a grant for Research on Emerging and Re-emerging Infectious Diseases, Research on Food Safety from the Ministry of Health, Labor and Welfare of Japan and a grant for Research on Health Science Focusing on Drug Innovation from The Japan Health Science Foundation.
We also thank Saori Kadowaki at the Akita Prefectural Center for Public Health and Environment for technical assistance.
Footnotes
Published ahead of print on 31 January 2007.
REFERENCES
- 1.Chan, M. C. W., J. J. Y. Sung, R. K. Y. Lam, P. K. S. Chan, N. L. S. Lee, R. W. M. Lai, and W. K. Leung. 2006. Fecal viral load and Norovirus-associated gastroenteritis. Emerg. Infect. Dis. 12:1278-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansman, G. S., R. Guntapong, Y. Pongsuwanna, K. Natori, K. Katayama, and N. Takeda. 2006. Development of an antigen ELISA to detect sapovirus in clinical stool specimens. Arch. Virol. 151:551-561. [DOI] [PubMed] [Google Scholar]
- 3.Hansman, G. S., K. Katayama, N. Maneekarn, S. Peerakome, P. Khamrin, S. Tonusin, S. Okitsu, O. Nishio, N. Takeda, and H. Ushijima. 2004. Genetic diversity of norovirus and sapovirus in hospitalized infants with sporadic cases of acute gastroenteritis in Chiang Mai, Thailand. J. Clin. Microbiol. 42:1305-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansman, G. S., M. Kuramitsu, H. Yoshida, K. Katayama, N. Takeda, H. Ushijima, G. Surenkhand, D. Gantolga, and C. Kuroiwa. 2005. Viral gastroenteritis in Mongolian infants. Emerg. Infect. Dis. 11:180-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansman, G. S., K. Natori, T. Oka, S. Ogawa, K. Tanaka, N. Nagata, H. Ushijima, N. Takeda, and K. Katayama. 2005. Cross-reactivity among sapovirus recombinant capsid proteins. Arch. Virol. 150:21-36. [DOI] [PubMed] [Google Scholar]
- 6.Hansman, G. S., K. Natori, H. Shirato-Horikoshi, S. Ogawa, T. Oka, K. Katayama, T. Tanaka, T. Miyoshi, K. Sakae, S. Kobayashi, M. Shinohara, K. Uchida, N. Sakurai, K. Shinozaki, M. Okada, Y. Seto, K. Kamata, N. Nagata, K. Tanaka, T. Miyamura, and N. Takeda. 2006. Genetic and antigenic diversity among noroviruses. J. Gen. Virol. 87:909-919. [DOI] [PubMed] [Google Scholar]
- 7.Hansman, G. S., N. Takeda, K. Katayama, E. T. Tu, C. J. McIver, W. D. Rawlinson, and P. A. White. 2006. Genetic diversity of Sapovirus in children, Australia. Emerg. Infect. Dis. 12:141-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansman, G. S., N. Takeda, T. Oka, M. Oseto, K. O. Hedlund, and K. Katayama. 2005. Intergenogroup recombination in sapoviruses. Emerg. Infect. Dis. 11:1916-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Hansman, G. S., D. Sano, Y. Ueki, T. Imai, T. Oka, K. Katayama, N. Takeda, and T. Omura. 2007. Sapovirus in water, Japan. Emerg. Infect. Dis. 13:133-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang, X., M. Wang, D. Y. Graham, and M. K. Estes. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 66:6527-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson, P. J., K. Bergentoft, P. A. Larsson, G. Magnusson, A. Widell, M. Thorhagen, and K. O. Hedlund. 2005. A nosocomial sapovirus-associated outbreak of gastroenteritis in adults. Scand. J. Infect. Dis. 37:200-204. [DOI] [PubMed] [Google Scholar]
- 11.Kageyama, T., S. Kojima, M. Shinohara, K. Uchida, S. Fukushi, F. B. Hoshino, N. Takeda, and K. Katayama. 2003. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 41:1548-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katayama, K., T. Miyoshi, K. Uchino, T. Oka, T. Tanaka, N. Takeda, and G. S. Hansman. 2004. Novel recombinant sapovirus. Emerg. Infect. Dis. 10:1874-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oka, T., K. Katayama, G. S. Hansman, T. Kageyama, S. Ogawa, F. T. Wu, P. A. White, and N. Takeda. 2006. Detection of human sapovirus by real-time reverse transcription-polymerase chain reaction. J. Med. Virol. 78:1347-1353. [DOI] [PubMed] [Google Scholar]