Abstract
There is an urgent need for improved diagnosis of leptospirosis, an emerging infectious disease which imparts a large disease burden in developing countries. We evaluated the use of Leptospira immunoglobulin (Ig)-like (Lig) proteins as a serodiagnostic marker for leptospirosis. Lig proteins have bacterial immunoglobulin-like (Big) tandem repeat domains, a moiety found in virulence factors in other pathogens. Sera from patients identified during urban outbreaks in Brazil reacted strongly with immunoblots of a recombinant fragment comprised of the second to sixth Big domains of LigB from L. interrogans serovar Copenhageni, the principal agent for transmission in this setting. Furthermore, the sera recognized an analogous LigB fragment derived from L. kirschneri serovar Grippotyphosa, a pathogenic serovar which is not endemic to the study area. The immunoblot assay detected anti-LigB IgM antibodies in sera from 92% (95% confidence interval, 85 to 96%) of patients during acute-phase leptospirosis. The assay had a sensitivity of 81% for sera from patients with less than 7 days of illness. Anti-LigB antibodies were found in sera from 57% of the patients who did not have detectable anti-whole-Leptospira responses as detected by IgM enzyme-linked immunosorbent assay and microagglutination test. The specificities of the assay were 93 to 100% and 90 to 97% among sera from healthy individuals and patients with diseases that have clinical presentations that overlap with those of leptospirosis, respectively. These findings indicate that the antibody response to this putative virulence determinant is a sensitive and specific marker for acute infection. The use of this marker may aid the prompt and timely diagnosis required to reduce the high mortality associated with severe forms of the disease.
Leptospirosis is a zoonotic disease caused by pathogenic spirochetes of the genus Leptospira (4, 19, 30). Infection occurs during exposure to animal reservoirs or an environment contaminated by their urine and produces a spectrum of clinical manifestations ranging from an undifferentiated febrile illness to life-threatening manifestations such as Weil's disease and severe pulmonary hemorrhage syndrome (4, 38, 41, 60). Mortality from severe forms of the disease is 5% to 40% (4, 27, 41). Prompt diagnosis is critical in preventing severe outcomes, since antibiotics are believed to provide the greatest benefit when initiated early in the course of illness (19, 63). Yet, early phase leptospirosis is often not identified or is diagnosed as other causes of acute febrile disease due to its nonspecific clinical presentation (26). Misdiagnosis of leptospirosis has become a significant problem as diseases with similar early symptoms, such as dengue, have reemerged in the same places (8, 21, 29). Identification of leptospirosis will therefore need to rely on a high index of clinical suspicion and the use of a rapid and specific laboratory test (21, 31).
However, the standard diagnostic method, the microscopic agglutination test (MAT), requires paired serum samples for proper interpretation and is not adequate for clinical management (12, 41). Whole-Leptospira-based serologic assays are commercially available in enzyme-linked immunosorbent assay (ELISA) and other rapid formats, yet clinical evaluations found that these assays have sensitivities of 28 to 72% during acute-phase illness (3, 15, 32, 56-59). Moreover, the sensitivity for these assays may be less than 25% for patients in the critical first week of illness (15), when treatment with antibiotic therapy may be most effective. PCR-based detection methods have been developed (14, 42, 44, 45, 54), but their use has been restricted to the reference laboratory setting, and they are unlikely to be implemented in developing countries, where the major public health burden of leptospirosis exists. Therefore, new strategies for diagnosis which can aid early case identification and timely administration of antimicrobial therapy need to be identified.
Virulence factors expressed during host infection are expected to elicit specific antibody responses and, thus, may serve as candidate markers for a recombinant protein-based serodiagnostic test. A novel family of surface-associated proteins, Leptospira immunoglobulin (Ig)-like proteins (LigA, LigB, and LigC) (28, 39, 47), which have bacterial Ig-like (Big) tandem-repeat domains found in virulence factors such as intimin of enteropathogenic Escherichia coli (34) and invasin of Yersinia pseudotuberculosis (24), have been identified. lig genes are present exclusively in pathogenic and not saprophytic Leptospira species. Furthermore, they are expressed in virulent strains but not in strains that have been attenuated by culture passaging (39). Lig proteins are expressed during host infection (39) and appear to induce strong antibody responses in patients (28, 39) and infected animals (28, 46, 47). However, previous studies were performed with limited numbers of leptospirosis patients (28, 39).
Leptospirosis is a major public health problem in Brazil, as it is the cause of large urban epidemics each year during seasonal periods of heavy rainfall (27, 50, 52). In this study, we evaluated the antibody response to recombinant Lig proteins in sera from Brazilian patients, and we present findings that indicate that Lig proteins are a sensitive and specific serodiagnostic marker for acute infection.
MATERIALS AND METHODS
Patients and control subjects.
The evaluation was performed with paired acute- and convalescent-phase sera from 95 laboratory-confirmed cases of leptospirosis which were identified during active hospital-based surveillance in the city of Salvador, Brazil, from March 1996 to February 2003. Laboratory-confirmed leptospirosis was defined according to the criteria for seroconversion, a fourfold rise in titer or a single titer of ≥1:800 in the MAT, or culture isolation of pathogenic Leptospira from a blood sample (27, 52). Acute-phase samples were collected during hospital admission (mean, 9.0 ± 3.8 days after onset of symptoms). Early-convalescent-phase samples were collected during a follow-up visit after hospital discharge (mean, 35.3 ± 26.8 days after onset of symptoms). Acute-phase samples from an additional 40 laboratory-confirmed cases which had a negative reaction in the whole-Leptospira-based IgM ELISA (Bio-Manguinhos, Rio de Janeiro, Brazil) were evaluated. Late-convalescent-phase samples were obtained from 58 patients during house visits performed 4 to 72 months after their hospitalization for leptospirosis.
Control serum samples used in the evaluation were from (i) 40 healthy individuals residing in northern California (courtesy of Michael Hendry, California State Department of Health, Berkeley, CA); (ii) 50 subjects who were randomly selected among 1,400 participants of a citywide serosurvey in Salvador, Brazil; and (iii) 75 residents of slum communities in Salvador, Brazil, for whom samples were collected at the time that a neighbor was identified as a leptospirosis case (52). Furthermore, serum samples from control patient groups which included 30 cases of Lyme disease from the United States (courtesy of Martin Schriefer, Centers for Disease Control and Prevention, Fort Collins, CO) and 30 cases of dengue, 30 cases of hepatitis, and 30 individuals with Venereal Disease Research Laboratory (VDRL) test-positive reactions from Salvador, Brazil, were evaluated. The study protocol was approved by the institutional review board committees of the Oswaldo Cruz Foundation, Brazilian Ministry of Health, and the New York Presbyterian Hospital.
Recombinant Lig proteins.
Recombinant techniques were used as previously described (39) to obtain a fragment, rLigB[131-649], which corresponds to amino acid sequence positions 131 to 649 of the LigB protein from L. interrogans serovar Copenhageni strain Fiocruz L1-130, an isolate obtained from a patient during an outbreak of leptospirosis in Salvador, Brazil (27, 43). Ni2+-nitrilotiracetic acid affinity chromatography was used to purify the His-tagged fusion protein. A second recombinant fragment, rLigB[20-581], was constructed for the portion from amino acid sequence positions 20 to 581 of the LigB protein from L. kirschneri serovar Grippotyphosa strain RM52 (39), an isolate obtained during an outbreak among swine in the United States. The fragment, fused to maltose binding protein, was purified by amylase resin chromatography (39).
Immunoblot assay.
Recombinant proteins were solubilized in a sample buffer composed of 62.5 mM Tris hydrochloride (pH 6.8), 10% glycerol, 5% 2-mercaptoethanol, and 2% sodium dodecyl sulfate (SDS) and were resolved by 12% SDS-polyacrylamide gel electrophoresis (1.5 μg/lane) in a discontinuous buffer system. Immunoblots were blocked in 0.05 M Tris-buffered saline, pH 7.4, 0.05% (vol/vol) Tween 20 (TBS-T) with 5% (wt/vol) nonfat dry milk. After being washed, the blots were probed with goat anti-human or anti-rat IgM or IgG antibodies conjugated to alkaline phosphatase (Sigma Chemical Co.) which were diluted 1:1,000 in TBS-T with 5% (wt/vol) nonfat dry milk. Immunoblots were developed in NBT/BCIP solution (Bio-Rad) for 30 min and scored as positive if a staining was visualized. In parallel, samples were analyzed in an ELISA (Bio-Manguinhos, Rio de Janeiro, Brazil) which detects IgM antibodies to whole-cell antigen extract obtained from a clinical isolate of L. interrogans serovar Copenhageni. The assay was performed according to the manufacturer's instructions. Leptospirosis patient and control subject samples were assigned randomly selected numerical codes and evaluated in sequential order according to these codes as a measure to blind assay operators to the identities of the sample sources.
Data analysis.
The sensitivity of the immunoblot assay was defined as the proportion of leptospirosis case samples that were positive in the assay. Specificity was defined as the proportion of control subject samples that were negative in the assay. Ninety-five-percent confidence intervals were calculated with the Epitable program in Epi Info version 6.04 software. Interviews of leptospirosis patients were performed to identify the onset of illness prior to hospitalization. The sensitivities of the assays were evaluated for intervals that corresponded to the period between the onset of the illness and the day during which samples were collected from patients. The performance of the anti-rLig immunoblot assay was compared with that of the whole-Leptospira-based IgM ELISA and MAT screening criteria for an agglutination titer of ≥1:100. The chi-square and Fisher's exact tests were used to determine whether differences between proportions were statistically significant (P < 0.05).
RESULTS
Reactivity of leptospirosis patient sera to recombinant L. interrogans and L. kirschneri LigB fragments.
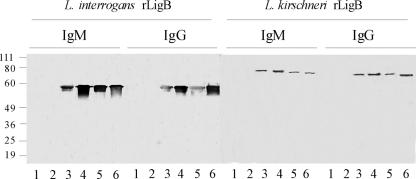
A recombinant fragment, rLigB[131-649], which is comprised of the second to sixth Big tandem-repeat domains of the LigB protein from L. interrogans strain Fiocruz L1-130, was purified. This strain belongs to serovar Copenhageni, which is the most common agent of urban leptospirosis in Brazil (27, 49). In immunoblot analyses, sera of leptospirosis cases from the city of Salvador, Brazil, demonstrated strong IgM and IgG antibody reactivities to rLigB[131-649] (Fig. 1). The amino acid sequence for this 57-kDa fragment is identical with the analogous portion (position 131 to 649) in the LigA molecule from the same strain (GenBank accession no. AY221109) (39). Furthermore, this fragment has amino acid sequence identities of 97, 96, and 96% with the corresponding LigB proteins in L. interrogans serovars Lai (GenBank accession no. NC004342), Pomona (GenBank accession no. AF534640), and Manilae (GenBank accession no. AB098517), respectively; of 63% with the corresponding portion of the LigB protein in L. borgspetersenii serovar Hardjo (GenBank accession no. YP_798783); and of 93% with the corresponding portion of the LigB protein in L. kirschneri serovar Grippotyphosa (GenBank accession no. AY190126). Of note, sera from leptospirosis cases reacted with rLigB[20-581] (Fig. 1), a 61.7-kDa fragment which is comprised of the first to fifth Big domains of LigB from L. kirschneri serovar Grippotyphosa, a serovar which has not been found to circulate at the surveillance area.
FIG. 1.
Anti-recombinant LigB antibodies in sera from leptospirosis patients. Membranes were prepared from SDS-polyacrylamide gel electrophoresis of recombinant fragments of L. interrogans LigB (rLigB[131-649]) and L. kirschneri LigB (rLigB[20-581]). The membranes were probed with sera from healthy control individuals (lanes 1 and 2) and from leptospirosis patients, obtained during their acute illness (lanes 3 to 6). The relative mobilities (in kDa) of molecular mass standards (Invitrogen) are shown on the left.
Evaluation of the recombinant LigB-based immunoblot.
Table 1 shows the reactivities of sera from leptospirosis cases and control subject groups in the immunoblot assay. The sensitivity of the IgM antibody response to rLigB[131-649] was 92% (95% confidence interval, 85 to 96%) in identifying leptospirosis during acute-phase illness. Moreover, anti-rLigB[131-649] IgM antibodies were detected in 57% of the leptospirosis case samples for which whole-Leptospira-based IgM ELISA results were negative. The specificities of the IgM antibody response to rLigB[131-649] were 100% (95% confidence interval, 93 to 100%) and 96% (95% confidence interval, 87 to 99%) among sera from healthy control individuals from a region of nonendemicity in the United States and the region of endemicity of Salvador, Brazil, respectively. As part of a case-control investigation (52), sera were collected from residents of high-risk slum communities at the time that a neighbor was identified to be a leptospirosis case. Among sera from these asymptomatic individuals, the reactivity of the immunoblot assay was 8%. The specificities of the IgM antibody response to rLigB[131-649] were 90% to 97% among sera from patients with diseases that have clinical presentations that overlap with those of leptospirosis (dengue and hepatitis) and with other spirochetal infections (Lyme disease and syphilis) (Table 1).
TABLE 1.
Detection of anti-recombinant LigB IgG and IgM antibodies in sera from leptospirosis patients and control subject groups as determined by immunoblotting
| Group | No. of samples tested | rLigB seroreactivity (no. positive reactions [%]) to:
|
|
|---|---|---|---|
| IgM | IgG | ||
| Confirmed leptospirosis cases | |||
| Acute phasea | 95 | 87 (92) | 81 (85) |
| Acute phase, IgM ELISA negativeb | 40 | 23 (57) | 21 (52) |
| Convalescent phasea | 95 | 69 (73) | 93 (98) |
| Healthy control individualsc | |||
| Region of nonendemicity | 40 | 0 (0) | 0 (0) |
| Region of endemicity | 50 | 2 (4) | 3 (6) |
| High-risk region of endemicity | 75 | 6 (8) | 9 (12) |
| Patient control groups | |||
| Dengue | 30 | 1 (3) | 0 (0) |
| Hepatitis | 30 | 3 (10) | 9 (30) |
| Lyme disease | 30 | 2 (7) | 4 (13) |
| VDRL test positive | 30 | 3 (10) | 6 (20) |
Paired serum samples from 95 laboratory-confirmed leptospirosis patients were evaluated. Acute and convalescent patients had sample collection means of 9.0 (±3.8) days and 35.3 (±26.8) days, respectively, after onset of symptoms.
Acute-phase samples from 40 confirmed leptospirosis patients, which had negative reactions in the whole-Leptospira-based IgM ELISA, were tested.
Samples were obtained from a serum bank for healthy individuals from northern California (region of nonendemicity), from randomly-selected subjects participating in a citywide serosurvey in Salvador, Brazil (region of endemicity), and from neighbors of leptospirosis cases residing in slum communities in Salvador, Brazil (high-risk region of endemicity).
The sensitivity of the IgG antibody response to rLigB[131-649] during acute-phase disease was lower (85%; 95% confidence interval, 77 to 91%) than the IgM antibody response. However, the sensitivity of the IgG antibody response increased to 98% during the convalescent phase. Although the specificity of the IgG antibody response was 100% among sera from healthy individuals from the United States, the specificity was lower among sera from residents of the region of endemicity of Salvador, Brazil (94%), and those from high-risk urban slum communities in the same city (88%). Use of the criterion of either a positive anti-rLigB[131-649] IgM or IgG antibody response did not improve the overall sensitivity (92%) or specificities (100 and 90% in sera from healthy individuals from the United States and Brazil, respectively) in comparison to those found for the anti-rLigB[131-649] IgM response alone.
Comparison of anti-Lig protein and whole-Leptospira antibody responses during leptospirosis.
Table 2 shows the sensitivities of anti-rLigB[131-649] IgM and IgG antibody immunoblotting and the IgM ELISA and MAT screening criteria of a titer of ≥1:100, which are standard methods used for detecting anti-whole-Leptospira antibody responses. During the first week of acute illness, a significantly higher proportion of sera from leptospirosis patients had anti-rLigB[131-649] IgM antibodies than whole-Leptospira antibodies as detected by the IgM ELISA and MAT (81% versus 52 and 33%, respectively; P = 0.049 and P = 0.002, respectively. Anti-rLigB[131-649] IgM antibody reactivity was found in more than 94% of sera from the leptospirosis patients during the second and third weeks of acute illness and was higher than the reactivities observed for IgM ELISA and MAT screening criteria during the same period.
TABLE 2.
Comparison of recombinant LigB-based immunoblot assay with standard diagnostic tests for leptospirosis
| Phase of illness and time period after onset | No. of samples tested | Results for standard diagnostic evaluation
|
rLigB immunoblot (no. of positive reactions [%]) for:
|
|||
|---|---|---|---|---|---|---|
| Median maximum reciprocal MAT titer (range) | No. of samples with reciprocal MAT titer of ≥100 (%) | IgM ELISA (no. of positive reactions [%]) | IgM | IgG | ||
| Acute phase (n = 95)a | ||||||
| 2 to 6 days | 21 | 0 (0-3,200) | 7 (33) | 11 (52) | 17 (81) | 13 (62) |
| 7 to 11 days | 55 | 400 (0-6,400) | 35 (64) | 47 (85) | 52 (94) | 51 (93) |
| 12 to 23 days | 19 | 800 (0-6,400) | 15 (79) | 14 (74) | 18 (95) | 17 (89) |
| Early convalescent phase (n = 95)a | ||||||
| 18 to 28 days | 42 | 3,200 (800-25,600) | 42 (100) | 41 (98) | 37 (88) | 40 (95) |
| 29 to 36 days | 33 | 1,600 (400-12,800) | 33 (100) | 32 (97) | 25 (76) | 33 (100) |
| 37 to 113 days | 20 | 1,600 (200-6,400) | 20 (100) | 20 (100) | 7 (35) | 20 (100) |
| Late convalescent phase (n = 58)b | ||||||
| 4 to 23 mos | 24 | 400 (0-800) | 20 (83) | 23 (96) | 4 (17) | 13 (54) |
| 24 to 47 mos | 17 | 400 (100-1,600) | 17 (100) | 7 (41) | 1 (6) | 3 (18) |
| 48 to 72 mos | 17 | 200 (200-800) | 15 (88) | 5 (29) | 1 (6) | 3 (18) |
Paired samples were obtained from 95 leptospirosis cases (n) in Salvador, Brazil. Early-convalescent-phase samples were obtained more than 14 days and less than 4 months after collection of acute-phase samples.
Late-convalescent-phase samples were obtained from leptospirosis patients 4 months to 72 months after discharge from hospital.
The proportions of patients with detectable anti-rLigB[131-649] IgM antibodies in their sera during convalescence decreased to 17% and 6% when followed 4 to 23 and 24 to 47 months, respectively, after their acute illness. In contrast, sera from 41% and 100% of patients had positive whole-Leptospira-based IgM ELISA and MAT reactions, respectively, when the patients were followed 24 to 47 months after their illness. IgM ELISA and MAT reactivity rates 48 to 72 months after acute illness remained elevated in comparison to those for the rLigB[131-649]-based immunoblot assay (30 and 88% versus 6%, respectively).
Anti-rLigB[131-649] IgG antibodies were detected in 62% of sera from leptospirosis patients in the first week, in more than 90% during the second and third weeks of acute illness, and in more than 95% during early convalescence (<4 months from onset of illness). Among patients followed 4 to 23 months after their illness, 54% had detectable anti-rLigB[131-649] IgG antibodies. This proportion decreased to 18% when patients were followed more than 2 years after their hospitalization for leptospirosis.
DISCUSSION
Identification of an effective serodiagnostic marker for infection has been the major barrier to identifying recombinant protein-based approaches to improve diagnosis of leptospirosis. Previous studies have focused on detecting antibody responses against a series of proteins which include LipL32 (6, 7, 20, 22, 23), LipL41 (20, 22, 37), and GroEL (20, 22, 48). However, to date, the performance of these recombinant-based serologic assays has not provided adequate sensitivity for identifying acute-phase leptospirosis. More recently, a novel family of surface-associated proteins, Lig proteins, were identified (28, 39, 47) which have Big tandem-repeat domains. These proteins were identified through screening of genomic DNA expression libraries with patient (28, 39) and infected animal sera (46) and appear to be preferentially expressed during host infection (39, 46). Initial studies found that acute leptospirosis produces robust antibody responses to Lig proteins (28, 39), albeit these evaluations were performed with small numbers of patient samples. Palaniappan et al. found that levels of antibodies to recombinant Lig fragments as detected by ELISA correlated with MAT titers in dogs (46). However, this study did not specifically determine the performance of the assay in diagnosing acute leptospirosis in these animals. In this study, we evaluated paired serum samples from Brazilian patients identified during active population-based surveillance for urban leptospirosis and found that antibody response to a recombinant Lig fragment was a highly sensitive and specific marker for acute leptospirosis in this setting.
The sensitivity of the immunoblot detection of anti-rLigB IgM antibodies was 92% in identifying acute leptospirosis. The assay's specificity was found to be high when the test was applied to serum samples from healthy individuals from California (100%), where leptospirosis is not endemic, and from health controls from the surveillance site for urban leptospirosis in Brazil (96%). Anti-Lig protein antibodies were found in 57% of the cases for which the standard assays, the IgM ELISA and MAT, did not detect anti-whole-Leptospira responses. More importantly, rLigB-based immunoblotting had a sensitivity of 81% in identifying leptospirosis during the first week of illness. These findings indicate that rLig-based serodiagnosis approaches may aid in detecting cases early in the course of illness when antimicrobial therapy provides the greatest benefit.
In addition, the use of Lig proteins may address a limitation associated with current serodiagnostic assays. Anti-whole-Leptospira antibodies as detected by IgM ELISA or MAT persist for years after acute leptospirosis (17, 33, 35, 51). Low specificity is a major barrier to the use of whole-Leptospira-based assays in areas of high endemicity and transmission (5, 13, 18, 25, 55, 61, 62). In this study, we found that 29% of sera from leptospirosis patients have positive whole-Leptospira-based IgM ELISA reactions 4 to 6 years after infection. In contrast, the rates of anti-LigB IgM antibody reactivity as detected in the immunoblot declined sharply one to three months after infection (35%) and decreased to 6% two years after the illness. As a caveat, the possibility of subclinical reinfection during the follow-up period, which would contribute to the reactivity rates observed during late-phase convalescence, cannot be excluded. Nevertheless, the kinetics of the anti-LigB IgM antibody response suggest that the use of defined recombinant Lig protein fragments will be a feasible approach to developing a highly specific serodiagnostic assay in high transmission settings. In fact, we found low rates (8%) of detectable anti-LigB IgM antibodies in samples which were collected from residents of high-risk slum communities at the time that a neighbor was identified as a leptospirosis case (Table 1).
Of note, high proportions of leptospirosis patients were found to have anti-Lig IgG antibodies during the acute phase of the illness. Among sera from patients presenting with less than 7 days of symptoms, 62% had anti-rLigB IgG antibodies as detected by the immunoblot assay. Similar findings have been observed with IgG antibody responses to other Leptospira proteins (10, 11, 20, 22). This may relate in part to a memory response in individuals with a prior exposure to leptospirosis. Alternatively, the robust IgG response to Lig and other Leptospira proteins may be due to an accelerated class switch phenomenon during the incubation period, which may range from 2 to 30 days, but is usually between 5 and 14 days (19). Both IgG and IgM antibodies to protein moieties are found during the early immune response to other spirochetal infections, such as Lyme disease and syphilis (16, 36, 53).
The evaluation was performed with patient populations in Brazil which were identified during surveillance for urban leptospirosis. Therefore, the study's observations will need to be confirmed in other regions where leptospirosis is endemic. The distribution of pathogenic Leptospira species and serovars varies geographically and according to epidemiological situations. However, lig genes are present in all pathogenic Leptospira species (28, 39, 47). Lig proteins are hypothesized to be a virulence determinant, since they have Big domains found in other bacterial virulence determinants (24, 34). They presumably are expressed in the spectrum of pathogenic Leptospira during host infection and elicit similar acute-phase antibody responses. In this evaluation, samples were obtained from hospitalized cases of leptospirosis. As with anti-whole-Leptospira antibody responses (1), anti-LigB antibody levels are likely to be positively correlated with disease severity. Further investigations will therefore need to specifically address the performance of Lig proteins as serodiagnostic markers in outpatient populations. Whole-Leptospira-based diagnostic methods appear to detect IgM antibodies against immunodominant carbohydrate epitopes (2, 19), such as “broad reactive antigen” (40). Although the study findings indicate that anti-Lig protein antibody response may be an improved serodiagnostic marker, direct comparisons of their performances will need to be made in studies in which similar assay formats are used.
A major goal will be to apply the anti-Lig protein antibody detection system in an easily performed, rapid-format system which is more suitable for rapid diagnosis than immunoblotting. The study's findings indicate that the first six Big domains of LigB proteins form a candidate antigen for such an assay. Previous studies found that the C-terminal Big domain regions of LigA (domains 7 to 12) and LigB (domains 7 to 11) are seroreactive as well (28, 39, 46). Inclusion of these fragments may potentially increase assay performance. A serodiagnostic assay will ideally use a recombinant LigB fragment derived from a single serovar which detects cross-reactive antibodies elicited during infection with other serovars. Predicted amino acid sequence identities are 92 to 96% among corresponding LigB[131-649] fragments from five L. interrogans and L. kirschneri serovars for which complete sequence information is available (GenBank accession no. YP000448, NC004342, AF534640, AB098517, and AY190126). Furthermore, we found that sera of leptospirosis patients from an area of L. interrogans serovar Copenhageni endemicity and transmission reacted with a recombinant LigB fragment derived from an L. kirschneri serovar that is not circulating in the region (Fig. 1). A single recombinant LigB fragment may therefore be capable of detecting cross-reactive antibody responses elicited during infections with L. interrogans and L. kirschneri, which account for the majority of serovars of public health importance (30). In contrast, the predicted amino acid sequence identity is 62% between rLigB[131-649] fragments from L. interrogans serovar Copenhageni and L. borgspetersenii serovar Hardjo (9) (GenBank accession no. YP_798783). Multivalent LigB fragments may be used as a diagnostic approach to identify infections due to this serovar, if the use of a single fragment cannot efficiently detect cross-reactive antibody responses.
In summary, the findings of this study indicate that the antibody response against a putative Leptospira virulence determinant, the Lig proteins, is a marker for acute infection. The results obtained with the immunoblot assay suggest that it may be feasible to apply recombinant Lig protein fragments in developing high-performing lateral flow or dipstick assays. The development of such assays would be an advance in addressing the under-reporting of this neglected disease and would permit the implementation of intervention strategies based on early case detection and timely initiation of antimicrobial therapy as a measure to prevent disease progression and the severe outcomes associated with leptospirosis.
Acknowledgments
This work was supported by grants from Bio-Manguinhos, the Oswaldo Cruz Foundation, the Brazilian Ministry of Health (09224-7), the Brazilian National Research Council (300.861/96-6, 350.052/95-6, and FINEP 4196086200), VA Medical Research Funds, and the National Institutes of Health (AI-052473, AI-034431, TW-00905, and TW-00919).
Footnotes
Published ahead of print on 14 March 2007.
REFERENCES
- 1.Abdulkader, R. C., E. F. Daher, E. D. Camargo, C. Spinosa, and M. V. da Silva. 2002. Leptospirosis severity may be associated with the intensity of humoral immune response. Rev. Inst. Med. Trop. Sao Paulo 44:79-83. [DOI] [PubMed] [Google Scholar]
- 2.Adler, B., and S. Faine. 1978. The antibodies involved in the human immune response to leptospiral infection. J. Med. Microbiol. 11:387-400. [DOI] [PubMed] [Google Scholar]
- 3.Bajani, M. D., D. A. Ashford, S. L. Bragg, C. W. Woods, T. Aye, R. A. Spiegel, B. D. Plikaytis, B. A. Perkins, M. Phelan, P. N. Levett, and R. S. Weyant. 2003. Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis. J. Clin. Microbiol. 41:803-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharti, A. R., J. E. Nally, J. N. Ricaldi, M. A. Matthias, M. M. Diaz, M. A. Lovett, P. N. Levett, R. H. Gilman, M. R. Willig, E. Gotuzzo, and J. M. Vinetz. 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 3:757-771. [DOI] [PubMed] [Google Scholar]
- 5.Blacksell, S. D., L. Smythe, R. Phetsouvanh, M. Dohnt, R. Hartskeerl, M. Symonds, A. Slack, M. Vongsouvath, V. Davong, O. Lattana, S. Phongmany, V. Keolouangkot, N. J. White, N. P. Day, and P. N. Newton. 2006. Limited diagnostic capacities of two commercial assays for the detection of Leptospira immunoglobulin M antibodies in Laos. Clin. Vaccine Immunol. 13:1166-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bomfim, M. R., A. Ko, and M. C. Koury. 2005. Evaluation of the recombinant LipL32 in enzyme-linked immunosorbent assay for the serodiagnosis of bovine leptospirosis. Vet. Microbiol. 109:89-94. [DOI] [PubMed] [Google Scholar]
- 7.Boonyod, D., Y. Poovorawan, P. Bhattarakosol, and C. Chirathaworn. 2005. LipL32, an outer membrane protein of Leptospira, as an antigen in a dipstick assay for diagnosis of leptospirosis. Asian Pac. J. Allergy Immunol. 23:133-141. [PubMed] [Google Scholar]
- 8.Bruce, M. G., E. J. Sanders, J. A. Leake, O. Zaidel, S. L. Bragg, T. Aye, K. A. Shutt, C. C. Deseda, J. G. Rigau-Perez, J. W. Tappero, B. A. Perkins, R. A. Spiegel, and D. A. Ashford. 2005. Leptospirosis among patients presenting with dengue-like illness in Puerto Rico. Acta Trop. 96:36-46. [DOI] [PubMed] [Google Scholar]
- 9.Bulach, D. M., R. L. Zuerner, P. Wilson, T. Seemann, A. McGrath, P. A. Cullen, J. Davis, M. Johnson, E. Kuczek, D. P. Alt, B. Peterson-Burch, R. L. Coppel, J. I. Rood, J. K. Davies, and B. Adler. 2006. Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc. Natl. Acad. Sci. USA 103:14560-14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman, A. J., B. Adler, and S. Faine. 1988. Antigens recognised by the human immune response to infection with Leptospira interrogans serovar hardjo. J. Med. Microbiol. 25:269-278. [DOI] [PubMed] [Google Scholar]
- 11.Chapman, A. J., C. O. Everard, S. Faine, and B. Adler. 1991. Antigens recognized by the human immune response to severe leptospirosis in Barbados. Epidemiol. Infect. 107:143-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cumberland, P., C. O. Everard, and P. N. Levett. 1999. Assessment of the efficacy of an IgM ELISA and microscopic agglutination test (MAT) in the diagnosis of acute leptospirosis. Am. J. Trop. Med. Hyg. 61:731-734. [DOI] [PubMed] [Google Scholar]
- 13.Cumberland, P., C. O. Everard, J. G. Wheeler, and P. N. Levett. 2001. Persistence of anti-leptospiral IgM, IgG and agglutinating antibodies in patients presenting with acute febrile illness in Barbados 1979-1989. Eur. J. Epidemiol. 17:601-608. [DOI] [PubMed] [Google Scholar]
- 14.de Abreu Fonseca, C., V. L. Teixeira de Freitas, E. Calo Romero, C. Spinosa, M. C. Arroyo Sanches, M. V. da Silva, and M. A. Shikanai-Yasuda. 2006. Polymerase chain reaction in comparison with serological tests for early diagnosis of human leptospirosis. Trop. Med. Int. Health 11:1699-1707. [DOI] [PubMed] [Google Scholar]
- 15.Effler, P. V., A. K. Bogard, H. Y. Domen, A. R. Katz, H. Y. Higa, and D. M. Sasaki. 2002. Evaluation of eight rapid screening tests for acute leptospirosis in Hawaii. J. Clin. Microbiol. 40:1464-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engstrom, S. M., E. Shoop, and R. C. Johnson. 1995. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 33:419-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everard, C. O., and S. Bennett. 1990. Persistence of leptospiral agglutinins in Trinidadian survey subjects. Eur. J. Epidemiol. 6:40-44. [DOI] [PubMed] [Google Scholar]
- 18.Everard, C. O., R. J. Hayes, and G. M. Fraser-Chanpong. 1985. A serosurvey for leptospirosis in Trinidad among urban and rural dwellers and persons occupationally at risk. Trans. R. Soc. Trop. Med. Hyg. 79:96-105. [DOI] [PubMed] [Google Scholar]
- 19.Faine, S., B. Adler, C. Bolin, and P. Perolat. 1999. Leptospira and leptospirosis, 2nd ed. MediSci, Melbourne, Australia.
- 20.Flannery, B., D. Costa, F. P. Carvalho, H. Guerreiro, J. Matsunaga, E. D. Da Silva, A. G. P. Ferreira, L. W. Riley, M. G. Reis, D. A. Haake, and A. I. Ko. 2001. Evaluation of recombinant Leptospira antigen-based enzyme-linked immunosorbent assays for the serodiagnosis of leptospirosis. J. Clin. Microbiol. 39:3303-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flannery, B., M. M. Pereira, L. de F. Velloso, C. de C. Carvalho, L. G. De Codes, G. de S. Orrico, C. M. Dourado, L. W. Riley, M. G. Reis, and A. I. Ko. 2001. Referral pattern of leptospirosis cases during a large urban epidemic of dengue. Am. J. Trop. Med. Hyg. 65:657-663. [DOI] [PubMed] [Google Scholar]
- 22.Guerreiro, H., J. Croda, B. Flannery, M. Mazel, J. Matsunaga, M. Galvao Reis, P. N. Levett, A. I. Ko, and D. A. Haake. 2001. Leptospiral proteins recognized during the humoral immune response to leptospirosis in humans. Infect. Immun. 69:4958-4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haake, D. A., G. Chao, R. L. Zuerner, J. K. Barnett, D. Barnett, M. Mazel, J. Matsunaga, P. N. Levett, and C. A. Bolin. 2000. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect. Immun. 68:2276-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamburger, Z. A., M. S. Brown, R. R. Isberg, and P. J. Bjorkman. 1999. Crystal structure of invasin: a bacterial integrin-binding protein. Science 286:291-295. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, M. A., H. Smith, P. Joeph, R. H. Gilman, C. T. Bautista, K. J. Campos, M. Cespedes, P. Klatsky, C. Vidal, H. Terry, M. M. Calderon, C. Coral, L. Cabrera, P. S. Parmar, and J. M. Vinetz. 2004. Environmental exposure and leptospirosis, Peru. Emerg. Infect. Dis. 10:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaur, I. R., R. Sachdeva, V. Arora, and V. Talwar. 2003. Preliminary survey of leptospirosis amongst febrile patients from urban slums of East Delhi. J. Assoc. Physicians India 51:249-251. [PubMed] [Google Scholar]
- 27.Ko, A. I., M. Galvao Reis, C. M. Ribeiro Dourado, W. D. Johnson, Jr., L. W. Riley, et al. 1999. Urban epidemic of severe leptospirosis in Brazil. Lancet 354:820-825. [DOI] [PubMed] [Google Scholar]
- 28.Koizumi, N., and H. Watanabe. 2004. Leptospiral immunoglobulin-like proteins elicit protective immunity. Vaccine 22:1545-1552. [DOI] [PubMed] [Google Scholar]
- 29.LaRocque, R. C., R. F. Breiman, M. D. Ari, R. E. Morey, F. A. Janan, J. M. Hayes, M. A. Hossain, W. A. Brooks, and P. N. Levett. 2005. Leptospirosis during dengue outbreak, Bangladesh. Emerg. Infect. Dis. 11:766-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levett, P. N. 2001. Leptospirosis. Clin. Microbiol. Rev. 14:296-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levett, P. N., S. L. Branch, and C. N. Edwards. 2000. Detection of dengue infection in patients investigated for leptospirosis in Barbados. Am. J. Trop. Med. Hyg. 62:112-114. [DOI] [PubMed] [Google Scholar]
- 32.Levett, P. N., S. L. Branch, C. U. Whittington, C. N. Edwards, and H. Paxton. 2001. Two methods for rapid serological diagnosis of acute leptospirosis. Clin. Diagn. Lab. Immunol. 8:349-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levett, P. N., and C. U. Whittington. 1998. Evaluation of the indirect hemagglutination assay for diagnosis of acute leptospirosis. J. Clin. Microbiol. 36:11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo, Y., E. A. Frey, R. A. Pfuetzner, A. L. Creagh, D. G. Knoechel, C. A. Haynes, B. B. Finlay, and N. C. Strynadka. 2000. Crystal structure of enteropathogenic Escherichia coli intimin-receptor complex. Nature 405:1073-1077. [DOI] [PubMed] [Google Scholar]
- 35.Lupidi, R., M. Cinco, D. Balanzin, E. Delprete, and P. E. Varaldo. 1991. Serological follow-up of patients involved in a localized outbreak of leptospirosis. J. Clin. Microbiol. 29:805-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magnarelli, L. A., J. W. Ijdo, S. J. Padula, R. A. Flavell, and E. Fikrig. 2000. Serologic diagnosis of Lyme borreliosis by using enzyme-linked immunosorbent assays with recombinant antigens. J. Clin. Microbiol. 38:1735-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mariya, R., P. Chaudhary, A. A. Kumar, E. Thangapandian, R. Amutha, and S. K. Srivastava. 2006. Evaluation of a recombinant LipL41 antigen of Leptospira interrogans serovar Canicola in ELISA for serodiagnosis of bovine leptospirosis. Comp. Immunol. Microbiol. Infect. Dis. 29:269-277. [DOI] [PubMed] [Google Scholar]
- 38.Marotto, P. C., C. M. Nascimento, J. Eluf-Neto, M. S. Marotto, L. Andrade, J. Sztajnbok, and A. C. Seguro. 1999. Acute lung injury in leptospirosis: clinical and laboratory features, outcome, and factors associated with mortality. Clin. Infect. Dis. 29:1561-1563. [DOI] [PubMed] [Google Scholar]
- 39.Matsunaga, J., M. A. Barocchi, J. Croda, T. A. Young, Y. Sanchez, I. Siqueira, C. A. Bolin, M. G. Reis, L. W. Riley, D. A. Haake, and A. I. Ko. 2003. Pathogenic Leptospira species express surface-exposed proteins belonging to the bacterial immunoglobulin superfamily. Mol. Microbiol. 49:929-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuo, K., E. Isogai, and Y. Araki. 2000. Occurrence of [→ 3)-β-d-Manp-(1 → 4)-β-d-Manp-(1 →]n units in the antigenic polysaccharides from Leptospira biflexa serovar patoc strain Patoc I. Carbohydr. Res. 328:517-524. [DOI] [PubMed] [Google Scholar]
- 41.McBride, A. J., D. A. Athanazio, M. G. Reis, and A. I. Ko. 2005. Leptospirosis. Curr. Opin. Infect. Dis. 18:376-386. [DOI] [PubMed] [Google Scholar]
- 42.Merien, F., G. Baranton, and P. Perolat. 1995. Comparison of polymerase chain reaction with microagglutination test and culture for diagnosis of leptospirosis. J. Infect. Dis. 172:281-285. [DOI] [PubMed] [Google Scholar]
- 43.Nascimento, A. L., A. I. Ko, E. A. Martins, C. B. Monteiro-Vitorello, P. L. Ho, D. A. Haake, S. Verjovski-Almeida, R. A. Hartskeerl, M. V. Marques, M. C. Oliveira, C. F. Menck, L. C. Leite, H. Carrer, L. L. Coutinho, W. M. Degrave, O. A. Dellagostin, H. El-Dorry, E. S. Ferro, M. I. Ferro, L. R. Furlan, M. Gamberini, E. A. Giglioti, A. Goes-Neto, G. H. Goldman, M. H. Goldman, R. Harakava, S. M. Jeronimo, I. L. Junqueira-de-Azevedo, E. T. Kimura, E. E. Kuramae, E. G. Lemos, M. V. Lemos, C. L. Marino, L. R. Nunes, R. C. de Oliveira, G. G. Pereira, M. S. Reis, A. Schriefer, W. J. Siqueira, P. Sommer, S. M. Tsai, A. J. Simpson, J. A. Ferro, L. E. Camargo, J. P. Kitajima, J. C. Setubal, and M. A. Van Sluys. 2004. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J. Bacteriol. 186:2164-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ooteman, M. C., A. R. Vago, and M. C. Koury. 2006. Evaluation of MAT, IgM ELISA and PCR methods for the diagnosis of human leptospirosis. J. Microbiol. Methods 65:247-257. [DOI] [PubMed] [Google Scholar]
- 45.Palaniappan, R. U., Y. F. Chang, C. F. Chang, M. J. Pan, C. W. Yang, P. Harpending, S. P. McDonough, E. Dubovi, T. Divers, J. Qu, and B. Roe. 2005. Evaluation of lig-based conventional and real time PCR for the detection of pathogenic leptospires. Mol. Cell. Probes 19:111-117. [DOI] [PubMed] [Google Scholar]
- 46.Palaniappan, R. U., Y. F. Chang, F. Hassan, S. P. McDonough, M. Pough, S. C. Barr, K. W. Simpson, H. O. Mohammed, S. Shin, P. McDonough, R. L. Zuerner, J. Qu, and B. Roe. 2004. Expression of leptospiral immunoglobulin-like protein by Leptospira interrogans and evaluation of its diagnostic potential in a kinetic ELISA. J. Med. Microbiol. 53:975-984. [DOI] [PubMed] [Google Scholar]
- 47.Palaniappan, R. U., Y. F. Chang, S. S. Jusuf, S. Artiushin, J. F. Timoney, S. P. McDonough, S. C. Barr, T. J. Divers, K. W. Simpson, P. L. McDonough, and H. O. Mohammed. 2002. Cloning and molecular characterization of an immunogenic LigA protein of Leptospira interrogans. Infect. Immun. 70:5924-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park, S. H., B. Y. Ahn, and M. J. Kim. 1999. Expression and immunologic characterization of recombinant heat shock protein 58 of Leptospira species: a major target antigen of the humoral immune response. DNA Cell Biol. 18:903-910. [DOI] [PubMed] [Google Scholar]
- 49.Pereira, M. M., M. G. Matsuo, A. R. Bauab, S. A. Vasconcelos, Z. M. Moraes, G. Baranton, and I. Saint Girons. 2000. A clonal subpopulation of Leptospira interrogans sensu stricto is the major cause of leptospirosis outbreaks in Brazil. J. Clin. Microbiol. 38:450-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romero, E. C., C. C. Bernardo, and P. H. Yasuda. 2003. Human leptospirosis: a twenty-nine-year serological study in Sao Paulo, Brazil. Rev. Inst. Med. Trop. Sao Paulo 45:245-248. [DOI] [PubMed] [Google Scholar]
- 51.Romero, E. C., C. R. Caly, and P. H. Yasuda. 1998. The persistence of leptospiral agglutinins titers in human sera diagnosed by the microscopic agglutination test. Rev. Inst. Med. Trop. Sao Paulo 40:183-184. [DOI] [PubMed] [Google Scholar]
- 52.Sarkar, U., S. F. Nascimento, R. Barbosa, R. Martins, H. Nuevo, I. Kalafanos, I. Grunstein, B. Flannery, J. Dias, L. W. Riley, M. G. Reis, and A. I. Ko. 2002. Population-based case-control investigation of risk factors for leptospirosis during an urban epidemic. Am. J. Trop. Med. Hyg. 66:605-610. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt, B. L., M. Edjlalipour, and A. Luger. 2000. Comparative evaluation of nine different enzyme-linked immunosorbent assays for determination of antibodies against Treponema pallidum in patients with primary syphilis. J. Clin. Microbiol. 38:1279-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segura, E. R., C. A. Ganoza, K. Campos, J. N. Ricaldi, S. Torres, H. Silva, M. J. Cespedes, M. A. Matthias, M. A. Swancutt, R. Lopez Linan, E. Gotuzzo, H. Guerra, R. H. Gilman, and J. M. Vinetz. 2005. Clinical spectrum of pulmonary involvement in leptospirosis in a region of endemicity, with quantification of leptospiral burden. Clin. Infect. Dis. 40:343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sehgal, S. C., P. Vijayachari, M. V. Murhekar, A. P. Sugunan, S. Sharma, and S. S. Singh. 1999. Leptospiral infection among primitive tribes of Andaman and Nicobar Islands. Epidemiol. Infect. 122:423-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smits, H. L., Y. V. Ananyina, A. Chereshsky, L. Dancel, A. F. R. F. Lai, H. D. Chee, P. N. Levett, T. Masuzawa, Y. Yanagihara, M. A. Muthusethupathi, E. J. Sanders, D. M. Sasaki, H. Domen, C. Yersin, T. Aye, S. L. Bragg, G. C. Gussenhoven, M. G. Goris, W. J. Terpstra, and R. A. Hartskeerl. 1999. International multicenter evaluation of the clinical utility of a dipstick assay for detection of Leptospira-specific immunoglobulin M antibodies in human serum specimens. J. Clin. Microbiol. 37:2904-2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smits, H. L., H. D. Chee, C. K. Eapen, M. Kuriakose, S. Sugathan, M. H. Gasem, C. Yersin, D. Sakasi, A. F. R. F. Lai, R. A. Hartskeerl, B. Liesdek, T. H. Abdoel, M. G. Goris, and G. C. Gussenhoven. 2001. Latex based, rapid and easy assay for human leptospirosis in a single test format. Trop. Med. Int Health 6:114-118. [DOI] [PubMed] [Google Scholar]
- 58.Smits, H. L., C. K. Eapen, S. Sugathan, M. Kuriakose, M. H. Gasem, C. Yersin, D. Sasaki, B. Pujianto, M. Vestering, T. H. Abdoel, and G. C. Gussenhoven. 2001. Lateral-flow assay for rapid serodiagnosis of human leptospirosis. Clin. Diagn. Lab. Immunol. 8:166-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smits, H. L., M. A. van der Hoorn, M. G. Goris, G. C. Gussenhoven, C. Yersin, D. M. Sasaki, W. J. Terpstra, and R. A. Hartskeerl. 2000. Simple latex agglutination assay for rapid serodiagnosis of human leptospirosis. J. Clin. Microbiol. 38:1272-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trevejo, R. T., J. G. Rigau-Perez, D. A. Ashford, E. M. McClure, C. Jarquin-Gonzalez, J. J. Amador, J. O. de los Reyes, A. Gonzalez, S. R. Zaki, W. J. Shieh, R. G. McLean, R. S. Nasci, R. S. Weyant, C. A. Bolin, S. L. Bragg, B. A. Perkins, and R. A. Spiegel. 1998. Epidemic leptospirosis associated with pulmonary hemorrhage—Nicaragua, 1995. J. Infect. Dis. 178:1457-1463. [DOI] [PubMed] [Google Scholar]
- 61.Vado-Solis, I., M. F. Cardenas-Marrufo, B. Jimenez-Delgadillo, A. Alzina-Lopez, H. Laviada-Molina, V. Suarez-Solis, and J. E. Zavala-Velazquez. 2002. Clinical-epidemiological study of leptospirosis in humans and reservoirs in Yucatan, Mexico. Rev. Inst. Med. Trop. Sao Paulo 44:335-340. [DOI] [PubMed] [Google Scholar]
- 62.Wagenaar, J. F., T. H. Falke, N. V. Nam, T. Q. Binh, H. L. Smits, F. G. Cobelens, and P. J. de Vries. 2004. Rapid serological assays for leptospirosis are of limited value in southern Vietnam. Ann. Trop. Med. Parasitol. 98:843-850. [DOI] [PubMed] [Google Scholar]
- 63.World Health Organization. 2003. Human leptospirosis: guidance for diagnosis, surveillance and control. World Health Organization, Malta.