Abstract
Trauma intensive care unit (TICU) patients requiring mechanical respiratory support frequently develop ventilator-associated pneumonia (VAP). Oral and oropharyngeal bacteria are believed to be responsible for many cases of VAP, but definitive evidence of this relationship is lacking. Earlier studies used conventional culture-based methods for identification of bacterial pathogens, but these methods are insufficient, as some bacteria may be uncultivable or difficult to grow. The purpose of this study was to use a culture-independent molecular approach to analyze and compare the bacterial species colonizing the oral cavity and the lungs of TICU patients who developed VAP. Bacterial samples were acquired from the dorsal tongue and bronchoalveolar lavage fluid of 16 patients. Bacterial DNA was extracted, and the 16S rRNA genes were PCR amplified, cloned into Escherichia coli, and sequenced. The sequencing data revealed the following: (i) a wide diversity of bacterial species in both the oral and pulmonary sites, some of them novel; (ii) known and putative respiratory pathogens colonizing both the oral cavity and lungs of 14 patients; and (iii) a number of bacterial pathogens (e.g., Dialister pneumosintes, Haemophilus segnis, Gemella morbillorum, and Pseudomonas fluorescens) in lung samples that had not been reported previously at this site when culture-based methods were used. Our data indicate that the dorsal surface of the tongue serves as a potential reservoir for bacterial species involved in VAP. Furthermore, it is clear that the diversity of bacterial pathogens for VAP is far more complex than the current literature suggests.
Ventilator-associated pneumonia (VAP) frequently occurs in patients requiring mechanical respiratory support, with incidence from 8% to 28% and mortality rates from 24% to 76%, depending on the population studied and the techniques used for the diagnosis of pneumonia (3). VAP is often associated with prolonged hospitalization of trauma patients, which can result in additional hospital charges of about $40,000 per patient (1).
The etiology of VAP is variable, depending on the time of onset, duration of hospitalization, population studied, and hospital setting (3). For example, Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae usually predominate in early-onset VAP. Aerobic gram-negative bacteria, including members of the family Enterobacteriaceae, have been isolated in both early- and late-onset pneumonia. Pseudomonas aeruginosa and Acinetobacter and Enterobacter species are often isolated from late-onset pneumonia. Trauma intensive care unit (TICU) patients are at high risk of infection with S. aureus, among a variety of other microorganisms. In the postsurgical population, H. influenzae and S. pneumoniae dominate in trauma patients but not in patients with other diagnoses (3, 5). In one study, oral anaerobic bacteria such as Prevotella, Veillonella, and Fusobacterium spp. were isolated from VAP patients (4).
Several different routes of infection by VAP pathogens have been proposed, but in most cases infection appears to result from aspiration of the oropharyngeal microflora or colonizing pathogens. Aspiration of secretions from the upper respiratory tract is facilitated by leakage of secretions around the endotracheal tube cuff, impaired swallowing and oral defense mechanisms, and the supine position of these patients (3, 12).
Epidemiological studies have addressed the role of oral bacteria in respiratory infections and suggest that respiratory pathogens colonize the oral cavity prior to entering the lower respiratory tract (7-9, 19-21). A recent review of studies examining oral and lung colonization suggests that, upon admission to the ICU, the mean prevalence of oral colonization with VAP-associated pathogens was 63% (2). The most common bacteria from the oral cavity in ICU studies included gram-negative rods such as Pseudomonas aeruginosa and Enterobacteriaceae and gram-positive cocci such as S. aureus.
Conventional cultivable methodology is insufficient for the identification of the full spectrum of VAP pathogens, as there are likely additional not-yet-cultivated species or difficult-to-grow species involved in VAP. In addition, based on the difficulties in sampling and culturing of anaerobic organisms, it is likely that the prevalence of these species in VAP has been underestimated. An approach extensively used for exploring microbial diversity is based on isolating DNA from the target environment, PCR amplifying the 16S rRNA gene, cloning the amplicons into Escherichia coli, and sequencing the cloned 16S rRNA gene inserts (14). These culture-independent molecular methods have been used to deduce the identities of novel phylotypes from the oral cavity in a variety of diseases and afflictions, such as gingivitis, dentoalveolar abscesses, periodontitis, acute necrotizing ulcerative periodontitis, noma (facial gangrene), halitosis, and caries (11, 13, 16, 17). The use of a molecular approach, which would facilitate the identification of oral and VAP pathogens that are difficult or presently impossible to detect by current culture-based methods, would add greatly to the understanding of the pathophysiology of VAP. The purpose of this study was to use such molecular methodologies to identify putative bacterial pathogens associated with VAP and to correlate these species with those detected in the oral cavity at the time of diagnosis of VAP.
MATERIALS AND METHODS
Patient recruitment and sampling.
Our study population consisted of 39 newly admitted TICU patients above the age of 17 (mean age, 40.0 ± 13.9 years) who were intubated and on mechanical ventilation. Demographic data demonstrated 82% male and 18% female patients. The ethnicities of those enrolled were 69% Caucasian, 28% African American, and 3% Asian.
We excluded patients with the following conditions: acute oral infection, significant preexisting health conditions upon admission, likely aspiration during intubation or prior to admission to the TICU, and failure to meet the criteria for VAP. Institutional review board-approved consent forms were signed by the patient's nearest relative or designated power of attorney. Patients were enrolled at the time that they were to undergo bronchoalveolar lavage (BAL) for diagnosis of VAP. Of the 39 patients, 22 were determined to have VAP, defined clinically as consisting of at least one of the following clinical symptoms suggestive of a lung infection: fever, tachycardia, leukocytosis, and a chest radiograph or computed tomography scan with clinical symptoms consistent with VAP. A BAL was completed, and the presence of a VAP pathogen concentration of ≥104 CFU/ml by routine culturing techniques confirmed the VAP diagnosis.
Oral swab samples were taken from the dorsal tongue within 12 h of a BAL and prior to, or at least 2 h after, routine oral care provided by the TICU staff. The oral cavity was examined for the state of oral hygiene, total number of teeth, and evidence of generalized gingivitis and periodontitis (Table 1).
TABLE 1.
Assessment of the oral cavities of VAP patients
| Subject no. | Oral hygienea | No. of teeth remaining | Generalized gingivitis | Periodontitisb |
|---|---|---|---|---|
| 02 | 1 | 29 | Yes | Mild, generalized |
| 03 | 3 | 26 | Yes | Mild, generalized |
| 12 | 3 | 28 | Yes | Mild, localized |
| 13 | 2 | 28 | Yes | None |
| 14 | 2 | 27 | Yes | Moderate, localized |
| 15 | 1 | 26 | Yes | Mild, generalized |
| 16 | 1 | 30 | Yes | None |
| 18 | NE | |||
| 19 | 1 | 28 | Yes | None |
| 20 | 2 | 28 | Yes | Moderate, generalized |
| 21 | 2 | 26 | Yes | Moderate, localized |
| 22 | 1 | 28 | No | None |
| 23 | 1 | 28 | Yes | Mild, generalized |
| 24 | 1 | 24 | Yes | Moderate, localized |
| 27 | 1 | 28 | Yes | Mild, generalized |
| 28 | NE |
General assessment of plaque: 1, slight; 2, moderate; 3, heavy. NE, not evaluated.
Ranges of periodontitis: mild, moderate, or severe and generalized or localized.
Tongue bacterial samples were acquired by wiping a sterile swab across both sides of the mid-dorsal region of the tongue. Swabs were placed individually in 1.5 ml of phosphate-buffered saline plus 0.04% sodium azide and put on ice before processing. BAL samples were taken by one of the trauma surgeons at the onset of suspected VAP infection. The patient was sedated, and the endoscope was introduced into the lung through the endotracheal tube. After the tip of the endoscope was wedged into the desired area of lung, 50 to 100 ml of sterile, nonbacteriostatic saline was instilled and withdrawn by a closed system connected to the suction channel of the endoscope. This specimen was sent immediately to the hospital clinical microbiology laboratory. About 2 to 3 ml of the BAL sample was removed aseptically, transferred to a screw-cap tube, and carried to our oral medicine research laboratory for processing within 2 h. Samples from both sites were analyzed by molecular methods to identify those bacterial species present.
Bacterial DNA preparation.
Oral swab samples were rotated for 4 to 5 h at room temperature to release bacteria from the swab into the solution. Bacterial DNA was isolated with a modification of the QIAamp DNA mini kit (QIAGEN, Valencia, CA). BAL samples were centrifuged at high speed (18,000 × g for 15 min; Sorvall centrifuge), and DNA was isolated from the cell pellet with the QIAamp DNA mini kit, as described above. DNA was stored at −20°C until analysis.
Amplification of the 16S rRNA gene by PCR.
The following bacterial universal primers were used (17): 9F forward primer, positions 9 to 27, 5′-GAGTTTGATYMTGGCTCAG-3′; 1541R reverse primer, positions 1541 to 1525, 5′-AAGGAGGTGWTCCARCC-3′. Bacterial DNA served as the template in a PCR using Taq polymerase (Invitrogen, Carlsbad, CA). PCR was performed in thin-walled tubes with the Gene Amp PCR System 9700 (PE Applied Biosystems, Foster City, CA) as follows: (i) 94°C, 4 min; (ii) 94°C, 45 s; (iii) 60°C, 45 s; (iv) 72°C, 90 s; (v) 72°C, 15 min; (vi) 4°C, hold. Steps ii, iii, and iv were repeated for 30 consecutive cycles. PCR products were analyzed by electrophoresis on a 1% agarose gel. DNA was stained by ethidium bromide and visualized with a UV transilluminator.
Construction of 16S rRNA clone libraries.
A 16S rRNA clone library was constructed for each sample as follows. Following electrophoresis of 5 μl of the PCR product from amplified DNA of each sample to visualize a band of the appropriate size, the remaining 45 μl of PCR product was dried, resuspended in 5 μl of water, and run on a 0.8% low-melting-point agarose gel in Tris-borate-EDTA buffer. The 16S rRNA band of the expected size was excised from the gel and purified by using spin columns (QIAquick gel extraction kit; QIAGEN, Valencia, CA) according to the manufacturer's instructions. The purified PCR product (3 to 4 μl) was cloned by a TA cloning method with a TOPO TA cloning kit (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The insert sizes of 50 clones were verified by PCR by using M13 universal primers. Clones with the correct-size inserts were analyzed by sequencing.
Sequencing of the 16S rRNA gene.
Purified PCR products were sequenced with an ABI Prism cycle sequencing kit using the BigDye Terminator cycle sequencing ready reaction kit according to the manufacturer's instructions (PE Applied Biosystems, Foster City, CA). The primers used were 9F (see above) and 533R (TTACCGCGGCTGCTG). Sequencing reactions were run on an ABI model 3100 DNA sequencer.
Data analysis of unrecognized inserts.
Clones (at least 50 per subject) with the correct-size insert of approximately 1,500 bases were analyzed. Approximately 500 bases were obtained first to determine identity or approximate phylogenetic position. Full sequences (about 1,500 bases obtained with five or six additional sequencing primers [15]) were obtained for novel species. For identification of closest relatives, sequences of the unrecognized inserts were compared to the 16S rRNA gene sequences of over 10,000 microorganisms in our database and the 100,000 sequences in the Ribosomal Database Project (http://wdcm.nig.ac.jp/RDP/html/index.html) and GenBank databases by BLAST (http://www.ncbi.nlm.nih.gov/). Programs for data entry, editing, sequence alignment, secondary structure comparison, similarity matrix generation, and phylogenetic tree construction have been written by Paster and Dewhirst (15). The similarity matrices were corrected for multiple base changes at single positions by the method of Jukes and Cantor (10). Similarity matrices were constructed from the aligned sequences by using only those sequence positions from which 90% of the strains had data. Phylogenetic trees were constructed by the neighbor-joining method of Saitou and Nei (18). TREECON, a software package for the Microsoft Windows environment, was used for the construction and drawing of evolutionary trees (22). Chimeric sequences were identified by using the Chimera check program of the Ribosomal Database Project, by treeing analysis, or base signature analysis. Species identification of chimeras was obtained, but the sequences were not subjected to phylogenetic analysis.
RESULTS
A wide diversity of bacterial species was detected from the tongue samples of each subject. All bacterial species, including 14 phylotypes that had not been previously identified, are shown in Table 2. These novel, not-yet-cultivated species belong to the genera Bacteroides, Dialister, Eubacterium, Gemella, Peptostreptococcus, Prevotella, and Selenomonas.
TABLE 2.
Bacterial species/phylotypes found in the oral cavities of subjects with VAP
| Species or phylotypea |
|---|
| Abiotrophia defectiva |
| Acinetobacter baumannii |
| Actinomyces lingnae |
| Actinomyces odontolyticus |
| Actinomyces radingae |
| Aerococcus christensenii |
| Anaerococcus lactolyticus D14154 |
| Anaerococcus tetradius AF542234 |
| Atopobium parvulum |
| Atopobium rimae |
| Bacteroidessp. clone CMC966 |
| β-Proteobacteriumsp. clone CMC756 |
| β-Proteobacterium vaginal clone rRNA373 |
| Campylobacter concisus |
| Campylobacter gracilis |
| Campylobacter showae |
| Capnocytophaga sp. clone BM058 |
| Capnocytophaga sputigena |
| Catonella clone EZ006 |
| Catonella morbi |
| Catonella sp. clone IS018B32 (cystic fibrosis) |
| Catonella sp. clone EZ006 |
| Catonella sp. oral clone BR063 |
| Clostridium celerecrescens AY458859 |
| Corynebacterium asperum/xerosis/amycolatum |
| Dialister clone rRNA287 |
| Dialister invisus |
| Dialister invisus |
| Dialister pneumosintes |
| Dialister sp. AF473837 |
| Dialistersp. clone CMC407 |
| Dialister vaginal clone rRNA287 AY959060 |
| Dialister vaginal clone rRNA387 AY959160 |
| Enterbacteraceaesp. oral clone CMC261 |
| Enterococcus faecalis |
| Escherichiasp. clone CMC357 |
| Eubacterium infirmum |
| Eubacterium saphenum |
| Eubacterium sp. clone BP2-88 |
| Eubacterium sp. clone DO016 |
| Eubacterium sp. clone EH006 |
| Eubacterium sp. clone JN088 |
| Eubacteriumsp. oral clone CMC125 |
| Eubacterium sp. oral clone JN088 |
| Eubacterium strain MDA2477 |
| Finegoldia magna |
| Firmicutessp. clone CMC1416 |
| Firmicutes sp. strain FTB41 (Oribacterium sinus) |
| Firmicutes vaginal clone FX93B4-11 AY995273 |
| Firmicutes vaginal clone rRNA115 AY958888 |
| Fusobacterium gonidoformans |
| Fusobacterium nucleatum |
| Gardnerella vaginalis |
| Gemella hemolysans |
| Gemella morbillorum |
| Gemella sanguinis |
| Gemellasp. clone CMC656 |
| Gemellasp. clone CMC663 |
| Gemella strain 1754-94 Y13363 |
| Gemella strain 933-88 |
| Granulicatella adiacens |
| Haemophilus haemolyticus |
| Haemophilus influenzae |
| Haemophilus parainfluenzae |
| Haemophilus segnis |
| Haemophilus sp. oral clone BJ095 |
| Lachnospiraceae genomo sp. clone C1 AY278618 |
| Lachnospiraceae oral clone MCE9_31 E3 AF481220 |
| Lachnospiraceae sp. clone MCE7_60 E1 |
| Lachnospiraceaesp. clone CMC255 |
| Lactobacillus acidophilus |
| Lactobacillus catenaforme |
| Lactobacillus gasseri |
| Lactobacillus kalixensis |
| Lactobacillus oral clone HT070 |
| Megasphaera micronuciformis clone BU057 |
| Megasphaera sp. clone BS073 |
| Megasphaera sp. oral clone CS025 |
| Micromonas micros |
| Mogibacterium timidum |
| Mogibacterium vescum AB021702 |
| Mycoplasma hominis |
| Mycoplasma salivarium |
| Neisseria bacilliformis clone AK105 |
| Neisseria clone AP60-53 AB028405 |
| Neisseria elongata |
| Neisseria flava/perflava |
| Neisseria flavescens |
| Neisseria mucosa |
| Neisseria pharyngis |
| Neisseria sp. clone BL026B07 AY806299 |
| Neisseria subflava/perflava |
| Peptoniphilus asaccharolyticus |
| Peptoniphilus lacrimalis AF542230 |
| Peptostreptococcus anaerobius |
| Peptostreptococcus clone CK035 |
| Peptostreptococcus hydrogenalis D14140 |
| Peptostreptococcus indolicus |
| Peptostreptococcus lactolyticus |
| Peptostreptococcus micros |
| Peptostreptococcus sp. clone CK035 |
| Peptostreptococcus sp. clone GMA-A52 |
| Peptostreptococcus sp. clone HE064 |
| Peptostreptococcus sp. clone IS040B03 |
| Peptostreptococcussp. clone CMC101 |
| Peptostreptococcussp. clone CMC259 |
| Peptostreptococcus sp. oral clone BS044/FL008 |
| Peptostreptococcus sp. oral clone CK035 |
| Peptostreptococcus strain CCUG 42997 |
| Prevotella clone AO009 |
| Prevotella clone GI030 |
| Prevotella clone PUS9.180 AJ012605 |
| Prevotella melaninogenica |
| Prevotella salivae |
| Prevotella sp. BI027 |
| Prevotella sp. clone 4.24k DQ016961 |
| Prevotellasp. clone CMC712 |
| Prevotella sp. clone DO039 |
| Prevotella sp. oral clone AO036 |
| Proteus mirabilis |
| Pseudomonas fluorescens |
| Pseudomonas tolaasii |
| Pseudoramibacter alactolyticus |
| Sedimentibactersp. clone CMC964 |
| Selenomonassp. clone CMC401 |
| Selenomonas sp. clone DM071 |
| Selenomonas sp. oral clone CS015 |
| Selenomonas sputigena |
| Shewanella algae |
| Shuttleworthia satelles |
| Solobacterium moorei |
| Staphylococcus aureus |
| Streptococcus anginosus |
| Streptococcus constellatus |
| Streptococcus cristatus |
| Streptococcus infantis |
| Streptococcus mitis biovar 2 |
| Streptococcus mitis/pneumoniae |
| Streptococcus oralis ATCC 35037 |
| Streptococcus sanguinis |
| Streptococcus sp. clone BW009 |
| Streptococcus sp. clone CH016 |
| Streptococcus sp. clone RP55-19 |
| Streptococcus sp. oral clone BP2-57 AB121930 |
| Streptococcus sp. clone P3 |
| Streptococcus sp. strain 12F |
| Streptococcus sp. strain H6 |
| Terrahaemophilus aromaticivorans |
| Veillonella parvula/dispar |
Boldface type indicates a novel phylotype.
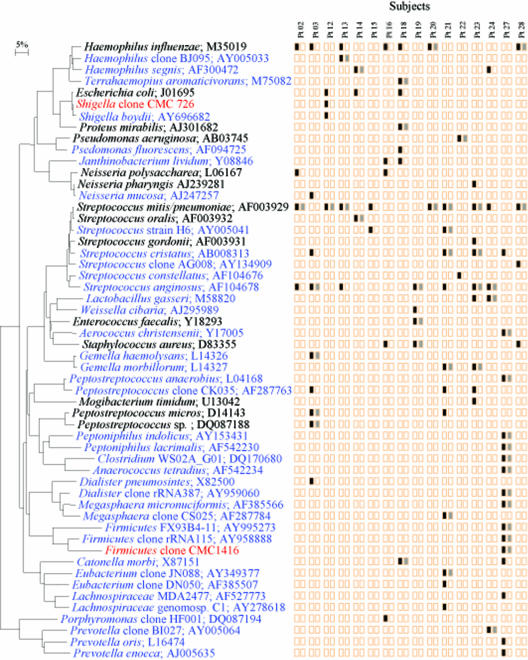
Known VAP pathogens such as H. influenzae, Escherichia sp., Streptococcus pneumoniae, Proteus mirabilis, and Pseudomonas sp. were identified in the oral cavity (Fig. 1). Putative VAP pathogens, at least one of the same species, colonized both the oral cavity and the lung in 14 of 16 subjects (88%). Eleven species in subject 27, six in subject 21, and four in subjects 23 and 3 were recovered from both oral and lung sites. In lung samples, 40 bacterial species that had not been reported previously in studies of VAP by culture-based methods were identified by clonal analysis (Fig. 1). These include Haemophilus sp., Terrahaemophilus aromaticivorans, Shigella sp., Pseudomonas fluorescens, Janthinobacterium lividum, Streptococcus sp., Gemella sp., Dialister sp., Megasphaera sp., Firmicutes sp., Lachnospiraceae sp., Porphyromonas sp., and Prevotella sp. Intriguingly, in subject 27, a male patient, Firmicutes, Megasphaera, and Dialister species, normally cultured from vaginal samples, were detected in both oral and BAL samples. Importantly, VAP-associated pathogens such as H. influenzae, S. pneumoniae, E. coli, Shigella sp., Neisseria meningitidis, and P. fluorescens were detected in the BAL samples by clonal analysis but not by conventional culture-based methods, as determined by the clinical microbiology laboratory at Carolinas Medical Center (Table 3).
FIG. 1.
Phylogenetic tree of bacterial species or phylotypes identified in BAL samples. Bacterial species or phylotype clones and GenBank accession numbers are shown. Species in blue are bacterial species previously unreported from BAL samples. Species in red are novel phylotypes identified in this study. Novel phylotypes are defined as those clones with 16S rRNA sequences having less than 99% similarity to the sequences of their closest relatives. Colored boxes: black, species or phylotype detected in BAL samples; gray, BAL species or phylotype detected in the oral cavity. The bar represents a 5% difference in nucleotide sequences. Pt, patient.
TABLE 3.
BAL sample bacterial species identified by clonal analysis but not by the culture-based method used by the clinical microbiology laboratory
| Subject no. | Bacterial species |
|---|---|
| 02 | Haemophilus influenzae |
| 03 | Dialister pneumosintes |
| 12 | Escherichia coli/Shigella flexneri |
| Shigella boydii | |
| Streptococcus mitis/pneumoniae | |
| 16 | Neisseria meningitidis/polysaccharea |
| 18 | Pseudomonas fluorescens |
| Haemophilus influenzae | |
| 21 | Streptococcus mitis/pneumoniae |
| 23 | Streptococcus mitis/pneumoniae |
| 24 | Streptococcus mitis/pneumoniae |
| 28 | Haemophilus influenzae |
| Streptococcus mitis/pneumoniae |
DISCUSSION
To our knowledge, there have been no prior studies using molecular methods to correlate oral bacterial flora with respiratory pathogens in VAP patients. VAP is often a clinical diagnosis without confirmation by bacterial culturing and subsequent identification of putative pathogens (3). Even when bronchoscopic protected brush or BAL cultures are obtained, over half of the patients invasively sampled have negative bacteriologic cultures (6). Indeed, identification of VAP pathogens may be hampered by the difficulty of in vitro cultivation. In addition, anaerobic bacterial isolation may fail due to the unavoidable exposure of bronchoscopic samples to oxygen. Inappropriate choices of selective and nonselective media can also impede bacterial recovery. Over 700 bacterial species in the human oral cavity have been identified, but more than half of these species have not yet been cultivated (14). There is no reason to expect that pathogens exist only among the cultivated segment of the population. The “uncultivables” should not be ruled out.
This study was designed to determine the correlation between the bacterial species colonizing the oral cavity and lungs of intubated patients in the TICU setting. There has been a general belief that oral pathogens are responsible for colonization of the lung and the development of VAP, but it has not been shown definitively.
The findings of this study demonstrate the strength and utility of clonal analysis of these clinical samples, in that both cultivable and not-yet-cultivated species were detected. We identified a wide range of bacterial diversity in the oral cavity and lung, including many species that are not typically detected in the oral cavity. We also identified novel VAP putative pathogens, previously uncultivable and unreported species. Eighty-eight percent of VAP patients had an overlap of VAP pathogens in the oral cavity and the lungs. This strongly supports the hypothesis that the oral cavity is a likely source of VAP pathogens.
Based on our clonal analyses, the diversity of bacteria identified in BAL fluid was, in general, far more complex than had been shown previously by conventional methods. For example, we would not have identified any of the anaerobes, because clinical laboratories report all such species as “usual oral flora” without speciation. Also, we found VAP pathogens in 56% of patients that were not identified by the clinical microbiology laboratory (Table 3), and this supports our contention that the identification of the etiology of VAP may be compromised by the use of only routine culture-based methods. Our clonal approach for BAL samples not only detected previously reported VAP pathogens, such as H. influenzae, S. pneumoniae, S. aureus, and P. aeruginosa, but also many other putative pathogens, some of which have not yet been cultivated (Fig. 1).
The healthy lung is free of bacteria, and a BAL sample would be expected to be aseptic. Given the clinical presentation, it is a reasonable assumption that, in a febrile patient with chest radiographic changes, the recovered predominant bacteria in a BAL sample would be responsible for the VAP. Clinical management and antibacterial therapy are based on this assumption, which is well supported by clinical evidence.
Most of the subjects enrolled in this study had been on antibiotic therapy prior to the development of VAP. Many patients in the TICU are on a narrow-spectrum cephalosporin as antibiotic coverage against penetrating wounds or as additional coverage for abdominal wounds, if the bowel is perforated. These patients develop VAP in spite of empirical antibiotic therapy. Therefore, the recovered bacteria are possible pathogens and may be antibiotic resistant. Although we may not have identified the full spectrum of bacterial species in the lung at the time of BAL, we have identified the predominant bacteria present at the time VAP was suspected.
We are not suggesting that clonal analysis should replace traditional culturing in the clinical setting for this clinical purpose, as culturing is necessary for antibiotic sensitivity testing of isolates. However, we have demonstrated that culture-based methods are not sufficient for accurate identification of the bacterial species associated with VAP. In addition, clinical microbiology laboratories do not routinely do sensitivity tests for organisms referred to as “usual oral flora,” which may include anaerobes involved in the disease process. It is not clear to what extent these “usual oral flora” are contaminants from the inner surface of the endotracheal tube (i.e., not from the lung) versus unidentified species from the lung that are not present in large enough numbers to be considered an important part of the disease process.
The present study provides valuable information concerning the etiology of VAP in this patient population. A better understanding of the sequential development of VAP and the role of oral bacteria is critical for the design of effective prophylactic and therapeutic interventions for this common and life-threatening condition.
Acknowledgments
We thank William Miles and the staff of the Trauma Intensive Care Unit at Carolinas Medical Center for help with patient identification and recruitment. We also thank Tainika Williams for excellent editorial work.
This work was supported by grant 201179 from the Health Services Foundation, Carolinas Medical Center.
Footnotes
Published ahead of print on 14 February 2007.
REFERENCES
- 1.Baker, A. M., J. W. Meredith, and E. F. Haponik. 1996. Pneumonia in intubated trauma patients. Microbiology and outcomes. Am. J. Respir. Crit. Care Med. 153:343-349. [DOI] [PubMed] [Google Scholar]
- 2.Brennan, M. T., F. Bahrani-Mougeot, P. C. Fox, T. P. Kennedy, S. Hopkins, R. C. Boucher, and P. B. Lockhart. 2004. The role of oral microbial colonization in ventilator-associated pneumonia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 98:665-672. [DOI] [PubMed] [Google Scholar]
- 3.Chastre, J., and J. Fagon. 2002. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 165:867-903. [DOI] [PubMed] [Google Scholar]
- 4.Doré, P., R. Robert, G. Grollier, J. Rouffineau, H. Lanquetot, J. M. Charrière, and J. L. Fauchère. 1996. Incidence of anaerobes in ventilator-associated pneumonia with use of a protected specimen brush. Am. J. Respir. Crit. Care Med. 153:1292-1298. [DOI] [PubMed] [Google Scholar]
- 5.Fagon, J. Y., J. Chastre, Y. Domart, J. L. Trouillet, J. Pierre, C. Darne, and C. Gibert. 1989. Nosocomial pneumonia in patients receiving continuous mechanical ventilation. Prospective analysis of 52 episodes with use of a protected specimen brush and quantitative culture techniques. Am. Rev. Respir. Dis. 139:877-884. [DOI] [PubMed] [Google Scholar]
- 6.Fagon, J. Y., J. Chastre, M. Wolff, C. Gervais, S. Parer-Aubas, F. Stephan, T. Similowski, A. Mercat, J. L. Diehl, J. P. Sollet, and A. Tenaillon. 2000. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann. Intern. Med. 132:621-630. [DOI] [PubMed] [Google Scholar]
- 7.Fourrier, F., B. Duvivier, H. Boutigny, M. Roussel-Delvallez, and C. Chopin. 1998. Colonization of dental plaque: a source of nosocomial infections in intensive care unit patients. Crit. Care Med. 26:301-308. [DOI] [PubMed] [Google Scholar]
- 8.Garrouste-Org, S. Chevret, G. Arlet, O. Marie, M. Rouveau, N. Popoff, and B. Schlemmer. 1997. Oropharyngeal or gastric colonization and nosocomial pneumonia in adult intensive care unit patients. A prospective study based on genomic DNA analysis. Am. J. Respir. Crit. Care Med. 156:1647-1655. [DOI] [PubMed] [Google Scholar]
- 9.Johanson, W. G., A. K. Pierce, and J. P. Sanford. 1969. Changing pharyngeal bacterial flora of hospitalized patients. Emergence of gram-negative bacilli. N. Engl. J. Med. 281:1137-1140. [DOI] [PubMed] [Google Scholar]
- 10.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, p. 21-132. In H. N. Munro (ed.), Mammalian protein metabolism. Academic Press, New York, NY.
- 11.Kazor, C. E., P. M. Mitchell, A. M. Lee, L. N. Stokes, W. J. Loesche, F. E. Dewhirst, and B. J. Paster. 2003. Diversity of bacterial populations on the tongue dorsa of patients with halitosis and healthy patients. J. Clin. Microbiol. 41:558-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kollef, M. H. 1999. The prevention of ventilator-associated pneumonia. N. Engl. J. Med. 340:627-634. [DOI] [PubMed] [Google Scholar]
- 13.Kroes, I., P. W. Lepp, and D. A. Relman. 1999. Bacterial diversity within the human subgingival crevice. Proc. Natl. Acad. Sci. USA 96:14547-14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paster, B. J., S. K. Boches, J. L. Galvin, R. E. Ericson, C. N. Lau, V. A. Levanos, A. Sahasrabudhe, and F. E. Dewhirst. 2001. Bacterial diversity in human subgingival plaque. J. Bacteriol. 183:3770-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paster, B. J., and F. E. Dewhirst. 1988. Phylogeny of campylobacters, wolinellas, Bacteroides gracilis, and Bacteroides ureolyticus by 16S ribosomal ribonucleic acid sequencing. Int. J. Syst. Bacteriol. 38:56-62. [Google Scholar]
- 16.Paster, B. J., W. A. Falkler, Jr., C. O. Enwonwu, E. O. Idigbe, K. O. Savage, V. A. Levanos, M. A. Tamer, R. L. Ericson, C. N. Lau, and F. E. Dewhirst. 2002. Prevalent bacterial species and novel phylotypes in advanced noma lesions. J. Clin. Microbiol. 40:2187-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paster, B. J., M. K. Russell, T. Alpagot, A. M. Lee, S. K. Boches, J. L. Galvin, and F. E. Dewhirst. 2002. Bacterial diversity in necrotizing ulcerative periodontitis in HIV-positive subjects. Ann. Periodontol. 7:8-16. [DOI] [PubMed] [Google Scholar]
- 18.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 19.Scannapieco, F. A., and A. W. Ho. 2001. Potential associations between chronic respiratory disease and periodontal disease: analysis of National Health and Nutrition Examination Survey III. J. Periodontol. 72:50-56. [DOI] [PubMed] [Google Scholar]
- 20.Scannapieco, F. A., E. M. Stewart, and J. M. Mylotte. 1992. Colonization of dental plaque by respiratory pathogens in medical intensive care patients. Crit. Care Med. 20:740-745. [DOI] [PubMed] [Google Scholar]
- 21.Valenti, W. M., R. G. Trudell, and D. W. Bentley. 1978. Factors predisposing to oropharyngeal colonization with gram-negative bacilli in the aged. N. Engl. J. Med. 298:1108-1111. [DOI] [PubMed] [Google Scholar]
- 22.Van de Peer, Y., and R. De Wachter. 1994. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput. Appl. Biosci. 10:569-570. [DOI] [PubMed] [Google Scholar]