Abstract
We report a case of rhino-orbital zygomycosis in a 43-year-old male with well-controlled diabetes mellitus. The patient initially received liposomal amphotericin B, but the infection continued to progress, so posaconazole treatment was begun and eventually led to the cure of his infection. The causative agent was identified as Apophysomyces elegans, an emerging cause of zygomycosis in immunocompetent hosts.
CASE REPORT
A 43-year-old male with a history of diabetes mellitus, well controlled (hemoglobin A1c, 5.2%) with oral hypoglycemic agents, was involved in a motor vehicle collision in June 2006. He was ejected from the automobile and sustained multiple injuries, including a right orbital fracture, multiple rib fractures, a thoracic vertebral body fracture causing a left pneumothorax, and a right tibia-fibular fracture. He was initially treated at a hospital facility. During the course of his first 2 weeks of hospitalization, he began to develop right orbital cellulitis, edema, proptosis, and decreased visual acuity in the right eye. At that time, he was placed on broad-spectrum antibacterial and antifungal agents, including clindamycin, daptomycin, imipenem, and amphotericin B. An ear, nose, and throat evaluation demonstrated no signs of necrotic tissue or fungal elements, and several attempts to decompress the orbit were unsuccessful. Imaging at the hospital, including computed tomography scanning and magnetic resonance imaging, demonstrated the opacification of the ethmoid and maxillary sinuses, most consistent with posttraumatic change and edema. The patient's condition and visual acuity continued to worsen, and he was subsequently transferred to the Emory University Hospital, Atlanta, GA, for further management.
Upon arrival, another ear, nose, and throat exam was performed, again with no signs of mucosal disease. Repeat imaging, however, demonstrated findings suggestive of invasive fungal disease, including marked edema, sinus opacification, proptosis of the right globe, and the extension of inflammatory changes into the bony orbit. The patient was urgently taken to the operating room for right orbital exenteration and partial right facial resection. Specimens were sent to pathology and microbiology laboratories. On frozen and surgical pathology sections, fungal elements were seen but were not further identified. While routine Gram staining and all cultures were negative, fungal cultures were not initially performed. As a result, the patient continued to receive antifungal therapy with liposomal amphotericin B at a dose of 5 mg/kg of body weight/day, as well as broad-spectrum antibacterial coverage with vancomycin and piperacillin-tazobactam.
For approximately 1 week after the initial debridement, the wound was examined daily and packed with amphotericin-soaked dressings. Fungal elements, including the visible regrowth of woolly-appearing colonies, and necrotic changes continued to be present upon inspection, and the wound was debrided daily at the bedside. During this week, the level of creatinine in the patient's serum increased from a baseline value of 0.8 mg/deciliter to a value of 1.8 mg/deciliter. After 13 days of liposomal amphotericin treatment and a total dose of approximately 4.5 g, renal function worsened and fungal elements continued to be found in the wound bed and on the bone of the zygomatic arch. The decision was made to switch to posaconazole (available under an Institutional Review Board-approved expanded-access protocol) at a dosage of 200 mg administered orally every 6 h with a fatty meal. Liposomal amphotericin treatment was stopped after the first 4 days of posaconazole therapy. On day 2 of posaconazole therapy, the patient was taken to the operating room for further orbitofacial debridement, which included the removal of the lateral wall of the maxillary sinus and part of the zygomatic process (Fig. 1). Cultures taken at this time yielded a fungus that was identified as Apophysomyces elegans.
FIG. 1.
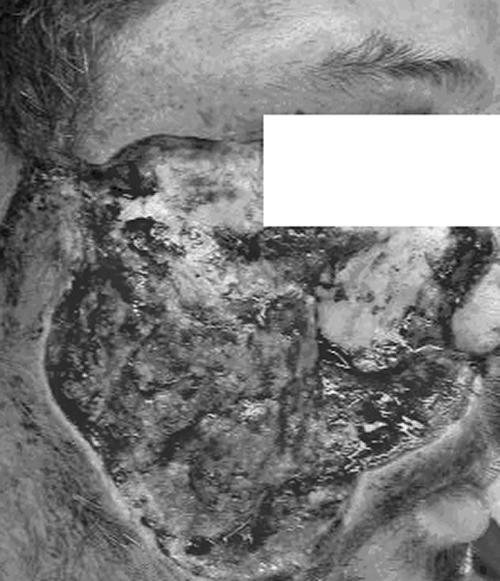
An intraoperative photo demonstrates the extent of required facial and orbital debridement.
After the second surgical debridement, no fungal elements were noted in the wound bed upon daily visual inspection. Repeat imaging of the orbits and brain was negative for any signs of further fungal invasion. The patient's serum creatinine level stabilized, and he tolerated the posaconazole well. On day 9 of posaconazole therapy, the patient was taken to the operating room a third time for partial reconstruction of the lower aspect of his face, leaving the orbital area exposed so that any recurrence of fungal disease could be easily identified. Cultures from this procedure were negative. The wound continued to heal well postoperatively, and physical and occupational therapists recommended that the patient be sent to a rehabilitation facility upon discharge. One month after admission to Emory University Hospital, he was transferred to a rehabilitation facility, where he continued to do well on posaconazole. Seven weeks after beginning posaconazole therapy, the patient returned for further reconstructive surgery, including flap coverage of the open orbit. Once the successful surgical debridement had occurred and no further fungal elements were seen, the patient received a total of 6 months of posaconazole treatment. He has continued to do well off of therapy for 2 months at the writing of this report.
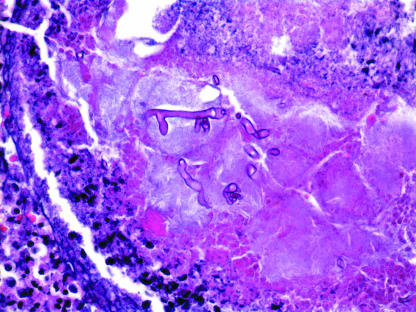
A microscopic examination of frozen and formalin-fixed tissue collected during the first surgical debridement demonstrated irregular-width, broad, ribbon-like, thin-walled hyphal fragments, branching at acute and right angles, with no septae (Fig. 2).
FIG. 2.
Hematoxylin- and eosin-stained slides showed thin-walled, ribbon-like, pauciseptate hyphae with 45- to 90-degree-angle branching. In addition to rare true septae, the folding of the thin-walled hyphae created the false impression of septae in some areas (magnification, ×400).
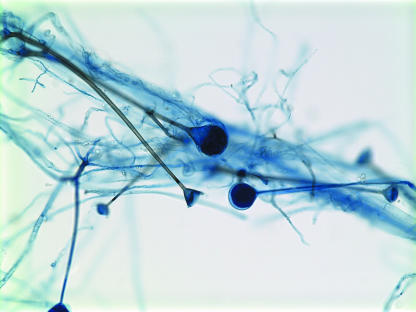
Inoculation with tissue collected at the second surgical debridement yielded the rapid growth of white, woolly-cottony colonies on Sabouraud dextrose agar at 25, 37, and 42°C. A microscopic examination of the isolate upon staining with lactophenol cotton blue demonstrated aseptate, nonconidiating mycelia with rhizoid formation. A lack of sporulation on routine media prompted the use of water culture (10), resulting in abundant sporulation at 37°C. Sporangiophores were long, slightly tapered, unbranched, and brown-green, with clear endoplasm and funnel-shaped apophyses (Fig. 3). This morphology was indicative of A. elegans. Antifungal susceptibility testing was performed using a standardized broth microdilution method (NCCLS M38-A) (3, 14). The MICs of amphotericin B and posaconazole were 0.25 and 0.006 μg/ml, respectively.
FIG. 3.
Lactophenol cotton blue staining of the water culture isolate demonstrates unbranched, slightly tapering, brown-green sporangiophores terminating in funnel-shaped apophyses. Rhizoids are present (magnification, ×400).
Rhino-orbital zygomycosis is a severe, life-threatening infection that occurs most commonly in patients with underlying immunocompromise and classically occurs in patients with poorly controlled diabetes mellitus, often with ketoacidosis (8). Over the last decade, zygomycosis caused by A. elegans has emerged as an important disease affecting immunocompetent hosts (19), especially following trauma (4, 6, 9). An environmental fungus, Apophysomyces is a member of the fungal class Zygomycetes, order Mucorales. Although zygomycetes are ubiquitous environmental pathogens, disease from Apophysomyces is rare.
Infection with zygomycetes causes rapidly progressive angioinvasive disease that is usually fatal without prompt intervention. Effective treatment requires a combination of surgical resection and debridement, the correction of the underlying disease process or immunocompromised state, and therapy with systemic antifungal agents (18). Presently, antifungal therapy includes mainly treatment with amphotericin B and its lipid derivatives. Treatment with amphotericin B is limited by dose-related toxicities, as well as the intravenous route of drug administration. Posaconazole, an oral azole antifungal with broad activity against a variety of yeasts and molds, including zygomycetes (15), has emerged as a safe and efficacious therapeutic alternative (5, 12). Posaconazole was recently approved for antifungal prophylaxis for high-risk patient populations (2, 16, 17); however, it has also been examined for use in salvage therapy for a variety of invasive fungal infections after the failure of or intolerance to other antifungals (5, 12, 18). van Burik et al. reported the largest series of zygomycosis cases treated with posaconazole as salvage therapy following amphotericin failure or intolerance (18). In this retrospective analysis of 91 cases, the success rate was 52.6% at 12 weeks (18), which is similar to the published response rates for amphotericin B (13). A case series report describing posaconazole salvage therapy for severely immunocompromised hosts with central nervous system infections noted the failure of posaconazole treatment in a patient with a brain abscess due to A. elegans and a Basidiomycetes sp. (11). This patient received 11 days of amphotericin therapy followed by 4 days of posaconazole treatment before eventually succumbing to his illness. The efficacy of posaconazole as a primary therapy for zygomycosis is presently being studied, but posaconazole will likely play a significant role in the future in the management of this disease.
Infection in immunocompromised hosts, such as those with poorly controlled diabetes with ketoacidosis, patients with hematologic malignancies, and those receiving stem cell transplantation, is usually caused by zygomycetes in the genera Mucor, Rhizopus, and Absidia (1) Clinically, these patients usually develop rhinocerebral, pulmonary, disseminated, or cutaneous infections, and mortality is highest among patients with disseminated disease (13). An observational case control study designed to examine risk factors for zygomycosis at a tertiary-care cancer center identified a rise in the incidence of this infection among high-risk patients at the center (7). While pulmonary disease was the most common type of infection in these patients, sinusitis and prophylaxis with voriconazole were risk factors associated with zygomycosis (7).
In contrast, immunocompetent hosts typically develop isolated rhinocerebral infections, acquired through either inhalation or direct inoculation as a result of trauma (9). In these cases, the causative organism is usually of the genus Apophysomyces. A recent literature review cited seven cases of rhinocerebral infection caused by A. elegans in immunocompetent patients, three of which occurred after head trauma (9). Our patient's case serves as a reminder of the relevance of increasingly common invasive fungal infections, even in patients without severe immunocompromise. It also highlights a potential niche for posaconazole in the management of zygomycoses. While the clinical presentations and outcomes of infections caused by A. elegans are similar to those of infections caused by the other zygomycetes and the treatment strategies for these infections are similar, this emerging trend of fungal infections emphasizes the need to be cognizant that zygomycosis may occur in an immunocompetent host, especially following trauma.
Acknowledgments
We express our gratitude to our patient and his family for agreeing to this publication and approving the manuscript.
Footnotes
Published ahead of print on 7 March 2007.
REFERENCES
- 1.Chayakulkeeree, M., M. A. Ghannoum, and J. R. Perfect. 2006. Zygomycosis: the re-emerging fungal infection. Eur. J. Clin. Microbiol. Infect. Dis. 25:215-229. [DOI] [PubMed] [Google Scholar]
- 2.Cornely, O., J. Maertens, D. Winston, J. Perfect, D. Helfgott, A. Ullmann, J. Holowiecki, D. Stockelberg, Y.-T. Goh, M. Petrini, T. Walsh, J. Gogate, C. Hardalo, and D. Angulo-Gonzalez. 2005. Posaconazole vs standard azole (FLU/ITRA) therapy for prophylaxis of invasive fungal infections (IFIs) among high-risk neutropenic patients: results of a randomized, multicenter trial. Blood 106:1844. [Google Scholar]
- 3.Espinel-Ingroff, A., A. Fothergill, J. Peter, M. G. Rinaldi, and T. J. Walsh. 2002. Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp.: NCCLS collaborative study. J. Clin. Microbiol. 40:3204-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Covarrubias, L., R. Bartlett, D. M. Barratt, and R. J. Wassermann. 2001. Rhino-orbitocerebral mucormycosis attributable to Apophysomyces elegans in an immunocompetent individual: case report and review of the literature. J. Trauma 50:353-357. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg, R. N., K. Mullane, J. A. van Burik, I. Raad, M. J. Abzug, G. Anstead, R. Herbrecht, A. Langston, K. A. Marr, G. Schiller, M. Schuster, J. R. Wingard, C. E. Gonzalez, S. G. Revankar, G. Corcoran, R. J. Kryscio, and R. Hare. 2006. Posaconazole as salvage therapy for zygomycosis. Antimicrob. Agents Chemother. 50:126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huffnagle, K. E., P. M. Southern, Jr., L. T. Byrd, and R. M. Gander. 1992. Apophysomyces elegans as an agent of zygomycosis in a patient following trauma. J. Med. Vet. Mycol. 30:83-86. [DOI] [PubMed] [Google Scholar]
- 7.Kontoyiannis, D. P., M. S. Lionakis, R. E. Lewis, G. Chamilos, M. Healy, C. Perego, A. Safdar, H. Kantarjian, R. Champlin, T. J. Walsh, and I. I. Raad. 2005. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J. Infect. Dis. 191:1350-1360. [DOI] [PubMed] [Google Scholar]
- 8.Lehrer, R. I., D. H. Howard, and P. S. Sypherd. 1980. Mucormycosis. Ann. Intern. Med. 93:93-108.6772062 [Google Scholar]
- 9.Liang, K. P., I. M. Tleyjeh, W. R. Wilson, G. D. Roberts, and Z. Temesgen. 2006. Rhino-orbitocerebral mucormycosis caused by Apophysomyces elegans. J. Clin. Microbiol. 44:892-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padhye, A. A., and L. Ajello. 1988. Simple method of inducing sporulation by Apophysomyces elegans and Saksenaea vasiformis. J. Clin. Microbiol. 26:1861-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitisuttithum, P., R. Negroni, J. R. Graybill, B. Bustamante, P. Pappas, S. Chapman, R. S. Hare, and C. J. Hardalo. 2005. Activity of posaconazole in the treatment of central nervous system fungal infections. J. Antimicrob. Chemother. 56:745-755. [DOI] [PubMed] [Google Scholar]
- 12.Raad, I. I., J. R. Graybill, A. B. Bustamante, O. A. Cornely, V. Gaona-Flores, C. Afif, D. R. Graham, R. N. Greenberg, S. Hadley, A. Langston, R. Negroni, J. R. Perfect, P. Pitisuttithum, A. Restrepo, G. Schiller, L. Pedicone, and A. J. Ullmann. 2006. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin. Infect. Dis. 42:1726-1734. [DOI] [PubMed] [Google Scholar]
- 13.Roden, M. M., T. E. Zaoutis, W. L. Buchanan, T. A. Knudsen, T. A. Sarkisova, R. L. Schaufele, M. Sein, T. Sein, C. C. Chiou, J. H. Chu, D. P. Kontoyiannis, and T. J. Walsh. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41:634-653. [DOI] [PubMed] [Google Scholar]
- 14.Singh, J., D. Rimek, and R. Kappe. 2005. In vitro susceptibility of 15 strains of zygomycetes to nine antifungal agents as determined by the NCCLS M38-A microdilution method. Mycoses 48:246-250. [DOI] [PubMed] [Google Scholar]
- 15.Torres, H. A., R. Y. Hachem, R. F. Chemaly, D. P. Kontoyiannis, and I. I. Raad. 2005. Posaconazole: a broad-spectrum triazole antifungal. Lancet Infect. Dis. 5:775-785. [DOI] [PubMed] [Google Scholar]
- 16.Ullmann, A., J. Lipton, D. Vesole, P. Chandrasekar, A. Langston, S. Tarantolo, H. Greinix, W. Azevedo, V. Reddy, C. Hardalo, H. Patino, and S. Durrant. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-716, Washington, DC, 16 to 19 December 2005.
- 17.Ullmann, A. J., and O. A. Cornely. 2006. Antifungal prophylaxis for invasive mycoses in high risk patients. Curr. Opin. Infect. Dis. 19:571-576. [DOI] [PubMed] [Google Scholar]
- 18.van Burik, J. A., R. S. Hare, H. F. Solomon, M. L. Corrado, and D. P. Kontoyiannis. 2006. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin. Infect. Dis. 42:e61-e65. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg, W. G., B. H. Wade, G. Cierny III, D. Stacy, and M. G. Rinaldi. 1993. Invasive infection due to Apophysomyces elegans in immunocompetent hosts. Clin. Infect. Dis. 17:881-884. [DOI] [PubMed] [Google Scholar]