Abstract
We describe a case of pustular dermatitis in a 15-year-old girl who had just returned from horseback riding camp. Based on gram staining, colony characteristics, biochemical reactions, and whole-cell fatty acid analysis, the causative agent was identified as Dermatophilus congolensis. The literature contains few reports of human infection with this organism.
CASE REPORT
A 15-year-old Caucasian girl with a history of recalcitrant verruca on both hands presented to the dermatology clinic with an eruption on her left inner thigh. The eruption began after the patient returned home from a 2-week session at a horseback riding camp in rural Michigan. The weather had been hot and humid, and the campers often rode bareback, wore shorts, and did not shower regularly. The eruption was slightly tender to touch but not painful or pruritic, and there was no burning or tingling in the area prior to the lesion's appearance. Examination of the skin revealed multiple erythematous papules and pustules over a large portion of the left medial thigh. A few other campers had similar lesions, but they were not as severe. Samples from the pustules were taken for bacterial and fungal cultures by swabbing the purulent discharge. Gram staining was not performed. The patient was placed on cephalexin at 500 mg four times a day for 10 days and was encouraged to wash with an antibacterial soap.
An organism was isolated in pure culture. The organism grew readily on blood and chocolate agars (Remel, Lenexa, KS) after overnight incubation at 35°C in 5% CO2. Colonies were slightly beta-hemolytic, yellowish, irregular, hard, and adherent to the agar surface and produced pits in the agar medium. Gram staining of the colonies showed gram-positive coccoid forms and large, long, irregular, tapering filaments, many with transverse septa (Fig. 1). The organism was catalase positive and urease positive. Based on these characteristics, the organism was presumptively identified as Dermatophilus congolensis. Definitive identification was performed by the Michigan Department of Community Health based on a variety of biochemical reactions and whole-cell fatty acid analysis with the Sherlock Microbial Identification System (MIDI, Inc., Newark, DE). The organism hydrolyzed casein and starch but not xanthine or tyrosine (Becton Dickinson, Sparks, MD). Nitrates were reduced, urease was produced, and gelatin was liquefied slowly (Remel, Lenexa, KS). The organism produced an alkaline reaction with peptonization in litmus milk (Becton Dickinson, Sparks, MD; prepared at the Michigan Department of Community Health [MDCH]) and grew in standard nutrient broth (Becton Dickinson, Sparks, MD; prepared at MDCH) but not in the presence of 6% sodium chloride. With Purple Broth Base containing 1% carbohydrates (Becton Dickinson, Sparks, MD; prepared at MDCH), dextrose was weakly fermented but xylose, mannitol, lactose, sucrose, maltose, salicin, inositol, sorbitol, arabinose, raffinose, rhamnose, galactose, and glycerol were not fermented. The organism was not acid fast and grew at 25°C and 35°C but did not grow at 45°C. The results of gas-liquid chromatography for whole-cell fatty acid analysis provided a single identification, D. congolensis, with a similarity index of 0.461, indicating a good match but potentially an atypical strain. The results of gas-liquid chromatography and other tests, taken together, provided sufficient evidence for a definitive identification.
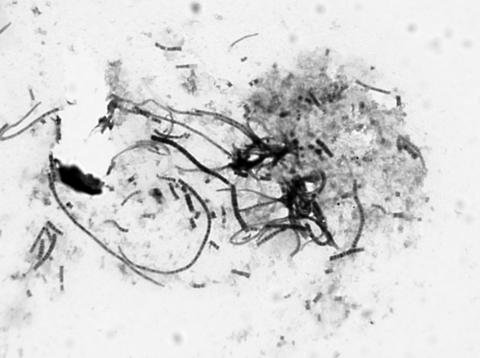
FIG. 1.
Gram staining of D. congolensis organisms from colonies on blood agar after 16 h of incubation at 35°C in 5% carbon dioxide (original magnification, ×1,300). Septate filaments and coccoid forms are visible.
The pustules resolved over 10 days with postinflammatory hyperpigmentation.
D. congolensis is an unusual gram-positive organism in the order Actinomycetales. In stains of clinical specimens, Dermatophilus is usually seen as characteristic branched filaments; however, the organism may be seen in any of the various stages of its life cycle (12, 14). Young filaments are 0.5 to 1.5 μm in diameter but enlarge as they produce layers of transverse and longitudinal septations and may ultimately reach 5 to 8 μm in diameter, surrounded by a mucoid capsule. The septations result in the formation of parallel chains and packets of coccoid cells (sporangia) that may contain up to eight cells in transverse section. In the presence of liquid, the sporangia (0.5 to 1.0 μm in diameter) are released and become flagellated, motile zoospores, the infective form of the organism. Under favorable conditions, the zoospores forms germ tubes that develop into branching filaments, the invasive form of the organism, and the cycle is repeated.
Dermatophilus is a facultative anaerobe that generally grows well on blood agar at 37°C in an atmosphere containing 5 to 10% CO2. After 24 h of incubation on blood agar, colonies are beta-hemolytic, small (0.5 to 1.0 mm), round, irregular, raised, rough, hard, and adherent with a depressed periphery. Pigmentation varies from grayish white to yellow to orange, depending on the strain. Hemolysis and production of aerial hyphae are more pronounced when the organism is cultured in a microaerophilic atmosphere (17). The organism does not grow on MacConkey agar, Sabouraud dextrose agar, or Lowenstein-Jensen medium (17). Its minimum growth requirements are not known.
Key biochemical characteristics of D. congolensis are shown in Table 1 (10). The organism is catalase positive and urease positive (11, 17). Casein and starch are decomposed, but xanthine and tyrosine are not. Gelatin is liquefied, and most strains reduce nitrate. Indole is not produced. In enriched broth, acid, but not gas, is produced from glucose and fructose. Sucrose, lactose, xylose, mannitol, dulcitol, salicin, and sorbitol are not fermented. Dermatophilus liberates significant amounts of keratinase when cultured on appropriate substrate medium (9). The cell wall contains meso-diaminopimelic acid but no arabinose or galactose. Madorose is present in whole-cell hydrolysates, and polar lipids include phosphatidylglycerol, diphosphatidylglycerol, and phosphatidylinositol. The 16S rRNA sequence for the organism has been published, so ribotyping may also be useful for identification.
TABLE 1.
Basic biochemical reactions for D. congolensis
| Test | Test resulta
|
|
|---|---|---|
| D. congolensis | Patient isolate | |
| Hemolysis | Beta in 3-7 days | Beta |
| Catalase | + | + |
| Urea, Christensen's | + | + |
| Nitrate reduction | − | − |
| Indole | − | − |
| Acid from: | ||
| Glucose | + | + |
| Xylose | − | − |
| Mannitol | − | − |
| Lactose | − | − |
| Sucrose | − | − |
| Maltose | − | − |
| Nutrient broth-0% NaCl | + | + |
| Nutrient broth-6% NaCl | − | − |
| Litmus milk | Peptonization | Peptonization |
| Growth at: | ||
| 25°C | − | − |
| 35°C | + | + |
| 42°C | − | − |
| Gelatin hydrolysis | + | + |
| Starch hydrolysis | + | + |
The data shown are from reference 10.
D. congolensis was first described from an exudative dermatitis in cattle in the Congo by van Saceghem in 1915 (16). D. congolensis is primarily known as an animal pathogen that causes acute, subacute, or chronic skin disease. It is found throughout the world but is most prevalent in tropical and subtropical regions and has a broad host range (17). Endemic and epidemic infections are most commonly reported in cattle, sheep, and horses. D. congolensis has also been isolated from a wide variety of other domestic and wild terrestrial and aquatic mammals (17). Outbreaks of D. congolensis infection have resulted in serious economic losses in many countries, primarily in the livestock and leather industries. Although effective immunity does occur, development of a vaccine has been difficult since it appears that acquired immunity is strain specific (2, 17).
The first cases of human infection with D. congolensis in the United States were reported in 1961 in four people who developed furunculosis after handling an infected deer in a wildlife reserve in Orange County, NY (4). Although infection may not be uncommon in humans, few additional cases have been reported, primarily in Africa and Australia. In all but two reports, the infected individuals had described contact with animals (1, 8). A review of the literature indicates that human disease caused by D. congolensis has a wide clinical spectrum (12, 15, 17). Infected individuals have presented with pitted keratolysis; pustules; erythematous, exudative, scaly lesions; intertriginous lesions with maceration and fissuring of the skin; and folliculitis. Subcutaneous nodules and hairy leukoplakia have been described in patients with immune deficiencies (1, 3). Asymptomatic infection involving the interdigital skin of the foot was coincidentally found in one case postmortem (15).
D. congolensis is sensitive to pH and osmotic changes, and there is no direct evidence that it multiplies in the external environment or is able to survive for long periods on the ground (12, 15, 17). Soil, therefore, is probably not a very important source of infection. Infections in humans seem to be governed by factors similar to those needed to produce disease in animals. Infection occurs mainly in keratinized tissues and remains localized in the upper layers of the skin. Most infections appear to result from mechanical transfer by direct contact with infected animals or animals that carry D. congolensis as a skin commensal. It is also possible that infection may be spread indirectly by debris from infected animals. Although D. congolensis has been isolated from the mouth parts of ticks and heavy tick infestations have been associated with D. congolensis infections in animals, the mode of transmission has not been definitively explained. It has been speculated that infection is transferred by feeding ticks; however, experimental studies have shown that healthy animals have self-limiting lesions while animals with concurrent tick infestations have more severe lesions that tend to become chronic because ticks interfere with effective immune responses of the host (2, 17). Flies, biting flies, and other biting insects have also been incriminated in the transmission of D. congolensis infection, but their role is not clear (17). Entry of the organism into the skin is facilitated by minor trauma. The microclimate of the skin is also important, as wetting of the skin under experimental conditions has been shown to help in the transmission of the disease (17). Infection generally involves only the living epidermis and does not affect the keratin of the stratum corneum or the dermis proper or the hair or wool of animals (5, 17). Invasion of the dermis proper is apparently inhibited by neutrophils that accumulate beneath the infected epidermis (5). The organism synthesizes a large number of products, including exoenzymes such as proteases, keratinases, and ceramidase, that may have a role in virulence and pathogenesis (7, 9, 17). When D. congolensis overcomes the barriers of the skin, the invasive filamentous form grows, fungus like, through the epidermis. Histopathological changes in the early stage of infection in animals include the appearance of exudate with a moderate influx of neutrophils and some lymphocytes at the base of the stratum corneum. Small vesicles form that cause separation of the cornified layers. Other histopathological changes include congestion, edema, and thickening of the stratum spinosum. Langerhans cells may be present and possibly play a role in bacterial antigen presentation and initiation of the host immune response in animals and humans (6, 15). The formation of thick scabs in animal infections is caused by repeated cycles of invasion and multiplication of the organism in the epidermis, rapid infiltration by neutrophils, regeneration of the epidermis, and invasion of the regenerated epidermis (2, 17). D. congolensis organisms in the scab then can serve as a source for new infections. If moisture is not present, the zoospores will not be released and may remain dormant in the dry scab for 12 months or longer (12, 15). While the disease is usually localized to the skin, invasion of deeper tissues has been reported to involve lymph nodes (cattle, goats, sheep, cats), muscles (cats, cattle), and subcutaneous tissues (lizards, cattle, cats).
Treatment of dermatophilosis in animals has been attempted with a wide variety of topical and parenteral antibiotics and other preparations but has been largely ineffective, probably because of the inability of topical antibiotics to reach organisms in the deep epidermis and the inability of parenteral antibiotics to reach the avascular upper epidermal layers (17). Human infections are apparently generally self-limiting and regress gradually without treatment but can recur if the skin remains moist for 10 to 16 h per day (8). Although methods of testing are not given, D. congolensis has been reported to be sensitive in vitro to penicillin, ampicillin, streptomycin, amikacin, neomycin, erythromycin, tetracycline, chloramphenicol, sulfonamides, and norfloxacin (8, 15). MICs were determined for the isolate in this case by Etest (AB Biodisk, Piscataway, NJ) on Mueller-Hinton sheep blood agar (Remel, Lenexa, KS). The organism did not grow on Mueller-Hinton II agar (Remel, Lenexa, KS). MICs for ampicillin (0.25 μg/ml), amikacin (4 μg/ml), clindamycin (0.75 μg/ml), erythromycin (0.023 μg/ml), penicillin G (0.125 μg/ml), tetracycline (0.064 μg/ml), and trimethoprim-sulfamethoxazole (0.023 μg/ml) were obtained. Antibiotic treatment of human cases has been without a clear-cut effect (1, 8, 12, 15).
Footnotes
Published ahead of print on 21 February 2007.
REFERENCES
- 1.Albrecht, R., S. Horowitz, E. Gilbert, R. Hong, J. Richard, and D. H. Connor. 1974. Dermatophilus congolensis chronic nodular disease in man. Pediatrics 53:907-913. [PubMed] [Google Scholar]
- 2.Ambrose, N., D. Lloyd, and J. C. Mallard. 1999. Immune responses to Dermatophilus congolensis infections. Parasitol. Today 15:295-300. [DOI] [PubMed] [Google Scholar]
- 3.Bunker, M. L., L. Chewning, S. E. Wang, and M. A. Gordon. 1988. Dermatophilus congolensis and “hairy” leukoplakia. Am. J. Clin. Pathol. 89:683-687. [DOI] [PubMed] [Google Scholar]
- 4.Dean, D., M. A. Gordon, C. W. Severinghaus, E. T. Kroll, and J. R. Reilly. 1961. Streptothricosis: a new zoonotic disease. N. Y. State J. Med. 61:1283-1287. [Google Scholar]
- 5.Ellis, T. M., G. G. Robertson, S. S. Sutherland, and A. R. Gregory. 1987. Cellular responses in the skin of merino sheep to repeated inoculation with Dermatophilus congolensis. Vet. Microbiol. 15:151-162. [DOI] [PubMed] [Google Scholar]
- 6.Ellis, T. M., S. S. Sutherland, and A. R. Gregory. 1989. Inflammatory cell and immune function in merino sheep with chronic dermatophilosis. Vet. Microbiol. 21:79-93. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Sánchez, A., R. Cerrato, J. Larrasa, N. C. Ambrose, A. Parra, J. M. Alonso, M. Z. Hermoso-de-Mendoza, J. M. Rey, and J. Hermoso-de-Mendoza. 2004. Identification of an alkaline ceramidase gene from Dermatophilus congolensis. Vet. Microbiol. 99:67-74. [DOI] [PubMed] [Google Scholar]
- 8.Gillum, R. L., S. M. H. Qadri, M. N. Al-Ahdal, D. H. Connor, and A. J. Strano. 1988. Pitted keratolysis: a manifestation of human dermatophilosis. Dermatologica 177:305-308. [DOI] [PubMed] [Google Scholar]
- 9.Hänel, H., J. Kalish, W. C. Marsch, and M. Buslau. 1991. Quantification of keratinolytic activity from Dermatophilus congolensis. Med. Microbiol. Immunol. 180:45-51. [DOI] [PubMed] [Google Scholar]
- 10.Hollis, D. G., and R. E. Weaver. 1981. Gram-positive organisms: a guide to identification. Special Bacteriology Section, Centers for Disease Control, Atlanta, GA.
- 11.Holt, J. G., N. R. Krieg, P. H. A. Sneath, J. T. Staley, and S. T. Williams. 1994. Bergey's manual of determinative bacteriology, ninth edition. William & Wilkins, Baltimore, MD.
- 12.Kaminski, G. W., and I. I. Suter. 1976. Human infection with Dermatophilus congolensis. Med. J. Aust. 1:443-447. [PubMed] [Google Scholar]
- 13.Reference deleted.
- 14.Robert, D. S. 1961. The life cycle of Dermatophilus dermatonomus, the causal agent of ovine mycotic dermatitis. Aust. J. Exp. Biol. Med. Sci. 39:463. [PubMed] [Google Scholar]
- 15.Towersey, L., E. DeCastro Soares Martins, A. T. Londero, R. J. Hay, P. J., Soares Filho, C. M. Takiya, C. C., Martins, and O. F. Gompertz. 1993. Dermatophilus congolensis human infection. J. Am. Acad. Dermatol. 29:351-354. [DOI] [PubMed] [Google Scholar]
- 16.van Saceghem, R. 1915. Dermatose contagieuse (impetigo contagieux). Bull. Soc. Pathol. Exot. Fil. 8:354-359. [Google Scholar]
- 17.Zaria, L. T. 1993. Dermatophilus congolensis infection (dermatophilosis) in animals and man! An update. Comp. Immun. Microbiol. Infect. Dis. 16:179-222. [DOI] [PubMed] [Google Scholar]