Abstract
Candida parapsilosis former groups II and III have recently been established as independent species named C. orthopsilosis and C. metapsilosis, respectively. In this report, 400 isolates (290 patients) previously classified as C. parapsilosis by conventional laboratory tests were screened by BanI digestion profile analysis of the secondary alcohol dehydrogenase gene fragment and by amplification fragment length polymorphism (AFLP). Thirty-three strains collected from 13 patients were identified as C. orthopsilosis, thus giving the first retrospective evidence that C. orthopsilosis was responsible for 4.5% of the infections/colonization attributed to C. parapsilosis. AFLP was proven to unambiguously identify C. orthopsilosis at the species level and efficiently delineate intraspecific genetic relatedness. A high percentage of polymorphic AFLP bands was observed for independent isolates collected from each patient. Statistical analysis of the pairwise genetic distances and bootstrapping revealed that clonal reproduction and recombination both contribute to C. orthopsilosis genetic population structure. AFLP patterns of sequential isolates obtained from two patients demonstrated that a successful strain colonization within the same patient occurred, as revealed by strain maintenance in various body sites. No association between AFLP markers and drug resistance was observed, and none of the clinical C. orthopsilosis isolates were found to produce biofilm in vitro.
Candida parapsilosis former groups II and III have been recently classified as separate species (Candida orthopsilosis and Candida metapsilosis) on the basis of extensive genomic differences and molecular phylogenetic analysis (31). The identification of the two new species is currently performed with the aid of DNA-based techniques (28, 31). A recent study on the complete sequences of mitochondrial DNA of the three species indicates that C. metapsilosis most likely diverged from a common ancestor prior to the split between C. orthopsilosis and C. parapsilosis (12). The latter species is believed to be well adapted to the human commensal environment, being frequently isolated from clinical samples and from various anatomical body sites (1, 15, 17, 20). Such a successful genetic asset is also reflected by C. parapsilosis' mainly clonal mode of reproduction, as shown by the low nucleotide variability observed within the species (10, 31), and could also account for its geographic spread (31). On the other hand, the low frequencies with which C. orthopsilosis and C. metapsilosis strains have been identified from clinical specimens have so far prevented a well-founded phenotypic and genotypic analysis (31).
In a molecular screening performed on 400 isolates initially classified as C. parapsilosis by conventional laboratory tests, we identified 33 strains that were misdiagnosed and were revealed to be C. orthopsilosis. The interest in a better characterization of the “psilosis” group relies on the epidemiology of C. parapsilosis infections, which are now assessed as the second/third most frequently occurring bloodstream infection in Europe, Canada, and Latin America (26, 27, 29), as well as on the occurrence of C. parapsilosis carriage by the hands of health care workers (3, 17). At present, little is known about genetic variability within these species. Sequence analysis of four gene fragments (31) showed a considerable nucleotide sequence diversity for nine independent C. orthopsilosis isolates, suggesting the presence of a variability greater than that demonstrated for the mainly clonal C. parapsilosis (10, 31). To validate this previous finding, amplification fragment length polymorphism (AFLP) analysis was used to evaluate C. orthopsilosis genetic diversity for a collection of 33 strains we isolated from 13 patients. AFLP was proven to be a suitable method for both species identification and strain typing, since its reproducible and high-resolution genotyping allows evaluation of strain relatedness as well as strain replacement/maintenance (2, 4, 11, 34). C. orthopsilosis isolates were also phenotyped by examining their abilities to produce biofilm and their susceptibilities to the most commonly used antifungal drugs.
This report provides a first insight into C. orthopsilosis epidemiology, demonstrating the existence of genetic diversity among isolates from different patients and successful strain colonization of various body sites within the same patient.
MATERIALS AND METHODS
Strains.
The Candida parapsilosis strain collection included 400 isolates obtained from 290 different individuals. Most of the isolates were provided by the Unità Operativa di Microbiologia, Ospedale Universitario, Pisa, Italy, and collected over an 8-year period (1998 to 2006). Isolates from different Italian hospitals were kindly provided by Giulia Morace (n = 83) (isolates from Bergamo, Cagliari, Catania, Milano, Novara, Palermo, Pavia, Treviso, Varese, and Verona) and Eugenio Pontieri (n = 5) (isolates from L'Aquila). C. orthopsilosis clinical isolates used in this study (Table 1) were originally identified as C. parapsilosis according to their biochemical profiles on API32 ID (bioMérieux, Marcy l'Etoile, France). Strains CP287 to -289 (originally named J960679/2, J950813, and 81/026) were a generous gift from F. C. Odds (Table 1). The collection included strains isolated from 11 different patients (P1 to P11) and sequential isolates collected from 2 patients (19 strains from P12 and 3 from P13). C. orthopsilosis ATCC 96139 and ATCC 96140, C. parapsilosis ATCC 22019, and C. metapsilosis ATCC 96143 were also included in the study as reference strains. Reference and clinical isolates of several Candida species, Cryptococcus neoformans, Saccharomyces cerevisiae, Aspergillus fumigatus, and A. terreus were included in the study (Table 2). All the isolates were maintained on Sabouraud agar (Liofilchem S.r.l., TE, Italy) for the duration of the study.
TABLE 1.
Details of Candida orthopsilosis clinical isolates used in this study
| Patient | Isolate no. | Site of isolationb | Area of origin | Date of isolation (day/mo/yr)b | Biofilm productiond | Susceptibility (MIC [μg/ml]) to indicated antifungal
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphotericin | Fluconazole | Itraconazole | Ketoconazole | 5-Flucytosine | Voriconazole | Caspofungin | ||||||
| P1 | CP25 | Nail | Pisa (Italy) | 16/08/2004 | A | 0.03 | 4 | 0.125 | 0.06 | 0.06 | 0.03 | 0.25 |
| P2 | CP47 | Skin | Pisa (Italy) | 05/09/2004 | A | 0.03 | 2 | 0.125 | 0.06 | 0.03 | 0.03 | 0.125 |
| P3 | CP85a | Catheter | L'Aquila (Italy) | U | A | 0.06 | 8 | 0.25e | 0.25 | 0.125 | 0.06 | 0.25 |
| P4 | CP125 | Nail | Pisa (Italy) | 01/05/2005 | A | 0.03 | 1 | 0.06 | 0.06 | 0.06 | 0.06 | 0.125 |
| P5 | CP185a | Blood | Varese (Italy) | U | A | 0.125 | 4 | 0.125e | 0.125 | 0.125 | 0.06 | 1 |
| P6 | CP287a | Toenail | Hong Kong | U | A | 16 | 0.125 | 0.125 | 0.06 | 0.06 | 0.06 | 0.25 |
| P7 | CP288a | Nail | Belgium | U | A | 0.03 | 4 | 0.125 | 0.06 | <0.03 | 0.03 | 0.125 |
| P8 | CP289a | U | NCPFc (United Kingdom) | U | A | 0.06 | 8 | 0.25e | 0.25 | >64f | 0.06 | 0.25 |
| P9 | CP296 | Skin | Pisa (Italy) | U | A | 0.03 | 2 | 0.06 | 0.03 | 0.03 | 0.03 | 0.125 |
| P10 | CP331 | Sputum | Pisa (Italy) | 16/01/2006 | A | 0.125 | 2 | 0.125 | 0.06 | 0.06 | 0.03 | 0.5 |
| P11 | CP344 | Catheter | Pisa (Italy) | 09/01/2006 | A | 0.03 | 2 | 0.125 | 0.06 | <0.03 | 0.016 | 0.125 |
| P12 | CP124 | Bronchial aspirate | Pisa (Italy) | 02/05/2005 | A | 0.03 | 8 | 0.25e | 0.125 | 0.03 | 0.06 | 0.125 |
| P12 | CP128 | Wound | Pisa (Italy) | 16/05/2005 | A | 0.03 | 8 | 0.25e | 0.06 | 0.06 | 0.03 | 0.125 |
| P12 | CP133 | Bronchial aspirate | Pisa (Italy) | 19/05/2005 | A | 0.06 | 8 | 0.25e | 0.125 | 0.06 | 0.06 | 0.25 |
| P12 | CP134 | Bronchial aspirate | Pisa (Italy) | 23/05/2005 | A | 0.03 | 8 | 0.25e | 0.125 | 0.03 | 0.06 | 0.125 |
| P12 | CP135 | Bronchial aspirate | Pisa (Italy) | 26/05/2005 | A | 0.03 | 8 | 0.25e | 0.125 | 0.03 | 0.06 | 0.125 |
| P12 | CP136 | Bronchial aspirate | Pisa (Italy) | 30/05/2005 | A | 0.06 | 8 | 0.25e | 0.125 | 0.06 | 0.06 | 0.25 |
| P12 | CP137 | Urine | Pisa (Italy) | 06/06/2005 | A | 0.06 | 8 | 0.25e | 0.125 | 0.06 | 0.06 | 0.25 |
| P12 | CP138 | Bronchial aspirate | Pisa (Italy) | 06/06/2005 | A | 0.06 | 8 | 0.125 | 0.125 | 0.06 | 0.06 | 0.25 |
| P12 | CP139 | Skin | Pisa (Italy) | 06/06/2005 | A | 0.06 | 8 | 0.25e | 0.125 | 0.06 | 0.06 | 0.25 |
| P12 | CP140 | Blood | Pisa (Italy) | 10/06/2005 | A | 0.06 | 8 | 0.25e | 0.125 | 0.06 | 0.06 | 0.25 |
| P12 | CP141 | Blood | Pisa (Italy) | 11/06/2005 | A | 0.03 | 4 | 0.125 | 0.05 | 0.03 | 0.03 | 0.125 |
| P12 | CP142 | Bronchial aspirate | Pisa (Italy) | 09/06/2005 | A | 0.06 | 4 | 0.125 | 0.06 | 0.06 | 0.03 | 0.125 |
| P12 | CP143 | Bronchial aspirate | Pisa (Italy) | 13/06/2005 | A | 0.03 | 4 | 0.125 | 0.06 | 0.06 | 0.03 | 0.125 |
| P12 | CP144 | Urine | Pisa (Italy) | 13/05/2005 | A | 0.03 | 4 | 0.125 | 0.06 | 0.03 | 0.03 | 0.125 |
| P12 | CP145 | Urine | Pisa (Italy) | 20/06/2005 | A | 0.03 | 4 | 1.125 | 0.06 | 0.06 | 0.03 | 0.125 |
| P12 | CP227 | Urine | Pisa (Italy) | 17/06/2005 | A | 0.03 | 4 | 0.125 | 0.06 | 0.06 | 0.03 | 0.125 |
| P12 | CP230 | Urine | Pisa (Italy) | 04/07/2005 | A | 0.03 | 8 | 0.25e | 0.125 | 0.06 | 0.06 | 0.125 |
| P12 | CP234 | Urine | Pisa (Italy) | 11/07/2005 | A | 0.03 | 8 | 0.25e | 0.125 | 0.03 | 0.06 | 0.125 |
| P12 | CP242 | Urine | Pisa (Italy) | 18/07/2005 | A | 0.03 | 16e | 0.25e | 0.5 | 0.06 | 0.125 | 0.125 |
| P13 | CP269 | Bronchial aspirate | Pisa (Italy) | 05/09/2005 | A | 0.06 | 4 | 0.125 | 0.125 | 0.06 | 0.06 | 0.25 |
| P13 | CP268 | Bronchial aspirate | Pisa (Italy) | 08/09/2005 | A | 0.06 | 4 | 0.125 | 0.125 | 0.03 | 0.06 | 0.125 |
| P13 | CP273 | Bronchial aspirate | Pisa (Italy) | 12/09/2005 | A | 0.06 | 4 | 0.125 | 0.125 | 0.03 | 0.06 | 0.25 |
Strains CP85 (originally named HEM22), CP185 (originally OSVE-L075), and CP287 to CP289 (originally J960679/2, J950813, and 81/026) were kindly provided by E. Pontieri, G. Morace, and F. C. Odds, respectively.
U, unknown.
NCPF, The National Collection of Pathogenic Fungi.
A, absent.
Dose-dependently susceptible.
Resistant.
TABLE 2.
Reference and clinical isolates used to validate AFLP as a species identification technique
| Isolate reference no.a, description | Site of isolation or description |
|---|---|
| C. parapsilosis isolates | |
| ATCC 22019 | Sprue sample |
| CBS 604 | Sprue sample |
| C. orthopsilosis isolates | |
| ATCC 96139 | Central venous pressure catheter tip |
| ATCC 69140 | Urine |
| C. metapsilosis isolates | |
| ATCC 69144 | Hand |
| CP5, clinical isolate | Sputum |
| C. albicans isolates | |
| DSM 1386 | Bronchomycosis sample |
| SC5314 | Laboratory strain |
| C. tropicalis isolate | |
| DSM 11953 | Bronchomycosis sample |
| C. dubliniensis isolates | |
| CPx, clinical isolate | Unknown |
| CP412, clinical isolate | Feces |
| C. lusitaniae isolates | |
| DSM 70102 | Urine |
| CP153, clinical isolate | Urine |
| C. rugosa isolates | |
| DSM 70761 | Beer |
| CP301C, clinical isolate | Feces |
| C. krusei isolate | |
| DSM 6128 | Sputum |
| C. glabrata isolate | |
| DSM11226 | Blood |
| C. famata isolate | |
| DSM 70590 | Harzer cheese |
| C. pulcherrima isolate | |
| DSM70336 | Red dates |
| S. cerevisiae isolate | |
| DSM 70449 | Top-fermenting beer yeast |
| C. neoformans isolate | |
| DSM 11959 | Cerebrospinal fluid |
| A. fumigatus isolate | |
| DSM 819 | Quality control strain |
| A. terreus isolate | |
| DSM 826 | Soil |
ATCC, strain obtained from the American Type Culture Collection; CBS, strain obtained from the Centraalbureau voor Schimmelcultures; DSM, strain obtained Deutsche Sammlung von Mikroorganismen und Zellkulturen.
DNA extraction.
Genomic DNA was extracted from yeast samples grown in Sabouraud broth (Liofilchem S.r.l., TE, Italy). Briefly, cells were harvested in stationary phase and lysed by vortexing the pellet for 3 min with 0.3 g of glass beads (0.45- to 0.52-mm diameter; Sigma, St. Louis, MO) in 200 μl of lysis buffer (100 mM Tris-HCl, pH 8.0, 2% Triton X-100 [vol/vol], 1% sodium dodecyl sulfate [wt/vol], and 1 mM EDTA) and 200 μl of 1:1 (vol/vol) phenol-chloroform. After vortexing, 200 μl of TE (10 mM Tris-HCl, pH 8.0, 1 mM EDTA) was added to the lysate; the mixture was microcentrifuged at full speed for 10 min and the aqueous phase transferred to a new tube. DNA was precipitated by the addition of 1 ml of ethanol. Samples were centrifuged and the pellet was suspended in 400 μl of TE containing 100 μg RNase (Sigma, St. Louis, MO). The mixture was incubated for 1 h at 37°C and subsequently treated with proteinase K (5 μl of a 20-mg/ml stock solution; Sigma) for 1 h at 65°C and for 30 min at 72°C. Phenol-chloroform treatment was repeated, and then DNA was precipitated with 1 ml of isopropanol and 10 μl of 4 M ammonium acetate, dried, and redissolved in 50 μl of TE, pH 8.0.
Molecular identification of C. orthopsilosis.
The two-step DNA-based identification test described by Tavanti et al. (31) and AFLP were used to screen C. parapsilosis isolates. Briefly, a 716-bp fragment of the SADH (secondary alcohol dehydrogenase) gene was amplified, purified, and digested with BanI. The different digestion patterns were used to identify the three species, since the C. parapsilosis, C. metapsilosis, and C. orthopsilosis SADH amplicons contain, respectively, one, three, and zero BanI restriction sites.
AFLP.
AFLP analysis was used to identify as well as to genotype C. orthopsilosis strains. Sequences of the adapters and preselective primers used for AFLP analysis were as described previously (4). Fifty nanograms of genomic DNA in 5.0 μl was added to 10 μl of restriction/ligation mixture (1.5× T4 DNA ligase buffer, 37.5 mM NaCl, 37.5 μg/ml of bovine serum albumin, 0.45 μM EcoRI adapter, 4.50 μM MseI adapter, 60 U of T4 DNA ligase, 3 U of EcoRI, and 3 U of MseI). 10× T4 DNA ligase buffer, bovine serum albumin, EcoRI, MseI, and T4 DNA ligase were obtained from New England BioLabs (Beverly, MA). After incubation overnight at 37°C, the reaction mixture was diluted by adding 60 μl TE and heated at 60°C for 10 min. Preselective PCR was undertaken using primers without extensions (core sequences). The AFLP adaptors and primers were obtained from SigmaGenosys (Sigma Aldrich). Five microliters of the diluted restriction/ligation product was added to 20 μl of the AFLP amplification core mixture (1.25× Promega GoTaq buffer, 0.25 mM deoxynucleotide triphosphates, 0.375 μM of the EcoRI core sequence, 0.375 μM of the MseI core sequence, and 0.625 U of GoTaq DNA polymerase). GoTaq DNA polymerase and its buffer were obtained from Promega (Promega, Madison, WI). Amplification was performed using the following conditions: 2 min at 72°C, followed by 20 cycles of 20 s at 94°C, 30 s at 56°C, and 2 min at 72°C. The PCR product was then diluted by the addition of 60 μl of sterile double-distilled water and frozen. A second PCR with the more selective primers (Cy5-labeled) EcoRI-AC and MseI-C (same concentrations as in the preselective PCR) was performed. The conditions were 2 min at 94°C, followed by 10 cycles consisting of 20 s at 94°C and 30 s at 66°C (with the annealing temperature decreasing 1°C for each successive cycle) and an extension of 2 min at 72°C. Subsequently, 25 cycles consisting of 20 s at 94°C, 30 s at 56°C, and 2 min at 72°C were performed, with a final incubation of 30 min at 60°C. The ramping rate from annealing temperature to 72°C was in both protocols set at 0.3°C/s. Samples were prepared by adding 3 μl deionized formamide to 3 μl of the selective PCR product. The mixture was heated at 96°C for 3 min and quenched on ice. Samples were run (900 min) on an ALFexpress instrument using ReproGel long-read gels, together with an ALFexpress sizer (Amersham Pharmacia Biotech AB, Uppsala, Sweden) (50 to 500 bases). Data, ranging from 50 bases to an estimated 800 bases, were exported as a tag image file format file and analyzed with TotalLab TL120 software package (Nonlinear Dynamics Ltd., United Kingdom). Dendrograms were built by the TL120 software using the unweighted-pair group method using arithmetic means (UPGMA). For each pair of isolates, a similarity index (SAB) was calculated, ranging between 0 (complete nonidentity) and 1.0 (identity). The TL120 software was also used to export a binary similarity file for the C. orthopsilosis strains to the AFLP-URV 1.0 program (33). To test for clonality within the C. orthopsilosis species, Nei's genetic distances were calculated after the method of Lynch and Milligan (18, 24) by use of the AFLP-URV 1.0 program, and the index of association (Ia) test was performed as described previously (32). In this analysis, the variance of the observed genetic distances between the 15 independent strains is compared with the distribution of variance calculated as Ia for 1,000 shuffled data sets. These distance matrices were computed by bootstrapping over the polymorphic AFLP loci by the AFLP-URV software.
Biofilm formation.
To test for biofilm production by C. orthopsilosis clinical isolates, cells were grown in Sabouraud broth, and the concentration of each suspension, microscopically determined, was adjusted to 1.5 × 107 cells/ml. Twenty microliters of yeast suspension was added to 180 μl of Sabouraud broth supplemented with 8% glucose in a 96-well polystyrene microtiter plate (Corning Inc., Corning, NY). Each strain was inoculated in replicate in eight wells. Negative controls were represented by eight wells containing 20 μl of distilled water added to 180 μl of medium. Plates were incubated at 37°C without agitation. After 24 h, the culture broth was aspirated and wells were washed twice with 200 μl of water then refilled with 200 μl of water. The optical density (OD) was then measured at 405 nm with a microplate reader (model 550; Bio-Rad Laboratories S.r.l., Milano, Italy). Biofilm production by each isolate was scored as absent (OD < 0.03), weak (0.03 ≤ OD < 0.08), moderate (0.08 ≤ OD < 0.16), or prolific (OD ≥ 0. 16).
Antifungal susceptibility.
The colorimetric broth microdilution method SensititreYeast One (Trek Diagnostic Systems Inc., Cleveland, OH) was used to evaluate C. orthopsilosis susceptibility to amphotericin B, fluconazole, ketoconazole, itraconazole, voriconazole, 5-flucytosine, and caspofungin, following the manufacturer's instructions. Briefly, suspensions were prepared to a final turbidity of 0.5 McFarland standard. Twenty microliters of each suspension was then diluted with 11 ml of YeastOne inoculum broth, and a 100-μl inoculum was transferred into each well of the Sensititre plate. Drug- and yeast-free controls were also included. Microplates were incubated at 35°C for 24 to 48 h. Quality control was ensured by testing the NCCLS-recommended strain C. parapsilosis ATCC 22019 (23).
RESULTS
Molecular identification of C. orthopsilosis.
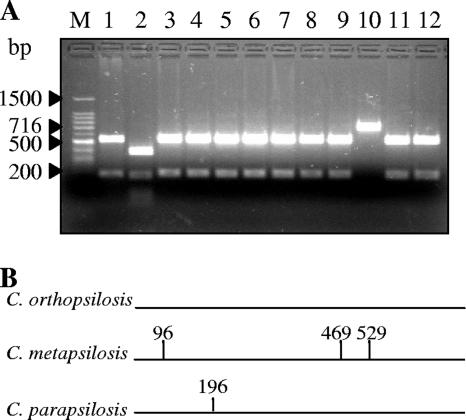
A collection of 400 clinical isolates identified as C. parapsilosis on the basis of conventional biochemical tests was screened to reveal the presence of C. orthopsilosis strains. Restriction analysis of the SADH PCR products and AFLP patterns was used to identify the species of the “psilosis” group. SADH amplicon analysis led to the identification of C. parapsilosis, C. metapsilosis, and C. orthopsilosis, as they contain, respectively, one, three, and zero BanI restriction sites (31). As shown in Fig. 1, C. orthopsilosis (lane 10) could be detected by the presence of a 716-bp DNA band (undigested fragment), while C. metapsilosis (lane 2) and C. parapsilosis (lanes 1, 3 to 9, 11, and 12) are revealed by typical four- and two-band patterns, respectively.
FIG. 1.
(A) Representative gel of BanI restriction analysis of SADH PCR products in 12 clinical isolates. Lanes: M, 100-bp DNA ladder molecular weight marker; 2, C. metapsilosis; 1, 3 to 9, 11, and 12, C. parapsilosis; 10, C. orthopsilosis (Cp 25). (B) Positions of BanI sites within the 716-bp SADH amplicon for the three species.
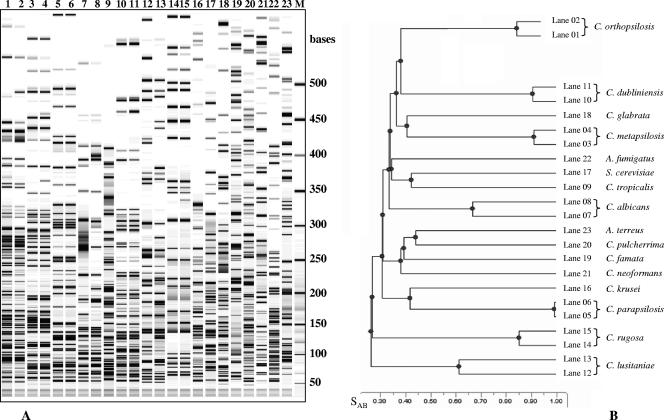
The ability of AFLP to identify C. orthopsilosis from various fungal species was tested with a variety of yeast and filamentous fungi, including reference and clinical isolates (Table 2). As shown in Fig. 2A, the AFLP profiles obtained for several Candida species (lanes 1 to 16 and 18 to 20), Saccharomyces cerevisiae (lane 17), Cryptococcus neoformans (lane 21), and Aspergillus spp. (lanes 22 and 23) were clearly distinctive for each of the species tested (SAB values always lower than 0.45) (Fig. 1B). Therefore, as already reported by Borst et al. (4), the high reproducibility of the AFLP profiles we obtained for the clinical isolates in comparison to the corresponding reference species ensured that the AFLP technique could be used for the unequivocal identification of C. orthopsilosis and C. metapsilosis as well as of the other 14 species (Table 2 and Fig. 2). The molecular screening of the strain collection revealed that C. orthopsilosis was responsible for 4.5% of the infections/colonization previously attributed to C. parapsilosis (13 independent isolates out of 400 strains obtained from 290 patients), with at least 4 of them responsible for deep-seated infection (patients P5, P10, P12, and P13; Table 1).
FIG. 2.
(A) AFLP profiles obtained from strains of 16 different fungal species. Lanes: 1 and 2, Candida orthopsilosis (ATCC 96139 and ATCC 96140); 3 and 4, C. metapsilosis (ATCC 96144 and CP5); 5 and 6, C. parapsilosis (ATCC 22019 and CBS 604); lanes 7 and 8, C. albicans (SC5314 and DSM 1386); 9, C. tropicalis (DSM 11953); 10 and 11, C. dubliniensis (CPx and CP142); 12 and 13, C. lusitaniae (DSM 70102 and CP153); 14 and 15, C. rugosa (DSM 70761 and CP301C); 16, C. krusei (DSM 6128); 17, S. cerevisiae (DSM 70449); 18, C. glabrata (DSM 11226); 19, C. famata (DSM 70590); 20, C. pulcherrima (DSM 70336); 21, Cryptococcus neoformans (DSM 11959); 22, Aspergillus fumigatus (DSM 819); 23, A. terreus (DSM 826); M, molecular weight marker (50- to 500-base ladder). (B) UPGMA dendrogram indicating genetic relatedness among the different fungal species. SAB values of 1 indicate undistinguishable strains.
Genetic variability within C. orthopsilosis isolates assessed by AFLP.
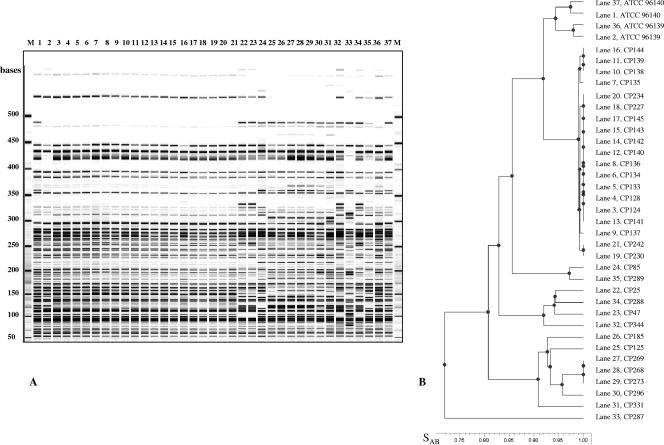
AFLP was used to evaluate genetic relatedness of 33 C. orthopsilosis strains encompassing independent isolates from 11 patients (P1 to P11) and sequential isolates collected from 2 patients (19 strains from P12 and 3 from P13). The distinctive AFLP profile exhibited by each of the strains isolated from different patients (P1 to P13) (Fig. 3, lanes 21 to 27 and 30 to 37) together indicated that a genetic diversity does exist within the C. orthopsilosis species. UPGMA analysis (Fig. 3B) showed that the independent isolates grouped into four major clusters, with the exception of one outgroup strain (CP287) collected in Hong Kong. The SAB values among the subgroups ranged from 0.81 to 0.86, while within each group they ranged from 0.91 to 0.97 (Fig. 3B). Two strains collected from two different countries (CP85 from a catheter in L'Aquila, Italy, and CP289 from the National Collection of Pathogenic Fungi [NCPF], United Kingdom) grouped together with SAB values of 0.97, as shown in the dendrogram reported in Fig. 3B. To estimate experimental error in AFLP analysis, genomic DNA was purified from reference strains ATCC 96139 and ATCC 96140 in separate experiments and analyzed by AFLP independently. Duplicate AFLP samples for each reference strain were then run at the opposite sides of the gel (Fig. 3A). As shown in Fig. 3B, AFLP patterns obtained for each of the reference strains clustered together, with SAB values of 0.97 and 0.98 for ATCC 961340 and ATCC 96139, respectively. Since such SAB values indicated the resolution limit of this technique, values of ≥0.97 were taken as an index of undistinguishable strains.
FIG. 3.
(A) AFLP profiles obtained from 33 clinical isolates of Candida orthopsilosis (lanes 3 to 35) and two C. orthopsilosis reference strains tested in duplicate. The loading order for the indicated lanes is as follows: 1, ATCC 96140; 2, ATCC 96139; 3 to 21, sequential isolates from patient P12; 22, CP25 (patient P1); 23, CP47 (patient P2); 24, CP85 (patient P3); 25, CP125 (patient P4); 26, CP185 (patient P5); 27 to 29, sequential isolates from patient P13; 30, CP296 (patient P9); 31, CP331 (patient P10); 32, CP344 (patient P11); 33, CP287 (patient P6); 34, CP288 (patient P7); 35, CP289 (patient P8); 36, ATCC 96139; 37, ATCC 69140; M, molecular weight marker (50- to 500-base ladder). (B) UPGMA dendrogram indicating genetic relatedness among the different C. orthopsilosis isolates. SAB values of 1 indicate undistinguishable strains.
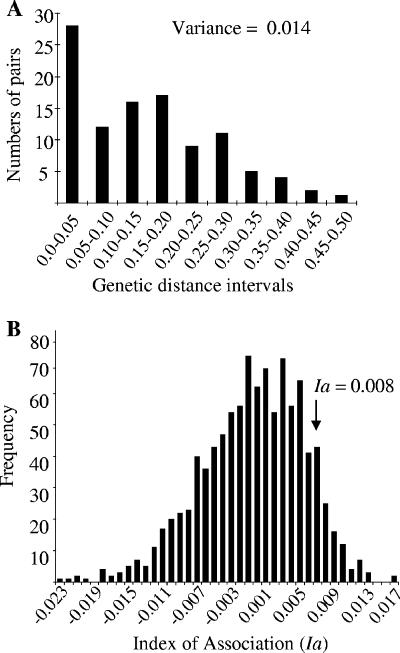
Within our C. orthopsilosis strain collection, 94 of the 124 (76%) AFLP markers were polymorphic, while the remaining 24% were monomorphic. The genetic distances between the 13 independent clinical isolates and the 2 reference strains of C. orthopsilosis, calculated according to the method of Lynch and Milligan (18), ranged from 0.05 to 0.47, with an average of 0.15 (variance, 0.014) (Fig. 4A). Their distributions were not completely symmetrical, and a relatively high number of close relatives was found (Fig. 4A). The observed data were used to simulate data expected for a recombining organism by bootstrapping. The Ia values of the observed distances were compared to the Ia distribution obtained for the bootstrapped data set: only 112 of the 1,000 shuffled data sets had Ia values higher than that for the observed data (P = 0.112) (Fig. 4B).
FIG. 4.
Distribution of pairwise comparisons of AFLP genotypes for 15 independent C. orthopsilosis isolates. (A) Bar graph of the frequency distribution of pairwise distances among AFLP genotypes. Recombining organisms show a normal distribution, while the high number of close relatives (n = 28) suggests that clonality also occurs (30). (B) Bar graph of the Ia values obtained for our data set.
Sequential isolates from the same patients (P12 and P13 in Fig. 3, lanes 2 to 21 and 27 to 29, respectively) were found to be indistinguishable (SAB values of 0.98 and 1.00 for P12 and P13, respectively) even when isolated from different body sites.
Biofilm formation.
None of the C. orthopsilosis clinical isolates were able to produce biofilm (Table 1). Among the reference strains, only ATCC 96140 was found to be a weak biofilm producer, while ATCC 96139 also failed to produce biofilm (data not shown).
Antifungal susceptibility.
All C. orthopsilosis isolates were found to be susceptible to amphotericin B, ketoconazole, voriconazole, and caspofungin (Table 1). Only one isolate, CP289, was resistant to flucytosine (MIC = >64 μg/ml) and dose-dependently susceptible to itraconazole. A few of the other strains, e.g., CP85, displayed a dose-dependent susceptibility to itraconazole (MIC = 0.25 μg/ml), and this phenotype was particularly evident for sequential isolates from P12 (12 out of 19), with the last strain, CP242, also showing a diminished susceptibility to fluconazole (MIC = 16 μg/ml) (Table 1).
DISCUSSION
This report provides evidence for the first time that C. orthopsilosis may be responsible for symptomatic mycoses in humans. The molecular screening of a large collection of C. parapsilosis isolates obtained from various hospitals in Italy showed that at least 4.5% of the infections/colonization attributed to C. parapsilosis was retrospectively recognized to be caused by C. orthopsilosis. In addition, C. orthopsilosis was found to be able to colonize different body sites, as clinical isolates were recovered from blood, nails, skin, lungs, and urine as well as from indwelling catheters, thus highlighting the clinical relevance of this species.
Two DNA-based methods were used to identify C. orthopsilosis. While BanI restriction analysis of SADH amplicons permits the identification of the species belonging to the “psilosis” group only (31), AFLP allowed the unequivocal identification of several fungal species, C. orthopsilosis and C. metapsilosis included, and served as a discriminative tool to evaluate the extent of intraspecific genetic variation. The AFLP technique was proven to be highly reproducible under standardized experimental conditions. The use of high-purity DNA is mandatory to prevent variations of the AFLP profiles (impure DNA leads to partial AFLP patterns [data not shown]), and the use of reference strains and molecular size markers facilitates interlaboratory comparison of the profiles. In this study, the same reference strain of C. parapsilosis, obtained from the United States (strain ATCC 22019) and from The Netherlands (strain CBS 604), was used as an internal control for AFLP species identification: the profile obtained for the two reference strains clustered together, with a SAB value of 0.99, thus validating the robustness of this methodology. The SAB values observed for C. parapsilosis, C. orthopsilosis, and C. metapsilosis ranged from 0.30 to 0.36, values which were similar to those found for other species (e.g., C. albicans/C. dubliniensis), thus confirming the reclassification of C. orthopsilosis and C. metapsilosis as separate species.
Despite the facts that the gold standard for typing both fungi and bacteria is the well-established multilocus sequence typing (MLST) method (5-9, 19, 22, 30) and that theoretically MLST could be applied to C. orthopsilosis (31), an MLST scheme has not yet been defined for this species. AFLP was preferred to genotype C. orthopsilosis isolates, since it offered the advantage of simultaneously identifying and genotyping each isolate studied. AFLP profiles obtained for C. orthopsilosis showed genetic diversity among independent isolates (patients P1 to P13), which grouped into four major clusters in the UPGMA dendrogram. Only the isolate from Hong Kong was clearly outgrouped. On the other hand, sequential C. orthopsilosis isolates obtained for P12 and P13 gave indistinguishable AFLP profiles for each patient. This finding clearly indicates that a single strain persisted in different body sites during the course of infection. Strain maintenance was also described for C. albicans vaginal-oral symptomatic infection, while during the healthy carrier status, the majority of women were found to carry unrelated strains in the two body niches (16).
The low percentage of monomorphic AFLP bands (24%) obtained for C. orthopsilosis suggested that recombination may occur, as predominantly selfing and/or clonal species show a much higher proportion of monomorphic randomly amplified polymorphic DNA or AFLP loci (for plant species, a range from 45% to 80% [13]; for C. parapsilosis, >80% [data not shown]). Moreover, statistical analysis of the pairwise genetic distances revealed that clonal reproduction in addition to recombination may occur within this species, as also suggested by bootstrapping and the Ia test.
None of the clinical isolates of C. orthopsilosis were found to produce biofilm in vitro, in contrast to what was found for C. parapsilosis (references 14 and 21 and data not shown). A similar trend was observed for C. metapsilosis isolates (95% of the strains were unable to produce biofilm [data not shown]). Since this virulence factor is known to promote fungal adhesion to prosthetic materials and to protect yeast cells from potential chemical (drug) and mechanical insults, the failure to produce extracellular matrix could contribute to the relatively low frequency of C. orthopsilosis isolation in the clinical setting. However, other adherence properties as well as different virulence determinants, such as proteinase/phospholipase production, should be evaluated for this species to better understand their possible contributions to C. orthopsilosis pathogenicity.
Finally, all C. orthopsilosis isolates were found to be susceptible to the most commonly used antifungals (amphotericin B, ketoconazole, voriconazole, and caspofungin), with only one strain, CP289, resistant to flucytosine and dose-dependently susceptible to itraconazole. In contrast to S. Park et al., who recently presented their data at the Interscience Conference on Antimicrobial Agents and Chemotherapy (25), we did not observe any intrinsic resistance to echinocandins in our C. orthopsilosis isolates. Among sequential isolates from P12, the majority were dose-dependently susceptible to itraconazole (12 out of 19), with the last isolate being characterized also by a diminished susceptibility to fluconazole. Such a difference in susceptibility was not associated with a different AFLP profile, as the patterns of all C. orthopsilosis isolates from P12 were indistinguishable by AFLP, at least with the primer pair used.
In summary, a major finding of this study relies on the demonstration that C. orthopsilosis may behave as a human pathogen able to cause symptomatic infections in different body sites. This result stresses the need for further investigation addressed towards defining C. orthopsilosis epidemiology by identifying its environmental reservoir, its routes of transmission, and the array of virulence attributes potentially favoring disease development that this yeast may express.
Acknowledgments
This study was supported by research grant no. 2005068754 from the Italian Ministero dell'Istruzione, dell'Università e della Ricerca.
We are grateful to Frank Odds, Giulia Morace, and Eugenio Pontieri, who provided us with C. orthopsilosis, C. parapsilosis, and C. metapsilosis isolates.
Footnotes
Published ahead of print on 28 February 2007.
REFERENCES
- 1.Almirante, B., D. Rodríguez, M. Cuenca-Estrella, M. Almela, F. Sanchez, J. Ayats, C. Alonso-Tarres, J. L. Rodriguez-Tudela, A. Pahissa, and the Barcelona Candidemia Project Study Group. 2006. Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: case-control population-based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 44:1681-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball, L. M., M. A. Bes, B. Theelen, T. Boekhout, R. M. Egeler, and E. J. Kuijper. 2004. Significance of amplified fragment length polymorphism in identification and epidemiological examination of Candida species colonization in children undergoing allogeneic stem cell transplantation. J. Clin. Microbiol. 2004. 42:1673-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonassoli, L. A., M. Bertoli, and T. I. E. Svidzinski. 2005. High frequency of Candida parapsilosis on the hands of healthy hosts. J. Hosp. Infect. 59:159-162. [DOI] [PubMed] [Google Scholar]
- 4.Borst, A., B. Theelen, E. Reinders, T. Boekhout, A. C. Fluit, and P. H. M. Savelkout. 2003. Use of amplified fragment length polymorphism analysis to identify medically important Candida species, including C. dubliniensis. J. Clin. Microbiol. 41:1357-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bougnoux, M. E., A. Tavanti, C. Bouchier, N. A. R. Gow, A. Magnier, A. D. Davidson, M. C. J. Maiden, C. d'Enfert, and F. C. Odds. 2003. Collaborative consensus for optimized multilocus sequence typing of Candida albicans. J. Clin. Microbiol. 41:5265-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dingle, K. E., F. M. Colles, D. R. A. Wareing, R. Ure, A. J. Fox, F. E. Bolton, H. J. Bootsma, R. J. L. Willems, R. Urwin, and M. C. Maiden. 2001. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 39:14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodgson, A. R., C. Pujol, D. W. Denning, D. R. Soll, and A. J. Fox. 2003. Multilocus sequence typing of Candida glabrata reveals geographically enriched clades. J. Clin. Microbiol. 41:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enright, M. C., B. G. Spratt, A. Kalia, J. H. Cross, and D. E. Bessen. 2001. Multilocus sequence typing of Streptococcus pyogenes and the relationships between emm type and clone. Infect. Immun. 69:2416-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enright, M. C., N. P. J. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fundyga, R. E., R. J. Kuykendall, W. Lee-Yang, and T. J. Lott. 2004. Evidence for aneuploidy and recombination in the human commensal yeast Candida parapsilosis. Infect. Genet. Evol. 4:37-43. [DOI] [PubMed] [Google Scholar]
- 11.Janssen, P., R. Coopman, G. Huys, J. Swings, M. Bleeker, P. Vos, M. Zabeau, and K. Kersters. 1996. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology 142:1881-1893. [DOI] [PubMed] [Google Scholar]
- 12.Kosa, P., M. Valach, L. Tomaska, K. H. Wolfe, and J. Nosek. 2006. Complete DNA sequences of the mitochondrial genomes of the pathogenic yeasts Candida orthopsilosis and Candida metapsilosis: insight into the evolution of linear DNA genomes from mitochondrial telomere mutants. Nucleic Acids Res. 34:2472-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krauss, A. L. 2000. Accurate gene diversity estimates from amplified fragment length by polymorphism (AFLP) markers. Mol. Ecol. 9:1241-1245. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn, D. M., P. K. Mukherjee, T. A. Clarke, C. Pujol, J. Chandra, R. A. Hajjeh, D. W. Warnock, D. R. Soll, and M. A. Ghannoum. 2004. Characterization of Candida parapsilosis in an outbreak setting. Emerg. Infect. Dis. 10:1074-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin, D., L.-C. Wu, M. G. Rinaldi, and P. F. Lehman. 1995. Three distinct genotypes within Candida parapsilosis from clinical sources. J. Clin. Microbiol. 33:1815-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockhart, S. R., B. D. Reed, C. L. Pierson, and D. R. Soll. 1996. Most frequent scenario for recurrent vaginitis is strain maintenance with “substrain shuffling”: demonstration by sequential DNA fingerprinting with probes Ca3, C1, and CARE2. J. Clin. Microbiol. 34:767-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lupetti, A., A. Tavanti, P. Davini, E. Ghelardi, V. Corsini, I. Merusi, A. Boldrini, M. Campa, and S. Senesi. 2002. Horizontal transmission of Candida parapsilosis candidemia in a neonatal intensive care unit. J. Clin. Microbiol. 40:2363-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch, M., and B. C. Milligan. 1994. Analysis of population genetic structure with RAPD markers. Mol. Ecol. 3:91-99. [DOI] [PubMed] [Google Scholar]
- 19.Maiden, M. C. J., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messer, S. A., R. N. Jones, and T. R. Fritsche. 2006. International surveillance of Candida spp. and Aspergillus spp.: report from the SENTRY Antimicrobial Surveillance Program (2003). J. Clin. Microbiol. 44:1782-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherje, P. K., and J. Chandra. 2004. Candida biofilm resistance. Drug Resist. Updates 7:301-309. [DOI] [PubMed] [Google Scholar]
- 22.Nallapareddy, S. R., R. W. Duh, K. V. Singh, and B. E. Murray. 2002. Molecular typing of selected Enterococcus faecalis isolates: pilot study using multilocus sequence typing and pulsed-field gel electrophoresis. J. Clin. Microbiol. 40:868-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved document M27-A2, 2nd ed. NCCLS, Wayne, PA.
- 24.Nei, M. 1987. Molecular evolutionary genetics. Columbia University Press, New York, NY.
- 25.Park, S., P. Paderu, and D. S. Perlin. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1755.
- 26.Peman, J., E. Canton, M. Gobernado, et al. 2005. Epidemiology and antifungal susceptibility of Candida species isolated from blood: results of a 2-year multicentre study in Spain. Eur. J. Clin. Microbiol. Infect. Dis. 24:23-30. [DOI] [PubMed] [Google Scholar]
- 27.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, and S. A. Messer. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J. Clin. Microbiol. 39:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pryce, T. M., S. Palladino, D. M. Price, D. J. Gardam, P. B. Campbell, K. J. Christiansen, and R. J. Murray. 2006. Rapid identification of fungal pathogens in BacT/ALERT, BACTEC, and BBL MGIT media using polymerase chain reaction and DNA sequencing of the internal transcribed spacer regions. Diagn. Microbiol. Infect. Dis. 54:289-297. [DOI] [PubMed] [Google Scholar]
- 29.Swinne, D., M. Watelle, C. Suetens, K. Mertens, P. A. Fonteyne, and N. Nolard. 2004. A one-year survey of candidemia in Belgium in 2002. Epidemiol. Infect. 132:175-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavanti, A., A. D. Davidson, E. M. Johnson, M. C. J. Maiden, D. J. Shaw, N. A. R. Gow, and F. C. Odds. 2005. Multilocus sequence typing for differentiation of strains of Candida tropicalis. J. Clin. Microbiol. 43:5593-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tavanti, A., A. D. Davidson, N. A. R. Gow, M. C. J. Maiden, and F. C. Odds. 2005. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J. Clin. Microbiol. 43:284-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor, W. T., D. M. Geiser, A. Burt, and V. Koufopanou. 1999. The evolutionary biology and population genetics underlying fungal strain typing. Clin. Microbiol. Rev. 12:126-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vekemans, X., T. Beauwens, M. Lemaire, and I. Roldan-Ruiz. 2002. Data from amplified fragment length polymorphism (AFLP) markers show indication of size homoplasy and of a relationship between degree of homoplasy and fragment size. Mol. Ecol. 11:139-151. [DOI] [PubMed] [Google Scholar]
- 34.Vos, P., R. Hogers, M. Bleeker, M. Reijans, T. van de Lee, M. Hornes, A. Freijters, J. Pot, J. Peleman, M. Kuiper, and M. Zabeau. 1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 23:4407-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]