Abstract
Specific antibodies against human immunodeficiency virus (HIV), usually used for diagnosis, almost invariably become detectable within 3 months of exposure. We report on a patient whose HIV infection was identified early by a combined antigen/antibody test, but seroconversion did not occur for 7 months, until the implementation of antiretroviral therapy.
CASE REPORT
A 37-year-old Korean man visited a medical clinic for fever and chills in February 2005. He had also experienced myalgia, weight loss, diarrhea, and easy fatigability. There were no specific findings in the physical examination, except for a skin rash and cervical lymphadenopathy. Routine laboratory examination revealed leukopenia (2,370 cells/mm3) and thrombocytopenia (93,000 cells/mm3), with a mild elevation in transaminase levels (aspartate aminotransferase and alanine aminotransferase levels, 83 and 82 IU/liter, respectively). Empirical antibiotics were administered for his sustained fever, but there was no clinical response for 10 days. Therefore, he was referred to a tertiary-care hospital in March 2005, and a screening test for human immunodeficiency virus (HIV) infection (AxSYM HIV Ag/Ab Combo; Abbott) was positive. He was unmarried, and his medical history and family history were unremarkable. He denied any history of intravenous drug use or blood transfusion. He also denied a history of homosexual contact and insisted that he had had no sexual contact for a few years.
In the Republic of Korea, all cases of HIV infection are confirmed by the Division of AIDS, Korea National Institute of Health. For confirmation of HIV infection, we performed an anti-HIV antibody enzyme-linked immunosorbent assay (ELISA; Vironostika HIV-Uni-Form II Plus O; bioMérieux, The Netherlands), a p24 antigen assay (Vironostika HIV-1 Antigen; bioMérieux), a particle agglutination assay (SERODIA HIV-1/2; Fujirebio Inc., Japan), and a Western blot assay (HIV Blot 2.2; Genelabs Diagnostics, Singapore) on 7 March 2005 (day 0). As shown in Table 1, the p24 antigen assay was reactive (sample-to-cutoff ratio [S/CO], 21.00). However, the anti-HIV antibody ELISA, the particle agglutination assay, and the Western blot assay showed negative results. The three HIV-specific antibody assays described above were repeated on five occasions up to 27 September 2005 (day 205), but all assays except for the anti-HIV antibody ELISA, performed on day 163 (S/CO, 1.04), were negative. During the follow-up, the serological status was explained to the patient and his informed consent was obtained for additional laboratory examinations beyond the routine diagnostic assays. The serum immunoglobulin G (IgG) titer against cytomegalovirus was positive (CMV IgG; Radium, Rome, Italy); and the levels of serum IgG, IgM, and IgA were within the reference ranges (COBAS Integra IgG/IgM/IgA; Roche Diagnostics). The serum IgD level was depressed until day 254 (radial immunodiffusion kit; Bindarid), and its recovery was observed 4 months after the initiation of highly active antiretroviral therapy (HAART) (day 310). The results of the p24 antigen assay and an HIV RNA quantification test (NucliSens HIV-1 RNA QT; bioMérieux) supported the persistent existence of a high HIV viral load in the plasma (1,100,000 to 13,000,000 copies/ml). The initial CD4 T-cell count was 208 cells/mm3, which declined to 20 cells/mm3 on day 163. The proportion of CD19 cells among the total lymphocytes, which represents the B-cell population, was depressed to 2.8% on day 122 (reference range, 6% to 22%).
TABLE 1.
Time course of laboratory findings
| Day | HIV serology
|
HIV RNA quantification (no. of copies/ml) | Cell countb
|
Serum Ig levelb
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV antigen ELISAa | Anti-HIV antibody ELISAa | Particle agglutination | Western blotting
|
No. of CD4+ cells/μl | No. of CD8+ cells/μl | Ratio (%) of CD19 cells/total lymphocytes (6-22%) | Total IgG (700-1,600 mg/dl) | Total IgA (40-400 mg/dl) | Total IgM (40-230 mg/dl) | Total IgD (0.94-3.58 mg/dl) | AntiCMV IgG (<0.9 negative; >1.1 positive) | ||||
| gag | pol | env | |||||||||||||
| −5 | 208 | 214 | |||||||||||||
| 0 | 21.00 | 0.42 | −d | − | − | − | |||||||||
| 12 | 4.52 | 0.48 | − | − | − | − | 8,900,000 | 89 | 250 | ||||||
| 80 | 3.78 | 0.59 | − | − | − | − | 1,100,000 | 28 | 313 | ||||||
| 122 | 8.30 | 0.64 | − | − | − | − | 4,800,000 | 32 | 272 | 2.80 | 1,569 | 520 | 152 | <0.45 | 4.7 |
| 163 | 16.34 | 1.04 | − | − | − | − | 13,000,000 | 20 | 137 | 3.32 | 1,770 | 541 | 141 | <0.45 | |
| 205c | 9.14 | 0.65 | − | − | − | − | 2,300,000 | 258 | 868 | 1,776 | 558 | 96 | <0.45 | ||
| 254 | 2.53 | 11.29 | 2+ | 24 | 31 | 120,160 | 310,000 | 190 | 398 | 11.90 | 2,187 | 633 | 111 | <0.45 | |
| 310 | 7.16 | 8.85 | 2+ | 24 | 31 | 41,120,160 | 7,900,000 | 59 | 210 | 24.20 | 2,094 | 805 | 80 | 1.82 | 10.1 |
All ELISA results are S/CO ratios; the result is positive if the ratio is ≥1.0.
Reference values are given in parentheses.
HAART had been implemented on day 177, but the compliance was not adequate on account of adverse reactions.
—, negative result.
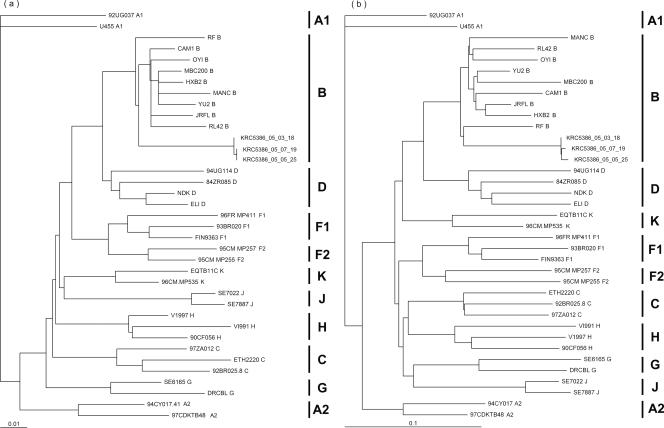
Serological tests may yield false-negative results for HIV type 1 (HIV-1) group N infections or infections caused by new and highly divergent HIV-1 variants (16). Therefore, it is important to analyze the genotypes of the viruses in patients with seronegative HIV-1 infections. We characterized the pol and env genes from three separate isolates obtained on days 12, 80, and 135. The genetic subtypes of the viruses were determined by phylogenetic analysis by using the neighbor-joining method of the PAUP* program (version 4). On the basis of the genetic alignment, we found that the isolates typically belonged to HIV-1 subtype B (Fig. 1) and the tetrameric motif at the tip of the V3 loop was GPGS (data not shown), which is the prevailing type in the Republic of Korea (11). The number of possible N-glycosylation sites (PNGSs) in the gp120 region was 21, which is lower than the median PNGS number for the Los Alamos HIV database M group (20).
FIG. 1.
Genetic subtype analysis of three separate isolates in pol sequences (a) and env sequences (b). Isolates KRC5386_05_03_18, KRC5386_05_05_25, and KRC5386_05_07_19 were isolated on day 12, day 80, and day 122, respectively. The tree was constructed by the neighbor-joining method with the PAUP* program (version 4), and the length of the horizontal line connecting one sequence to another is proportional to the divergence between the sequences. The scale represents genetic distance. The bootstrap values supporting each of the internal branches defining a subtype or subsubtype are shown.
The symptoms and signs, except for the mild generalized weakness and weight loss, were alleviated without specific treatment within a few weeks of the identification of the HIV-1 infection, and the thrombocytopenia and mild aminotransferase level elevation became normalized. However, HAART was implemented on 30 August 2005 (day 177), because the low CD4 T-cell count and high viral load persisted. After 2 months of HAART (day 254), anti-HIV antibody seroconversion was confirmed by ELISA, particle agglutination assay, and Western blot assay. Subsequently, the CD4 T-cell count increased to 349 cells/mm3 on day 582, about 1 year after the implementation of HAART (data not shown).
The diagnosis of HIV infection by the detection of HIV-specific antibody is not possible if infected individuals do not produce HIV-specific antibodies (2) or in cases of infection with distant HIV variants (16). Otherwise, seronegativity is attributed to the aggressive disease course and the associated immunological dysfunction in patients whose disease course is rapid and severe (10, 13). However, the loss of antibody cannot be distinguished from the lack of antibody formation because antibody testing is first performed late in the course of the disease in most reported seronegative cases (7, 10, 13, 17). In actuality, seroreversion occurs in very rare instances (14), although incomplete HIV-1 antibody evolution and seroreversion have been observed in acutely infected individuals who receive early antiretroviral therapy (5).
No information about the prior serological status of our patient was available. Therefore, we cannot differentiate clearly whether the seronegativity of our patient represents a prolonged window period of primary HIV infection (PHI) or seroreversion in the context of waning immunological function in far advanced AIDS. We could not observe a dynamic evolution of the Western blot seroconversion pattern of PHI, a clue to which is provided in part by the delayed appearance of p31 after the appearance of the major bands (p24, gp41, gp120/160). Furthermore, our patient failed to obtain CD4 T-cell recovery and viral load stabilization spontaneously. However, we believe that our case favors PHI with a severe presentation rather than advanced AIDS, because the patient recovered from the initial presenting symptoms and signs, which were compatible with acute HIV syndrome without specific medical treatment, and there was no newly identified AIDS-defining condition over 6 months without HAART, despite the persistent deterioration of the CD4 T-cell count. In actuality, nadir CD4 T-cell counts are known to be quite variable in patients undergoing seroconversion (3) and anecdotal cases of very severe PHI have been reported (9). Similar features have also been described in nonhuman primates during passages with chimeric simian/human immunodeficiency viruses (12).
Regarding the Western blot assay, the median time interval between the appearance of major bands and the appearance of p31 was reported to be 35 days (range, 23 to 47 days) (6). The time interval between seroconversion and the previous Western blot assay was 49 days in our case, and we suppose that we failed to observe the sequential appearance of major bands and p31 due to the relatively long test interval in the late phase of the diagnostic procedures. The seronegativity of our patient might be attributed to a high viral antigen load, which resulted in the formation of an antigen-antibody complex and a nonreactive result by antibody testing. However, it is not feasible to explain the seronegativity simply by the overwhelming amount of antigen bound to antibodies because an even higher viral load than that seen during the seronegative period was observed by a definitely positive Western blot assay result late in the course of our observations (day 310). The explanation of an antigen-antibody complex is also incompatible with the current consensus that seroreversion is very rare. Other findings suggesting PHI rather than far advanced AIDS were that the serum levels of IgG, IgM, and IgA indicating humoral immunity were within the reference ranges; and positive IgG responses to cytomegalovirus demonstrated the ability of the patient to maintain an appropriate antibody response against a virus that causes persistent latent infection. However, it is clear that the humoral immunity of our patient was relatively suppressed when the HIV infection was identified, because the B-cell population, as represented by the CD19-positive proportion of the total lymphocytes, was decreased and the serum level of IgD, which was not detected initially, increased after the implementation of HAART, although it is well known that IgD levels, as well as IgG, IgA, and IgM levels, are elevated from the early stages of HIV-1 infection as a result of both specific and polyclonal B-cell activation (1).
Combined antigen/antibody HIV assays are increasingly used for routine screening for HIV infection because these assays greatly contribute to reducing the diagnostic window in patients with recent HIV infection (15). Furthermore, as well as having a higher sensitivity in the early seroconversion phase, the combined antigen/antibody detection assay permits the additional identification of infected patients, albeit very rarely, during the late stage of disease in patients who have antigenemia and impaired synthesis of anti-HIV antibody; for these patients, third-generation assays may give false-negative results (19). However, a second diagnostic window for combined antigen/antibody HIV assays has been reported in association with a reduction in sensitivity to antibody compared with the sensitivities of third-generation tests (4, 8) and a reduction in sensitivity for antigen compared with that of the dedicated HIV p24 antigen test (18). Although this phenomenon is likely rare, such cases illustrate a sensitivity issue with the combined antigen/antibody HIV assay and emphasize the importance of additional testing and follow-up sampling for patients who have clinically suspected HIV infection but who are not reactive by the combined antigen/antibody HIV assay (18).
We have reported on a patient with HIV-1 infection with delayed seroconversion identified early by a combined antigen/antibody HIV screening test. Our patient owes the early identification of HIV infection to the advanced mechanism of the screening test. However, even highly advanced testing systems carry their own pitfalls, as mentioned above, and the application and interpretation of HIV testing require prudent consideration of clinical information.
Nucleotide sequence accession numbers.
The HIV RNA sequences have been assigned GenBank accession numbers DQ885604 to DQ885609.
Footnotes
Published ahead of print on 7 March 2007.
REFERENCES
- 1.Abelian, A., K. Burling, P. Easterbrook, and G. Winter. 2004. Hyperimmunoglobulinemia and rate of HIV type 1 infection progression. AIDS Res. Hum. Retrovir. 20:127-128. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso, A. R., C. Goncalves, D. Pascoalinho, C. Gil, A. F. Ferreira, I. Bartolo, and N. Taveira. 2004. Seronegative infection and AIDS caused by an A2 subsubtype HIV-1. AIDS 18:1071-1074. [DOI] [PubMed] [Google Scholar]
- 3.Dorrucci, M., A. N. Phillips, B. Longo, and G. Rezza. 2005. Changes over time in post-seroconversion CD4 cell counts in the Italian HIV-Seroconversion Study: 1985-2002. AIDS 19:331-335. [PubMed] [Google Scholar]
- 4.Gurtler, L., A. Muhlbacher, U. Michl, H. Hofmann, G. G. Paggi, V. Bossi, R. Thorstensson, R. G.-Villaescusa, A. Eiras, and J. M. Hernandez. 1998. Reduction of the diagnostic window with a new combined p24 antigen and human immunodeficiency virus antibody screening assay. J. Virol. Methods 75:27. [DOI] [PubMed] [Google Scholar]
- 5.Kassutto, S., M. N. Johnston, and E. S. Rosenberg. 2005. Incomplete HIV type 1 antibody evolution and seroreversion in acutely infected individuals treated with early antiretroviral therapy. Clin. Infect. Dis. 40:868-873. [DOI] [PubMed] [Google Scholar]
- 6.Kleinman, S., M. P. Busch, L. Hall, R. Thomson, S. Glynn, D. Gallahan, H. E. Ownby, and A. E. Williams. 1998. False-positive HIV-1 test results in a low-risk screening setting of voluntary blood donation. JAMA 280:1080-1085. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Rico, P., C. Pedersen, P. Skinhoj, C. Nielsen, and B. O. Lindhardt. 1995. Rapid development of AIDS in an HIV-1-antibody-negative homosexual man. AIDS 9:95-96. [PubMed] [Google Scholar]
- 8.Meier, T., E. Knoll, M. Henkes, G. Enders, and R. Braun. 2001. Evidence for a diagnostic window in fourth generation assays for HIV. J. Clin. Virol. 23:113. [DOI] [PubMed] [Google Scholar]
- 9.Michael, N. L., A. E. Brown, R. F. Voigt, S. S. Frankel, J. R. Mascola, K. S. Brothers, M. Louder, D. L. Birx, and S. A. Cassol. 1997. Rapid disease progression without seroconversion following primary human immunodeficiency virus type 1 infection—evidence for highly susceptible human hosts. J. Infect. Dis. 175:1352-1359. [DOI] [PubMed] [Google Scholar]
- 10.Montagnier, L., C. Brenner, S. Chamaret, D. Guetard, A. Blanchard, J. de Saint Martin, J. D. Poveda, G. Pialoux, and M. L. Gougeon. 1997. Human immunodeficiency virus infection and AIDS in a person with negative serology. J. Infect. Dis. 175:955-959. [DOI] [PubMed] [Google Scholar]
- 11.Oh, M. D., S. W. Park, U. Kim, H. B. Kim, Y. J. Choe, E. Kim, and K. Choe. 2002. Determination of genetic subtypes of HIV type 1 isolated from Korean AIDS patients. AIDS Res. Hum. Retrovir. 18:1229-1233. [DOI] [PubMed] [Google Scholar]
- 12.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I. W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reimer, L., S. Mottice, C. Schable, P. Sullivan, A. Nakashima, M. Rayfield, R. Den, and C. Brokopp. 1997. Absence of detectable antibody in a patient infected with human immunodeficiency virus. Clin. Infect. Dis. 25:98-100. [DOI] [PubMed] [Google Scholar]
- 14.Roy, M. J., J. J. Damato, and D. S. Burke. 1993. Absence of true seroreversion of HIV-1 antibody in seroreactive individuals. JAMA 269:2876-2879. [PubMed] [Google Scholar]
- 15.Saville, R. D., N. T. Constantine, F. R. Cleghorn, N. Jack, C. Bartholomew, J. Edwards, P. Gomez, and W. A. Blattner. 2001. Fourth-generation enzyme-linked immunosorbent assay for the simultaneous detection of human immunodeficiency virus antigen and antibody. J. Clin. Microbiol. 39:2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon, F., P. Mauclere, P. Roques, I. Loussert-Ajaka, M. C. Muller-Trutwin, S. Saragosti, M. C. Georges-Courbot, F. Barre-Sinoussi, and F. Brun-Vezinet. 1998. Identification of a new human immunodeficiency virus type 1 distinct from group M and group O. Nat. Med. 4:1032-1037. [DOI] [PubMed] [Google Scholar]
- 17.Soriano, V., F. Dronda, A. Gonzalez-Lopez, F. Chaves, R. Bravo, M. Gutierrez, A. Heredia, and I. Hewlett. 1994. HIV-1 causing AIDS and death in a seronegative individual. Vox Sang. 67:410-411. [DOI] [PubMed] [Google Scholar]
- 18.Speers, D., P. Phillips, and J. Dyer. 2005. Combination assay detecting both human immunodeficiency virus (HIV) p24 antigen and anti-HIV antibodies opens a second diagnostic window. J. Clin. Microbiol. 43:5397-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber, B. 1998. Multicenter evaluation of the new automated Enzymun-Test Anti-HIV 1 + 2 + subtype O. J. Clin. Microbiol. 36:580-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, M., B. Gaschen, W. Blay, B. Foley, N. Haigwood, C. Kuiken, and B. Korber. 2004. Tracking global patterns of N-linked glycosylation site variation in highly variable viral glycoproteins: HIV, SIV, and HCV envelopes and influenza hemagglutinin. Glycobiology 14:1229-1246. [DOI] [PubMed] [Google Scholar]