Abstract
Infections by strains belonging to the O3:K6 pandemic clone of Vibrio parahaemolyticus are prevalent in southern Thailand, and serovariants of these strains have also been detected. V. parahaemolyticus strains lacking important virulence genes (tdh and trh) were isolated from 6.5 to 10.9% of clinical specimens during the period from 2000 to 2003. In order to understand whether changes to the characteristics of V. parahaemolyticus occur during infection, 10 isolates collected from each of 63 patients who presented with diarrhea at the Hat Yai hospital from 2003 to 2004 were examined for the presence of the tdh and trh genes, the O:K serotype, and genetic markers for the pandemic clone. A total of 42 patients (66.7%) yielded identical isolates (homogeneous populations), and 21 of the patients (33.3%) yielded isolates that differed in at least one character from the other isolates (heterogeneous populations). The DNA fingerprints (examined by arbitrarily primed PCR and pulsed-field gel electrophoresis) of some, but not all, of the heterogeneous populations from single patients were indistinguishable. The results indicated that some patients were infected with a unique strain and that in vivo changes (tdh deletion or serotype conversion) might have occurred in certain individuals. It is therefore important to bear in mind that epidemiological studies based on the analysis of a single colony from a single patient might lead to misleading conclusions. Finally, the present study did not rule out the possibility that isolates lacking tdh and trh have unknown virulence mechanisms other than the tdh and trh genes.
Vibrio parahaemolyticus is a marine bacterium that causes seafood-borne gastroenteritis after the consumption of contaminated raw, or partially cooked, fish or shellfish (29). The disease is often associated with abdominal cramps, nausea, vomiting, headache, occasional diarrhea, mild fever, and chills. The incubation period ranges from 4 to 96 h (3). Most clinical isolates of V. parahaemolyticus produce a major virulence factor, known as the thermostable direct hemolysin (TDH) (25), which is responsible for the hemolytic activity, called the Kanagawa phenomenon (KP), displayed on special blood agar (Wagatsuma agar). Another virulence factor, the TDH-related hemolysin (TRH), which is generally associated with some KP-negative strains of V. parahaemolyticus, is also involved in food-poisoning outbreaks (8, 10). TDH and TRH are encoded by the tdh and trh genes, respectively. The trh gene sequence can vary from strain to strain to some extent, but trh gene sequences can be clustered into two subgroups, represented by trh1 and trh2 (12). Almost all clinical isolates carry tdh, trh, or both genes, whereas only 1 to 2% of environmental isolates possess these genes (12, 23). Therefore, a strain carrying tdh, trh, or both genes is considered a virulent strain (17).
Since 1996, an increased incidence of gastroenteritis due to V. parahaemolyticus O3:K6 has been reported in many Asian countries (15), the United States (15), Europe (14), South America (7), and Africa (1). These O3:K6 strains carry the tdh gene, but not the trh gene, and show identical DNA fingerprints when examined by an arbitrarily primed-PCR (AP-PCR) method (15). Therefore, these strains are considered to be a pandemic clone. A molecular typing method, known as group-specific PCR (GS-PCR), has detected nucleotide variations within the 1,364-bp toxRS region that are unique to the pandemic clone. Examination of recent clinical strains using this method led to the finding of pandemic clones belonging to non-O3:K6 serotypes, e.g., O1:K25 and O4:K68 (2, 4, 15). These serotypes might have diverged from the O3:K6 clone (2, 15). In addition, one of the open reading frames (ORFs) of the f237 phage genome, ORF8, was proposed as a marker that might be useful for detection of the pandemic clone (9, 16). Many GS-PCR-positive non-O3:K6 strains carry ORF8, but ORF8 is considered to be less specific to the pandemic clone than the GS-PCR-positive reaction (2, 5). We define pandemic strains here as those carrying the tdh gene, lacking the trh gene, and yielding GS-PCR-positive reactions.
Since the emergence of the pandemic strains, a surveillance program for V. parahaemolyticus has been established in the southern part of Thailand. Analysis of V. parahaemolyticus isolates collected from the Songklanagarind and Hat Yai hospitals from December 1998 to December 1999 showed that 76 to 87% were pandemic strains, some of which belonged to non-O3:K6 serotypes (O1:K25, O1:K41, and O4:K12) (13, 27). The pandemic strains accounted for 64, 68, and 69% of the V. parahaemolyticus strains isolated at the Hat Yai hospital in the years 2000, 2001, and 2002, respectively. During this 3-year surveillance period, we found that 10.9, 6.5, and 9.8% of V. parahaemolyticus isolates from this hospital carried neither the tdh nor the trh gene, respectively.
In routine clinical investigations of V. parahaemolyticus infection, one non-sucrose-fermenting colony is picked from one thiosulfate-citrate-bile salts-sucrose (TCBS) agar plate inoculated with a specimen from a diseased individual. However, analysis of a single isolate per patient may lead to misdiagnosis of the etiological agent due to sampling error if the infecting microbial population is not homogeneous. We hypothesized that the isolation of strains lacking the tdh and trh genes from individuals exhibiting symptoms of V. parahaemolyticus illness might be most likely due to the simultaneous consumption and proliferation of virulent and avirulent strains in a human host, followed by isolation of an avirulent strain lacking both tdh and trh by chance, by deletion of the virulence genes (tdh and trh) from virulent strains in vivo, or by mediation of V. parahaemolyticus illness by a novel tdh and trh mutant-independent mechanism. We also hypothesized that in vivo serotype conversion may contribute to the diversity of serotypes currently recognized in the pandemic strain. Variability of the serotype, toxin gene profile, and markers for the pandemic clone among the isolates from a single patient, if any, affects epidemiological investigation of V. parahaemolyticus infection. To examine these possibilities, we investigated the variability of V. parahaemolyticus isolates from single patients in southern Thailand.
MATERIALS AND METHODS
Sample collection and bacteriology.
A rectal swab was obtained from each diarrhea patient in the Hat Yai hospital, Songkhla Province, Thailand, between 2003 and 2004. Each sample was plated on MacConkey, Salmonella-Shigella, and TCBS agars (Difco). After overnight incubation at 37°C, samples showing growth predominantly on TCBS were selected. Ten non-sucrose-fermenting colonies were randomly picked from each plate. The isolates were identified as V. parahaemolyticus by standard biochemical tests and confirmed by PCR targeted to the toxR gene.
toxR investigation and virulence gene detection.
The test isolate was grown in Luria-Bertani (LB) broth containing 1% NaCl with shaking (160 rpm) at 37°C overnight. One milliliter of the broth culture was centrifuged, and the bacterial cells were washed with sterile saline (0.85% NaCl) and then suspended in it. The cell suspension was boiled for 10 min, and the supernatant was obtained by centrifugation, diluted 10-fold in distilled water, and used as the template for PCR amplification. To investigate the toxR gene, PCR with the primers T4 and T7 was performed as described previously (11). To determine the presence of the tdh and trh genes, the template was prepared as described above. PCR was carried out with the primers D3 and D5 and primers R2 and R6, respectively (24).
O:K serotype.
The O:K serotype of the test isolate was determined by the slide agglutination test using anti-O and anti-K antibodies (Denka Seiken, Tokyo, Japan). Briefly, the test isolate was grown in tryptic soy broth containing 3% NaCl at 37°C for 18 h, and the bacterial cells were suspended in saline (3% NaCl). The bacterial cell suspension was subjected to agglutination with specific anti-K antibodies for the K serotype determination. For the O serotype determination, the bacterial cell suspension was autoclaved at 121°C for 30 min. Autoclaved bacterial cells were subjected to agglutination with specific anti-O antibodies.
GS-PCR.
GS-PCR was carried out with the primers GS-VP1 and GS-VP2 (15). Briefly, the PCR template was prepared in the manner described above. The PCR mixture consisted of 1.5 mM MgCl2, 0.125 mM concentrations of deoxynucleoside triphosphates (dNTPs), 0.2 μM concentrations of each primer, 0.5 U of Taq DNA polymerase in storage buffer A, and 2.5 μl of DNA template in a 20-μl volume. Amplification was performed with a single cycle at 96°C for 5 min, followed by 25 cycles of denaturation at 96°C for 1 min, annealing at 45°C for 2 min, and extension at 72°C for 3 min, with a final extension at 72°C for 7 min.
ORF8 detection.
The presence of ORF8 was determined by PCR with the primers VP36RF8U and VP36RF8L (13). Briefly, a 20-μl volume contained 2 mM MgCl2, 0.2 mM concentrations of dNTPs, 0.5 μM concentrations of each primer, 0.5 U of Taq DNA polymerase in storage buffer A, and 2.0 μl of DNA template. The amplification conditions consisted of 1 cycle at 96°C for 5 min, followed by 25 cycles of denaturation at 94°C for 1 min, annealing at 53°C for 1 min, and extension at 72°C for 1 min, with a final cycle at 72°C for 7 min.
AP-PCR.
DNA was extracted by a standard phenol-chloroform extraction method (22). AP-PCR was performed with primers 2 (5′-GTTTCGCTCC-3′) and 4 (5′-AAGAGCCCGT-3′) as described previously (15). Briefly, amplification was performed in a 30-μl mixture composed of 0.33 mM concentrations of dNTPs (TaKaRa Biochemicals, Tokyo, Japan), 25 ng of template DNA, 2.5 U of Ex Taq (TaKaRa), 0.83 pmol of primer, and 1× Ex Taq Buffer (TaKaRa). The PCR was performed in a thermal cycler (Program Temp Control System PC-808; Astec Co.). The thermocycle was started with a cycle at 95°C for 4 min. This was followed by 45 cycles of denaturation at 95°C for 1 min, annealing at 36°C for 1 min, and extension at 72°C for 2 min, in which a transition time of 5 min was set between the denaturation and annealing, annealing and extension, and extension and denaturation steps. The thermocycle finished with one cycle at 72°C for 7 min. The amplification products were analyzed by electrophoresis in a 1.5% agarose gel.
PFGE.
V. parahaemolyticus was grown in LB broth supplemented with 1% NaCl at 37°C overnight with continuous shaking (160 rpm). A 1-ml sample of culture was centrifuged and resuspended in 150 μl of SE buffer (75 mM NaCl and 25 mM EDTA [pH 8.0]). An agarose plug was prepared by mixing equal volumes of the bacterial suspension with melted agarose (2% low-melting-point agarose in a buffer consisting of 10 mM Tris-HCl [pH 7.5], 10 mM MgCl2, and 0.1 mM EDTA) and was transferred to a disposable plug mold (Bio-Rad Laboratories, Hercules, CA). After solidification, lysis was achieved with 950 μl of lysis solution (containing 50 mM Tris-HCl [pH 8.0], 50 mM EDTA, 1% N-laurylsarcosine, and 1 mg of proteinase K per ml), and the DNA was cleaved by the NotI restriction enzyme (TOYOBO Co., Ltd., Osaka, Japan) at 37°C overnight. The lambda DNA ladder marker (Bio-Rad) was incubated at 50°C for 10 min before being loaded into the gel. The digested DNA fragments and DNA markers were separated in 1% Pulse-Field Certified agarose (Bio-Rad Laboratories) using 0.5× Tris-borate-EDTA buffer on a CHEF-DRIII system (Bio-Rad). Electrophoresis was performed at 6 V/cm with a field angle of 120° at 14°C. The pulse times were 1 to 18 s for 36 h. After electrophoresis in a 1% pulsed-field gel electrophoresis (PFGE) agarose gel, the gel was stained with ethidium bromide, and DNA was visualized with a UV transilluminator.
Southern blot hybridization.
DNA probes specific to the tdh, trh1, and trh2 genes were excised from recombinant plasmids (12, 23); purified by using agarose gel electrophoresis and a DNA extraction kit (QIAGEN); and then labeled with digoxigenin (Roche Diagnostics) according to the manufacturer's specifications. HindIII-digested total bacterial DNA was subjected to electrophoresis in a 1% agarose gel and transferred to a nylon membrane (Boehringer Manheim). The hybridization was carried out under high-stringency conditions (at 37°C for the tdh probe and at 30°C for the trh1 and trh2 probes). The hybridized probes were detected by using a DNA detection kit (Roche Diagnostics) according to the manufacturer's specifications.
Stability of the serotype and genotype of isolates in vivo and in vitro.
To examine whether changes in the serotype or genotype of V. parahaemolyticus isolates from patients could occur in vivo, three isolates from patient group 16—PSU1681, PSU1690, and PSU1683—and three additional new isolates with various toxin gene profiles or serotypes—PSU1958, PSU2056, and PSU2490—were examined before and after passage through a rabbit. Detailed characteristics of the test isolates are given in Table 2. Each isolate was grown in LB agar, and the bacterial cells were washed twice and suspended in 1% NaCl at 104, 109, or 1012 CFU/ml. Then, 3 ml of bacterial suspension was mixed with 50 g of rabbit food grain. Individual New Zealand White rabbits approximately 3 months old were fasted for 24 h, then fed with 50 ml of 5% sodium bicarbonate solution to neutralize stomach acid, and then allowed to feed on contaminated food grain. Rabbits were challenged with increasing doses of V. parahaemolyticus once a week for 3 weeks. Feces were collected 24 h before the feeding and every 6 h for 4 days after the feeding. Collected feces were enriched in alkaline peptone water and plated on TCBS agar. After incubation at 37°C for 24 h, non-sucrose-fermenting isolates were identified as V. parahaemolyticus as described above, and their serotypes and toxin gene profiles (i.e., the presence or absence of the tdh and trh genes) were also determined as described above.
TABLE 2.
V. parahaemolyticus isolates used to examine the serotype and toxin gene profile stability in vivo and in vitro and the result of in vivo study using rabbits
| Source and isolate | Characteristics
|
Recovery of administered isolate from rabbit stoolc | ||
|---|---|---|---|---|
| Serotype | Virulence gene patternb | GS-PCR | ||
| Patient group 16 | ||||
| PSU1681 | O3:K6 | tdh+trh− | + | - |
| PSU1690 | O4:K8 | tdh+trh− | + | - |
| PSU1683 | O4:K8 | tdh+trh− | - | + (6) |
| Hat Yaia hospital | ||||
| PSU1958 | O3:K6 | tdh+trh+ | - | - |
| PSU2056 | O11:KUT | tdh+trh+ | - | + (12)d |
| PSU2490 | O4:K55 | tdh−trh+ | - | + (6, 18, 24) |
Clinical specimens isolated in 2005.
Gene patterns: tdh+, tdh positive; trh−, trh negative; tdh−, tdh negative; trh+, trh positive.
+, Recovered; −, not recovered. When the isolate was recovered, the time (in hours) of stool specimen collection is indicated in parentheses.
A serotype change was observed for some of the isolates from the indicated fecal specimen.
To investigate whether in vitro subculture techniques could influence the serotype or genotype of the isolates, the three isolates from patient group 16 (PSU1681, PSU1690, and PSU1683) and one of the new isolates (PSU2056) were continuously subcultured by using a TCBS agar plate. An inoculated isolate was grown on this medium at 37°C for 24 h and transferred to the new TCBS agar plate for 50 passages. The serotype and the toxin gene profile were determined for each of the five colonies taken from each passage.
RESULTS
Ten colonies were randomly selected from the non-sucrose-fermenting colonies on TCBS agar derived from each of the 63 patients, and all of the isolates were identified as V. parahaemolyticus. A total of 629 V. parahaemolyticus isolates acquired from the 63 patients were examined for the presence of the tdh gene, the trh gene, and ORF8; the GS-PCR result; the O:K serotypes; and the AP-PCR profile. The results are summarized in Table 1.
TABLE 1.
Characteristics of V. parahaemolyticus isolates from 63 patients in the Hat Yai hospital from 2003 to 2004a
| Patient group | No. of patient specimens | No. of V. parahaemolyticus isolates (no. of isolates per patient group)b | Presence of gene
|
GS-PCR result | ORF8 result | O:K serotypec | AP-PCR patternsd | |
|---|---|---|---|---|---|---|---|---|
| tdh | trh | |||||||
| 1 | 16 | 160 | + | - | + | + | 3:6 | Identical |
| 2 | 6 | 60 | + | - | + | - | 3:6 | Identical |
| 3 | 8 | 80 | + | - | + | - | 1:25 | Identical |
| 4 | 3 | 30 | + | - | + | + | 1:UT | Identical |
| 5 | 3 | 30 | - | - | - | - | 11:UT | Identical |
| 6 | 3 | 30 | + | - | - | - | 4:8 | Identical |
| 7 | 2 | 20 | + | - | + | + | 4:68 | Identical |
| 8 | 1 | 10 | + | - | - | - | 8:21 | Identical |
| 9 | 2 | 10 (9, 1) | + | - | + | + | 3:6 | Identical |
| 10 (1, 9) | + | - | + | - | 3:6 | |||
| 10 | 1 | 9 | + | + | - | - | 4:67 | Identical |
| 1 | - | + | - | - | 4:67 | |||
| 11 | 1 | 9 | + | - | + | + | 1:25 | Identical |
| 1 | + | - | + | + | 1:UT | |||
| 12 | 1 | 9 | + | + | - | - | 3:56 | Identical |
| 1 | + | + | - | - | R:56 | |||
| 13 | 2 | 6 (5, 1e) | + | - | - | - | 4:8 | Nonidentical |
| 13 (5, 8) | + | - | - | - | 1:56 | |||
| 14 | 1 | 5 | + | - | - | - | 3:5 | Nonidentical |
| 5 | + | - | - | - | 4:8 | |||
| 15 | 1 | 6 | + | + | - | - | 11:UT | Nonidentical |
| 3 | + | + | - | - | 3:56* | |||
| 1 | + | + | - | - | R:56* | |||
| 16 | 1 | 3 | + | - | + | - | 3:6* | Nonidentical |
| 2 | + | - | + | - | 4:8* | |||
| 5 | + | - | - | - | 4:8 | |||
| 17 | 1 | 1 | - | + | - | - | 1:UT | Nonidentical |
| 1 | - | + | - | - | 3:UT | |||
| 8 | + | + | - | - | 5:UT | |||
| 18 | 1 | 9 | + | - | + | - | 1:25 | Nonidentical |
| 1 | - | - | - | - | 4:67 | |||
| 19 | 3 | 13 (9, 2, 2) | + | - | + | + | 3:6 | Nonidentical |
| 17 (1, 8, 8) | + | - | - | - | 4:8 | |||
| 20 | 2 | 12 (8, 4) | + | - | + | + | 3:6 | Nonidentical |
| 8 (2, 6) | + | - | - | - | 3:5 | |||
| 21 | 1 | 3 | + | - | + | + | 3:6 | Nonidentical |
| 1 | + | - | - | - | 3:7 | |||
| 6 | + | - | - | - | 4:8 | |||
| 22 | 1 | 5 | + | - | + | - | 1:25* | Nonidentical |
| 1 | + | - | + | + | 3:6* | |||
| 2 | + | - | - | - | 3:5* | |||
| 2 | + | - | - | - | 3:UT* | |||
| 23 | 1 | 3 | + | - | + | + | 3:6 | Nonidentical |
| 7 | + | + | - | - | 11:UT | |||
| 24 | 1 | 9 | + | - | + | + | 4:68 | Nonidentical |
| 1 | - | - | - | - | 11:UT | |||
+, present; -, absent.
The first, second, or third number in parentheses corresponds to the number of isolates from patient specimen 1, 2, or 3, respectively, that shared the same characteristics within each patient group. The total number of isolates collected per patient can be obtained by adding the first, second, or third number in parentheses in the first row of patient group data in column 3 to the corresponding first, second, or third number in parentheses in the second row of patient group data in the column. The total number or isolates per patient specimen is thus equal to 10, with the exception of the data for one patient in group 13 (see footnote e).
UT, untypeable; R, rough. *, identical AP-PCR patterns for the indicated serotype were observed within a patient group (see footnote d for exceptions observed for patient group 22).
Identical, all 10 isolates showed identical AP-PCR patterns; nonidentical, the AP-PCR patterns were not identical for the subgroups in the patient group except as follows: for patient group 22, AP-PCR patterns for O1:K25 and O3:K6 were identical, and AP-PCR patterns for O3:K5 and O3:KUT were identical, but the AP-PCR patterns for O1:K25 and O3:K6 differed from the AP-PCR patterns for O3:K5 and O3:KUT.
Only nine isolates were obtained from this specimen.
We classified the patients into 24 groups based on the characteristics of the isolates, as shown in Table 1. Patient groups 1 to 8 yielded 10 isolates per patient, for which all characteristics were identical. We designated these as the homogeneous patient groups, and they accounted for 42 of the 63 patients (66.7%). All of the isolates from 39 patients among the homogeneous patient groups possessed the tdh gene. However, those from three patients (patient group 5) lacked the tdh and trh genes, and all of these isolates belonged to the same serotype, O11:K untypeable (UT).
We designated the other patient groups (9 to 24) as the heterogeneous patient groups, in which at least 1 of the 10 isolates from a single patient differed in at least one of the characteristics examined. Interestingly, although all 10 isolates from patient groups 9 to 12 had identical AP-PCR profiles in each group, they differed in one of the other characteristics. The isolates from patient group 9 appeared to belong to the pandemic clone because they were tdh positive and GS-PCR positive, had the O3:K6 serotype, and had identical AP-PCR and PFGE genomic fingerprints (data not shown). However, the occurrence of ORF8 was variable since it was only detected in 9 of the 10 isolates from one patient and in 1 of 10 isolates from the second patient in the group. Similarly, 1 of the 10 isolates from patient group 10 lacked the tdh gene. All of the isolates from this patient group had identical AP-PCR fingerprints and similar PFGE profiles (data not shown). These results indicate that ORF8 (patient group 9) and the tdh gene (patient group 10) might have been lost during the infection. Patient groups 11 and 12 consisted of one and nine isolates that differed slightly in their serotypes: O1:KUT and O1:K25 in patient group 11 and OR:K56 and O3:K56 in patient group 12.
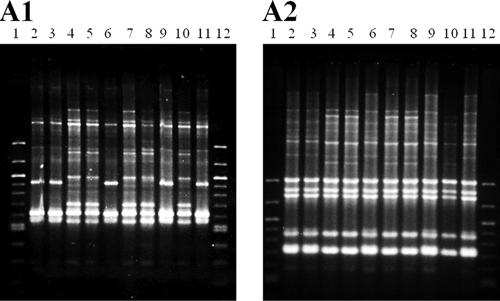
The remaining heterogeneous patient groups (13 to 24) harbored isolates that exhibited nonidentical AP-PCR patterns within each group. The isolates from these 16 patients also differed in their serotypes within the groups, with between two and four different serotypes detected in each. More importantly, the isolates with differing serotypes exhibited different virulence gene patterns in four of the patient groups: tdh negative, trh positive (tdh− trh+) and tdh+ trh+ in patient group 17, tdh+ trh− and tdh+ trh+ in patient group 23, and tdh+ trh− and tdh− trh− in patient groups 18 and 24. In the last two patient groups, the tdh-positive genotype was correlated with the GS-PCR-positive genotype. Patient group 16 contained isolates that differed in both the GS-PCR genotype and the serotype. Within this group, the GS-PCR genotype correlated with the AP-PCR profile (Fig. 1) and the PFGE pattern (Fig. 2) regardless of the serotype. In addition, the DNA fingerprints of the GS-PCR-positive isolates in patient group 16 (three O3:K6 isolates and two O4:K8 isolates) and those of the GS-PCR-positive isolates in patient group 22 (five O1:K25 isolates and one O3:K6 isolate) were indistinguishable (data not shown). These DNA fingerprints were different from those of the GS-PCR-negative isolates from patient group 22 (two O3:K5 isolates and two O3:KUT isolates [data not shown]).
FIG. 1.
AP-PCR profiles of 10 isolates of V. parahaemolyticus from patient group 16. Lanes 1 and 12 (molecular weight markers, 100-bp ladder; New England Biolabs, Ipswich, MA); lanes 2, 3, and 6, O3:K6, GS-PCR-positive isolates; lanes 4, 5, 7, 8, and 10, O4:K8, GS-PCR-negative isolates; lanes 9 and 11, O4:K8, GS-PCR-positive isolates. Panels A1 and A2 show the results obtained with primers 2 and 4, respectively.
FIG. 2.
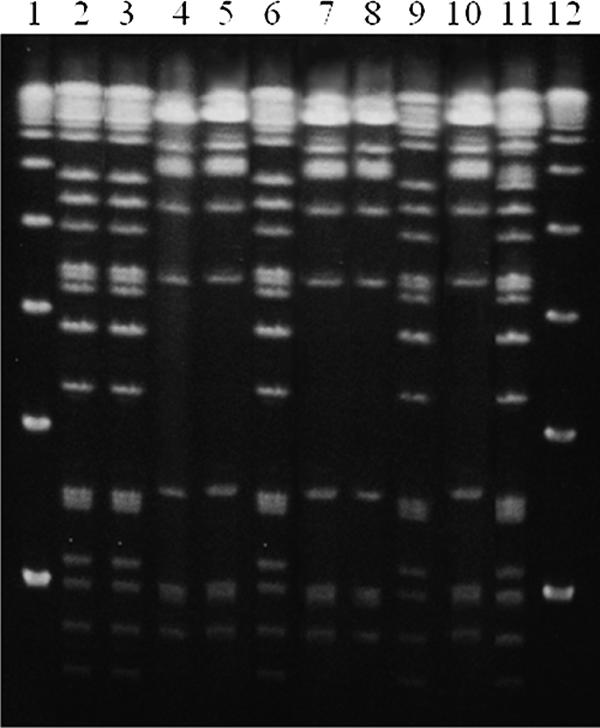
PFGE profiles of 10 isolates of V. parahaemolyticus from patient group 16. Lanes 1 and 12 (molecular weight markers, lambda ladder; Bio-Rad Laboratories); lanes 2, 3, and 6, O3:K6, GS-PCR-positive isolates; lanes 4, 5, 7, 8, and 10, O4:K8, GS-PCR-negative isolates; lanes 9 and 11, O4:K8, GS-PCR-positive isolates.
Altogether, 87.4, 0.5, or 7% of the 629 isolates from the 63 patients possessed the tdh gene alone, the trh gene alone, or both genes, respectively. The remaining 5.1% (32 isolates) lacked both genes. In all, 70% of the V. parahaemolyticus isolates (440 of 629) were GS-PCR positive; these isolates belonged to serotypes O3:K6 (43.7%), O1:K25 (16.4%), O1:KUT (5.0%), O4:K68 (4.6%), and O4:K8 (0.3%). A total of 62% of the GS-PCR-positive isolates carried ORF8.
The in vitro and in vivo stabilities of serotypes and toxin gene profiles in selected isolates were investigated (Table 2). Each of the six isolates listed in Table 2 was fed to a fasted rabbit. The rabbits fed with PSU1683 from patient group 16 and the two rabbits fed with recent isolates PSU2056 and PSU2490 at a concentration of 1012 CFU/ml produced V. parahaemolyticus-positive stools (Table 2). Fifty to eighty isolates from each of the V. parahaemolyticus-positive stool samples were examined for their serotypes and toxin gene profiles. A serotype change, but no change in the toxin gene profile, was observed in one of the three V. parahaemolyticus-positive cases. Three of the fifty isolates from the rabbit fed PSU2056 (serotype O11:KUT) had undergone a serotype change to O11:K15. These three isolates showed the same AP-PCR fingerprints as those of the isolates that had not undergone a serotype change (data not shown).
V. parahaemolyticus isolates PSU1681, PSU1690, PSU1683, and PSU2056 (Table 2) were used in the in vitro stability test. None of the tested isolates showed changes in their serotypes or toxin gene profiles during continuous subculture for 50 passages.
DISCUSSION
Only a small portion of the V. parahaemolyticus isolates in the environment are thought to be virulent and to carry virulence genes (tdh or trh), whereas most clinical isolates are virulent (12, 17, 23). We have accumulated data to support this notion; only 16 virulent isolates were obtained from 13 of the 302 seafood samples in our survey carried out between the years 2000 and 2002 (28). In addition, we have confirmed that a single seafood sample can be contaminated by different strains of V. parahaemolyticus; for example, we isolated V. parahaemolyticus belonging to five serotypes (O3:K6, O4:KUT, O11:KUT, O rough:KUT, and O2:KUT) from one oriental hard clam sample (V. Vuddhakul, unpublished). In the present study, we found that 597 of 629 isolates obtained from 63 patients possessed either tdh or trh or both virulence genes, suggesting that they were virulent isolates and able to proliferate in human hosts. This was the basis for the assumption that isolates in clinical specimens are virulent and represent a homogeneous population.
Recent observations that some clinical isolates lack the tdh and trh genes (18, 27) prompted us to examine whether the isolates in a single patient specimen were homogeneous or if mixed infection could explain the occurrence of clinical isolates lacking tdh and trh. Here we showed that up to 33% of the patient specimens contained heterogeneous populations of V. parahaemolyticus. The isolates obtained from the patients in groups 18 and 24 were a mixture of strains containing or lacking one or both of the virulence genes tdh and trh (Table 1). This result indicates that V. parahaemolyticus infections may be mixed and supports the possibility of accidental isolation of an avirulent isolate, rather than a virulent isolate, from a patient specimen.
However, 30 isolates from the three patient specimens in patient group 5 carried neither the tdh nor the trh gene when examined by PCR, and their absence was confirmed by Southern blot hybridization (data not shown). No known enteric pathogen was isolated from these patients. We therefore cannot rule out the possibility that these isolates have unknown virulence mechanisms other than the tdh and trh genes. However, it is possible that this could be explained by deletion of the tdh or trh gene during infection. The isolates in this group are under investigation to clarify their pathogenicity in patients.
Our observations also suggest that in vivo changes in the genotype of V. parahaemolyticus may contribute to strain heterogeneity in clinical specimens. In patient group 9, one and nine isolates from two patients, respectively, lacked ORF8, although other isolates from the same patients carried ORF8 and the DNA fingerprints of all isolates were indistinguishable. ORF8 is contained in a lysogenic filamentous phage. This phage may be unstable and may have been lost during infection in these patients. Similarly, the possibility that the tdh gene might be deleted during infection was raised. All 10 isolates from patient group 10 had identical characteristics, including DNA fingerprints, except for the possession of the tdh gene. The tdh gene is located in a transposon-like structure, which contains an insertion sequence-like element named ISV (26). Therefore, this genetic structure could mediate tdh deletion. We have detailed molecular genetic evidence to support the theory that there is an insertion sequence-mediated tdh deletion at least in the isolate in patient group 10 (M. Kamruzzaman, unpublished data). We suspect that the tdh deletion took place in vivo rather than in vitro after isolation because the toxin gene genotypes of the isolates were stable during maintenance of the culture.
In addition, the distribution of serotypes and genotypes within the isolates from patient groups 11, 12, and 16 suggest the possibility of in vivo serotype changes. In patient group 16 (one patient), three O3:K6, GS-PCR-positive isolates and two O4:K8, GS-PCR-positive isolates showed identical DNA fingerprints, including PFGE profiles. In contrast, five O4:K8, GS-PCR-negative isolates from this patient group showed different DNA fingerprints from the O4:K8, GS-PCR-positive isolates (Fig. 1 and 2). It is possible that this patient was infected with a single GS-PCR-positive strain and that some host factors affected the genes expressing the O and K antigens located at the cell surface. Patient groups 11 and 12 consisted of one and nine isolates that differed slightly in their serotypes: O1:KUT and O1:K25 in patient group 11 and OR:K56 and O3:K56 in patient group 12. The O1:KUT and OR:K56 serotypes might have been derived from the O1:K25 and O3:K56 serotypes, respectively. However, the possibility of infection by strains with different serotypes in a single patient cannot be ruled out. We therefore examined whether a serotype change or loss of the tdh and trh genes in V. parahaemolyticus can occur in vitro and in vivo. In the in vitro experiment with TCBS medium, no serotype change or loss of tdh or trh was detected in isolates from patient group 16 and other recent isolates, suggesting that the genotype and serotype are stable under routine culture conditions. However, we did observe a serotype change, KUT to K15, in one of test isolates that induced V. parahaemolyticus-positive stools in a rabbit model. A considerable proportion of environmental isolates of V. parahaemolyticus belongs to the KUT serotype (Vuddhakul, unpublished). The K antigen is the outermost antigen of V. parahaemolyticus. The in vivo conversion of the O11:KUT isolate to O11:K15 might be an adaptive change to avoid host immunity since different K antigens have been reported to confer different levels of immune protection (6, 20). It is important to note that rabbits are probably less susceptible to V. parahaemolyticus infections than humans. As much as 3 × 1012 CFU of test isolate, which is 106 times higher than the human infective dose, incorporated into rabbit food caused only softened stools but no diarrhea symptoms in the present study. No change in O antigen or deletion of tdh or trh was observed after passage through the rabbit model; however, our sample size may not have been large enough to detect such events. In vivo serotype change merits further study.
The ORF8 of a filamentous phage, f237, has been reported to be associated with O3:K6 and other pandemic serotypes (9, 16, 21). However, Bhuiyan et al. (2) and Okura et al. (19) failed to detect ORF8 in some pandemic strains (GS-PCR-positive strains). We found here that up to 38.4% of GS-PCR-positive isolates were ORF8 negative. Therefore, ORF8 might not be an appropriate genetic marker for pandemic strains. As discussed above, the possible instability of the phage can explain the absence of ORF8 in these GS-PCR-positive strains.
In total, 70% of all V. parahaemolyticus isolates were GS-PCR positive and thus considered pandemic strains. These belonged mostly to O3:K6, followed by the O1:K25, O4:K68, and O1:KUT serotypes. This result was consistent with the findings of our survey carried out in the Hat Yai hospital in 2000 to 2004 (Vuddhakul, unpublished) and indicates that this trend has remained unchanged for at least 5 years in the study area.
In conclusion, we showed here that V. parahaemolyticus isolates obtained from 33% of the patient specimens were not homogeneous and that this was due, in part, to mixed infection by two or more different strains. Our observations of the distribution of genotypes and serotypes in Hat Yai hospital patients also suggests that in vivo virulence gene deletion and in vivo serotype conversion may play a role in generating heterogeneity after infection. The present study did not rule out the possibility that V. parahaemolyticus lacking both virulence markers tdh and trh may have other unknown virulence mechanisms responsible for diarrhea in patients.
Acknowledgments
This study was supported, in part, by funds from the Government Budget, National Science and Technology Development Agency, of Thailand; the Commission on Higher Education; a Grant-in-Aid from the Ministry of Health, Labor, and Welfare of Japan (H17-Sinkou-ippan-019); and a Grant for International Health Cooperation Research (18-5) from the Ministry of Health, Labor, and Welfare of Japan.
We thank the animal housing facility of the Faculty of Science, Prince of Songkla University, for providing New Zealand White rabbits. We also thank Brian Hodgsen for reading the manuscript.
Footnotes
Published ahead of print on 7 March 2007.
REFERENCES
- 1.Ansaruzzaman, M., M. Lucas, J. L. Deen, N. A. Bhuiyan, X. Y. Wang, A. Safa, M. Sultana, A. Chowdhury, G. B. Nair, D. A. Sack, L. von Seidlein, M. K. Puri, M. Ali, C. L. Chaignat, J. D. Clemens, and A. Barreto. 2005. Pandemic serovars (O3:K6 and O4:K68) of Vibrio parahaemolyticus associated with diarrhea in Mozambique: spread of the pandemic into the African continent. J. Clin. Microbiol. 43:2559-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhuiyan, N. A., M. Ansaruzzaman, M. Kamruzzaman, K. Alam, N. R. Chowdhury, M. Nishibuchi, S. M. Faruque, D. A. Sack, Y. Takeda, and G. B. Nair. 2002. Prevalence of the pandemic genotype of Vibrio parahaemolyticus in Dhaka, Bangladesh and the significance of its distribution across different serotypes. J. Clin. Microbiol. 40:284-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blake, P. A., R. E. Weaver, and D. G. Hollis. 1980. Diseases of humans (other than cholera) caused by vibrios. Annu. Rev. Microbiol. 34:341-367. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhury, N. R., S. Chakraborty, B. Eampokalap, W. Chaicumpa, M. Chongsa-Nguan, P. Moolasart, R. Mitra, T. Ramamurthy, S. K. Bhattacharya, M. Nishibuchi, Y. Takeda, and G. B. Nair. 2000. Clonal dissemination of Vibrio parahaemolyticus displaying similar DNA fingerprint but belonging to two different serovars (O3:K6 and O4:K68) in Thailand and India. Epidemiol. Infect. 125:17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhury, A., M. Ishibashi, V. D. Thiem, D. T. N. Tuyet, T. V. Tung, B. T. Chien, L. von Seidlein, D. G. Canh, J. Clemens, D. D. Trach, and M. Nishibuchi. 2004. Emergence and serovar transition of Vibrio parahaemolyticus pandemic strains isolated during a diarrhea outbreak in Vietnam between 1997 and 1999. Microbiol. Immunol. 48:319-327. [DOI] [PubMed] [Google Scholar]
- 6.Glynn, A. A., and C. J. Howard. 1970. The sensitivity to complement of strains of Escherichia coli related to their K antigens. Immunology 18:331-346. [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Escalona, N., V. Cachicas, C. Acevedo, M. L. Rioseco, J. A. Vergara, F. Cabello, J. Romero, and R. T. Espejo. 2005. Vibrio parahaemolyticus diarrhea, Chile, 1998 and 2004. Emerg. Infect. Dis. 11:129-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honda, S., I. Goto, I. Minematsu, N. Ikeda, N. Asano, M. Ishibashi, Y. Kinoshita, M. Nishibuchi, T. Honda, and T. Miwatani. 1987. Gastroenteritis due to Kanagawa negative Vibrio parahaemolyticus. Lancet i:331-332. [DOI] [PubMed] [Google Scholar]
- 9.Iida, T., A. Hattori, K. Tagomori, H. Nasu, R. Naim, and T. Honda. 2001. Filamentous phage associated with recent pandemic strains of Vibrio parahaemolyticus. Emerg. Infect. Dis. 7:477-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly, M. T., and E. M. D. Stroh. 1989. Urease-positive, Kanagawa-negative Vibrio parahaemolyticus from patients and the environment in the Pacific Northwest. J. Clin. Microbiol. 27:2820-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, Y. B., J. Okuda, C. Matsumoto, N. Takahashi, S. Hashimoto, and M. Nishibuchi. 1999. Identification of Vibrio parahaemolyticus strains at the species level by PCR targeted to the toxR gene. J. Clin. Microbiol. 37:1173-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishishita, M., N. Matsuoka, K. Kumagai, S. Yamasaki, Y. Takeda, and M. Nishibuchi. 1992. Sequence variation in the thermostable direct hemolysin-related hemolysin (trh) gene of Vibrio parahaemolyticus. Appl. Environ. Microbiol. 58:2449-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laohaprertthisan, V., A. Chowdhury, U. Kongmuang, S. Kalnauwakul, M. Ishibashi, C. Matsumoto, and M. Nishibuchi. 2003. Prevalence and serodiversity of the pandemic clone among the clinical strains of Vibrio parahaemolyticus isolated in southern Thailand. Epidemiol. Infect. 130:395-406. [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Urtaza, J., A. Lozano-Leon, A. DePaola, M. Ishibashi, K. Shimada, M. Nishibuchi, and E. Liebana. 2004. Characterization of pathogenic Vibrio parahaemolyticus isolates from clinical sources in Spain and comparison with Asian and North American pandemic isolates. J. Clin. Microbiol. 42:4672-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto, C., J. Okuda, M. Ishibashi, M. Iwanaga, P. Garg, T. Rammamurthy, H. C. Wong, A. DePaola, Y. B. Kim, M. J. Albert, and M. Nishibuchi. 2000. Pandemic spread of an O3:K6 clone of Vibrio parahaemolyticus and emergence of related strains evidenced by arbitrarily primed PCR and toxRS sequence analyses. J. Clin. Microbiol. 38:578-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasu, H., T. Iida, T. Sugahara, Y. Yamaichi, K. Park, K. Yokoyama, K. Makino, H. Shinagawa, and T. Honda. 2000. A filamentous phage associated with recent pandemic Vibrio parahaemolyticus O3:K6 strains. J. Clin. Microbiol. 38:2156-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishibuchi, M., and J. B. Kaper. 1995. Thermostable direct hemolysin gene of Vibrio parahaemolyticus: a virulence gene acquired by a marine bacterium. Infect. Immun. 63:2093-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okuda, J., M. Ishibashi, E. Hayakawa, T. Nishino, Y. Takeda, A. K. Mukhopadhyay, S. Garg, S. K. Bhattacharya, G. B. Nair, and M. Nishibuchi. 1997. Emergence of a unique O3:K6 clone of Vibrio parahaemolyticus in Calcutta, India, and isolation of strains from the same clonal group from Southeast Asian travelers arriving in Japan. J. Clin. Microbiol. 35:3150-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okura, M., R. Osawa, A. Iguchi, E. Arakawa, J. Terajima, and H. Watanabe. 2003. Genotypic analyses of Vibrio parahaemolyticus and development of a pandemic group-specific multiplex PCR assay. J. Clin. Microbiol. 41:4676-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opal, S., A. Cross, and P. Gemski. 1982. K antigen and serum sensitivity of rough Escherichia coli. Infect. Immun. 32:956-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osawa, R., A. Iguchi, E. Arakawa, and H. Watanabe. 2002. Genotyping of pandemic Vibrio parahaemolyticus O3:K6 still open to question. J. Clin. Microbiol. 40:2708-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 23.Shirai, H., H. Ito, T. Hirayama, Y. Nakabayashi, K. Kumagai, Y. Takeda, and M. Nishibuchi. 1990. Molecular epidemiologic evidence for association of thermostable direct hemolysin (TDH) and TDH-related hemolysin of Vibrio parahaemolyticus with gastroenteritis. Infect. Immun. 58:3568-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tada, J., T. Ohashi, N. Nishimura, Y. Shirasaki, H. Ozaki, S. Fukushima, J. Takano, M. Nishibuchi, and Y. Takeda. 1992. Detection of thermostable direct hemolysin gene (tdh) and the thermostable direct hemolysin-related hemolysin gene (trh) of Vibrio parahaemolyticus by polymerase chain reaction. Mol. Cell Probes 6:477-487. [DOI] [PubMed] [Google Scholar]
- 25.Takeda, Y. 1983. Thermostable direct hemolysin of Vibrio parahaemolyticus. Pharmacol. Ther. 19:123-146. [DOI] [PubMed] [Google Scholar]
- 26.Terai, A., K. Baba, H. Shirai, O. Yoshida, Y. Takeda, and M. Nishibuchi. 1991. Evidence for insertion sequence-mediated spread of the thermostable direct hemolysin gene among Vibrio species. J. Bacteriol. 173:5036-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vuddhakul, V., A. Chowdhury, V. Laohaprertthisan, P. Pungrasamee, N. Patararungrong, P. Thianmontri, M. Ishibashi, C. Matsumoto, and M. Nishibuchi. 2000. Isolation of a pandemic O3:K6 clone of a Vibrio parahaemolyticus strain from environmental and clinical sources in Thailand. Appl. Environ. Microbiol. 66:2685-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vuddhakul, V., S. Soboon, W. Sunnghiran, S. Kaewpiboon, A. Chowdhury, M. Ishibashi, Y. Nakaguchi, and M. Nishibuchi. 2006. Distribution of virulent and pandemic strains of Vibrio parahaemolyticus in three molluscan shellfish species (Meretrix meretrix, Perna viridis, and Anadara granosa) and their association with food-borne disease in southern Thailand. J. Food Prot. 69:2615-2620. [DOI] [PubMed] [Google Scholar]
- 29.Yeung, P. S., and K. J. Boor. 2004. Epidemiology, pathogenesis, and prevention of food-borne Vibrio parahaemolyticus infections. Foodborne Pathog. Dis. 1:74-88. [DOI] [PubMed] [Google Scholar]