Abstract
Urethral and endocervical swabs and self-collected vaginal swabs (SCVSs) and urine specimens are all used as samples for diagnosis of urogenital infection with Chlamydia trachomatis. We have now determined chlamydial organism load in matched specimens from different anatomic sites and examined its relation to clinical signs and symptoms in men and women. Organism load was measured with assays based on the ligase chain reaction or real-time PCR analysis. The mean organism loads in 58 infected men were 1,200 and 821 elementary bodies (EBs) per 100 μl of sample for first-void urine (FVU) and urethral swabs, respectively (P > 0.05). Organism load in FVU samples or urethral swabs was positively associated with symptoms (P < 0.01) and clinical signs (P < 0.01) in men. The mean organism loads in 73 infected women were 2,231, 773, 162, and 47 EBs/100 μl for endocervical swabs, SCVSs, urethral swabs, and FVU samples, respectively (P < 0.001 for each comparison). Only the presence of multiple symptoms or clinical signs was associated with organism load in women. These results show that FVU is a suitable noninvasive sample type for men, given the fact that its chlamydial load did not differ significantly from that of urethral swabs. Given their higher organism load compared with FVU, SCVSs are the preferred noninvasive sample type for women.
Urogenital infection with Chlamydia trachomatis is the most commonly reported bacterial sexually transmitted infection (STI) and continues to be a major public health problem worldwide (4, 35). Given that most chlamydial infections are asymptomatic in both men and women, they often remain undiagnosed and untreated and therefore provide a reservoir for the disease (33). Infection of the upper genital tract may lead to complications, such as epididymitis in men and pelvic inflammatory disease in women. The inflammation and subsequent tissue scarring associated with the latter can lead to more serious sequelae (4).
Effective control of chlamydial infection within a population requires early diagnosis and prompt treatment of asymptomatic individuals (28). Targeted and regular screening is also recommended for people in high-risk groups or with a past history of genital chlamydial infection (14). The most common sites of infection in women are the cervix and urethra. Infected cells are shed from the endocervix into the vagina and are present in vaginal secretions. Infected epithelial cells from the urethra and the associated C. trachomatis elementary bodies (EBs) can also be detected in first-void urine (FVU) (3, 16). Potentially suitable clinical specimens for detection of chlamydial infection in women thus include urethral, vaginal, and endocervical swabs, self-inserted tampons, and FVU samples (3, 12). For screening programs, noninvasive specimens, such as vaginal swabs, tampons, and FVU, are preferable to invasive urethral and endocervical swabs because they overcome several barriers associated with the traditional diagnostic pathway (5, 11). Sensitivity of C. trachomatis detection with vaginal swabs has been shown to be similar to that with endocervical swabs or FVU samples (12, 22, 31). In men, C. trachomatis infects the urethral mucosa, which can be sampled effectively by collection of either urethral swabs or FVU, the latter being noninvasive (8-10).
We have now quantified chlamydial organism load in matched specimens from different anatomic sites of infected men and women in order to compare the respective yields. In addition, we examined the possible relations between chlamydial organism load in matched samples and either patient symptoms or clinical signs.
MATERIALS AND METHODS
Study participants.
Patients attending the Department of Genitourinary Medicine, Addenbrooke's Hospital, Cambridge, United Kingdom, for genital infection and STI testing between September 1998 and January 1999 were recruited into the study. The study was approved by the Cambridge Local Research Ethics Committee, informed consent was obtained from all study participants, and human experimentation guidelines of the relevant institutions were followed in the conduct of clinical research. Patient-reported symptoms and clinical signs were recorded at the time of the consultation, and these data were obtained retrospectively for analysis. A total of 1,654 patients (653 men and 1,001 women) participated in the prevalence and organism load analyses. The numbers of participants who were ineligible or declined participation in the study were not recorded.
For the purpose of analysis, three global variables were created: patient-reported symptoms, clinical signs, and traced STI contacts. Patient-reported symptoms for men included dysuria, urethral itching, urethral irritation, and urethral discharge. Patient-reported symptoms for women included vaginal irritation, abnormal vaginal discharge, irregular vaginal bleeding, dysuria, and pelvic or lower abdominal pain. Clinical signs for men (physician diagnosed) included urethral discharge, moist urethral meatus, genital lesions (genital warts, molluscum contagiosum, and ulceration), and a urethral smear with five or more polymorphonuclear leukocytes per high-power field (PMNLs/HPF). For women, clinical signs included mucopurulent cervical discharge, cervical contact bleeding, cervical motion tenderness, pelvic or adnexal tenderness, and infections other than Chlamydia (including candidiasis and bacterial vaginosis).
Specimen collection and testing.
All swab samples were collected with the LCx specimen swab as part of the collection kit provided by the manufacturer (Abbott Laboratories, Abbott Park, IL). Clinic nurses collected the urethral sample from male patients by inserting the LCx swab 1 to 2 cm into the urethra. Endocervical and urethral swabs were collected routinely from female patients by attending physicians. Female patients were given simple instructions (with a diagram) (22) on how to obtain a self-collected vaginal swab (SCVS); these samples were collected prior to speculum examination by inserting the swab into the vagina and rotating it for 15 to 30 s. Each patient was also asked to collect ∼20 ml of FVU after not having urinated for at least 1 h. As part of the routine screening protocol, all women were tested for infection with Candida species (vaginal microscopy and culture), Trichomonas vaginalis (vaginal wet-film microscopy and culture), Neisseria gonorrhoeae (urethral and endocervical microscopy and culture), and C. trachomatis (enzyme immunoassay). Urethral, vaginal, and endocervical smears from all women were subjected to Gram staining for determination of the number of PMNLs/HPF by microscopy, with ≥5 PMNLs/HPF for the urethra and ≥21 PMNLs/HPF for the vagina or cervix being considered abnormal.
For the research analysis, urethral and endocervical swabs, SCVSs, and FVU samples were screened with the ligase chain reaction (LCR)-based Chlamydia trachomatis LCx assay (Abbott Laboratories), which targets a conserved region of the cryptic plasmid. At the collection site, each swab was placed into the LCx swab specimen transport tube containing 0.5 ml of specimen transport buffer. FVU and swab specimens were immediately stored at 2 to 8°C and screened by LCR within 78 h of collection, well within the storage period of 60 days recommended by the test manufacturer. The transport tubes were incubated for 15 min at 97°C in a dry heat block. Similarly, the cell pellet obtained from 1 ml of a FVU specimen by centrifugation for 15 min at 17,000 × g (Megafuge 1.0R; Hereaus, Osterode, Germany) was dissolved in 1 ml of LCx urine extraction buffer and heat treated as for the swab specimens. All assays were performed by experienced laboratory technicians with 100 μl of sample per amplification according to the manufacturer's instructions (16, 31). The remaining extracts of positive swabs and positive urine samples were stored at −80°C and tested by quantitative LCR (QLCR) within 2 months of collection. These samples were thus frozen and thawed once prior to testing by QLCR. All positive swab and urine extracts were again stored at −80°C until tested by real-time quantitative PCR (QPCR) analysis in September 2006 (two freeze-thaw cycles).
Samples from individual patients that yielded discrepant results either among specimen types or between routine clinical testing and research testing underwent repeat LCx testing at serial dilutions (up to 1/10) in LCx urine extraction buffer in order to reduce the potential effect of inhibitors present in some specimens.
Quantitation of the EB standard.
A partially purified preparation of EBs from C. trachomatis (serovar L1), which is readily propagated in buffalo green monkey kidney cells, was provided by I. Clarke (University of Southampton, United Kingdom) and used as the standard for EB quantitation. Although most of the infected patients were likely to harbor C. trachomatis serovars D through K, the primer sets for both the LCR and QPCR assays correspond to conserved regions of the C. trachomatis cryptic plasmid and are therefore able to detect all C. trachomatis serovars (16, 21, 31). The EB concentration of the standard was determined by electron microscopy. In brief, samples of the diluted standard were placed in the cavities of an EM90 rotor and centrifuged at 90,700 × g for 5 min in an Airfuge ultracentrifuge (Beckman Coulter, Fullerton, CA) onto 300-mesh copper grids coated with silicon monoxide and carbon. The grids were then stained with 2% phosphotungstic acid and examined with a Philips CM100 electron microscope (Philips Electron Optics, Eindhoven, The Netherlands) at different magnifications (×2,200, ×2,950, and ×3,900). Three different areas of each grid containing 30 to 150 EBs were photographed. A total of 24 photomicrographs obtained from the various dilutions of the standard was analyzed for the number of EBs present. The standard used throughout the study had a mean concentration of 1.08 × 1010 EBs/ml (standard deviation [SD], 1.54 × 109).
Quantitative assay for measurement of EB concentration.
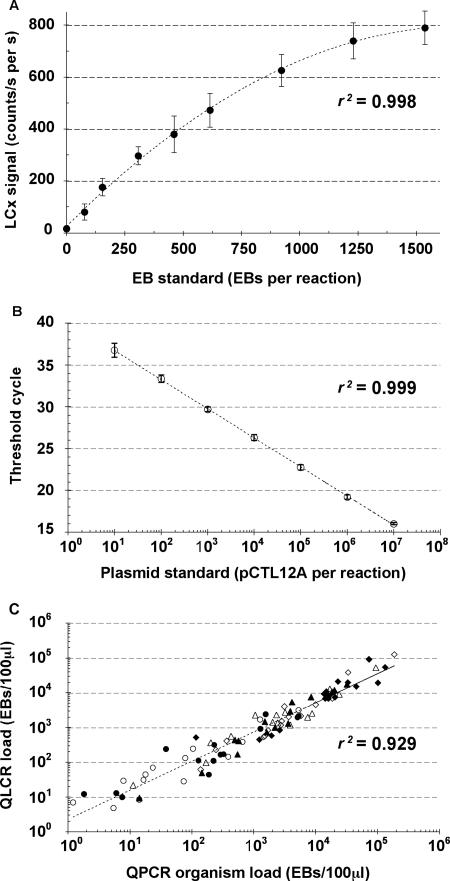
Reduced numbers of amplification cycles of the LCR (32, 28, 25, and 23 cycles instead of the standard 40 cycles) were used to generate linear standard curves covering a wide range of EB concentrations with the modified LCx assay. Specimens processed for the QLCR assay were diluted by 1/2 in LCx specimen buffer before testing. For each measurement run, a standard curve was generated, and the organism load for each sample was plotted. Samples with an organism load falling outside of the linear range of the standard curve were retested with the use of a more appropriate number of amplification cycles. The standard curves for the four different amplification protocols (32, 28, 25, or 23 cycles) were linear from 1 to 150, 100 to 1,500, 1,000 to 15,000, and 10,000 to 150,000 EBs per reaction (100 μl per amplification), respectively. Ten standard curves at 28 cycles were constructed for the EB standard on different days with LCx kits of different lots. Each curve was generated from nine different concentrations of EBs (0 to 1,500 per test) in duplicate (Fig. 1A). The coefficient of variation for the 10 curves was <15%.
FIG. 1.
Comparison between QLCR and QPCR assays. (A) Reproducibility of the QLCR standard curve at 28 cycles. Ten standard curves at 28 cycles were constructed for the EB standard on different days with LCx kits of different lots. Each curve was generated from nine different concentrations of EBs (0 to 1,500 per reaction) in duplicate. Data points are means ± standard errors (error bars) and yielded a correlation coefficient (r2) of 0.998. (B) Reproducibility of 11 QPCR standard curves performed in duplicate with seven serial 10-fold dilutions of the pCTL12A plasmid (r2 = 0.999). (C) Correlation between QLCR and QPCR results (r2 = 0.929). Urethral swabs (closed triangles) and FVU specimens (open triangles) from males and urethral swabs (closed circles), FVU specimens (open circles), SCVSs (open diamonds), and endocervical swabs (closed diamonds) from females are shown.
Real-time quantitative PCR assay.
A subset of the matched LCx swab and FVU extracts stored at −80°C were quantified by real-time QPCR analysis in 2006. Nucleic acid contained in 100 μl of LCx extract was purified by ethanol precipitation (24), dissolved in 100 μl of nuclease-free water, and used as a sample for QPCR testing. Real-time QPCR was performed by a modified version of the previously described method (21); 20 μl of sample was added to a reaction mixture volume of 50 μl, instead of 5 μl of sample to a reaction mixture volume of 25 μl. Amplification was performed with an Mx4000 multiplex quantitative PCR system (Stratagene, La Jolla, CA) according to the following protocol: one cycle of 50°C for 2 min and 95°C for 10 min, followed by 45 cycles of 95°C for 30 s (instead of 15 s) and 60°C for 1 min. The plasmid pCTL12A (kindly provided by I. Clarke) was purified as described previously (21), quantified by measurement of absorbance at 260 nm, and used to generate the reference curve (Fig. 1B).
Correlation between QLCR and QPCR.
Although we had access to the LCx test after it was no longer commercially available, a highly reproducible (r2 = 0.999) QPCR method (21) was used to confirm the validity of the QLCR results. The EB standard and LCx extracts from 15 matched sets each of male and female specimens were quantified by QPCR. Three dilutions of the EB standard were quantified relative to the plasmid standard in duplicate runs. The results showed that there were 7.72 ± 0.68 (mean ± SD) plasmid copies per EB, consistent with previously obtained values (21, 32). This value was used to convert plasmid copies into equivalent EBs per 100 μl. Analysis of the 15 matched sets each of male and female specimens revealed a pronounced correlation (r2 = 0.929) between EB counts determined by QLCR and those determined by QPCR (Fig. 1C).
Statistical analysis.
The 95% confidence interval (95% CI) for organism load in each of the various specimen types was calculated from the geometric mean and SD obtained for the specimen population. Organism loads of matched samples were compared with each other by the paired Student t test or analysis of variance. Two-sample t tests with natural log transformation were used to determine association between patient-reported symptoms or clinical signs and organism load. The relation between the number of PMNLs/HPF and organism load was evaluated with Pearson's correlation coefficient (r). The concordance between organism loads determined for different specimen types at a given anatomic site was assessed with the paired Wilcoxon rank-sum test. Pairs were considered discordant if the organism loads differed by >10 ranks and by at least 2.0 log units. The one-tailed McNemar's test for correlated proportions was performed for paired sample types, and the resulting P value, standard error, and 95% CI were calculated. A P value of 0.05 was considered statistically significant.
RESULTS
Identification of infected patients.
Of the 653 male and 1,001 female patients screened for C. trachomatis, 81 men (12.4%) and 83 women (8.3%) were identified as positive by LCx testing. The age of the screened patients ranged from 16 to 69 years, with a median of 27 years. Matched specimens from LCx-positive subjects were obtained for 58 of these 81 men (72%) and 73 of the 83 women (88%); most of the remaining subjects declined to provide a third urethral swab required for LCx testing. A patient was defined as LCx positive if a specimen from at least one anatomic site yielded a positive result. Of the 58 positive men with matched specimens, only two individuals tested negative with urethral swabs but positive with FVU specimens. No female patient with matched specimens was positive at only one anatomic site (Table 1). Indeed, most of the infected female patients (59 of 73 [80.8%]) yielded positive results with all four specimen types. The highest sensitivity for female specimens was obtained with endocervical swabs (72 of 73 [98.6%]), followed by SCVSs (69 of 73 [94.5%]) and urethral swabs (68 of 73 [93.2%]); the lowest sensitivity was observed with FVU specimens (62 of 73 [84.9%]). Sensitivity with endocervical swabs was significantly higher than that with FVU specimens (P = 0.0039), but it did not differ significantly from that with the other two sample types.
TABLE 1.
Detection of C. trachomatis infection in 73 women with four matched specimen types
| Parameter |
C. trachomatis infection detected in the following specimen typea:
|
No. (%) of infected female patients | |||
|---|---|---|---|---|---|
| Endocervical swab | Urethral swab | SCVS | FVU sample | ||
| + | + | + | + | 59 (80.8) | |
| + | + | − | − | 3 (4.1) | |
| + | − | + | − | 4 (5.5) | |
| + | + | + | − | 4 (5.5) | |
| + | + | − | + | 1 (1.4) | |
| + | − | + | + | 1 (1.4) | |
| − | + | + | + | 1 (1.4) | |
| Sensitivity (%)b | 72/73 (98.6; 96.0-100) | 68/73 (93.2; 87.4-98.9) | 69/73 (94.5; 89.3-99.7) | 62/73 (84.9; 76.7-93.1) | |
+, C. trachomatis infection detected; −, C. trachomatis infection not detected.
The percentage and 95% CI (as a range) are shown in parentheses. The sensitivity of C. trachomatis detection with endocervical swabs was significantly higher than that with FVU (P = 0.0039; 83.6% agreement) but did not differ significantly from that with SCVSs (P = 0.1797; 93.2% agreement) or with urethral swabs (P = 0.1025; 91.8% agreement). The sensitivity with FVU specimens was significantly lower than that with vaginal swabs (P = 0.0196; 87.8% agreement) or with urethral swabs (P = 0.0339, 89.0% agreement), but that with vaginal swabs did not differ significantly from that with urethral swabs (P = 0.7389; 87.7% agreement).
Organism load in infected men.
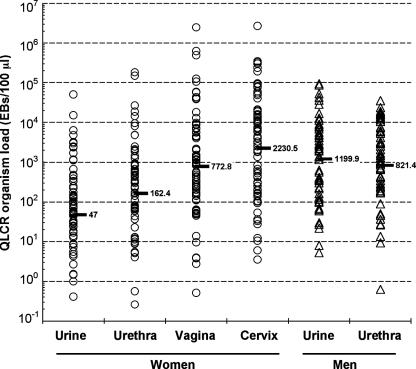
We analyzed the organism load data in terms of both the distribution of chlamydial load according to sex and specimen type and the load ranking for each specimen type. The distribution of organism load according to specimen type is shown in Fig. 2. The mean load for the 58 infected males was 1,200 EBs/100 μl (95% CI, 13.2 to 108,880) for FVU specimens and 821 EBs/100 μl (95% CI, 5.6 to 117,995) for urethral swabs; the difference was not significant (P = 0.16). Ranking analysis showed that the proportion of discordant pairs for FVU specimens and urethral swabs was only 3.45% (95% CI, −1.2 to 8.1%; P = 0.85).
FIG. 2.
Organism load in matched specimens from men and women infected with C. trachomatis. The geometric means are indicated by the horizontal bars. The P value for comparison between urine and urethral specimens of men was 0.16 (paired t test), whereas those for comparisons between urine and urethra, urethra and vagina, and vagina and cervix for women were all <0.001 (analysis of variance).
Organism load in infected women.
The approach used above to analyze organism load in infected men was used to analyze organism load in the four different specimen types for the 73 infected women. The mean organism loads were 47 (95% CI, 0.2 to 8718), 162 (95% CI, 0.6 to 40,831), 773 (95% CI, 3.1 to 194,308), and 2,231 (95% CI, 4.9 to 1,009,737) EBs/100 μl for FVU, urethral swabs, SCVSs, and endocervical swabs, respectively (Fig. 2). The difference in load between any pair of specimen types for women was statistically significant (P < 0.001). The organism loads for two distinct infection sites, the urethra and cervix, were examined for women. Ranking analysis revealed that the proportion of discordant pairs either between FVU specimens and urethral swabs or between endocervical swabs and SCVSs was 11%. For matched cervical and urethral swabs, however, the proportion of discordant pairs was 33% (95% CI, 20.8 to 45.0; P = 0.16).
Relation between organism load and either symptoms or clinical signs.
The relation of chlamydial infection to patient-reported symptoms, clinical signs, or traced STI contacts was examined by multivariate logistic regression modeling, with significant associations being shown in Table 2. Of these various associated variables, the presence of one or more clinical signs or that of one or more symptoms was associated with organism load of FVU specimens or urethral swabs in men (Table 3). Both the presence of dysuria and the number of urethral PMNLs/HPF were associated with organism load of FVU specimens but not with that of urethral swabs in men. Of the four specimen types in women, the organism load of only SCVSs or cervical swabs was significantly correlated with the corresponding number of PMNLs/HPF. Whereas individual symptoms or clinical signs were not significantly associated with organism load in women (data not shown), when the analysis was restricted to only those clinical features suggestive of cervicitis or pelvic inflammatory disease, a significant association with organism load was apparent (Table 3). The presence of at least two specific symptoms (vaginal discharge, irregular bleeding, or pelvic or abdominal pain) or of at least one of a group of clinical signs (mucopurulent cervical discharge, cervical motion tenderness, or pelvic or adnexal tenderness) was associated with organism load of both FVU specimens and cervical swabs. The physician diagnosis of nonspecific cervicitis was positively associated with organism load for all four sample types.
TABLE 2.
Clinical signs and symptoms associated with the presence of C. trachomatis infection by multivariate analysis
| Variable or clinical sign or symptom | Female patients
|
Male patients
|
||||||
|---|---|---|---|---|---|---|---|---|
| Crude ORa | P for crude OR | Adjusted ORb | P for adjusted OR | Crude ORc | P for crude OR | Adjusted OR | P for adjusted OR | |
| Age group | ||||||||
| <30 yr vs ≥30 yr | 4.20 (1.76-10.1) | 0.0005 | 4.13 (1.88-9.07) | 0.0002 | ||||
| <25 yr vs ≥25 yr | 2.71 (1.47-5.00) | 0.001 | 1.23 (0.66-2.32) | 0.514 | ||||
| Patient-reported symptoms | ||||||||
| Symptomatic vs asymptomatic | 1.91 (1.04-3.50) | 0.033 | 5.67 (2.81-11.5) | <0.0001 | ||||
| Dysuria | 4.28 (1.87-9.82) | 0.0002 | 3.92 (1.42-10.9) | 0.0086 | 8.27 (4.35-15.7) | <0.0001 | 4.42 (1.51-12.1) | 0.0011 |
| Urethral itching | NA | 6.15 (1.80-21.0) | 0.0011 | 13.7 (2.96-63.0) | 0.0008 | |||
| Vaginal/urethral irritation | 0.14 (0.02-1.04) | 0.026 | 2.17 (0.97-4.89) | 0.055 | ||||
| Pelvic or abdominal pain | 2.63 (1.32-5.24) | 0.004 | 3.39 (1.40-8.18) | 0.0066 | NA | |||
| Reported abnormal discharge | ||||||||
| Patient reported vs none | 3.38 (1.47-7.80) | 0.004 | 5.50 (2.33-13.0) | <0.0001 | ||||
| Doctor reported vs none | 3.15 (1.33-7.45) | 0.009 | 3.53 (1.29-9.61) | 0.014 | 14.3 (3.82-53.5) | <0.0001 | 7.60 (1.30-44.3) | 0.024 |
| Doctor or patient reported vs none | 7.37 (3.40-16.0) | <0.0001 | 4.78 (1.99-11.5) | 0.0005 | 10.2 (4.58-22.8) | <0.0001 | 4.27 (1.51-12.1) | 0.0063 |
| Clinical signs | ||||||||
| PMNLs/HPF | 1.04 (1.02-1.07) | 0.0008 | 8.27 (4.35-15.7) | <0.0001 | 4.42 (1.81-10.8) | 0.0011 | ||
| NSU or NSC vs neitherd | 3.71 (2.00-6.87) | <0.0001 | 3.98 (1.87-8.47) | 0.0003 | 15.2 (7.07-32.6) | <0.0001 | 7.56 (2.98-19.2) | <0.0001 |
| Cervical contact bleeding | 13.2 (4.24-41.0) | <0.0001 | 11.7 (2.28-60.5) | 0.0032 | NA | |||
| Moist urethral meatus | NA | 7.66 (3.17-18.5) | <0.0001 | 5.95 (1.86-19.0) | 0.0027 | |||
| Coinfections | ||||||||
| Molluscum contagiosum | 21.3 (3.8-119.5) | <0.0001 | 53.0 (6.04-466) | 0.0003 | ||||
| Genital warts | 2.15 (0.95-4.90) | 0.062 | 6.11 (2.30-16.2) | 0.0003 | ||||
| One or more infections | 1.86 (1.01-3.41) | 0.043 | ||||||
| Traced STI contacts | ||||||||
| C. trachomatis contact | 13.3 (4.86-36.2) | <0.0001 | 9.08 (2.50-32.9) | 0.0008 | 4.05 (1.43-11.5) | 0.0048 | 13.5 (3.32-54.8) | 0.0003 |
| NSU contact | 2.70 (1.11-6.54) | 0.023 | NA | |||||
OR, odds ratio. Numbers in parentheses after odds ratios represent the 95% CI. NA, not applicable to gender.
Only those variables that remained significant after adjustment for other variables in the model are included.
Lack of data for the crude odds ratio is due to the low prevalence of the variable.
NSU, nonspecific urethritis; NSC, nonspecific cervicitis.
TABLE 3.
Association between organism load in matched specimens and clinical signs or symptoms in men or women infected with C. trachomatis
| Symptom or clinical sign | Organism load (no. of EBs/100 μl)
|
Associationb (P value) | Correlationc (r) | |||||
|---|---|---|---|---|---|---|---|---|
| Symptom or sign absent
|
Symptom or sign present
|
|||||||
| Mean | πLa | πUa | Mean | πL | πU | |||
| Male FVU samples | ||||||||
| Dysuria | 513 | 8.4 | 31,445 | 2,080 | 27.3 | 158,198 | 0.0057 | |
| ≥1 clinical sign | 64 | 2.5 | 1,658 | 1,394 | 18.7 | 103,985 | 0.0074 | |
| ≥1 symptom | 240 | 3.5 | 16,541 | 1,772 | 27.8 | 113,006 | 0.0012 | |
| Nonspecific urethritis | 113 | 2.6 | 4,978 | 2,143 | 47.8 | 96,028 | <0.0001 | |
| PMNLs/HPF | 0.0024 | 0.3346 | ||||||
| Male urethral swabs | ||||||||
| Dysuria | 357 | 0.9 | 141,210 | 1,237 | 19.0 | 80,402 | 0.0930 | |
| ≥1 clinical sign | 8 | 0.0 | 3,586 | 953 | 9.5 | 95,416 | 0.0012 | |
| ≥1 symptom | 43 | 0.2 | 720 | 1,339 | 20.2 | 88,823 | <0.0001 | |
| Nonspecific urethritis | 88 | 0.2 | 38,407 | 1,300 | 18.5 | 91,418 | 0.0133 | |
| PMNLs/HPF | 0.0830 | 0.2276 | ||||||
| Female FVU samples | ||||||||
| ≥2 symptoms or ≥ 1 signd | 27 | 0.2 | 3,205 | 114 | 0.5 | 25,084 | 0.0174 | |
| Nonspecific cervicitise | 21 | 0.1 | 5,105 | 99 | 1.0 | 9,574 | 0.0095 | |
| PMNLs/HPF | 0.0901 | 0.1945 | ||||||
| Female urethral swabs | ||||||||
| ≥2 symptoms or ≥ 1 sign | 106 | 0.4 | 27,610 | 188 | 0.9 | 40,301 | 0.3577 | |
| Nonspecific cervicitis | 69 | 0.3 | 1,130 | 262 | 1.4 | 49,178 | 0.0295 | |
| PMNLs/HPF | 0.0507 | 0.2165 | ||||||
| SCVSs | ||||||||
| ≥2 symptoms or ≥ 1 sign | 1,495 | 4.8 | 466,401 | 3,605 | 8.6 | 1,508,762 | 0.1878 | |
| Nonspecific cervicitis | 250 | 0.9 | 71,998 | 2,018 | 3.5 | 1,155,912 | 0.0036 | |
| PMNLs/HPF | 0.0025 | 0.3377 | ||||||
| Endocervical swabs | ||||||||
| ≥2 symptoms or ≥1 sign | 314 | 0.5 | 187,138 | 1,826 | 5.6 | 592,437 | 0.0157 | |
| Nonspecific cervicitis | 545 | 3.3 | 88,965 | 8,866 | 39 | 2,021,196 | <0.0001 | |
| PMNLs/HPF | <0.0001 | 0.4406 | ||||||
πL and πU, lower and upper limits, respectively, of the 95% CI for the mean organism load, rounded to the nearest decimal point, in the 58 infected male patients with both sample types and 73 infected female patients with all four sample types.
Association between organism load in matched specimens and clinical signs or symptoms was evaluated by two-sample t tests with natural log transformation. P values that are statistically significant (P ≤ 0.05) are shown in bold type.
Correlation of organism load with PMNLs/HPF was assessed with Pearson's correlation coefficient (r).
Women reporting two or more specific symptoms (vaginal discharge, irregular bleeding, or pelvic or abdominal pain) or manifesting one or more specific clinical signs (mucopurulent cervical discharge, cervical motion tenderness, or pelvic or adnexal tenderness).
The diagnosis of nonspecific cervicitis was based on the presence of a mucopurulent cervical discharge or >40 PMNLs/HPF on the endocervical smear (or both).
DISCUSSION
The C. trachomatis positivity rate of 9.9% for the study population, which was recruited from patients at a genitourinary medicine clinic, is consistent with that reported previously for the United Kingdom (1). Although all the quantitative data for organism load analyzed in the present study were obtained with a QLCR assay, the QLCR data for a subset of samples were found to be highly correlated with corresponding QPCR results. The good correlation between the QLCR and QPCR data indicates that there was no loss of the target during the storage of samples at −80°C prior to performance of the latter assay. With the use of matched specimens, we have shown that, for men, urethral swabs offer no yield advantage over FVU specimens because the organism load did not differ significantly between these two specimen types (821 and 1,200 EBs/100 μl, respectively). Our results support those of previous studies suggesting that FVU is a more practical and acceptable specimen type for detection of C. trachomatis in men and is a better diagnostic specimen for screening of asymptomatic individuals (6, 8). For women, the endocervical swab has been the standard sample for most tests approved by the U.S. Food and Drug Administration. Recently, however, the sensitivity for detection of chlamydial or gonococcal infection with vaginal swabs was shown to be similar to that with endocervical swabs (5, 7, 26). In the present study, endocervical swabs contained the highest C. trachomatis organism load (2231 EBs/100 μl), followed by SCVSs (773 EBs/100 μl), urethral swabs (162 EBs/100 μl), and FVU specimens (47 EBs/100 μl).
The low organism load for female FVU specimens, which was markedly less than that for male FVU specimens or for any other female specimen type, is consistent with evidence that amplified enzyme immunoassays and even some nucleic acid amplification-based tests, especially those without a concentration step during sample processing, struggle to achieve adequate sensitivity with female FVU specimens as the diagnostic specimen (27, 29). The female urethra is only 3 to 4 cm in length, with its major distal portion being lined by stratified squamous epithelium (30). In contrast, the anterior male urethra is ∼16 cm in length and is lined mostly by pseudostratified columnar epithelium (30). Given that Chlamydia species typically infect columnar cells and do not show tropism for squamous cells (25), these differences may account for the lower organism load apparent in female FVU specimens compared to the organism load in male FVU specimens. Cell culture has suggested that ∼40% of women are infected with C. trachomatis solely in the endocervix, without true urethral infection (15). In such women, periurethral contamination from endocervical C. trachomatis is likely responsible for the chlamydial particles detected in FVU specimens by nucleic acid amplification-based tests. These specimens would also be expected to have a lower C. trachomatis organism load than those from women with true urethral infection. Vaginal swabs had the second-highest organism load for female specimen types, with values much higher than those for FVU specimens in the present study, making SCVSs the superior noninvasive sample for women. This conclusion also applies to the use of SCVSs for tests with lower sensitivity, such as enzyme immunoassay-based or rapid tests, for which a FVU specimen is also a suboptimal female sample.
Analysis of various patient-reported symptoms or clinical signs and their relations to organism load revealed that, for men, the organism load of FVU specimens was positively associated with dysuria, the number of urethral PMNLs/HPF, one or more patient-reported symptoms, and one or more clinical signs. The organism load of male urethral swabs did not show a significant association with either dysuria or the number of PMNLs/HPF, but it was positively associated with one or more patient-reported symptoms and one or more clinical signs. These differences between male urethral swabs and FVU specimens might be accounted for by suboptimal sampling of the urethral mucosa during swab collection as a result of the pain caused by this procedure, especially in individuals with an inflamed urethra.
In females, the association of FVU or endocervical organism load with multiple patient-reported symptoms or clinical signs characteristic of cervicitis or pelvic inflammatory disease suggests that symptomatic upper genital tract infection is more likely to occur in women with a higher chlamydial load. The association between nonspecific cervicitis and organism load in all four specimen types also demonstrates that women with a higher chlamydial load are more likely to display clinical evidence of infection. Female FVU specimens and urethral loads were not correlated with the corresponding number of PMNLs/HPF, whereas both vaginal and endocervical loads were significantly correlated with this parameter, consistent with previous findings that local organism load correlates with inflammation at the same site (18).
The organism load of some of the specimens in the present study may have been underestimated for two main reasons. First, the routine screening swabs were collected before the research swabs. This imposed order of specimen collection might thus have reduced the organism load in the research swabs if the initial screening swabs depleted available infected cells or chlamydial particles. This effect might be least relevant for SCVSs, for which the sampling area is much greater and the potential for depletion of chlamydial particles with repeated swabbing is therefore reduced. Second, it is possible that EB counts of certain samples might have been underestimated because of the presence of inhibitory substances. In addition, the study was performed with subjects attending a genitourinary medicine clinic, with approximately half of them being symptomatic. The distribution of organism load by anatomic site and the relationship of load to symptoms may be different in a largely asymptomatic population, such as those individuals attending young people's sexual health clinics.
Our findings may help to direct current screening strategies (17) and to guide diagnostics manufacturers in selecting the most appropriate sample types during test development (34). Noninvasive collection of FVU as a specimen would minimize patient discomfort and overcome some of the difficulties associated with screening a male population (23). The use of SCVSs might also help to increase the acceptability of screening programs for women, given that recent studies have shown that these samples are highly acceptable and perform as well as, if not better than, FVU specimens and endocervical swabs (13, 26). Evidence in support of screening based on home sampling with SCVSs in women and FVU specimens in men has been presented previously (2, 20), and our findings support the choice of these specimen types. In addition, vaginal swab specimens are easier to handle during collection and transport and require fewer steps to process compared to FVU specimens. Commonly encountered sampling problems for FVU specimens include the uncertain time since the last void, variable sample volume, and potential for dilution of the FVU fraction, especially in women (19). These issues can be overcome with the use of SCVSs, although caution may be needed in women who clean or douche vaginally immediately before sample collection because such action might deplete chlamydial particles and lead to a false-negative result. Finally, on the basis of our findings, we recommend that chlamydia screening programs adopt the use of FVU specimens in men and SCVSs in women as the most appropriate noninvasive specimen types.
Acknowledgments
This work was supported by a grant (049366/Z/96/Z) from The Wellcome Trust, United Kingdom.
We thank the nursing staff and physicians of the Genitourinary Medicine Clinic, Addenbrooke's Hospital, Cambridge, United Kingdom, who participated in patient care, specimen collection, and data gathering; I. Clarke for providing the C. trachomatis-infected buffalo green monkey kidney cell line and the plasmid pCTL12A; J.-P. Allain of the University of Cambridge for helpful advice and discussion on data analysis as well as assistance with writing and revision of the manuscript; and M. Cheang of the University of Manitoba for performing statistical analysis.
Footnotes
Published ahead of print on 21 March 2007.
REFERENCES
- 1.Adams, E. J., A. Charlett, W. J. Edmunds, and G. Hughes. 2004. Chlamydia trachomatis in the United Kingdom: a systematic review and analysis of prevalence studies. Sex. Transm. Infect. 80:354-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, B., F. Olesen, J. K. Moller, and L. Ostergaard. 2002. Population-based strategies for outreach screening of urogenital Chlamydia trachomatis infections: a randomized, controlled trial. J. Infect. Dis. 185:252-258. [DOI] [PubMed] [Google Scholar]
- 3.Buimer, M., G. J. van Doornum, S. Ching, P. G. Peerbooms, P. K. Plier, D. Ram, and H. H. Lee. 1996. Detection of Chlamydia trachomatis and Neisseria gonorrhoeae by ligase chain reaction-based assays with clinical specimens from various sites: implications for diagnostic testing and screening. J. Clin. Microbiol. 34:2395-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2002. Sexually transmitted diseases treatment guidelines. Morb. Mortal. Wkly. Rep. 51:32-53. [Google Scholar]
- 5.Chernesky, M. A., E. W. Hook III, D. H. Martin, J. Lane, R. Johnson, J. A. Jordan, D. Fuller, D. E. Willis, P. M. Fine, W. M. Janda, and J. Schachter. 2005. Women find it easy and prefer to collect their own vaginal swabs to diagnose Chlamydia trachomatis or Neisseria gonorrhoeae infections. Sex. Transm. Dis. 32:729-733. [DOI] [PubMed] [Google Scholar]
- 6.Chernesky, M. A., D. Jang, H. Lee, J. D. Burczak, H. Hu, J. Sellors, S. J. Tomazic-Allen, and J. B. Mahony. 1994. Diagnosis of Chlamydia trachomatis infections in men and women by testing first-void urine by ligase chain reaction. J. Clin. Microbiol. 32:2682-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernesky, M. A., and D. E. Jang. 2006. APTIMA transcription-mediated amplification assays for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev. Mol. Diagn. 6:519-525. [DOI] [PubMed] [Google Scholar]
- 8.Chernesky, M. A., H. Lee, J. Schachter, J. D. Burczak, W. E. Stamm, W. M. McCormack, and T. C. Quinn. 1994. Diagnosis of Chlamydia trachomatis urethral infection in symptomatic and asymptomatic men by testing first-void urine in a ligase chain reaction assay. J. Infect. Dis. 170:1308-1311. [DOI] [PubMed] [Google Scholar]
- 9.Chernesky, M. A., D. H. Martin, E. W. Hook, D. Willis, J. Jordan, S. Wang, J. R. Lane, D. Fuller, and J. Schachter. 2005. Ability of new APTIMA CT and APTIMA GC assays to detect Chlamydia trachomatis and Neisseria gonorrhoeae in male urine and urethral swabs. J. Clin. Microbiol. 43:127-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook, R. L., S. L. Hutchison, L. Ostergaard, R. S. Braithwaite, and R. B. Ness. 2005. Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhoeae. Ann. Intern. Med. 142:914-925. [DOI] [PubMed] [Google Scholar]
- 11.Gaydos, C. A., M. Theodore, N. Dalesio, B. J. Wood, and T. C. Quinn. 2004. Comparison of three nucleic acid amplification tests for detection of Chlamydia trachomatis in urine specimens. J. Clin. Microbiol. 42:3041-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hook, E. W. III, K. Smith, C. Mullen, J. Stephens, L. Rinehardt, M. S. Pate, and H. H. Lee. 1997. Diagnosis of genitourinary Chlamydia trachomatis infections by using the ligase chain reaction on patient-obtained vaginal swabs. J. Clin. Microbiol. 35:2133-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh, Y. H., M. R. Howell, J. C. Gaydos, K. T. McKee, Jr., T. C. Quinn, and C. A. Gaydos. 2003. Preference among female Army recruits for use of self-administrated vaginal swabs or urine to screen for Chlamydia trachomatis genital infections. Sex. Transm. Dis. 30:769-773. [DOI] [PubMed] [Google Scholar]
- 14.Hu, D., E. W. Hook III, and S. J. Goldie. 2004. Screening for Chlamydia trachomatis in women 15 to 29 years of age: a cost-effectiveness analysis. Ann. Intern. Med. 141:501-513. [DOI] [PubMed] [Google Scholar]
- 15.Jones, R. B., B. P. Katz, B. van der Pol, V. A. Caine, B. E. Batteiger, and W. J. Newhall. 1986. Effect of blind passage and multiple sampling on recovery of Chlamydia trachomatis from urogenital specimens. J. Clin. Microbiol. 24:1029-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, H. H., M. A. Chernesky, J. Schachter, J. D. Burczak, W. W. Andrews, S. Muldoon, G. Leckie, and W. E. Stamm. 1995. Diagnosis of Chlamydia trachomatis genitourinary infection in women by ligase chain reaction assay of urine. Lancet 345:213-216. [DOI] [PubMed] [Google Scholar]
- 17.Mabey, D., R. W. Peeling, and M. D. Perkins. 2001. Rapid and simple point of care diagnostics for STIs. Sex. Transm. Infect. 77:397-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marrazzo, J. M., R. E. Johnson, T. A. Green, W. E. Stamm, J. Schachter, G. Bolan, E. W. Hook III, R. B. Jones, D. H. Martin, M. E. St Louis, and C. M. Black. 2005. Impact of patient characteristics on performance of nucleic acid amplification tests and DNA probe for detection of Chlamydia trachomatis in women with genital infections. J. Clin. Microbiol. 43:577-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moncada, J., J. M. Chow, and J. Schachter. 2003. Volume effect on sensitivity of nucleic acid amplification tests for detection of Chlamydia trachomatis in urine specimens from females. J. Clin. Microbiol. 41:4842-4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostergaard, L., B. Andersen, J. K. Moller, and F. Olesen. 2000. Home sampling versus conventional swab sampling for screening of Chlamydia trachomatis in women: a cluster-randomized 1-year follow-up study. Clin. Infect. Dis. 31:951-957. [DOI] [PubMed] [Google Scholar]
- 21.Pickett, M. A., J. S. Everson, P. J. Pead, and I. N. Clarke. 2005. The plasmids of Chlamydia trachomatis and Chlamydophila pneumoniae (N16): accurate determination of copy number and the paradoxical effect of plasmid-curing agents. Microbiology 151:893-903. [DOI] [PubMed] [Google Scholar]
- 22.Polaneczky, M., C. Quigley, L. Pollock, D. Dulko, and S. S. Witkin. 1998. Use of self-collected vaginal specimens for detection of Chlamydia trachomatis infection. Obstetr. Gynecol. 91:375-378. [DOI] [PubMed] [Google Scholar]
- 23.Robertson, P., and O. E. Williams. 2005. Young, male, and infected: the forgotten victims of chlamydia in primary care. Sex. Transm. Infect. 81:31-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook, J., and D. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed., appendix 8, p. 12. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 25.Schachter, J. 1999. Infection and disease epidemiology, p. 139-169. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, DC.
- 26.Schachter, J., M. A. Chernesky, D. E. Willis, P. M. Fine, D. H. Martin, D. Fuller, J. A. Jordan, W. Janda, and E. W. Hook III. 2005. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex. Transm. Dis. 32:725-728. [DOI] [PubMed] [Google Scholar]
- 27.Schachter, J., E. W. Hook, D. H. Martin, D. Willis, P. Fine, D. Fuller, J. Jordan, W. M. Janda, and M. Chernesky. 2005. Confirming positive results of nucleic acid amplification tests (NAATs) for Chlamydia trachomatis: all NAATs are not created equal. J. Clin. Microbiol. 43:1372-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scholes, D., A. Stergachis, F. E. Heidrich, H. Andrilla, K. K. Holmes, and W. E. Stamm. 1996. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N. Engl. J. Med. 334:1362-1366. [DOI] [PubMed] [Google Scholar]
- 29.Skidmore, S., P. Horner, A. Herring, J. Sell, I. Paul, J. Thomas, E. O. Caul, M. Egger, A. McCarthy, E. Sanford, C. Salisbury, J. Macleod, J. A. Sterne, and N. Low for the Chlamydia Screening Studies (ClaSS) Project. 2006. Vulvovaginal-swab or first-catch urine specimen to detect Chlamydia trachomatis in women in a community setting? J. Clin. Microbiol. 44:4389-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Standring, S., H. Ellis, J. C. Healy, D. Johnson, A. Williams, P. Collins, and C. Wigley. 2005. Gray's anatomy—the anatomical basis of clinical practice, 39th ed., p. 94-95. Elsevier Churchill Livingstone, London, United Kingdom.
- 31.Stary, A., B. Najim, and H. H. Lee. 1997. Vulval swabs as alternative specimens for ligase chain reaction detection of genital chlamydial infection in women. J. Clin. Microbiol. 35:836-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tam, J. E., C. H. Davis, R. J. Thresher, and P. B. Wyrick. 1992. Location of the origin of replication for the 7.5-kb Chlamydia trachomatis plasmid. Plasmid 27:231-236. [DOI] [PubMed] [Google Scholar]
- 33.van Valkengoed, I. G., M. J. Postma, S. A. Morre, A. J. van den Brule, C. J. Meijer, L. M. Bouter, and A. J. Boeke. 2001. Cost effectiveness analysis of a population based screening programme for asymptomatic Chlamydia trachomatis infections in women by means of home obtained urine specimens. Sex. Transm. Infect. 77:276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vickerman, P., C. Watts, M. Alary, D. Mabey, and R. W. Peeling. 2003. Sensitivity requirements for the point of care diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in women. Sex. Transm. Infect. 79:363-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. 2001. Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. WHO/HIV_AIDS/2001.02; WHO/CDS/CSR/EDC/2001.10. http://www.who.int/hiv/pub/sti/who_hiv_aids_2001.02.pdf.