Abstract
Lymphadenopathy (LN) is the most common clinical manifestation of acute acquired toxoplasma infection in humans. The diagnosis of toxoplasmic lymphadenitis (TL) is established by serological methods and/or lymph node biopsy. In the United States, the differential agglutination (of acetone [AC]-fixed versus that of formalin [HS]-fixed tachyzoites) test (AC/HS test) has primarily been used in assessments of pregnant women as a component of the toxoplasma serological profile to distinguish between recently acquired infections and infections acquired in the distant past. We studied the AC/HS test in patients with TL to define its usefulness in diagnosing individuals presenting with LN and to determine its kinetics after the onset of LN. One hundred nine consecutive patients (158 serum samples) diagnosed serologically and by lymph node biopsy as having TL were studied. Specific patterns in the AC/HS test were noted to be dependent on the time from the clinical onset of LN (COLN). Acute AC/HS patterns were observed for more than 75% of patients who according to their histories had developed their TL within 6 months after COLN. Acute patterns were not observed beyond the 12th month except for a single patient for whom an acute pattern (400/800) persisted to the 13th month after COLN. Equivocal patterns were observed up to 36 months after COLN. Nonacute patterns were observed only for serum samples drawn at least 13 months after COLN. A nonacute pattern in an individual at less than 12 months after COLN should suggest an etiology other than TL. In such cases, investigation for alternative causes, including malignancy, should be instigated.
Because lymphadenopathy (LN) can be caused by a wide variety of infectious, immunological, neoplastic, iatrogenic, and other miscellaneous causes, it can be a source of high anxiety to patients and their health care providers (1). Of particular concern are those patients whose LN has been present for more than 6 weeks and less than 12 months (1). In those settings, malignancy and infection need to be ruled out first, since they are among the most common etiologies. The diagnostic approach to patients with LN includes serological assays (in the attempt to identify infectious causes), radiological studies (to distinguish localized from generalized LN), fine needle aspiration (to diagnose malignancies), and most helpfully, complete excisional lymph node biopsy (to diagnose infection or malignancy by histological analysis, immunochemistry testing, stains for different organisms, and/or cultures) (14).
Toxoplasma gondii is among the well-recognized and often underdiagnosed infectious causes of LN (5). Toxoplasmosis usually results in a solitary, painless, cervical or occipital and nonsuppurative enlarged lymph node (10). However, toxoplasmosis can also cause localized LN outside the head and neck areas or diffuse or generalized LN (10, 14).
Toxoplasmic lymphadenitis (TL) is most often diagnosed by lymph node biopsy and/or serological assays (7, 11, 12, 15). Pathological features diagnostic of TL include a reactive follicular hyperplasia, irregular clusters of epithelioid histiocytes encroaching on and blurring the margins of the germinal centers, and focal distention of sinuses with monocytoid cells (6). The presence of these histological abnormalities alone, when typical, can suffice for the diagnosis. However, to increase the diagnostic yield, serological testing is recommended both in patients with the classical histological features and in those patients with atypical histological findings (6, 8). It is noteworthy that fine needle aspiration is rarely useful for the diagnosis, since it allows visualization of only a few isolated cells and does not permit the evaluation of lymph node architecture necessary to detect the classical histological features observed for TL (6, 8).
The kinetics and time dependency of serological tests from the clinical onset of LN (COLN) have been demonstrated previously (7, 11, 12, 15). In patients who present with typical clinical manifestations of TL (a solitary, painless, nonsuppurative occipital or cervical LN of less than 6 weeks in duration), serological tests alone may suffice to make the final diagnosis of TL and thus avoid the need for lymph node biopsy (7, 11, 12, 15). However, it has been suggested that the threshold to perform a lymph node biopsy should be relatively low, and any deviation from the typical clinical or serological presentation should prompt a search for causes other than toxoplasmosis (12).
The roles of individual serological tests (11, 15) and of various combinations of tests (7, 12) for the diagnosis of TL have previously been reported. In the present study, we report the precise kinetics of the differential agglutination (of acetone [AC]-fixed versus that of formalin [HS]-fixed tachyzoites) test (AC/HS test) and its value for the diagnosis of TL. The AC/HS test has also been used extensively in our laboratory as a confirmatory test for the diagnosis of the acute infection in pregnant women (16).
MATERIALS AND METHODS
One hundred sixty serum samples from 111 consecutive, nonpregnant, nonimmunocompromised patients with TL were studied. Patients with a history of recurrent LN were excluded (two patients). The remaining 109 patients (158 serum samples) each had a single episode of TL. Their sera had been submitted to the Toxoplasma Serology Laboratory of the Palo Alto Medical Foundation (PAMF-TSL) between January 1994 and May 2005 because of the clinical history of new onset and first episode of clinically significant LN. LN was self-reported by the patients to their health care providers and/or detected upon physical examination by their physicians. Physicians provided the dates of onset of LN and demographics of their patients. Data were analyzed according to the month following COLN that the serum sample was drawn. The interval between COLN and the time the serum was drawn was rounded off to the nearest month (i.e., patients whose sera were collected within 15 days of onset of LN were assigned to the “0-month” time point, and those whose sera were collected ≥15 days after onset of LN were assigned to the “1-month” time point).
The diagnosis of TL was made according to results in the toxoplasma serological profile (TSP) consistent with a recently acquired infection (12) together with lymph node biopsy findings suggestive of TL for 57 (52.3%) of the 109 patients (6, 11, 12). For the remaining 52 (47.7%) patients, the diagnosis of TL was by the results of the TSP alone as previously reported (11, 12). Each of the 109 patients had a serological test result consistent with a diagnosis of TL. The TSP is comprised of the Sabin-Feldman dye test (17), double sandwich immunoglobulin M (IgM) enzyme-linked immunosorbent assay (ELISA) (13), IgA ELISA (18), IgE ELISA (22), IgE immunosorbent agglutination assay (22), and the AC/HS test (3). Each test was performed and interpreted as previously described (12). A TSP consistent with a recently acquired infection (i.e., high titers in the dye test, i.e., ≥1,024; positive IgG [20], IgA, and IgE findings; and an acute pattern in the AC/HS test) is strongly suggestive of TL and is essentially found only in patients for whom LN was detected within 2 to 3 months of the sampling of the serum (12).
AC/HS test.
In the early 1980s, it was observed that the agglutination of AC-treated T. gondii tachyzoites was remarkably and surprisingly different from that of HS-fixed parasites (4, 19). Sera from patients with a recently acquired infection tended to agglutinate both the HS and AC parasite suspensions, whereas sera from patients with infection acquired in the distant past tended to have higher titers in the HS agglutination test and lower or negative titers in the AC agglutination test. This phenomenon has been attributed to the variation in IgG profiles in response to shifting Toxoplasma surface antigens as the infection progresses from an acute to a more chronic stage.
To perform an AC/HS test, the AC and HS antigens are prepared and standardized at the Institut de Puériculture de Paris (3, 4, 19). Suggested dilutions for each antigen are provided for each lot and are validated at PAMF-TSL. Two sets of serum dilutions are prepared in microtiter plates. The serum is serially diluted in 2-mercaptoethanol to avoid interference by both specific and nonspecific IgM agglutinins. The first dilution for the AC test is 1:100. If this test is positive, a titer of 50 IU/ml is assigned. The first dilution for the HS test is 1:2,000. If this test is positive, a titer of 100 IU/ml is assigned. Agglutination is visually graded after overnight incubation. Quality assurance and controls are routinely performed at PAMF-TSL, and blind samples are frequently exchanged with the laboratory of P. Thulliez in Paris to ensure that both laboratories obtain same results from patients known to be recently or chronically infected.
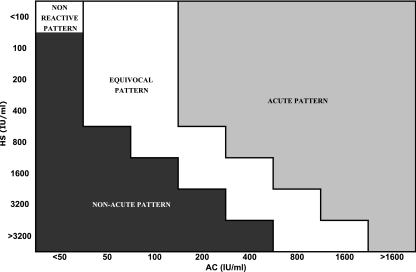
The AC/HS test was interpreted as previously described by comparing antibody titers obtained using the HS antigen with those obtained using the AC antigen (3). Certain agglutination patterns in the AC/HS test (i.e., “acute”) are more commonly observed for patients with a recently acquired T. gondii infection, whereas other patterns (i.e., “nonacute”) are more commonly observed for patients with an infection acquired in the distant past. (The final result of each AC/HS test was reported as follows: if the titer in the AC test was 400 IU/ml and that in the HS test was 100 IU/ml, the result was reported as 400/100 [Fig. 1].) It should be emphasized that the terms “acute” and “nonacute” refer solely to the interpretation of the agglutination pattern of the AC/HS test and not to whether the patient actually had a recently acquired infection (3). The AC/HS tests were performed and read without prior knowledge of the clinical histories of the patients.
FIG. 1.
Interpretative criteria for the differential agglutination (AC/HS) test.
RESULTS
The mean age of the patients was 34 years (range, 7 to 76). The female/male ratio was 1.1.
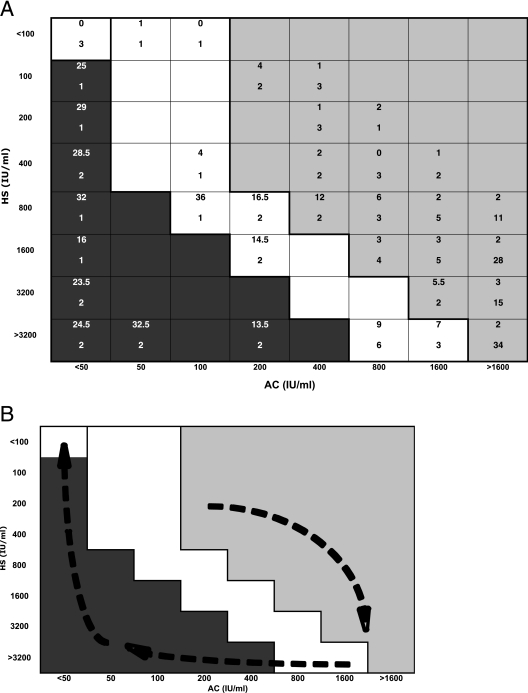
AC/HS test results for each of the 109 patients are shown in Fig. 2A. A summary of these results is presented in Table 1. The overall kinetics of the AC/HS test results for patients with TL observed in Fig. 2A followed a “clockwise” pattern of evolution on the interpretation chart, as diagrammatically represented in Fig. 2B.
FIG. 2.
(A) AC/HS test results for 109 patients (158 serum samples) with toxoplasmic LN. In each box, the top number is the median time from the onset of LN to the sampling of the first serum. The bottom number is the number of patients who had that AC/HS test pattern. (B) Diagrammatic representation of the approximate “clockwise” evolution of the AC/HS pattern in patients with TL.
TABLE 1.
Toxoplasma differential agglutination (AC/HS) patterns for 109 patients (158 serum samples) following onset of toxoplasmic LN
| Time from onset to serum sampling (mo)a | No. of serum samples | No. (%) with indicated AC/HS pattern
|
|||
|---|---|---|---|---|---|
| Acute | Nonacute | Equivocal | Nonreactive | ||
| 0 | 12 | 9 (75) | 0 (0) | 1 (8.3) | 2 (16.7) |
| 1 | 32 | 31 (96.9) | 0 (0) | 1 (3.1) | 0 (0) |
| 2 | 38 | 38 (100) | 0 (0) | 0 (0) | 0 (0) |
| 3 | 15 | 13 (86.7) | 0 (0) | 2 (13.3) | 0 (0) |
| 4 | 12 | 10 (83.3) | 0 (0) | 2 (16.7) | 0 (0) |
| 5 | 6 | 6 (100) | 0 (0) | 0 (0) | 0 (0) |
| 6 | 7 | 7 (100) | 0 (0) | 0 (0) | 0 (0) |
| 7 | 5 | 3 (60) | 0 (0) | 2 (40) | 0 (0) |
| 8-12 | 8 | 4 (50) | 0 (0) | 4 (50) | 0 (0) |
| 13-20 | 11 | 1 (9.1) | 6 (54.5) | 4 (36.4) | 0 (0) |
| >20 | 12 | 0 (0) | 8 (66.7) | 3 (25) | 1 (8.3) |
| Total | 158 | 0 | 0 | 0 | 0 |
Intervals from the onset of lymphadenopathy to the date that serum samples were collected (rounded off to the nearest month) are shown.
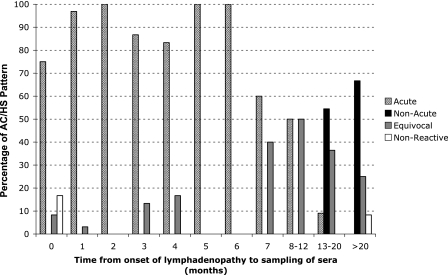
Acute AC/HS patterns were observed for more than 75% of patients for whom COLN was noted within 6 months prior to the time the first serum sample was obtained. The longest interval between COLN and serum sampling that was associated with an acute AC/HS pattern was 13 months. Acute patterns were not observed for 10 patients (12 serum samples) for whom LN was noted more than 13 months prior to serum sampling (Table 1 and Fig. 3).
FIG. 3.
Proportions of AC/HS test pattern results for 109 patients (158 serum samples) according to the time period in months following COLN.
Nonacute patterns were not observed prior to 13 months after COLN (Fig. 3 and Table 1). Certain nonacute patterns were observed for patients whose COLN had been observed more than 18 months before (<50/400, <50/200, and <50/100 in four serum samples corresponding to three patients) (Fig. 2A).
The equivocal patterns 50/<100 and 100/<100 (two serum samples from two patients) were observed only within the first month after COLN. Other equivocal patterns were observed only ≥3 months after COLN. An equivocal pattern, 100/800, was observed 36 months after COLN (one serum sample) (Fig. 2A).
The nonreactive pattern <50/<100 was observed both for very early infection (i.e., ≤15 days after COLN) and for late infection (i.e., 35 months after COLN) (Fig. 2A).
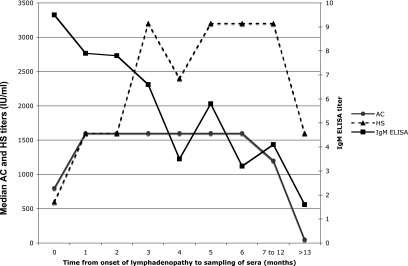
To determine the kinetics of the individual AC and HS tests, the AC test and the HS test results were considered separately and are plotted against the time from COLN to serum sampling in Fig. 4. Both tests appear to have lower values at less than 1 month or more than 12 months following COLN. Between 3 and 12 months, the AC appears to be stable and does not exhibit significant variations, but the HS test appears to have a decrease around 3 months followed by a rebound that plateaus between 5 and 12 months.
FIG. 4.
Individual kinetics of antibody responses to the AC-fixed tachyzoite and HS-fixed tachyzoite antigens and of the IgM ELISA titers for 109 patients (158 serum samples) following COLN.
Of 25 serum samples from patients whose LN had been detected 13 or more months earlier and had a nonacute AC/HS test result, 11 (44%) had a positive IgM ELISA result.
DISCUSSION
Several serological assays, including the TSP (12) and the avidity test (11, 15), have been reported as useful for the diagnosis of TL. TSP results consistent with a recently acquired infection are strongly suggestive of TL and are essentially found only for patients whose LN was detected within 2 to 3 months prior to the sampling of the serum (12). For these patients, an excisional biopsy or fine needle aspiration of the lymph node is usually not indicated unless the LN persists, other symptoms develop, or some other diagnosis is being considered. TSP results consistent with an infection acquired in the distant past essentially exclude T. gondii as the causative agent of LN that occurred within the 3 months prior to the drawing of the serum sample (12). For these patients, additional laboratory tests, including an excisional lymph node biopsy, should be strongly considered to exclude the diagnosis of etiologies other than toxoplasmosis. Equivocal results in the TSP are more difficult to interpret. In such cases, T. gondii should neither be ruled out nor be implicated as the cause of the patient's LN; the diagnosis will have to rely on other laboratory tests, including lymph node biopsy (12).
The avidity test alone has also been reported to be useful for patients with LN (11, 15). For patients with recent-onset LN (i.e., onset within the past 3 months), high avidity test results should caution physicians against attributing recently detected LN to toxoplasmosis (11). In theses cases, lymph node biopsy may be necessary to clarify the cause. Although low or equivocal avidity test results tend to occur for patients with a recently acquired infection, they cannot be used alone to diagnose recently acquired infection in patients with LN. Low or equivocal avidity test results may be observed for patients who have been infected with T. gondii for several months or even a few years (11, 15).
Acute pattern results in the AC/HS test were observed only for patients whose COLN was within 13 months of serum sampling. Thus, for patients whose LN has been present for more than 13 months and who have acute pattern results in the AC/HS, T. gondii is most likely not responsible for the LN, despite the fact that the AC/HS test results suggest that T. gondii infection has been recently acquired. Equivocal results in the AC/HS test were more difficult to interpret, since they were observed for a wide range of time periods following COLN (from 0 to 36 months). Conversely, nonacute results were observed only for patients for whom COLN has been observed more than 12 months from serum sampling. Thus, for patients whose LN has been documented to be present for less than 1 year and who have a nonacute pattern in the AC/HS test, the pursuit of etiologic agents other than T. gondii needs to be continued, and strong consideration should be given to an excisional lymph node biopsy.
The kinetics of the antibodies against the individual antigens (AC and HS) in the AC/HS test resemble those of the dye test observed for patients with LN (12). Both the AC and the HS test results appear to be low initially but then seem to rise and persist for several months. Subsequently, as observed with the dye test, both the AC and the HS test titers decline. However, in contrast with the dye test, where the decrease in antibody responses occurs around month 5 or 6, the decrease in the antibody responses to the AC and HS tests is observed around months 12 and 13. It is likely that this unique late decline is responsible for the enhanced power of the AC/HS test in ruling out an acute infection that occurred in the previous 12 months.
The IgM test used alone as a diagnostic tool for the diagnosis of TL should be performed and interpreted with caution. In general, the IgM antibodies appear earlier and decline more rapidly than IgG antibodies in patients with TL (12). However, IgM titers may persist for years after the acute infection for some patients, and commercially available assays vary considerably in terms of reliability (2, 9, 21). In fact, the 11 patients who had positive IgM ELISA titers would erroneously have been interpreted as having results consistent with recently acquired toxoplasmosis; each had a nonacute AC/HS test result, establishing that the infection was acquired 13 months or more before the sampling of the sera. As we have reported previously, IgM antibody titers were high within the first 4 weeks after the COLN, they gradually decreased from the beginning of month 2 until month 4, and they appeared to rebound in month 5. Thereafter, they gradually declined. However, as expected, some patients' IgM titers remained positive afterwards (12). Positive IgM test results for patients with TL must be followed by confirmatory testing performed at reference laboratories and should not necessarily be interpreted as consistent with a recently acquired T. gondii infection.
For a patient who presents with LN, a nonacute pattern in the AC/HS test rules out T. gondii as the etiologic agent. Whereas a high avidity test result for a patient with LN excludes an infection acquired within 3 to 5 months from the time the serum sample was drawn (11, 15), our data suggest that a nonacute pattern from the AC/HS test is unique in that it excludes infection acquired within the prior 13 months. Thus, the AC/HS test's window for excluding a recently acquired infection appears to be remarkably longer than that for the avidity test (3). This suggests that the AC/HS test may be of special value for women for whom the diagnosis of acute infection is being considered. Unfortunately, only two reference laboratories (one in the United States and one in Paris, France) perform the AC/HS test. Studies are needed to confirm whether the 13-month time window for the exclusion of an acute infection we observed for patients with LN also applies to cases of infection during pregnancy and, if so, whether the data should provide an impetus for industry to develop kits to make feasible the wider availability of the AC/HS test.
Footnotes
Published ahead of print on 21 February 2007.
REFERENCES
- 1.Bazemore, A. W., and D. R. Smucker. 2002. Lymphadenopathy and malignancy. Am. Fam. Physician 66:2103-2110. [PubMed] [Google Scholar]
- 2.Bobic, B., D. Sibalic, and O. Djurkovic-Djakovic. 1991. High levels of IgM antibodies specific for Toxoplasma gondii in pregnancy 12 years after primary toxoplasma infection. Gynecol. Obstet. Investig. 31:182-184. [DOI] [PubMed] [Google Scholar]
- 3.Dannemann, B. R., W. C. Vaughan, P. Thulliez, and J. S. Remington. 1990. Differential agglutination test for diagnosis of recently acquired infection with Toxoplasma gondii. J. Clin. Microbiol. 28:1928-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desmonts, G., and J. S. Remington. 1980. Direct agglutination test for diagnosis of Toxoplasma infection: method for increasing sensitivity and specificity. J. Clin. Microbiol. 11:562-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doganci, L., M. Tanyuksel, E. R. Araz, B. A. Besirbellioglu, U. Erdem, C. A. Ozoguz, N. Yucel, and A. Ciftcioglu. 2006. A probable outbreak of toxoplasmosis among boarding school students in Turkey. Clin. Microbiol. Infect. 12:672-674. [DOI] [PubMed] [Google Scholar]
- 6.Dorfman, R. F., and J. S. Remington. 1973. Value of lymph-node biopsy in the diagnosis of acute acquired toxoplasmosis. N. Engl. J. Med. 289:878-881. [DOI] [PubMed] [Google Scholar]
- 7.Durlach, R. A., F. Kaufer, L. Carral, and J. Hirt. 2003. Toxoplasmic lymphadenitis—clinical and serologic profile. Clin. Microbiol. Infect. 9:625-631. [DOI] [PubMed] [Google Scholar]
- 8.Eapen, M., C. F. Mathew, and K. P. Aravindan. 2005. Evidence based criteria for the histopathological diagnosis of toxoplasmic lymphadenopathy. J. Clin. Pathol. 58:1143-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liesenfeld, O., C. Press, J. G. Montoya, R. Gill, J. L. Isaac-Renton, K. Hedman, and J. S. Remington. 1997. False-positive results in immunoglobulin M (IgM) toxoplasma antibody tests and importance of confirmatory testing: the Platelia Toxo IgM test. J. Clin. Microbiol. 35:174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCabe, R. E., R. G. Brooks, R. F. Dorfman, and J. S. Remington. 1987. Clinical spectrum in 107 cases of toxoplasmic lymphadenopathy. Rev. Infect. Dis. 9:754-774. [DOI] [PubMed] [Google Scholar]
- 11.Montoya, J. G., H. B. Huffman, and J. S. Remington. 2004. Evaluation of the immunoglobulin G avidity test for diagnosis of toxoplasmic lymphadenopathy. J. Clin. Microbiol. 42:4627-4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montoya, J. G., and J. S. Remington. 1995. Studies on the serodiagnosis of toxoplasmic lymphadenitis. Clin. Infect. Dis. 20:781-790. [DOI] [PubMed] [Google Scholar]
- 13.Naot, Y., and J. S. Remington. 1980. An enzyme-linked immunosorbent assay for detection of IgM antibodies to Toxoplasma gondii: use for diagnosis of acute acquired toxoplasmosis. J. Infect. Dis. 142:757-766. [DOI] [PubMed] [Google Scholar]
- 14.Pasternack, M. S., and M. N. Swartz. 2005. Lymphadenitis and lymphangitis, p. 1204-1214. In G. Mandell, J. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. Churchill Livingstone, London, United Kingdom.
- 15.Paul, M. 1999. Immunoglobulin G avidity in diagnosis of toxoplasmic lymphadenopathy and ocular toxoplasmosis. Clin. Diagn. Lab. Immunol. 6:514-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Remington, J. S., R. McLeod, P. Thuilliez, and G. Desmonts. 2006. Toxoplasmosis. In J. S. Remington, J. O. Klein, C. B. Wilson, and C. Baker (ed.), Infectious diseases of the fetus and newborn infant, 6th ed. Elsevier Saunders, Philadelphia, PA.
- 17.Sabin, A. B., and H. A. Feldman. 1948. Dyes as microchemical indicators of a new immunity phenomenon affecting a protozoan parasite (toxoplasma). Science 108:660-663. [DOI] [PubMed] [Google Scholar]
- 18.Stepick-Biek, P., P. Thulliez, F. G. Araujo, and J. S. Remington. 1990. IgA antibodies for diagnosis of acute congenital and acquired toxoplasmosis. J. Infect. Dis. 162:270-273. [DOI] [PubMed] [Google Scholar]
- 19.Thulliez, P., J. S. Remington, F. Santoro, G. Ovlaque, S. Sharma, and G. Desmonts. 1986. A new agglutination test for the diagnosis of acute and chronic toxoplasma infection. Pathol. Biol. 34:173-177. (In French.) [PubMed] [Google Scholar]
- 20.Volker, H., M. Sigmund, M. Kropff, T. Hurter, J. Kemnitz, C. J. Kirkpatrick, and P. Hanrath. 1991. Myocarditis caused by Toxoplasma gondii and Aspergillus fumigatus after orthotopic heart transplantation. Z. Kardiol. 80:359-362. (In German.) [PubMed] [Google Scholar]
- 21.Wilson, M., D. A. Ware, and K. W. Walls. 1987. Evaluation of commercial serodiagnostic kits for toxoplasmosis. J. Clin. Microbiol. 25:2262-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong, S. Y., M.-P. Hadju, R. Ramirez, P. Thulliez, R. McLeod, and J. S. Remington. 1993. The role of specific immunoglobulin E in diagnosis of acute toxoplasma infection and toxoplasmosis. J. Clin. Microbiol. 31:2952-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]