Abstract
We reevaluated Enterobacteriaceae disk diffusion breakpoints for the tetracyclines published in the Clinical and Laboratory Standards Institute (CLSI) document M100-S16, which were (susceptible/resistant) ≥19 mm/≤14 mm for tetracycline, ≥16 mm/≤12 mm for doxycycline, and ≥19 mm/≤14 mm for minocycline. A collection of 504 recent clinical isolates of Enterobacteriaceae were tested against these tetracycline compounds by disk diffusion and broth microdilution methods according to CLSI guidelines. Regression line and scattergram plot analyses determined intermethod accuracy for current disk diffusion breakpoints and showed excellent r values of 0.91 to 0.95. However, error rates (minor/major [false-resistant]/very major [false-susceptible]) were 14.9/0.8/0.0% for tetracycline, 11.5/0.4/0.0% for doxycycline, and 30.6/0.7/0.0% for minocycline and only 4.4/0.0/0.0% for tetracycline, 5.6/0.0/0.2% for doxycycline, and 8.3/0.0/0.3% for minocycline when proposed breakpoints were modified to (susceptible/resistant) ≥15 mm/≤11 mm for tetracycline, ≥14 mm/≤10 mm for doxycycline, and ≥16 mm/≤12 mm for minocycline. Listed modifications were recently approved by the CLSI (M100-S17).
The tetracyclines were the initial group or class of “broad-spectrum” antimicrobials to be described, dating from 1944, when chlortetracycline was isolated from Streptomyces aureofaciens (5, 12). This compound was clinically introduced 4 years later, and other similar agents, such as oxytetracycline (from Streptomyces rimosus) and tetracycline (derived by dehalogenation of chlortetracycline), were discovered or produced by synthetic processes from 1950 through 1953. The more commonly used long-acting derivatives, doxycycline and minocycline, were described between 1965 and 1972 (1, 2, 6, 11, 18).
These compounds are complex polycycline structures with a carboxamide at the C-2 position. Substitutions producing the various agents in the class were generally minimal (tetracycline as the base molecule), usually occurring at positions C-5, -6, and -7 (examples, for doxycycline, a loss of a hydroxyl group [deoxy] at the C-6β position; and for minocycline, an addition of a dimethylamino group at position C-7) (5, 6). These chemical alterations change the lipophilicity, with the more hydrophilic agents (tetracycline and oxytetracycline) being least active. Minocycline (most lipophilic) has been generally recognized as the most potent agent in this class, followed by doxycycline. The tetracyclines are usually bacteriostatic, but the minimal bactericidal concentration may be only fourfold greater than the measured MIC (6).
Since the early days of standardized susceptibility testing methods (Clinical and Laboratory Standards Institute [CLSI], formerly the National Committee for Clinical Laboratory Standards [NCCLS]), the testing of tetracyclines used a 30-μg tetracycline disk as the class representative (3). The initial NCCLS interpretive tables were found in the M2-A and M2-A2 disk diffusion standards published before 1980 (13, 14), each table containing only tetracycline interpretive zone diameters (susceptible at ≥19 mm [MIC correlate at ≤4 μg/ml]; resistant at ≤14 mm [MIC correlate at ≥12 μg/ml]). When the annual supplemental table program was initiated in 1981 (15), only tetracycline was listed, with an accompanying statement (footnote p) that read, “Tetracycline is the class disk for all tetracyclines, and the results can be applied to chlortetracycline, demeclocycline, doxycycline, methocycline, minocycline, and oxytetracycline. However, some in vitro data show that certain organisms may be more susceptible to doxycycline and minocycline than to tetracycline.” The following year (1982), in NCCLS M2-A2 S2 (16), interpretive disk diffusion criteria for doxycycline (susceptible at ≥16 μg/ml; resistant at ≤12 mm) and minocycline (susceptible at ≥19 mm; resistant at ≤14 mm) were added to the tables. These criteria and their MIC correlations have not been altered in more than two decades, since these guidelines were adopted based on a single-laboratory study comparing the disk diffusion results for the three tetracycline derivatives (3).
In more recent years, the value of minocycline or a derivative has been rediscovered for the treatment of methicillin-resistant Staphylococcus aureus, multidrug-resistant Enterobacteriaceae, and resistant Acinetobacter spp. (4, 19); and doxycycline has been successfully applied to the therapy of vancomycin-resistant enterococci for more than 10 years (6). These emerging resistance events necessitated expanded testing of this class, with subsequent reports of discords between MIC and disk diffusion results. In this study, the Acinetobacter and Polymyxin Working Group of the CLSI Antimicrobial Susceptibility Testing Subcommittee addressed these concerns via a structured, multicenter comparison of three tetracyclines tested by reference MIC and standardized disk diffusion methods (7-10) against contemporary strains of Enterobacteriaceae.
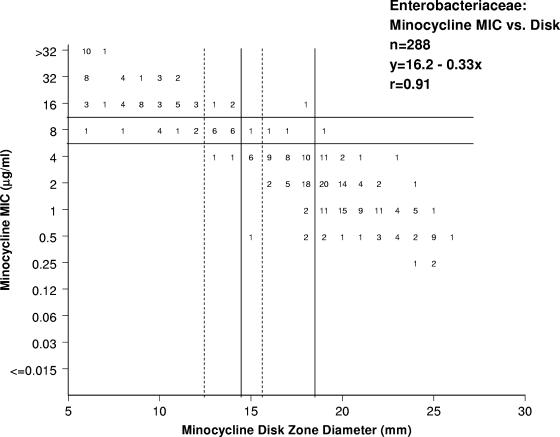
The study was designed utilizing multiple laboratories, with Enterobacteriaceae selected from current clinical isolates at five geographically diverse locations. The participating laboratories were as follows: Centers for Disease Control and Prevention (Atlanta, GA); Duke University (Durham, NC); JMI Laboratories (North Liberty, IA); Loyola University Medical Center (Maywood, IL); and Massachusetts General Hospital (Boston, MA). A total of 504 nonduplicated strains were identified locally and tested against tetracycline and doxycycline, including Citrobacter freundii (51 strains), Citrobacter koseri (7 strains), Citrobacter spp. (1 strain); Enterobacter cloacae (55 strains), Enterobacter aerogenes (12 strains), Enterobacter spp. (2 strains), Escherichia coli (110 strains), Hafnia alvei (1 strain), indole-positive Proteae (33 strains), Klebsiella oxytoca (55 strains), Klebsiella pneumoniae (110 strains), Pantoea agglomerans (1 strain), Proteus mirabilis (29 strains), Salmonella spp. (4 strains), Shigella spp. (4 strains), Serratia marcescens (28 strains), and Serratia liquifaciens (1 strain); while a 288-organism subset was evaluated against minocycline (Fig. 1).
FIG. 1.
Correlation between MIC results and disk diffusion inhibition zone diameters for minocycline. Solid lines indicate year 1982 to 2006 CLSI breakpoints (9), while broken lines show the proposed (CLSI, 2007) disk diffusion breakpoints (10).
The organisms were tested for susceptibility by CLSI methods in cation-adjusted Mueller-Hinton medium prepared in frozen-form broth microdilution panels (TREK Diagnostics, Cleveland, OH) (7-10). Each institution utilized the current Mueller-Hinton agar lot in use at that facility for disk diffusion testing. Quality control strains (Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853) were concurrently tested on ≥5 occasions by each participant for the three tetracyclines and control agents (gentamicin and tobramycin); all results (100.0%) except for gentamicin (96.2% by MIC tests only) were within published quality control ranges as recommended by the CLSI (9, 10). The inoculum colony counts for the broth microdilution method averaged 3.8 × 105 CFU/ml across all participant sites. This protocol design conforms to the NCCLS M23-A2 guideline document recommendations (15).
Tetracycline, doxycycline, and minocycline MIC and disk diffusion breakpoints established for Enterobacteriaceae by the CLSI (9) were applied for all pathogens evaluated in the present study (Table 1). The broth microdilution and disk diffusion results for each drug were also compared by regression and error-rate bounding analyses (17). Scattergrams correlating MICs and zone diameters around disks were constructed (Fig. 1) and analyzed by the error-rate bounding method (17) to maximize intermethod agreement for the MIC breakpoints established by the CLSI (9). Generally, the goal of such calculations is to minimize false-susceptible (very major) errors for the disk diffusion test to ≤1.5% and intermethod minor and total errors to ≤10.0% (17). Since tetracycline disk results may be used to predict the susceptibilities for doxycycline and minocycline, the investigators also evaluated the correlation between tetracycline disk diffusion inhibition zones and those of doxycycline and minocycline (cross-resistance analysis). Initial analyses examined MIC and zone diameter results for serious discords (susceptible to resistant or vice versa), with discordant results repeated to assess reproducibility.
TABLE 1.
Breakpoints (current and proposed) for three tetracyclines with the summary of intermethod error ratesa
| Antimicrobial agent (no. of tests/no. of resistant strains) | Current CLSI (2006) breakpoints
|
Proposed CLSI (2007) breakpoints
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Criterion (mm)
|
Error rate (%)
|
Criterion (mm)
|
Error rate (%)
|
|||||||
| Susceptible | Resistant | VM | Ma | Mib | Susceptible | Resistant | VM | Ma | Mi | |
| Tetracycline (504/140) | ≥19 | ≤14 | 0.0 | 0.8 | 14.9 | ≥15 | ≤11 | 0.0 | 0.0 | 4.4 |
| Doxycycline (504/120) | ≥16 | ≤12 | 0.0 | 0.4 | 11.5 | ≥14 | ≤10 | 0.2 | 0.0 | 5.6 |
| Minocycline (288/60) | ≥19 | ≤14 | 0.0 | 0.7 | 30.6 | ≥16 | ≤12 | 0.3 | 0.0 | 8.3 |
VM, very major (false-susceptible) error; Ma, major (false-resistant) error; Mi, minor errors (discords involving an intermediate interpretation).
Underlined error rates indicate unacceptable levels of intermethod discord (NCCLS, M23-A2) (17).
The susceptibility and resistance rates were, respectively, 67.6 and 27.8% for tetracycline, 68.5 and 23.8% for doxycycline, and 70.5 and 20.8% for minocycline. Figure 1 shows the correlation between one tetracycline (minocycline example) MIC and disk diffusion inhibitory zone diameters using both the current (9) and proposed (10) breakpoints. The proposed disk diffusion breakpoints were 2 to 4 mm smaller than the year 2006 CLSI (9) zone diameters (Table 1), and the adjustment of the breakpoints eliminated the major errors and produced a significant decrease in the unacceptable rate of minor errors (from 11.5 to 30.6% to 4.4 to 8.3% for all tetracyclines). The most elevated error rates were for minocycline (30.6%) (Fig. 1) and the proposed breakpoint changes reduced total error from 31.3% to only 8.6%. The discordant results were well distributed among the species/organism groups evaluated, with a slight predominance among S. marcescens and indol-positive Proteae when using either the 2006 CLSI breakpoints or the proposed breakpoints (data not shown).
With these proposed breakpoint modifications, no major errors were observed and a single very major error was observed for doxycycline (0.2%) and minocycline (0.3%). When the number of resistant strains was used as the denominator, the rates of very major errors were 0.8% for doxycycline and 1.7% for minocycline, each well below acceptable limits. The results of this study also demonstrated that tetracycline disk diffusion results can still be used to predict susceptibilities for doxycycline and minocycline (data not shown) using these modified breakpoints (10). Among isolates susceptible to tetracycline by disk diffusion assay, 98.4% were susceptible and 1.4% considered intermediate to doxycycline. When tested with minocycline, 95.7% of tetracycline-susceptible strains were categorized as susceptible and 3.5% as intermediate.
The CLSI disk diffusion breakpoints published from 1982 to 2006 (9, 16) provided reduced levels of intermethod interpretive accuracy when tetracycline, doxycycline, and minocycline were tested against Enterobacteriaceae, while the proposed adjustments in the disk diffusion breakpoints provide acceptable intermethod agreement (17). The tetracycline class breakpoints established based on the results of this study were presented and approved by the CLSI Subcommittee on Antimicrobial Susceptibility in June 2006 and published in CLSI document M100-S17 (10). These breakpoints currently provide improved guidance for susceptibility testing with the tetracyclines by disk diffusion methods, if needed for testing of emerging multidrug-resistant Enterobacteriaceae.
Footnotes
Published ahead of print on 14 March 2007.
REFERENCES
- 1.Agwuh, K. N., and A. MacGowan. 2006. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 58:256-265. [DOI] [PubMed] [Google Scholar]
- 2.Allen, J. C. 1976. Minocycline. Ann. Intern. Med. 85:482-487. [DOI] [PubMed] [Google Scholar]
- 3.Barry, A. L., D. L. Draper, and M. K. Wong. 1978. Aerobic and anaerobic susceptibility tests with three tetracyclines. Reassessment of the “class concept” of disk testing. Am. J. Clin. Pathol. 70:821-825. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, P. A. 2004. Tigecycline: a first in class glycylcycline. Clin. Microbiol. Newslett. 26:163-168. [Google Scholar]
- 5.Bryskier, A. 2005. Tetracyclines, p. 642-651. In A. Bryskier (ed.), Antimicrobial agents: antibacterials and antifungals, vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 6.Chopra, I., and M. Roberts. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65:232-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2006. M2-A9. Performance standards for antimicrobial disk susceptibility tests; approved standard, 9th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Clinical and Laboratory Standards Institute. 2006. M7-A7. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Clinical and Laboratory Standards Institute. 2006. M100-S16, Performance standards for antimicrobial susceptibility testing; 16th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
- 10.Clinical and Laboratory Standards Institute. 2007. M100-S17. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
- 11.Cunha, B. A., C. M. Sibley, and A. M. Ristuccia. 1982. Doxycycline. Ther. Drug Monit. 4:115-135. [DOI] [PubMed] [Google Scholar]
- 12.Duggar, B. 1948. Aureomycin, a product of the continuing search for new antibiotics. Ann. N. Y. Acad. Sci. 51:177-181. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 1976. Approved standard M2-A. Performance standards for antimicrobic disc susceptibility tests, 1st ed. National Committee for Clinical Laboratory Standards, Villanova, PA.
- 14.National Committee for Clinical Laboratory Standards. 1979. Approved standard M2-A2. Performance standards for antimicrobic disc susceptibility tests, 2nd ed. National Committee for Clinical Laboratory Standards, Villanova, PA.
- 15.National Committee for Clinical Laboratory Standards. 1981. First supplement, M2-A2S. Performance standards for antimicrobic disk susceptibility tests, 2nd ed. National Committee for Clinical Laboratory Standards, Villanova, PA.
- 16.National Committee for Clinical Laboratory Standards. 1982. Second supplement, M2-A2 S2. Performance standards for antimicrobic disk susceptibility tests, 2nd ed. National Committee for Clinical Laboratory Standards, Villanova, PA.
- 17.National Committee for Clinical Laboratory Standards. 2001. Approved guideline M23-A2. Development of in vitro susceptibility testing criteria and quality control parameters, 2nd ed. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 18.Neu, H. C. 1978. A symposium on the tetracyclines: a major appraisal. Introduction. Bull. N. Y. Acad. Med. 54:141-155. [PMC free article] [PubMed] [Google Scholar]
- 19.Woodford, N., P. M. Tierno, Jr., K. Young, L. Tysall, M. F. Palepou, E. Ward, R. E. Painter, D. F. Suber, D. Shungu, L. L. Silver, K. Inglima, J. Kornblum, and D. M. Livermore. 2004. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A β-lactamase, KPC-3, in a New York medical center. Antimicrob. Agents Chemother. 48:4793-4799. [DOI] [PMC free article] [PubMed] [Google Scholar]