Abstract
We previously hypothesized that efficient translation of influenza virus mRNA requires the recruitment of P58IPK, the cellular inhibitor of PKR, an interferon-induced kinase that targets the eukaryotic translation initiation factor eIF2α. P58IPK also inhibits PERK, an eIF2α kinase that is localized in the endoplasmic reticulum (ER) and induced during ER stress. The ability of P58IPK to interact with and inhibit multiple eIF2α kinases suggests it is a critical regulator of both cellular and viral mRNA translation. In this study, we sought to definitively define the role of P58IPK during viral infection of mammalian cells. Using mouse embryo fibroblasts from P58IPK−/− mice, we demonstrated that the absence of P58IPK led to an increase in eIF2α phosphorylation and decreased influenza virus mRNA translation. The absence of P58IPK also resulted in decreased vesicular stomatitis virus replication but enhanced reovirus yields. In cells lacking the P58IPK target, PKR, the trends were reversed—eIF2α phosphorylation was decreased, and influenza virus mRNA translation was increased. Although P58IPK also inhibits PERK, the presence or absence of this kinase had little effect on influenza virus mRNA translation, despite reduced levels of eIF2α phosphorylation in cells lacking PERK. Finally, we showed that influenza virus protein synthesis and viral mRNA levels decrease in cells that express a constitutively active, nonphosphorylatable eIF2α. Taken together, our results support a model in which P58IPK regulates influenza virus mRNA translation and infection through a PKR-mediated mechanism which is independent of PERK.
Influenza virus is a significant health problem worldwide and has the potential to become a major public threat, especially if a highly virulent strain, such as the circulating H5N1 (36), to which humans are immunogenically naive, emerges. If the immune system of the host is naive to the emerging virus' surface antigens, specifically the hemagglutinin and neuraminidase glycoproteins, and the virus bypasses both the innate and adaptive immune responses to gain entry into cells, then its virulence will be determined by the interactions between the other viral and cellular genes and the proteins they encode (23, 44).
Much of influenza virus' fitness depends on its ability to ensure that viral mRNAs are efficiently translated by the cellular machinery. Translational control is exerted at several steps in the influenza virus life cycle. Thus, the virus has evolved strategies of translational control that take advantage of its requirement for cap-dependent translation initiation, as well as the recruitment of host cell proteins to aid in the preferential translation of viral mRNA (22). All influenza virus mRNAs contain host cell RNA sequences, including the m7G cap at their 5′ ends, which are obtained though a “cap-stealing” mechanism and function as primers for the viral RNA-dependent RNA-polymerase (46, 47). Following this scavenging of 5′ ends from cellular mRNAs, the uncapped cellular transcripts are degraded (32). This degradation of cellular mRNA may contribute to the dramatic shutoff of cellular protein synthesis that is observed in influenza virus-infected cells. Influenza virus has also exploited other mechanisms to cause the selective translation of viral mRNAs (17, 26, 27). The short, conserved 5′ untranslated regions of influenza virus mRNAs increase the translational efficiency of viral transcripts (25). In addition, cellular proteins, including GRSF-1, may act in trans to further increase the translation of influenza virus proteins (45).
However, the cellular response to influenza virus infection has evolved to counteract the virus' mechanisms to bolster viral protein synthesis. One strategy of virus-infected cells is to globally inhibit translation initiation through the phosphorylation of the alpha subunit of eukaryotic initiation factor 2 (eIF2α) (25). There are at least four cellular eIF2α kinases, with the interferon-induced, double-stranded RNA (dsRNA)-activated, protein kinase R (PKR) playing a prominent role in virus-infected cells. PKR is a 68-kDa serine-threonine protein kinase ubiquitously expressed at low levels in many mammalian tissues (30). PKR is activated by dsRNA, an intermediate produced during the life cycle of many viruses (41). Phosphorylation of eIF2α at S51 results in the inhibition of translation initiation due to a block in eIF2B-mediated exchange of GDP for GTP, rendering eIF2 in an inactive form bound to GDP. This limits the amount of functional eIF2-GTP-Met-tRNA available to initiate translation (11).
Since viral translation is dependent upon functional eIF2 for translation initiation, viruses have developed strategies to prevent or reverse PKR-dependent eIF2α phosphorylation. Some viruses encode molecules that interfere with dsRNA binding to PKR, thus preventing PKR dimerization and autophosphorylation. These include adenovirus VAI RNA, reovirus capsid protein σ3, and influenza virus nonstructural (NS1) protein (24, 37, 39). Other viruses directly inhibit PKR dimerization. The hepatitis C virus (HCV) protein NS5A binds to PKR, physically interfering with its ability to dimerize (14). Rather than encoding a viral protein to block PKR activity, influenza virus infection activates a cellular protein, P58IPK, to inhibit PKR (14, 35). P58IPK interacts with a region of PKR that spans the ATP-binding region in the C-terminal catalytic domain (amino acids 244 to 296) (15) and is involved in its ability to dimerize. The interaction of P58IPK with this region of PKR prevents PKR dimerization and autophosphorylation (55), thus interfering with PKR's ability to phosphorylate eIF2α and inhibiting translation initiation in response to viral infection.
Not only is P58IPK activated by influenza virus infection, but it is also induced during the unfolded protein response (UPR), a stress response initiated by protein overload in the endoplasmic reticulum (ER). This induction is possible because the UPR increases the level of two transcription factors, ATF6 and XBP1, and these molecules interact with the ER stress response element within P58IPK's promoter region. P58IPK has been shown to interact with and inhibit PERK, an ER-localized eIF2α kinase (58, 61), thus enabling translation to ensue. Recent evidence has also shown that P58IPK has PERK-independent functions and mediates the cytosolic degradation of misfolded proteins delayed at the ER translocon (43).
Although P58IPK's interaction with other proteins has been studied with mammalian cells, its function during virus infection has not been examined. Interestingly, the role of plant P58IPK homologues during virus infection has been studied. During tobacco etch virus and tobacco mosaic virus infections in plants lacking P58IPK, increased host death and reduced viral titer were observed, suggesting P58IPK was required for virulence (6). Does P58IPK have a similar role during virus infection in eukaryotic cells? To answer this question, we recently generated P58IPK-null mice on a C57BL/6 background (31). These animals exhibit a diabetic phenotype by 4 months of age due to the disruption in ER homeostasis. However, this phenotype was not as severe as that seen with mice lacking PERK (19) or containing constitutively active, nonphosphorylatable eIF2α (49), both of which are downstream of P58IPK.
In this study, we define the role of P58IPK during influenza virus infection by showing that in embryonic fibroblasts derived from P58IPK−/− mice, there is reduced viral mRNA translation, which was correlated with increased levels of eIF2α phosphorylation. We further show that P58IPK has an effect not only on influenza virus mRNA translation but also on replication of other RNA viruses. Finally we analyzed influenza virus infections with mouse embryo fibroblasts (MEFs) lacking proteins that act downstream of P58IPK, further defining the signaling pathway by which P58IPK functions during viral infection.
MATERIALS AND METHODS
Cells and viruses.
P58IPK knockout (KO) (31), PKR KO (62), PERK KO (19), and their corresponding wild-type (WT) MEFs were grown as monolayers in high-glucose Dulbecco's modified Eagle's medium (hgDMEM) supplemented to contain 10% heat-inactivated fetal calf serum (HyClone Laboratories, Logan, UT), 2 mM l-glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 10 μM 2-mercaptoethanol, 50 units/ml penicillin G, and 50 μg/ml streptomycin sulfate. MEFs expressing WT or mutant (S51A) eIF2α (49) were maintained as monolayers in hgDMEM supplemented to contain 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 1× essential amino acids, 10 μM 2-mercaptoethanol, 50 units/ml penicillin G, and 50 μg/ml streptomycin sulfate. Murine L929 cells were maintained as suspension cultures as described previously (28). The A/WSN/33 (WSN) strain of influenza virus was grown in Madin-Darby bovine kidney cells as described previously (12). Vesicular stomatitis virus (VSV) strain Indiana was utilized for MEF infections as described previously (2). Reovirus strain Dearing, clone 8 (c8) and clone 87 (c87) are prototypic laboratory strains. Purified virions were prepared by CsCl density gradient centrifugation of extracts from cells infected with third-passage L929 cell lysate stocks (13). Intermediate subvirion particles were prepared by treating virions with chymotrypsin (42). Because some MEFs restrict reovirus uncoating (18), MEF infections were performed with intermediate subvirion particles.
Virus infections.
Near-confluent monolayers of cells were mock infected or infected with influenza virus diluted in infection medium (hgDMEM supplemented to contain 2% heat-inactivated calf serum, 2 mM l-glutamine, 50 units/ml penicillin G, 50 μg/ml streptomycin sulfate, and 50 mM HEPES) to the indicated multiplicity of infection (MOI). After 45 min of adsorption at 4°C, virus and medium were removed. Fresh infection medium was added to the cells, and infections were allowed to proceed at 37°C until the indicated time postinfection (p.i.). For VSV infections, P58IPK KO or WT MEFs were infected at the indicated MOI with virus diluted in serum-free DMEM. After 45 min of adsorption at 37°C, virus and medium were removed and cells were washed twice with phosphate-buffered saline (PBS). Fresh growth medium was then added to the cells, and infection proceeded at 37°C. At 24 h p.i., supernatants were collected and progeny virion production was assayed by standard plaque assay on baby hamster kidney (BHK) cells (2). P58IPK KO or WT MEFs were infected with the specified reovirus strain at an MOI of 2 PFU/cell, and adsorption was allowed to proceed for 1 h on ice at 4°C. After adsorption, cells were concentrated by low-speed centrifugation and resuspended in fresh medium. Virions and cells were then added to dram vials containing 1 ml of cold medium at cell densities to result in near-confluent monolayers. The remaining samples were incubated at 37°C until the desired time point was reached. Harvested samples were subjected to three cycles of freezing and thawing and titrated by plaque assay on L929 cells (59). Viral yields were calculated according to the following formula: log10 yieldt=x = log10 (PFU/ml)t=0, where t is time and x is the time p.i.
Analysis of protein synthesis.
At the indicated times p.i., mock- or influenza virus-infected cells were labeled with 30 μCi of EXPRESS35S protein labeling mix (Perkin-Elmer, Wellesley, MA) in methionine- and cysteine-free hgDMEM for 30 min at 37°C. Cells were then washed twice with ice-cold Hanks balanced salt solution and lysed in disruption buffer (0.5% Triton X-100, 50 mM KCl, 50 mM NaCl, 20 mM Tris-HCl [pH 7.5], 1 mM EDTA, 10% glycerol, 1× Complete protease inhibitor [Roche, Indianapolis, IN], 25 mM β-glycerophosphate, 1 mM Na3VO4). Levels of radioactivity for each sample were determined by trichloroacetic acid precipitation and scintillation counting. Lysates were boiled in an equal volume of 2× electrophoresis buffer (3.3% sodium dodecyl sulfate [SDS], 2.4 M β-mercaptoethanol, 16.7% glycerol, 13 mM Tris-HCl [pH 6.8], 8.3% water-saturated bromophenol blue) and separated by SDS-polyacrylamide gel electrophoresis (PAGE). As a loading control, identical counts per minute were loaded into each well. Following autoradiography, the amount of viral protein synthesis was determined by laser densitometry (Imagequant 5.1; Molecular Dynamics/GE Healthcare, Piscataway, NJ).
Analysis of eIF2α and PKR phosphorylation in influenza virus-infected cells.
Following influenza virus infection, cells were lysed at the indicated times p.i., as described above. Total protein content was determined for clarified cell lysates using the BCA protein assay kit (Pierce, Rockford, IL). Lysates were separated by SDS-PAGE, with the same amount of total protein being loaded into each lane, and then transferred to nitrocellulose paper. Immunoblots were blocked for 1 h in PBS containing 0.5% Tween 20 and 5% nonfat dry milk, washed in PBS containing 0.05% Tween 20, and incubated at 4°C overnight with rabbit anti-eIF2α[pS51] phospho-specific antibody (Biosource International, Camarillo, CA) or a mouse antibody recognizing full-length eIF2α (Santa Cruz Biotechnology, Santa Cruz, CA) in PBS containing 0.5% Tween 20 and 1% nonfat dry milk. Membranes were washed and incubated for 2 h with horseradish peroxidase-conjugated donkey anti-mouse or anti-rabbit immunoglobulin G (Jackson Immunoresearch, West Grove, PA), and bound antibodies were detected with the ECL Western blotting detection reagent (Amersham Biosciences/GE Healthcare). Membranes were subsequently reprobed using a mouse antiactin antibody (MP Biochemicals, Irvine, CA). The amounts of pS51 eIF2α, total eIF2α, and actin were quantitated by densitometry (Imagequant 5.1). To determine the relative amount of phosphorylated eIF2α, the eIF2α bands were normalized to their corresponding actin bands and the ratio between phosphorylated and total eIF2α was calculated. Western blotting for total PKR (Santa Cruz Biotechnology) and pT451 PKR (Biosource International) was performed as described by the product analysis sheets.
Quantitative RT-PCR.
At the indicated times p.i., cells were lysed in solution D (4 M guanidinium thiocyanate, 25 mM sodium citrate, 0.5% sarcosyl, 0.1 M β-mercaptoethanol), and total RNA was isolated using the RNeasy kit (QIAGEN, Valencia, CA). The quantity of total RNA was determined by spectrophotometry using the NanoDrop ND-1000 fluorospectrometer (Wilmington, DE). Contaminating DNA was removed by treating samples with RNase-free DNase and removal reagents (Ambion, Inc., Austin, TX). Reverse transcription was performed using TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA). Real-time PCR (RT-PCR) was performed on the ABI 7500 real-time PCR system, using TaqMan chemistry (Applied Biosystems). Each target was run in quadruplicate, with 20-μl reaction volumes of TaqMan 2× PCR Universal master mix (Applied Biosystems). Proprietary glyceraldehyde-3-phosphate dehydrogenase mRNA and 18S rRNA were chosen as endogenous controls to normalize quantification of the target. Quantification of each gene, relative to the calibrator, was calculated by the instrument, using the equation 2ΔCT(infected) − ΔCT(mock) within the Applied Biosystems Sequence Detection Software, version 1.3. minor groove binding (MGB) probes for the WSN M and NP genes were designed using the mRNA sequence for each with Primer Express 3.0 (Applied Biosystems) using the recommended parameters. The MGB probe and primer sets for each gene are as follows: WSN M1 (forward, TCGTCGCTTTAAATACGGTTTG; reverse, AGCATTCTGCTGTTCCTTTCG; probe, 6-carboxyfluorescein-TTCTACGGAAGGAGTGCCA); WSN NP (forward, TGGCACTCCAATTTGAATGATG; reverse, TCCATTCCTGTGCGAACAAG; probe, 6-carboxyfluorescein-AACTTACCAGAGGACAAGAG).
RESULTS
P58IPK promotes influenza virus protein synthesis.
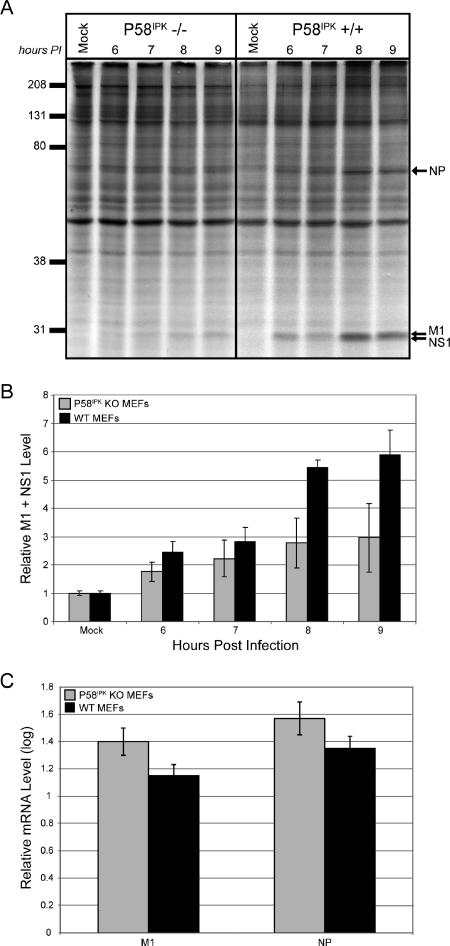
To directly test the hypothesis that P58IPK acts as a regulator of viral mRNA translation, we infected MEFs generated from P58IPK-null mice and their WT littermate controls with the mouse-adapted influenza virus strain WSN at an MOI of 2 PFU/cell. Although determining the levels of viral yield will elucidate the effects of P58IPK on viral infection and replication, we are seeking to test the effects of P58IPK on translational control during influenza virus infection, since it has been shown that P58IPK perturbs eIF2α phosphorylation in an in vitro yeast system (15, 55). Thus, we began by observing rates of viral protein synthesis, which were assessed at multiple times p.i. by labeling infected cells with [35S]methionine and analyzing total protein synthesis by SDS-PAGE. A representative experiment is shown in Fig. 1A, and the results from three independent experiments were averaged for quantitative representation in Fig. 1B. The synthesis of three viral proteins, nucleocapsid (NP), matrix (M1), and NS1, is indicated. This analysis revealed that viral protein synthesis was decreased approximately twofold in P58IPK KO MEFs compared to protein synthesis rates in the corresponding WT MEFs. In these experiments, we did not observe the shutoff of host-cell protein synthesis that is frequently observed in influenza virus-infected cells. This is because host-cell shutoff is dependent on MOI and is observed only at higher MOIs (16, 17). To determine if the decreased viral translation observed in the P58IPK KO MEFs was a consequence of a reduction in viral mRNA, we measured the amount of steady-state viral mRNA at a similar time p.i. At 8 h p.i., the levels of viral mRNA were similar between P58IPK KO and WT MEFs for two different viral transcripts, those of M1 and NP (Fig. 1C). Thus, we conclude that the presence of P58IPK results in increased viral mRNA translation.
FIG. 1.
The presence of P58IPK increases viral mRNA translation during influenza virus infection. P58IPK KO and WT MEFs were mock infected or infected with the WSN strain of influenza virus at an MOI of 2 PFU/cell. (A) Cells were labeled with [35S]methionine for 30 min at the indicated times p.i. Cells were lysed, and labeled proteins were analyzed by SDS-10% PAGE and autoradiography. The positions of the major viral proteins NP, M1, and NS1 are indicated. (B) Densitometry analysis of three independent experiments. The density of the M1-plus-NS1 band was normalized to the density of the entire lane, with each bar representing the mean ± standard deviation. (C) Total RNA was isolated from the cells at 8 h p.i. and reverse transcribed to generate cDNA. Quantitative RT-PCR was used to determine the amount of viral M1 and NP mRNA in each sample.
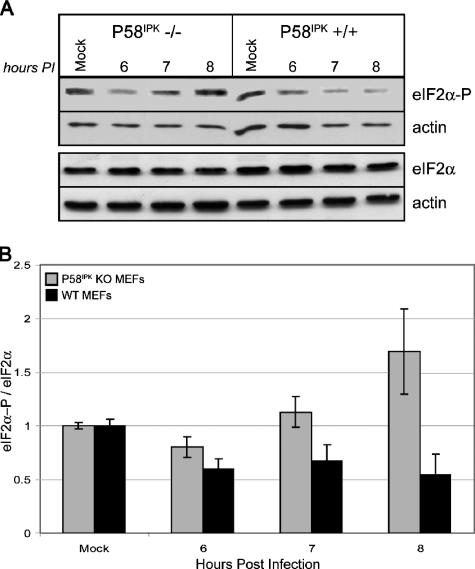
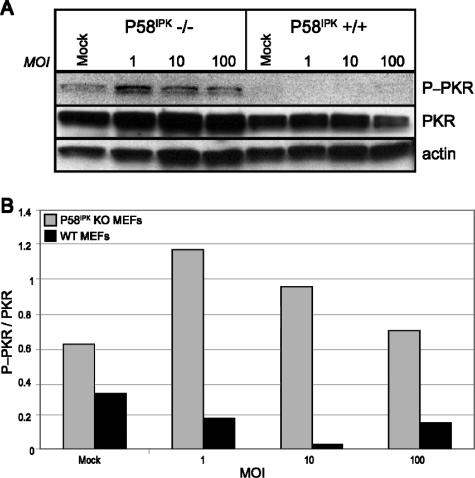
To determine if viral protein synthesis is inhibited in the P58IPK KO MEFs because of an increase in eIF2α phosphorylation, we examined the relative level of pS51 eIF2α by Western blot analysis (Fig. 2A). The results from two independent experiments are quantified in Fig. 2B. At 8 h p.i., we observed almost a fourfold increase in eIF2α phosphorylation in the P58IPK KO MEFs relative to the level in WT MEFs. Thus, the rate of viral mRNA translation is inversely correlated to levels of eIF2α phosphorylation. To determine if increased eIF2α phosphorylation in P58IPK KO MEFs is correlated to increased PKR activity, we examined the levels of pT451 PKR by Western blot analysis (Fig. 3). At 8 h p.i., there are drastically increased levels of phosphorylated PKR in P58IPK KO MEFs across multiple MOIs, indicating that the lack of P58IPK results in increased PKR activation. Using the same lysates, we also determined that PERK was phosphorylated in neither P58IPK KO nor WT MEFs during influenza virus infection (data not shown). These results suggest that influenza virus utilizes the cellular protein P58IPK to inhibit PKR, ensuring the efficient translation of viral mRNA.
FIG. 2.
The lack of P58IPK causes increased eIF2α phosphorylation during influenza virus infection. (A) P58IPK KO and WT MEFs were mock infected or infected with the WSN strain of influenza virus at an MOI of 2 PFU/cell. Cells were lysed at the indicated times p.i., and equivalent concentrations of protein were subjected to SDS-12.5% PAGE. The levels of phosphorylated eIF2α, total eIF2α, and actin were determined by immunoblot analysis. (B) Densitometry analysis of two independent experiments. The densities of the eIF2α or eIF2α-P bands were normalized to their corresponding actin bands. Total eIF2α phosphorylation was determined by dividing the normalized eIF2α-P value by the eIF2α value, with each bar representing the mean ± standard deviation.
FIG. 3.
The lack of P58IPK causes increased PKR phosphorylation during influenza virus infection. (A) P58IPK KO and WT MEFs were mock infected or infected with the WSN strain of influenza virus at a MOI of 1, 10, or 100 PFU/cell. Cells were lysed at 8 h p.i., and equivalent concentrations of protein were subjected to SDS-10% PAGE. The levels of phosphorylated PKR, total PKR, and actin were determined by immunoblot analysis. (B) Densitometry analysis. The density of the P-PKR band was normalized to the density of the PKR band, which is represented graphically.
VSV replication is decreased in the absence of P58IPK.
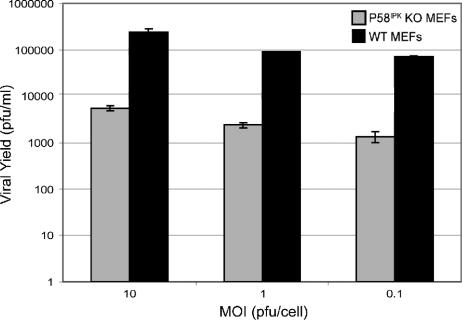
In all of our infections with influenza virus, we used the rates of viral protein synthesis as the readout for P58IPK's effect on replication. However, small changes in viral mRNA translation could have a large impact on viral replication. Since we previously showed that P58IPK enhances influenza virus mRNA translation, we next sought to determine how P58IPK affects viral replication using VSV. Like influenza virus, VSV is an enveloped virus with a negative-sense RNA genome. Regardless of the MOI used to initiate infection, VSV produced 100 times more progeny virions in the presence of P58IPK than in its absence (Fig. 4). This is in agreement with the findings of Balachandran et al. (2), in which VSV replicated to higher levels in PKR KO MEFs, the downstream, inhibitory target of P58IPK.
FIG. 4.
VSV replication is more efficient in the presence of P58IPK. P58IPK KO and WT MEFs were infected with VSV at an MOI of 10, 1, or 0.1 PFU/cell. At 24 h p.i., progeny virion production was determined by standard plaque assay on BHK-21 cells. Each result represents the mean activity for two independent experiments ± standard deviation.
Reovirus replication is enhanced in the absence of P58IPK.
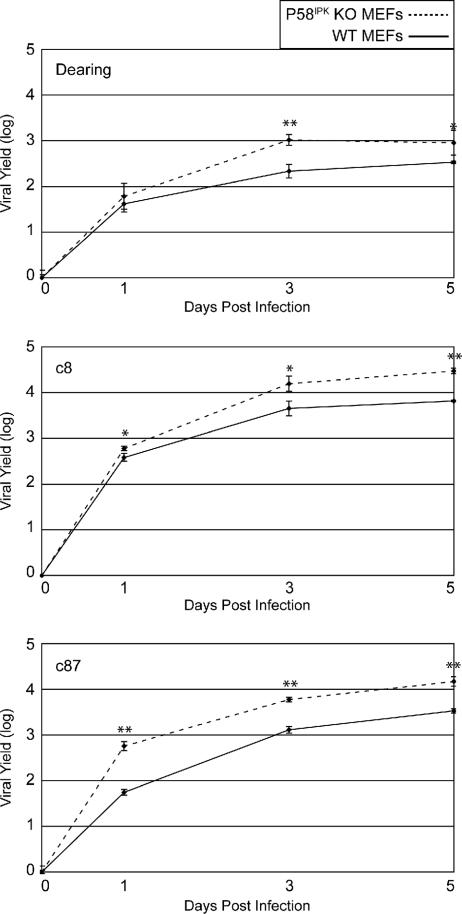
In contrast to most other viruses, including influenza virus and VSV, reovirus replicates more efficiently under conditions in which eIF2α is phosphorylated (51, 52). Furthermore, infection with some reovirus isolates resulted in the decrease of P58IPK expression (51). These results suggest that P58IPK might inhibit reovirus replication. To test this, we compared the single-cycle growth kinetics and final yields of three different reovirus isolates in the WT and P58IPK KO MEFs (Fig. 5). While the replication kinetics of all three strains of reovirus were similar with both types of MEFs, final yields were higher in the P58IPK KO MEFs. The increased yields in the absence of P58IPK were most apparent during c8 and c87 infections, isolates documented to decrease P58IPK expression and induce eIF2α phosphorylation (51). Since eIF2α phosphorylation is hypothesized to be beneficial to reovirus replication and reovirus yields were greater in the absence of P58IPK, these results support the idea that P58IPK functions to decrease eIF2α phosphorylation during viral infections.
FIG. 5.
The absence of P58IPK results in increased reovirus replication. P58IPK KO (dotted line) and WT (solid line) MEFs were infected with reovirus strain Dearing, c8, or c87 at a multiplicity of 2 PFU/cell. Infectious virus present at 0, 1, 3, and 5 days p.i. was measured by plaque assay on L929 cells. Each result represents the mean activity for three independent experiments ± standard deviation. P values from a two-tailed t test assuming nonequal variance are indicated (*, P ≤ 0.05; **, P ≤ 0.005).
Increased PKR-mediated eIF2α phosphorylation leads to decreased influenza virus mRNA translation.
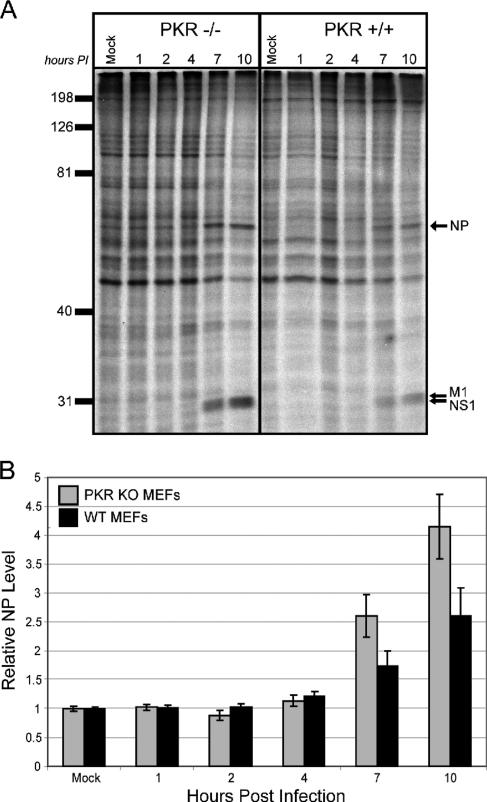
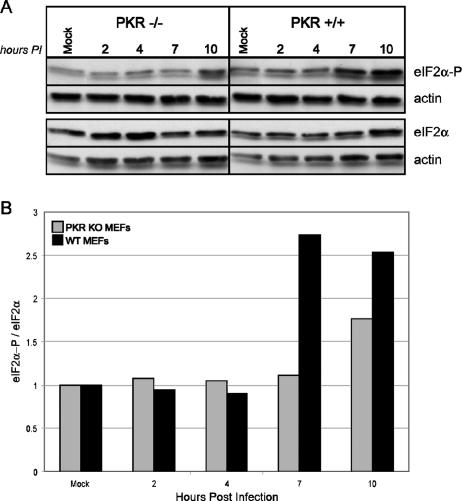
We have demonstrated that the absence of P58IPK leads to reduced influenza virus mRNA translation. Since P58IPK is an inhibitor of PKR, we expected that influenza virus protein synthesis would increase in the absence of this eIF2α kinase, similar to increased VSV replication in PKR KO MEFs (2). To test this, we infected MEFs derived from WT and PKR−/− mice (62) with the mouse-adapted strain of influenza virus, WSN, at an MOI of 1 PFU/cell. We assessed viral protein synthesis in PKR KO and WT MEFs at multiple times p.i. by metabolic labeling with [35S]methionine, followed by separation of proteins by SDS-PAGE. A representative experiment is shown in Fig. 6A and quantified in Fig. 6B. By 7 h p.i., we found that the rates of viral protein synthesis increase in the absence of the antiviral eIF2α kinase PKR. Since the steady-state levels of viral mRNA are similar in the WT and PKR KO MEFs (data not shown), we conclude that the increased quantities of viral proteins observed in the PKR KO MEFs are a consequence of increased rates of influenza virus translation. To determine whether the increased rate of viral protein synthesis in the PKR KO MEFs correlates with lower levels of influenza virus-induced eIF2α phosphorylation, we used the same lysates to examine the levels of total and phosphorylated eIF2α by immunoblot analysis. As a loading control, each blot was also probed against actin (Fig. 7A). The quantification of these results is represented in Fig. 7B. Since PKR is one of the major eIF2α kinases and is known to be activated as a consequence of influenza virus infection (27), we were not surprised to find decreased levels of phosphorylated eIF2α in influenza virus-infected PKR KO MEFs compared to those in WT MEFs. Differences in the extent of eIF2α phosphorylation were not apparent until 7 h p.i., most likely indicating the time needed for influenza virus infection to activate PKR, which then phosphorylates eIF2α. However, by 10 h p.i., levels of eIF2α phosphorylation rise in the PKR KO MEFs, indicating that another eIF2α kinase, such as GCN2, may be responsible for late eIF2α phosphorylation during influenza virus infection (4).
FIG. 6.
The lack of PKR increases viral mRNA translation during influenza virus infection. PKR KO and WT MEFs were mock infected or infected with the WSN strain of influenza virus at an MOI of 1 PFU/cell. (A) Cells were labeled with [35S]methionine for 30 min at the indicated times p.i. Cells were lysed, and labeled proteins were analyzed by SDS-10% PAGE and autoradiography. The positions of the major viral proteins NP, M1, and NS1 are indicated. (B) Densitometry analysis for three independent experiments. The density of the NP band was normalized to the density of the entire lane, with each bar representing the mean ± standard deviation.
FIG. 7.
The presence of PKR causes increased eIF2α phosphorylation during influenza virus infection. (A) PKR KO and WT MEFs were mock infected or infected with the WSN strain of influenza virus at an MOI of 1 PFU/cell. Cells were lysed at the indicated times p.i., and equivalent concentrations of protein were subjected to SDS-12.5% PAGE. The levels of phosphorylated eIF2α, total eIF2α, and actin were determined by immunoblot analysis. (B) Densitometry analysis. The densities of the eIF2α or eIF2α-P bands were normalized to their corresponding actin bands. Total eIF2α phosphorylation was determined by dividing the normalized eIF2α-P value by the eIF2α value, which is represented graphically.
PERK does not impact influenza virus protein synthesis.
In addition to PKR, there are at least three other cellular eIF2α kinases, PERK, GCN2, and HRI (11). Of these, the ER-stress-induced eIF2α kinase PERK has been implicated in the regulation of viral infections. PERK activation has been demonstrated during infection with a variety of enveloped viruses, including HCV, herpes simplex virus, bovine viral diarrhea virus, and Japanese encephalitis virus (8, 9, 21, 53), but there has been no evidence that influenza virus infection activates PERK. Since influenza virus is enveloped and the synthesis of its surface glycoproteins could increase ER lumenal content and activate the UPR, we hypothesized that PERK, through its ability to phosphorylate and inactivate eIF2α, might impact influenza virus protein synthesis. Utilizing MEFs derived from PERK−/− mice and their WT littermate controls (19), we observed no differences in levels of influenza virus protein synthesis or viral mRNA (data not shown) in the presence or absence of PERK. Furthermore, we observed an overall decrease in eIF2α phosphorylation in MEFs lacking PERK (data not shown). Together these data indicate that although PERK contributes to eIF2α phosphorylation in influenza virus-infected cells, it does not affect viral mRNA translation, implying that viral mRNA translation may be independent of PERK-mediated eIF2α phosphorylation.
Influenza virus protein synthesis and mRNA levels are decreased in cells that contain a constitutively active, nonphosphorylatable eIF2α.
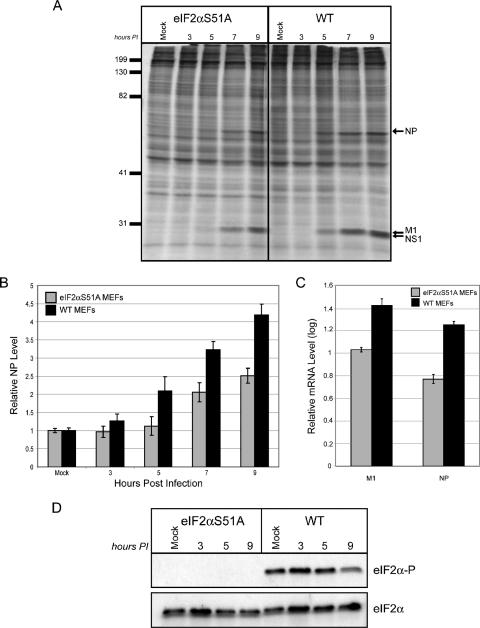
Using WT MEFs and cells devoid of either P58IPK or PKR, we have shown that influenza virus protein synthesis is inversely correlated to levels of phosphorylated eIF2α. However, this correlation was not observed in the presence or absence of PERK. To directly assess how eIF2α phosphorylation affects rates of viral protein synthesis, we infected WT MEFs or MEFs in which S51 of eIF2α had been mutated by homologous recombination to nonphosphorylatable A51 (eIF2αS51A MEFs) (49). This mutation results in constitutively active eIF2α because there is no phosphorylation of the α subunit at A51 (Fig. 8D). If eIF2α phosphorylation is inhibitory to influenza virus protein synthesis, we predicted that viral protein synthesis would increase in the mutant eIF2αS51A MEFs. However, when viral protein synthesis was assessed at various times p.i., we found that the synthesis of viral proteins was decreased in the MEFs containing constitutively active eIF2α (Fig. 8A). Quantification of the data revealed that there is an approximate twofold decrease in viral protein synthesis in the eIF2αS51A mutant MEFs relative to translation rates in the WT MEFs (Fig. 8B). To determine whether mRNA translation per se was decreased in the presence of constitutively active eIF2α, we assessed the level of steady-state viral mRNA at a similar time postinfection. We found that at 9 h p.i, the levels of viral mRNA are lower in the eIF2αS51A MEFs than in the WT MEFs. The difference in viral transcript levels between the two cell types is approximately 0.4 logs, corresponding to an n-fold change of 2.5 (Fig. 8C). Thus, the difference in viral transcript levels is almost identical to that observed for rates of viral protein synthesis between eIF2αS51A and WT MEFs. Furthermore, when levels of NP protein synthesis at 9 h p.i. are normalized to mRNA levels at the same time p.i., the values are the same for both eIF2αS51A and WT MEFs, meaning that translational efficiencies are similar for the two cell types. Collectively, these data imply that eIF2α phosphorylation may not affect levels of influenza virus mRNA translational efficiency when there are drastic differences in basal levels of eIF2α phosphorylation between two different cell types.
FIG. 8.
The lack of phosphorylatable eIF2α causes decreased levels of influenza virus protein synthesis and viral mRNA. eIF2αS51A and WT MEFs were mock infected or infected with the WSN strain of influenza virus at an MOI of 1 PFU/cell. (A) Cells were labeled with [35S]methionine for 30 min at the indicated times p.i. Cells were lysed and analyzed by SDS-10% PAGE and autoradiography. The positions of major viral proteins NP, M1, and NS1 are indicated. (B) Densitometry analysis for three independent experiments. The density of the NP band was normalized to the density of the entire lane, with each bar representing the mean ± standard deviation. (C) Total RNA was isolated from the cells at 9 h p.i. and reverse transcribed to generate cDNA. Quantitative RT-PCR was used to determine the amount of viral M1 and NP mRNA in each sample. (D) Cell lysates from the experiment in panel A were analyzed by SDS-12.5% PAGE. The levels of phosphorylated eIF2α and total eIF2α were determined by immunoblot analysis.
DISCUSSION
In this study, we show that the cellular protein P58IPK plays a novel role in the antiviral response to certain RNA viruses, with an emphasis on influenza virus. First, it has been shown that P58IPK is activated upon infection with influenza virus (34). Second, P58IPK binds to and inhibits PKR (15, 33, 35). Third, we now show that cells devoid of P58IPK exhibit lower levels of viral mRNA translation. Finally, P58IPK promotes viral mRNA translation through PKR inhibition, correlated to decreased levels of eIF2α phosphorylation. This is the first direct evidence of the phenotype of P58IPK KO MEFs during viral infection, suggesting that a role of P58IPK during viral infection is to promote virulence.
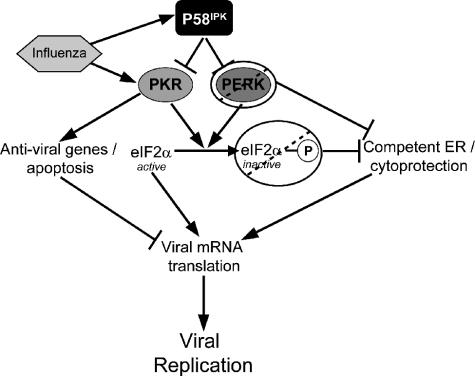
Based on the data from viral infections with a number of different KO cell lines, we present an increasingly complex model of translational control during influenza virus infection (Fig. 9). During influenza virus infection, P58IPK and PKR are activated (40). Activated PKR both phosphorylates eIF2α and can activate a number of antiviral genes, such as IRF3 and NF-κB (54, 60), as well as apoptosis (1, 56). Together, these effects cause a decrease in viral mRNA translation and replication; but in the absence of PKR, there is increased viral mRNA translation and replication and decreased eIF2α phosphorylation. Further, the converse is true in the absence of P58IPK. That is, when P58IPK is absent, PKR inhibition is lessened during viral infection, resulting in decreased viral mRNA translation and replication and increased eIF2α phosphorylation. Though P58IPK is an inhibitor of PERK, the absence of PERK did not have an effect on viral mRNA translation even though there were decreased levels of eIF2α phosphorylation in the absence of PERK. Furthermore, when a mutation was introduced to render eIF2α nonphosphorylatable, we observed decreased levels of viral protein synthesis and decreased viral mRNA levels.
FIG. 9.
Model of the role of P58IPK during influenza virus infection. In the presence of influenza virus infection, PKR and P58IPK are activated. In the presence of P58IPK, PKR inhibition is increased, resulting in decreased levels of eIF2α phosphorylation, antiviral genes, and apoptosis, resulting in increased influenza virus mRNA translation. When PERK is absent or eIF2α cannot be phosphorylated, ER homeostasis is compromised, resulting in poor viral mRNA translation in relation to decreased eIF2α phosphorylation levels. During the viral life cycle, changes in viral mRNA translation will amplify changes in viral replication. See the text for further details.
With regard to the inverse correlation between eIF2α phosphorylation and viral mRNA translation, the results which we observed in the PERK KO and eIF2αS51A MEFs are inconsistent with the results in the P58IPK KO and PKR KO MEFs; that is, we observed an inverse relationship between viral mRNA translation and eIF2α phosphorylation in the P58IPK KO and PKR KO MEFs but not in the PERK KO and eIF2αS51A MEFs. A possible explanation for this discrepancy is that in the complete absence of eIF2α phosphorylation or PERK, the cells may lack a competent ER to deal with the increased stresses of additional viral protein synthesis. As described by Lu et al. (38), basal levels of eIF2α phosphorylation contribute to cytoprotection, which preconditions the cells to deal with additional stresses, such as viral infection. Basal eIF2α phosphorylation is vital to normal cell physiology because it adapts the cell for ER stress via the coordination of an integrated stress response. Furthermore, mutations in PERK and eIF2α impair cell survival during additional stresses (5). EIF2α phosphorylation is also needed to activate NF-κB during ER stress (10), which may also be vital in eliciting other antiviral responses. It has been shown that a number of different viral infections cause ER stress, such as VSV, herpes simplex virus, reovirus, and HCV (3, 9, 51, 57). Further, it has been proposed that the influenza hemagglutinin glycoprotein may cause an overload of the ER when malfolded (7, 29), possibly inducing the UPR. Although mutant cells lacking PERK or phosphorylatable eIF2α have lower levels of eIF2B sequestration, a condition that would normally promote increased mRNA translation (48), in these mutant cells, this is offset by the lack of cytoprotection, resulting in the lack of efficient mRNA translation. Furthermore, since the ER stress response is negatively perturbed in these mutant cells (20, 49), any translational enhancement due to decreased eIF2α phosphorylation may be neutralized due to the lack of ER homeostasis. However, in cells containing PKR or lacking P58IPK, there is increased eIF2α phosphorylation, leading to increased eIF2B sequestration, and there is sufficient cytoprotection and ER homeostasis; thus, viral mRNA translation can be correlated to eIF2α phosphorylation levels. In order to test this hypothesis, a more careful dissection of cytoprotection during virus infection, which is beyond the scope of the present study, will need to be performed. We should also indicate that a reason that we observed different kinetics of eIF2α phosphorylation among the different mutant MEFs may be that each set of MEFs comes from a different parental mouse strain. Furthermore, while P58IPK MEFs are primary, PERK MEFs are transformed with simian virus 40 T antigen, and PKR and eIF2α MEFs are immortalized from continual passage.
Although the model presented above is with regard to influenza virus infection, the model still holds true for VSV infection. Balachandran et al. (2) showed that VSV replicates to higher levels in the absence of PKR, and we now show that it replicates to higher levels in the presence of P58IPK, analogous to the results with influenza virus mRNA translation in the absence of PKR or presence of P58IPK. With respect to reovirus, it has recently been shown that reovirus infection induces and benefits from ER stress, characterized by increased levels of eIF2α phosphorylation (51, 52). Further, it has been shown that during infection with host shutoff-inducing strains of reovirus, the σ3 protein binds dsRNA, limiting PKR activation in areas where viral proteins are synthesized (50). Since P58IPK is an inhibitor of eIF2α kinases, it follows that reovirus replicates to higher levels in the absence of P58IPK, as we have shown in this study.
Overall, our results complement those which were observed for virally infected plants lacking P58IPK. That is, there were increased levels of eIF2α phosphorylation associated with decreased viral replication in plants lacking P58IPK. Furthermore, the expression of nonphosphorylatable eIF2α blocked cell death in these plants. In contrast to the case with animal cells, cell death in plants is not an innate immune response to viral infection, implying that P58IPK is required to limit cell death and promote viral replication during infection (6). Therefore, we show that not only does P58IPK aid in the progression of viral infection in plants, but also in a mammalian system. We have recently begun to perform influenza virus infections with P58IPK−/− mice, and we have preliminary results showing that in the absence of P58IPK, there is an increased antiviral response at times early in infection, which may result in decreased viral replication. Furthermore, there was increased mortality in P58IPK−/− mice, analogous to the studies with plants lacking P58IPK (A. G. Goodman and M. G. Katze, unpublished data). Further work with influenza-infected P58IPK−/− mice and the cell lines described in this paper using high-throughput functional genomics will lead to a more definitive mechanism by which P58IPK functions as a virulence factor in a mammalian system.
Acknowledgments
We thank Heather Harding and David Ron for PERK KO and WT MEFs, Donalyn Scheuner and Randy Kaufman for eIF2αS51A and WT MEFs, and Robert Silverman and Bryan Williams for PKR KO and WT MEFs.
This work was supported by NIH grants T32CA09229 (A.G.), R01AI022646 (A.G., O.P., S.P., M.T., M.J.K., and M.G.K.), T32HL07741 (J.S.), R01AI045990 (L.S.), and 5R01CA086431-08 (S.B. and G.B).
Footnotes
Published ahead of print on 13 December 2006.
REFERENCES
- 1.Balachandran, S., C. N. Kim, W.-C. Yeh, T. W. Mak, and G. N. Barber. 1998. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J. 17:6888-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. [DOI] [PubMed] [Google Scholar]
- 3.Baltzis, D., L. K. Qu, S. Papadopoulou, J. D. Blais, J. C. Bell, N. Sonenberg, and A. E. Koromilas. 2004. Resistance to vesicular stomatitis virus infection requires a functional cross talk between the eukaryotic translation initiation factor 2α kinases PERK and PKR. J. Virol. 78:12747-12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlanga, J. J., I. Ventoso, H. P. Harding, J. Deng, D. Ron, N. Sonenberg, L. Carrasco, and C. de Haro. 2006. Antiviral effect of the mammalian translation initiation factor 2alpha kinase GCN2 against RNA viruses. EMBO J. 25:1730-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bi, M., C. Naczki, M. Koritzinsky, D. Fels, J. Blias, N. Hu, H. P. Harding, I. Novoa, M. Varia, J. Raleigh, D. Scheuner, R. J. Kaufman, J. Bell, D. Ron, B. G. Wouters, and C. Koumenis. 2006. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 24:3470-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilgin, D. D., Y. Liu, M. Schiff, and S. P. Dinesh-Kumar. 2003. P58IPK, a plant ortholog of double-stranded RNA-dependent protein kinase PKR inhibitor, functions in viral pathogenesis. Dev. Cell 4:651-661. [DOI] [PubMed] [Google Scholar]
- 7.Braakman, I., H. Hoover-Litty, K. R. Wagner, and A. Helenius. 1991. Folding of influenza hemagglutinin in the endoplasmic reticulum. J. Cell Biol. 114:401-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, S. W., and P. A. Egan. 2005. Hepatitis C virus envelope proteins regulate CHOP via induction of the unfolded protein response. FASEB J. 19:1510-1512. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, G., Z. Feng, and B. He. 2005. Herpes simplex virus 1 infection activates the endoplasmic reticulum resident kinase PERK and mediates eIF-2α dephosphorylation by the γ134.5 Protein. J. Virol. 79:1379-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng, J., P. D. Lu, Y. Zhang, D. Scheuner, R. J. Kaufman, N. Sonenberg, H. P. Harding, and D. Ron. 2004. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol. Cell. Biol. 24:10161-10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dever, T. E. 2002. Gene-specific regulation by general translation factors. Cell 108:545-556. [DOI] [PubMed] [Google Scholar]
- 12.Etkind, P. R., and R. M. Krug. 1975. Purification of influenza viral complementary RNA: its genetic content and activity in wheat germ cell-free extracts. J. Virol. 16:1464-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furlong, D. B., N. L. Nibert, and B. N. Fields. 1988. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral proteins. J. Virol. 62:246-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gale, M., Jr., M. J. Korth, N. M. Tang, S.-L. Tan, D. A. Hopkins, T. E. Dever, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1997. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology 230:217-227. [DOI] [PubMed] [Google Scholar]
- 15.Gale, M., Jr., S.-L. Tan, M. Wambach, and M. G. Katze. 1996. Interaction of the interferon-induced PKR protein kinase with inhibitory proteins P58IPK and vaccinia virus K3L is mediated by unique domains: implications for kinase regulation. Mol. Cell. Biol. 16:4172-4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garfinkel, M. S., and M. G. Katze. 1993. Translational control by influenza virus: selective translation is mediated by sequences within the viral mRNA 5′-untranslated region. J. Biol. Chem. 268:22223-22226. [PubMed] [Google Scholar]
- 17.Garfinkel, M. S., and M. G. Katze. 1992. Translational control by influenza virus: selective and cap-dependent translation of viral mRNAs in infected cells. J. Biol. Chem. 267:9383-9390. [PubMed] [Google Scholar]
- 18.Golden, J. W., J. Linke, S. Schmechel, K. Thoemke, and L. A. Schiff. 2002. Addition of exogenous protease facilitates reovirus infection in many restrictive cells. J. Virol. 76:7430-7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harding, H. P., H. Zeng, Y. Zhang, R. Jungries, P. Chung, H. Plesken, D. D. Sabatini, and D. Ron. 2001. Diabetes mellitus and exocrine pancreatic dysfunction in Perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7:1153-1163. [DOI] [PubMed] [Google Scholar]
- 20.Harding, H. P., Y. Zhang, A. Bertolotti, H. Zeng, and D. Ron. 2000. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5:897-904. [DOI] [PubMed] [Google Scholar]
- 21.Jordan, R., L. Wang, T. M. Graczyk, T. M. Block, and P. R. Romano. 2002. Replication of a cytopathic strain of bovine viral diarrhea virus activates PERK and induces endoplasmic reticulum stress-mediated apoptosis of MDBK cells. J. Virol. 76:9588-9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kash, J. C., A. G. Goodman, M. J. Korth, and M. G. Katze. 2006. Hijacking of the host-cell response and translational control during influenza virus infection. Virus Res. 119:111-120. [DOI] [PubMed] [Google Scholar]
- 23.Kash, J. C., T. M. Tumpey, S. C. Proll, V. Carter, O. Perwitasari, M. J. Thomas, C. F. Basler, P. Palese, J. K. Taubenberger, A. Garcia-Sastre, D. E. Swayne, and M. G. Katze. 2006. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature 443:578-581.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katze, M. G., D. DeCorato, B. Safer, J. Galabru, and A. G. Hovanessian. 1987. Adenovirus VAI RNA complexes with the 68,000 Mr protein kinase to regulate its autophosphorylation and activity. EMBO J. 6:689-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katze, M. G., B. M. Detjen, B. Safer, and R. M. Krug. 1986. Translational control by influenza virus: suppression of the kinase that phosphorylates the alpha subunit of initiation factor eIF-2 and selective translation of influenza viral mRNAs. Mol. Cell. Biol. 6:1741-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katze, M. G., and R. M. Krug. 1990. Translational control in influenza virus-infected cells. Enzyme 44:265-277. [DOI] [PubMed] [Google Scholar]
- 27.Katze, M. G., J. Tomita, T. Black, R. M. Krug, B. Safer, and A. G. Hovanessian. 1988. Influenza virus regulates protein synthesis during infection by repressing the autophosphorylation and activity of the cellular 68,000-Mr protein kinase. J. Virol. 62:3710-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kedl, R., S. Schmechel, and L. Schiff. 1995. Comparative sequence analysis of the reovirus S4 genes from 13 serotype 1 and serotype 3 field isolates. The J. Virol. 69:552-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozutsumi, Y., M. Segal, K. Normington, M. J. Gething, and J. Sambrook. 1988. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332:462-464. [DOI] [PubMed] [Google Scholar]
- 30.Ladiges, W., J. Morton, C. Blakely, and M. Gale. 2000. Tissue specific expression of PKR protein kinase in aging B6D2F1 mice. Mech. Ageing Dev. 114:123-132. [DOI] [PubMed] [Google Scholar]
- 31.Ladiges, W. C., S. E. Knoblaugh, J. F. Morton, M. J. Korth, B. L. Sopher, C. R. Baskin, A. MacAuley, A. G. Goodman, R. C. LeBoeuf, and M. G. Katze. 2005. Pancreatic β-cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum molecular chaperone gene P58IPK. Diabetes 54:1074-1081. [DOI] [PubMed] [Google Scholar]
- 32.Lamb, R. A., and R. M. Krug. 1996. Orthomyxoviridae: the viruses and their replication, p. 1353-1395. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven, Philadelphia, PA.
- 33.Lee, T. G., N. Tang, S. Thompson, J. Miller, and M. G. Katze. 1994. The 58,000-dalton cellular inhibitor of the interferon-induced double-stranded RNA-activated protein kinase (PKR) is a member of the tetratricopeptide repeat family of proteins. Mol. Cell. Biol. 14:2331-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee, T. G., J. Tomita, A. G. Hovanessian, and M. G. Katze. 1990. Purification and partial characterization of a cellular inhibitor of the interferon-induced protein kinase of Mr 68,000 from influenza virus-infected cells. Proc. Natl. Acad. Sci. USA 87:6208-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, T. G., J. Tomita, A. G. Hovanessian, and M. G. Katze. 1992. Characterization and regulation of the 58,000-dalton cellular inhibitor of the interferon-induced, dsRNA-activated protein kinase. J. Biol. Chem. 267:14238-14243. [PubMed] [Google Scholar]
- 36.Li, K. S., Y. Guan, J. Wang, G. J. D. Smith, K. M. Xu, L. Duan, A. P. Rahardjo, P. Puthavathana, C. Buranathai, T. D. Nguyen, A. T. S. Estoepangestie, A. Chaisingh, P. Auewarakul, H. T. Long, N. T. H. Hanh, R. J. Webby, L. L. M. Poon, H. Chen, K. F. Shortridge, K. Y. Yuen, R. G. Webster, and J. S. M. Peiris. 2004. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 430:209-213. [DOI] [PubMed] [Google Scholar]
- 37.LLoyd, R. M., and A. J. Shatkin. 1992. Translational stimulation by reovirus polypeptide σ3: substitution for VAI RNA and inhibition of phosphorylation of the α subunit of eukaryotic initiation factor 2. J. Virol. 66:6878-6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu, P. D., C. Jousse, S. J. Marciniak, Y. Zhang, I. Novoa, D. Scheuner, R. J. Kaufman, D. Ron, and H. P. Harding. 2004. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 23:169-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu, Y., M. Wambach, M. G. Katze, and R. M. Krug. 1995. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the eIF-2 translation initiation factor. Virology 214:222-228. [DOI] [PubMed] [Google Scholar]
- 40.Melville, M. W., S.-L. Tan, M. Wambach, J. Song, R. I. Morimoto, and M. G. Katze. 1999. The cellular inhibitor of the PKR protein kinase, P58IPK, is an influenza virus-activated co-chaperone that modulates heat shock protein 70 activity. J. Biol. Chem. 274:3797-3803. [DOI] [PubMed] [Google Scholar]
- 41.Meurs, E., K. L. Chong, J. Galabru, N. Thomas, I. Kerr, B. R. G. Williams, and A. G. Hovanessian. 1990. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell 62:379-390. [DOI] [PubMed] [Google Scholar]
- 42.Nibert, M. L., and B. N. Fields. 1992. A carboxy-terminal fragment of protein mu 1/mu 1C is present in infectious subvirion particles of mammalian reoviruses and is proposed to have a role in penetration. J. Virol. 66:6408-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oyadomari, S., C. Yun, E. A. Fisher, N. Krelinger, G. Kreibich, M. Oyadomari, H. P. Harding, A. G. Goodman, H. Harant, J. L. Garrison, J. Taunton, M. G. Katze, and D. Ron. 2006. Co-translocational degradation protects the stressed endoplasmic reticulum from misfolded client protein overload. Cell 126:727-739. [DOI] [PubMed] [Google Scholar]
- 44.Palese, P. 2004. Influenza: old and new threats. Nat. Med. 10:S82-S87. [DOI] [PubMed] [Google Scholar]
- 45.Park, Y.-W., J. Wilusz, and M. G. Katze. 1999. Regulation of eukaryotic protein synthesis: selective influenza viral mRNA translation is mediated by the cellular RNA-binding protein GRSF-1. Proc. Natl. Acad. Sci. USA 96:6694-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plotch, S. J., M. Bouloy, and R. M. Krug. 1979. Transfer of 5′-terminal cap of globin mRNA to influenza viral complementary RNA during transcription in vitro. Proc. Natl. Acad. Sci. USA 76:1618-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plotch, S. J., M. Bouloy, I. Ulmanen, and R. M. Krug. 1981. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell 23:847-858. [DOI] [PubMed] [Google Scholar]
- 48.Proud, C. G. 2005. eIF2 and the control of cell physiology. Semin. Cell Dev. Biol. 16:3-12. [DOI] [PubMed] [Google Scholar]
- 49.Scheuner, D., B. Song, E. McEwen, C. Liu, R. Laybutt, P. Gillespie, T. Saunders, S. Bonner-Weir, and R. J. Kaufman. 2001. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7:1165-1176. [DOI] [PubMed] [Google Scholar]
- 50.Schmechel, S., M. Chute, P. Skinner, R. Anderson, and L. Schiff. 1997. Preferential translation of reovirus mRNA by a σ3-dependent mechanism. Virology 232:62-73. [DOI] [PubMed] [Google Scholar]
- 51.Smith, J. A., S. C. Schmechel, A. Raghavan, M. Abelson, C. Reilly, M. G. Katze, R. J. Kaufman, P. R. Bohjanen, and L. A. Schiff. 2006. Reovirus induces benefits from a cellular integrated stress response. J. Virol. 80:2019-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith, J. A., S. C. Schmechel, B. R. G. Williams, R. H. Silverman, and L. A. Schiff. 2005. Involvement of the interferon-regulated antiviral proteins PKR and RNase L in reovirus-induced shutoff of cellular translation. J. Virol. 79:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su, H. L., C. L. Liao, and Y. L. Lin. 2002. Japanese encephalitis virus infection initiates endoplasmic reticulum stress and an unfolded protein response. J. Virol. 76:4162-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, and A. García-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 74:7989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan, S.-L., M. J. Gale, Jr., and M. G. Katze. 1998. Double-stranded RNA-independent dimerization of interferon-induced protein kinase PKR and inhibition of dimerization by the cellular P58IPK inhibitor. Mol. Cell. Biol. 18:2431-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang, N. M., M. J. Korth, M. Gale, Jr., M. Wambach, S. D. Der, S. K. Bandyopadhyay, B. R. G. Williams, and M. G. Katze. 1999. Inhibition of double-stranded RNA- and tumor necrosis factor alpha-mediated apoptosis by tetratricopeptide repeat protein and cochaperone, P58IPK. Mol. Cell. Biol. 19:4757-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tardif, K. D., G. Waris, and A. Siddiqui. 2005. Hepatitis C virus, ER stress, and oxidative stress. Trends Microbiol. 13:159-163. [DOI] [PubMed] [Google Scholar]
- 58.Van Huizen, R., J. L. Martindale, M. Gorospe, and N. J. Holbrook. 2003. P58IPK, a novel endoplasmic reticulum stress-inducible protein and potential negative regulator of eIF2α signaling. J. Biol. Chem. 278:15558-15564. [DOI] [PubMed] [Google Scholar]
- 59.Virgin, H. W. T., R. Bassel-Duby, B. N. Fields, and K. L. Tyler. 1988. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J. Virol. 62:4594-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, X., M. Li, H. Zheng, T. Muster, P. Palese, A. A. Beg, and A. Garcia-Sastre. 2000. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 74:11566-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan, W., C. L. Frank, M. J. Korth, B. L. Sopher, I. Novoa, D. Ron, and M. G. Katze. 2002. Control of PERK eIF2α kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc. Natl. Acad. Sci. USA 99:15920-15925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang, Y.-L., L. F. L. Reis, J. Pavlovic, A. Aguzzi, R. Schäfer, A. Kumar, B. R. G. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14:6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]