Abstract
Human immunodeficiency virus type 1 (HIV-1) transmission by the parenteral route is similar to mucosal transmission in the predominance of virus using the CCR5 coreceptor (R5 virus), but it is unclear whether blood dendritic cells (DCs), monocytes, or T cells are the cells initially infected. We used ex vivo HIV-1 infection of sorted blood mononuclear cells to model the in vivo infection of blood leukocytes. Using quantitative real-time PCR to detect full-length HIV-1 DNA, both sorted CD11c+ myeloid and CD11c− plasmacytoid DCs were more frequently infected than other blood mononuclear cells, including CD16+ or CD14+ monocytes or resting CD4+ T cells. There was a strong correlation between CCR5 coreceptor use and preferential DC infection across a range of HIV-1 isolates. After infection of unsorted blood mononuclear cells, HIV-1 was initially detected in the CD11c+ DCs and later in other leukocytes, including clustering DCs and activated T cells. DC infection with R5 virus was productive, as shown by efficient transmission to CD4+ T cells in coculture. Blood DCs infected with HIV-1 in vitro and cultured alone expressed only low levels of multiply spliced HIV-1 RNA unless cocultured with CD4+ T cells. Early selective infection of immature blood DCs by R5 virus and upregulation of viral expression during DC-T-cell interaction and transmission provide a potential pathway for R5 selection following parenteral transmission.
Dendritic cells (DCs) and Langerhans cells (LCs) are specialized cells in mucosa and skin that are among the first leukocytes to encounter antigens and migrate to the lymph nodes to elicit T-cell responses. Human immunodeficiency virus type 1 (HIV-1) is able to use these migratory cells to rapidly gain access to activated T cells in draining lymph nodes to establish infection. Following delivery of simian immunodeficiency virus (SIV) to the vagina, infection of LCs occurs in the stratified squamous epithelium of the vagina and exocervix (28, 66), and using a human ex vivo skin model, there is preferential carriage of HIV-1 using the CCR5 coreceptor (R5 virus) by LCs in the epidermis (32-34, 56). Although selective entry of R5 HIV-1 into LCs occurs in squamous epithelium, there are few CD4+ T cells in the epidermis compared to lamina propria and submucosa. In T-cell-rich sites, early and direct virus replication in CD4+ T cells, as suggested by infection and depletion in the gastrointestinal tract after SIV (41, 44) and HIV infection (3, 14, 36), may bypass DC involvement. Using in situ hybridization to detect infection, activated and resting CD4+ T cells rather than DCs are the primary cells infected after SIV infection via the rectal route and only later was infection seen in DCs or macrophages (75). A similar pattern was demonstrated following SIV infection of tonsillar tissue in macaques (67). Preferential passage of R5 HIV-1 across the columnar epithelium selects for R5 virus (47), and preferential infection of CD4+ T cells may also be facilitated by greater expression of CCR5 on mucosal CD4+ T cells compared to blood T cells (27). Although it is clear that both LCs and CD4+ T cells may provide cellular mechanisms for HIV selection across mucosa, the mechanism for selective transmission of R5 virus after parenteral exposure is not known.
We initially proposed a “virus carriage” model for cultured blood DCs (6) and DCs migrating from skin (53) that allowed for efficient carriage and transfer from DCs to T cells with minimal productive infection in DCs. In this model, CD4+ T cells are the primary site of virus production, and DCs are passive carriers of infectious virions, rather than sources of replicating HIV-1. Subsequent identification of DC-SIGN (19), a C-type lectin receptor (CLR) highly expressed on monocyte-derived DCs (MDDCs), allowed characterization of this pathway of virus carriage and transfer from uninfected MDDCs to CD4+ T cells. Although “transinfection” by a CLR-mediated path (38) allows for DC-mediated HIV-1 entry into CD4+ T cells in the absence of DC infection, transinfection does not explain early selective transmission of R5 virus (72), since DC-SIGN binds all HIV-1 and SIV isolates (52) and all viral phenotypes, including both X4 and R5.
Selective transmission of HIV-1 is a feature of virus transmission whatever the route of transmission (11, 59). This suggests a common cellular or molecular pathway that is independent of the route of infection. Recent studies of infection of blood DCs examined the role of DC infection and entry of HIV-1 into CD4+ T cells. In these studies, DCs were cultured with cytokines, including interleukin 3 and granulocyte-macrophage colony-stimulating factor or alpha interferon, before infection, and both X4 and R5 HIV-1 productively infected plasmacytoid DCs (pDCs) and myeloid DCs (mDCs) (42, 63) were transferred to CD4+ T cells (13). DC survival is improved by the addition of cytokines (30, 31, 35), but culture is also associated with maturational changes in the DCs (10, 46). Alteration in chemokine receptor expression occurs in both LCs (74) and DCs (39) during culture; therefore, cultured DCs may not accurately represent the nonactivated cells found in tissue or the DCs migrating through lymphatics to draining lymph nodes during homeostasis (21).
Parenteral transmission is associated with exposure of blood leukocytes to HIV-1 of different tropism and would be expected to result in infection and transmission of both R5 and X4 virus. Parenteral transmission, like mucosal transmission, selects for R5 virus (11). To determine the contribution of DC infection to this viral selection during parenteral infection, we compared HIV-1 entry of different HIV-1 isolates into subpopulations of blood leukocytes isolated directly without culture. We hypothesized that circulating blood DCs may select for transmission of R5 virus and are permissive to infection by R5 but not X4 virus. We found that uncultured DCs were more permissive for productive infection with R5 HIV-1 than CD4+ T cells or monocytes and that most infected DCs can transfer R5 HIV-1 to CD4+ T cells consistent with a predominant if not exclusive infectious pathway.
MATERIALS AND METHODS
Preparation and sorting of blood leukocyte subpopulations.
Peripheral blood mononuclear cells (PBMCs) were isolated over Ficoll Hypaque gradients (Pharmacia, Uppsala, Sweden) from fresh buffy coats (Melbourne Red Cross Blood Transfusion Service). Lymphocytes and monocytes were separated by elutriation. PBMCs (5 × 108 to 12 × 108 cells) were loaded into a standard chamber in a J6M centrifuge (Beckman, Fremont, CA), and elutriation was performed at 2,200 rpm at 4°C. The CD14+ and CD16+ monocyte/DC populations were sorted from directly labeled monocyte fractions and the CD4+ T cells from the pooled lymphocyte fractions labeled with nonsaturating concentrations of antibodies to CD4 and HLA-DR (Becton Dickinson, Palo Alto, CA). Cells in the monocyte elutriation fractions were depleted with antibodies to lineage markers CD3 (OKT3), CD14 (3C10), CD16 (3G8), and CD19 (FMC63). The cells were washed in fluorescence-activated cell sorting wash (phosphate-buffered saline, 1 mM EDTA, 1% normal human serum) and incubated with magnetic activated cell sorting (MACS) goat anti-mouse immunoglobulin G beads (Miltenyi Biotec, Bergisch Gladbach, Germany) at 4°C before depletion using MACS columns and magnets. The negative fraction was labeled with HLA-DR (BDIS, Palo Alto, CA) and sorted for HLA-DR+ lineage-negative (lin−) cells by flow cytometry (FCM) (FACS II [Becton Dickinson] or MoFlo CLS [Cytomation, Fort Collins, CO]). Subpopulations of pDCs and mDCs were sorted using antibodies to CD11c (BDIS) or CD1b/c (clone B-B5; Biosource International, Camarillo, CA), a marker for myeloid DC and LC precursors (29). For cells sorted after infection, PBMCs were harvested, and aliquots were stained with directly conjugated HLA-DR and CD4 or CD14 and CD16 (BDIS), fixed either in 80% methanol or 1% formaldehyde (Ultrapure; Polyscience, Warrington, PA) for 30 min on ice before sorting by FCM. Lineage+ MACS-selected (MACS+) cells were labeled with directly conjugated HLA-DR and CD3 antibodies after blocking with 10% mouse serum and the large CD3+ DR+ cells and clusters sorted by FCM. DCs were sorted from lin− cells negatively selected by MACS and labeled with HLA-DR and CD11c before sorting for HLA-DR+ lin− DCs or separate CD11c+ mDCs and CD11c− pDCs by FCM. Single cells were selected by forward scatter, side scatter, and pulse width to exclude doublets.
HIV-1 isolates.
Infection with HIV-1 included primary isolates that had been passaged a limited number of times (md#8, md#20, md#14, md#15, asx, mam4, AH1, AH2, and AH5 were provided by S. Crowe, Burnet Institute, Victoria, Australia, and M-tropic HIV-1 isolate 676-200 and X4 isolate 228-200 were provided by D. McPhee, Burnet Institute); laboratory-adapted X4 isolate IIIb, M-tropic R5 isolate Ba-L, and the molecular clones NL4-3 and AD8 (AIDS Reagent Repository, NIH) and chimeric virus NL-AD8env (M. Martin, NIH, Bethesda, MD).
Virus culture and infection.
HIV-1 isolates were passaged in mitogen-activated PBMCs in RF10 (10 mg/ml phytohemagglutinin in RPMI 1420 with 10% fetal calf serum, 2 mM glutamine, 25 mg/ml gentamicin) with interleukin 2 (10 U/ml) (Boehringer Mannheim, Mannheim, Germany). Supernatants were filtered (0.2-μm filter; Schleicher and Schuell, Keene, NH), stored at −70°C, and treated with 50 U/ml RNase-free DNase (Boehringer Mannheim, Mannheim, Germany) in 10 mM MgCl2 at 37°C for 30 min. The 50% tissue culture infective dose was determined in 3-day-activated PBMCs. Coreceptor usage was determined using “ghost” cells expressing CCR5 or CXCR4 as described previously (49). The 50% tissue culture infective doses of virus stocks were between 2 × 103 and 5 × 105 IU/ml. In each experiment, the sorted leukocyte subpopulations pooled from two donors (0.5 × 106 to 2 × 106) were incubated with 50 μl of virus at 4°C and 37°C for 2 h in round-bottom 96-well microtiter plates (Nunc, Naperville, IL). Cells were washed three times with fluorescence-activated cell sorting wash and once with RF10 culture medium. In some experiments, RF10 was added to the culture to dilute the cells to 0.5 × 106 to 1 × 106 cells/ml and culture continued for 24 to 36 h. Cells were harvested for PCR as described previously (6), and lysates were stored at −20°C before PCR analysis.
Quantitative PCR.
Semiquantitative PCR for HIV-1 was performed as described previously (6). The band intensity on radiographic film was compared to standards created with infected cell lines U1 and ACH2 using a scanning densitometer (Bio-Rad Laboratories, Hercules, CA). Real-time PCR was performed using molecular beacons as described previously (40). HIV-1 primers (SL19 [5′ TCT CTA GCA GTG GCG CCC GAA CA 3′] and SL20 [5′ TCT CCT TCT AGC CTC CGC TAG TC 3′]) and the matching molecular beacon [5′ 6-carboxyfluorescein-CGG GAG TAC TCA CCA GTC GCC GCC CCT CGC CCT CCC G-4-(4′-dimethylaminophenylazo) benzoic acid-3′] that cross the primer binding site and long terminal repeat were used to detect only full-length reverse transcripts. Primers for CCR5 (LK46 [5′GCT GTG TTT GCG TCT CTC CCA GGA 3′] and LK47 [5′ CTC ACA GCC CTG TGC CTC TTC TTC 3′]), and the molecular beacon recognizing the sequence 5′ GAG AAC GGT GAA TGA AGA GCA GAC AG 3′ were used to quantitate genomic copies. Amplifications were performed using a Perkin-Elmer 7700 (Perkin-Elmer, Branchburg, NJ). Copy number was determined from cycle number using HIV-1 and DNA standards created using 8E5 cell line and expressed as the number of HIV-1 copies per 105 cells.
Quantitative real-time PCR for multiply spliced RNA.
RNA was prepared from sorted DCs or DC-T-cell cocultures after infection with HIV-1 using a guanidinium lysis buffer and oligo(dT) beads (Dynal, Oslo, Norway) according to the manufacturer's instructions. Reverse transcription and real-time PCR were performed as described previously (40) but was modified to include random hexamers (Pharmacia, Uppsala, Sweden) in the reverse transcription reaction mixture to increase sensitivity.
Virus transfer and reverse transcriptase assays.
Sorted CD4+ T cells, CD14+ monocytes, and DCs were infected with HIV-1 by resuspending the pelleted cells in the virus-containing medium at a cell concentration of 2 × 106 cells/ml to give multiplicities of infection between 0.01 and 0.25. Cells were washed immediately after infection or were diluted to 0.5 × 106 to 1 × 106 cells/ml and cultured overnight before they were washed and added to CD4+ T cells in cocultures with the superantigen staphylococcal enterotoxin B (SEB) (40 ng/ml) (Sigma, St. Louis, MO). Infected cells (5 × 104) were added to 2 × 105 PBMCs in flat-bottom microtiter plates (Nunc, Naperville, IL), medium was changed twice weekly, and virus production was determined by a reverse transcriptase assay as previously described (6).
Statistical analysis.
Generation of box plots, nonparametric statistical analysis (Wilcoxon signed-rank test), and correlations (Spearman) were performed using the statistical program R.
RESULTS
Blood DCs sorted without culture from fresh blood are functional in mixed leukocyte cultures.
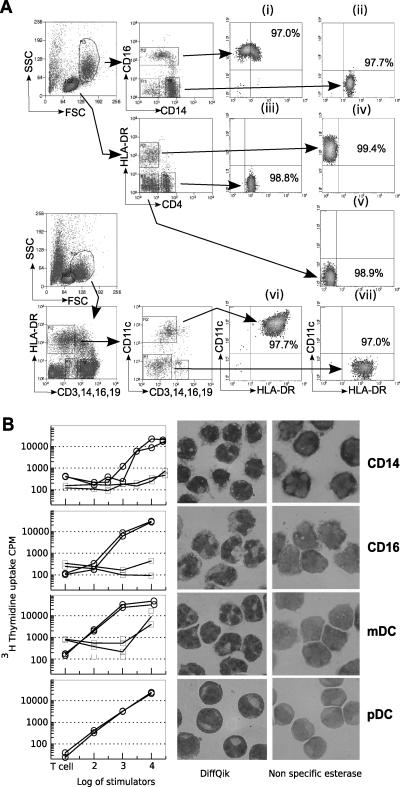
Blood leukocytes were sorted into lymphocyte, DC, and monocyte subpopulations (Fig. 1A). Lymphocytes were gated by size and side scatter and sorted using CD4 and HLA-DR antibodies into B, CD4+, and CD8+/NK cells. Monocytes were divided into CD14+ and CD16+ monocytes. Blood DCs were sorted from lineage-depleted populations and divided by CD11c or CD1b/c expression using the lineage markers that have been well described (48). The purity of sorted cells was determined by FCM and was routinely greater than 97%. Directly isolated DCs stimulated an allogeneic response (Fig. 1B). As few as 1,000 mDCs isolated by sorting for the lin−, HLA-DR+, CD1c+ population were able to stimulate proliferative responses in resting CD4+ T cells. The CD16+ monocyte populations and the pDCs required more cells to generate similar thymidine uptake but were more stimulatory than the CD14+ monocytes. Staining for the monocyte marker nonspecific esterase showed no detectable expression in pDCs but some low-level and heterogeneous expression in the mDC and CD16+ monocyte populations that include both DC and monocyte subpopulations (60). These experiments demonstrated that the sorting methods produced highly enriched populations of functional DCs. Both the phenotype and expression of CXCR4 and CCR5 on similarly defined populations of uncultured DCs have been described previously (39, 43). In these experiments, antibody to CD16 included in the lineage cocktail would deplete the CD16 DCs, including the small number expressing DC-SIGN (15, 65). DC-SIGN is also expressed on subpopulations of plasmacytoid DCs and increased after culture (65). These DC populations bind HIV gp120 only by C-type lectin-independent mechanisms (69), indicating the absence of detectable DC-SIGN and the CLR found on tissue DCs (71).
FIG. 1.
Isolation and function of blood leukocytes. (A) PBMCs were sorted by flow cytometry before or after HIV-1 infection. Monocytes were sorted into subpopulations expressing CD16+ (i) or CD14+ (ii). Lymphocytes were sorted by expression of HLA-DR and CD4 into CD4+ T cells (iii) and B cells (iv) and CD8+/NK cells (v). For sorting of DCs, the lineage-negative cells were enriched by MACS depletion with CD3, CD14, CD16, and CD19 and sorted as large lineage-negative HLA-DR+ cells and further divided by expression of CD1c or CD11c into mDCs (vi) and pDCs (vii), respectively. Representative purity is shown for sorted populations. SSC, side scatter; FSC, forward scatter. (B) Directly isolated and sorted populations were added in graded doses to allogeneic (open circle) or syngeneic (faint square) purified CD4+ T cells. Sorted cells were stained for nonspecific esterase and with Giemsa stain (Diff Quik). mDC, myeloid DCs; pDC, plasmacytoid DCs.
Quantitative analysis of HIV-1 entry into sorted PBMCs.
Given that prolonged culture may change expression of chemokine receptors (39, 74) and HIV-1 susceptibility, resulting from DC maturation during HIV-1 exposure, we pulsed sorted PBMCs (described above) with R5 HIV-1 for 2 h at 37°C before washing the cells and culturing them in virus-free medium.
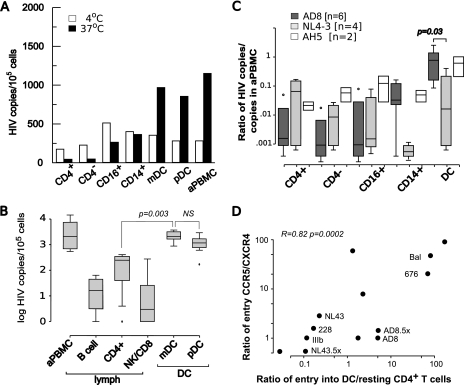
Where possible, the lin− HLA-DR+ DCs were further divided into CD11c+ mDCs and CD11c− pDCs, but as shown in Fig. 2B, similar entry of HIV-1 into total DC and DC subpopulations was found. The highest quantity of HIV-1 DNA was found in mDCs and pDCs compared to monocytes and resting CD4+ T cells (Fig. 2A). To further quantitate the relative frequency of infection of DCs compared to those of lymphocytes and monocytes, we next infected sorted cells with R5 HIV-1AD8. HIV-1 DNA in the mDCs sorted by CD1c (29) were compared to pDCs and lymphocytes (Fig. 2B). The concentrations of HIV-1 DNA in DC subpopulations infected with HIV (CD1c− HIV-1 DNA concentration median, 2,104 copies/105 cells; CD1c+ HIV-1 DNA median, 1,183 copies/105 cells) were similar to that of 1-day-activated autologous PBMCs (HIV-1 median, 2,078 copies/105 cells) and significantly higher than those of CD4+ T cells (median, 249 copies/105 cells; P = 0.00005) and NK/CD8+ T cells (median, 13.0 copies/105 cells; P = 0.0014) and B cells (median, 16.3 copies/105 cells; P = 0.0009).
FIG. 2.
Quantitation of HIV-1 infection of sorted blood leukocytes. (A) Cells were sorted by flow cytometry, infected with HIV-1AD8 for 2 h, and washed, and HIV entry was determined after culture for a further 24 h. For each experiment, infection was at 4°C and 37°C, and the positive controls were autologous PBMCs activated overnight with superantigen SEB (aPBMC). Representative data from one of six experiments are shown. The values are the means for replicate cultures. (B) Entry of R5 HIV-1AD8 into mDC and pDC subpopulations. Lin− HLA-DR+ cells were sorted into mDCs (HLA-DR+ CD1c+) and pDCs (HLA-DR+ CD1c−) and infected with R5 virus. Data are presented in a box and whisker plot where the edges of the boxes are the 25th and 75th percentiles, the horizontal line in the box is the median, the “whiskers” represent the 10th and 90th percentiles, and the dots indicate outliers. Box plots represent summary data from five separate experiments. Both mDCs and pDCs had significantly more infected cells than B cells (DR+ CD4−), CD4+ T cells (CD4+ DR−), or NK/CD8+ lymphocytes (CD4− DR−). aPBMC, SEB-activated PBMCs; NS, not statistically significant. (C) Comparison of entry of R5 and X4 viruses into blood leukocytes. Data are presented in a box and whisker plot where the edges of the boxes are the 25th and 75th percentiles, the horizontal line is the median, the “whiskers” represent the 10th and 90th percentiles, and the dots indicate outliers. Box plots represent summary data from six separate experiments using HIV-1 isolates R5 HIV-1AD8, X4 HIV-1NL4-3, and a patient isolate (AH5). The copy number in each cell population was standardized by comparing the copy number for the same isolate in a similar number of activated autologous PBMCs (aPBMC). Entry of HIV-1AD8 into DCs (defined as lin− DR+ cells) was significantly higher than entry of HIV-1NL4-3 (P = 0.03) and entry into other leukocytes. (D) Correlation between coreceptor usage and selective entry into DCs. Sorted DCs (defined as lin− DR+ cells), activated PBMCs, and sorted resting CD4+ T cells were infected with HIV-1 that had been characterized by entry into ghost cells expressing CCR5 or CXCR4. The ratio of entry by CCR5/CXCR4 was determined by FCM for each isolate (y axis) and plotted against the ratio of entry in panel D.
DCs and resting CD4+ T cells were then infected with either R5 or X4 virus (Fig. 2C and B). In these experiments, because DC numbers were limited, we compared bulk DCs to other blood leukocytes. We reasoned that an increase in susceptibility to X4 isolates that has been reported for cultured pDCs (50, 63) would be evident in the bulk DC population, as pDCs represented 50 to 70% of the DCs. To allow for differences in viral titers of the different patient isolates and the considerable host-dependent variability in infection by patient isolates (9), data are expressed as a ratio of HIV-1 DNA in the cells of interest to HIV-1 DNA in autologous activated PBMCs. Compared to R5 virus, the X4 HIV-1 isolates had significantly lower concentrations of HIV-1 DNA in the DCs. The efficiency of entry of X4 isolates into DCs was not significantly different to infection of resting CD4+ T cells. The primary patient isolate AH5 entered cells with similar efficiency to the R5 lab isolate AD8 (Fig. 2C). Significantly more DCs were infected with HIV-1AD8 than with HIV-1NL4-3 (P = 0.03). The sorted CD16+ or CD14+ monocytes were infected at a level similar to that of CD4+ T cells and significantly less than that of DCs (P = 0.016).
In the next experiment, the relationship between viral tropism and infection of CD4+ T cells was directly determined. For the different HIV-1 isolates, infection of cell lines expressing CCR5 or CXCR4 (ghost cells) was compared to infection of primary cells by quantitation of HIV-1 DNA. Preferential infection of DCs was determined by the frequency of entry into DCs compared to autologous resting CD4+ T cells. Across the isolates there was a clear correlation (0.82; P = 0.0002) between preferential infection in DCs by R5 viruses compared to CD4+ T cells and their ability to use CCR5 (Fig. 2D).
In summary, these experiments showed preferential infection with R5 and not X4 strains compared to CD4+ T cells and that this preferential infection was evident with both pDCs and mDCs. Further, the rate of R5 infection in DCs is significantly higher than in CD16+ or CD14+ monocytes or resting CD4+ T cells.
Selective infection of DCs during infection of unfractionated PBMCs.
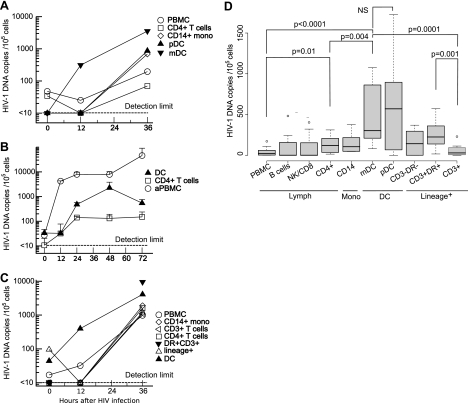
To determine whether HIV-1 infection of DCs also occurred under more physiological conditions where there is competition between leukocytes for HIV-1 binding, we infected unseparated PBMCs for 2 h with HIV-1 and sorted the leukocyte subpopulations up to 36 h after infection. Some HIV-1 entry into mDCs was detected at the 12-h time point and to a higher level than that for the pDCs (Fig. 3A). HIV-1 DNA was detected in the bulk PBMC populations and in the mDCs at 12 h but not in other populations. To exclude the possibility that the early detection of HIV-1 DNA reflected different kinetics and more rapid reverse transcription in DCs, the time course for detection of HIV-1 DNA was compared for sorted populations of DCs, resting CD4+ T cells, and SEB-activated PBMCs. High levels of HIV-1 DNA were found in activated PBMCs within 12 h (Fig. 3B), but the kinetics of appearance of full-length provirus were similar in DCs and resting CD4+ T cells. We concluded that the higher number of DCs carrying HIV-1 DNA early after PBMC infection results from preferential HIV-1 infection of the DCs rather than differences in the rates of reverse transcription and generation of full-length viral DNA.
FIG. 3.
Preferential entry of HIV-1 into DCs in unseparated PBMCs. Freshly isolated syngeneic PBMCs were infected for 2 h with R5 HIV-1 and DCs sorted after different culture periods. Cells were separated into lineage-negative and lin+ cells by MACS, and the DCs were sorted by FCM into separate cell populations after methanol fixation. Resting CD4+ T cells and monocytes were sorted by FCM directly from PBMCs. Viral entry was quantified by HIV-1 DNA copies/105 cells. The limit of detection of the assay was 10 copies/105 cells. (A) Distribution of R5 HIV-1 in sorted lymphocytes, monocytes (mono), and DCs immediately after infection and following 12 and 36 h in culture. Representative results from one of six similar experiments are shown. (B) Comparison of kinetics of entry and reverse transcription in DCs compared to resting and activated T cells. Representative results from one of four experiment are shown. The mean plus standard deviation (error bar) is shown for replicate values at each time point. aPBMC, autologous PBMCs activated overnight with superantigen SEB. (C) Distribution of HIV-1 DNA in single cells and in large cells or clusters expressing CD3 and HLA-DR. PBMCs were infected with R5 virus and sorted as described above for panel A. MACS+ cells were further labeled with CD3+ and DR+, and large cells and clusters were sorted for DC-T-cell clusters or activated T cells. Data are representative of three experiments. (D) PBMCs were infected with HIV-1AD8, and labeled cells were sorted after 36 h as described above for panels A and C, but formalin fixation was used before sorting to reduce cell clustering during sorting and increase yield. Lineage-positive cells were sorted by DR and CD3 expression to define activated T cells and DC-T-cell clusters (DR+ CD3+). Data are presented in a box and whisker plot where the edges of the boxes are the 25th and 75th percentiles, the horizontal line in the box is the median, the “whiskers” represent the 10th and the 90th percentiles, and the dots indicate outliers. The box plots show pooled results from eight donors in four separate experiments. NS, not statistically significant.
Reduced recovery of DCs sorted from cultured PBMCs was found at 12 and 36 h of culture (approximately 50% and 10%, respectively). This could be attributed to several factors, including reduced survival of DCs compared to lymphocytes as previously described for both plasmacytoid (26) and myeloid subpopulations of DCs during culture (12). In addition, formation of clusters of DCs and T cells as previously described for syngeneic and allogeneic cultures (5, 53, 68) may result in reduced recovery of single DCs. DC-T-cell clusters form during syngeneic or allogeneic culture (54, 73); however, we avoided allogeneic clustering by culturing cells from each donor separately and pooled before negative selection and sorting, but clustering and removal of DCs in the lineage+ population during MACS may account for some of the progressive loss of DCs. Methanol fixation improves performance of DNA PCR but favors clumping and reduces the isolation of single-cell populations (61), so experiments shown in Fig. 3D were performed with cells sorted after formalin fixation. The cells selected by lineage markers, including CD3 (MACS+ cells), were restained with directly labeled antibodies to HLA-DR and CD3, and HIV-1 DNA was determined in the CD3+ and DR+ CD3+ cells that will include both DC-T-cell conjugates and clustered activated T cells. At 0 and 12 h, there were too few DR+ CD3+ cells to quantitate viral load, but by 36 h, this fraction contained sufficient cells to show significantly higher frequency of HIV-1 than in the CD4+ T cells or CD3+ cells (Fig. 3C). Culture-associated increase in CD16 expression on monocytes allowed sorting of only one population of CD14+ CD16+ monocytes.
To block spreading infection, we added the protease inhibitor (PI) indinavir at 0.1 mM to the PBMCs at the end of the 2-h infection. In control experiments, this concentration of indinavir blocked spreading infection in PBMCs (data not shown). The addition of PI did not significantly change the distribution of HIV-1, suggesting that spreading infection to T cells from DCs was not occurring at these early time points. No significant difference between the DC subpopulations was found 36 h after infection, but the concentrations of HIV-1 DNA in both pDCs (HIV-1 DNA median, 570 copies/105 cells) and mDCs (median, 302 copies/105 cells) were significantly higher than in the CD4+ T cells (median, 120 copies/105 cells; P = 0.004 mDCs; P = 0.08 pDCs), CD3+ cells (median, 30 copies/105 cells; P ≤ 0.03) or the unseparated PBMCs (median, 23 copies/105 cells; P ≤ 0.03) (Fig. 3D).
DCs are productively infected with HIV-1 and transfer R5 virus to CD4+ T cells.
To determine whether the infected DCs contained replication-competent virus, DCs, CD14+ monocytes, and activated PBMCs were infected overnight with different R5 and X4 HIV-1 isolates and then activated PBMCs were added to determine whether differences in viral entry resulted in differential virus transfer from DCs to activated T cells. Spreading infection in these cocultures occurred following infection of DCs or activated PBMCs but not following infection of CD14+ monocytes (Fig. 4A). Importantly, both R5 and X4 viruses were transferred from DCs to the T cells, consistent with the nonspecific transfer previously observed with cultured primary DCs (6) or emigrant skin DCs (53). In our experiments, SEB-activated PBMCs were used in the cocultures to minimize the observed differences in the ability of DCs and monocytes to stimulate T cells (Fig. 1).
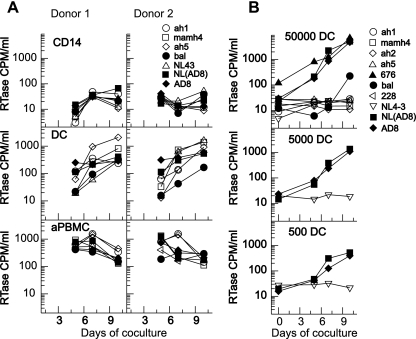
FIG. 4.
Productive infection and viral transfer by DCs. The frequency of infection and efficiency of transfer of HIV-1 from DCs to T cells was determined using HIV-1-infected DCs cocultured with T cells. Multiple HIV-1 strains were used to infect DCs for 18 h (A) or 2 h (B). The multiple HIV-1 strains included the following: primary isolates ah1 mamh4, ah2, ah5, 676, and 228; laboratory isolate Bal; and molecularly cloned virus NL4-3, AD8, and NL(AD8) The R5 isolates are shown as filled symbols; dualtropic R5X4 and X4 tropic are shown as open circles. RTase, reverse transcriptase. (A) DCs and CD14+ monocytes from two donors and control activated PBMCs (aPBMC, PBMCs activated with SEB for 3 days) were infected with HIV-1 and cultured overnight before the infected cells were washed and added to activated T cells to allow for differences in the stimulatory capacity of DCs and monocytes. Reverse transcriptase production in the supernatant of the cocultures was measured from day 5 to 10. All HIV-1 isolates were transferred to T cells, including R5 (closed symbols) and X4 or dualtropic R5X4 HIV-1 (open symbols) using an overnight incubation with virus. (B) Different numbers of DCs were infected with HIV-1 for 2 h before washing and coculture with activated T cells. Virus production was monitored by reverse transcriptase assay. R5 isolates are shown as filled symbols, and X4 isolates are shown as open symbols. A total of 50,000 DCs were infected with a panel of primary and lab isolates (top panel). A reduced number of DCs were pulsed with cloned HIV-1AD8, HIV-1NL4-3, and HIV-1NL(AD8) (middle and lower panels), and virus production in supernatant was measured from days 3 to 10. The transfer of HIV-1 from as few as 500 DCs to activated T cells suggests that the frequency of infection in the DCs is greater than 1 in 500 cells. None of the X4 virus was transferred to T cells following the short virus exposure.
We then determined whether the short 2-h infection of uncultured DCs (that we had shown was associated with preferential R5 infection by quantification of HIV-1 DNA) would show specificity of viral transfer from DCs to T cells. Preferential transfer of R5 virus was found with DCs pulsed with virus for 2 h and washed well before coculture with activated T cells (Fig. 4B). Titration of DC number suggests that as few as 500 DCs were able to establish productive infection in T cells, suggesting a high frequency of productive infection (Fig. 4B). The viral isolates that were transferred from DCs to T cells were the same isolates that infected DCs as measured by HIV-1 DNA (data not shown). The lack of transfer of X4 virus following a short virus incubation suggests that there is little if any transfer of virus by transinfection when the uncultured DCs are infected.
HIV-1 expression in DCs is increased during T-cell activation.
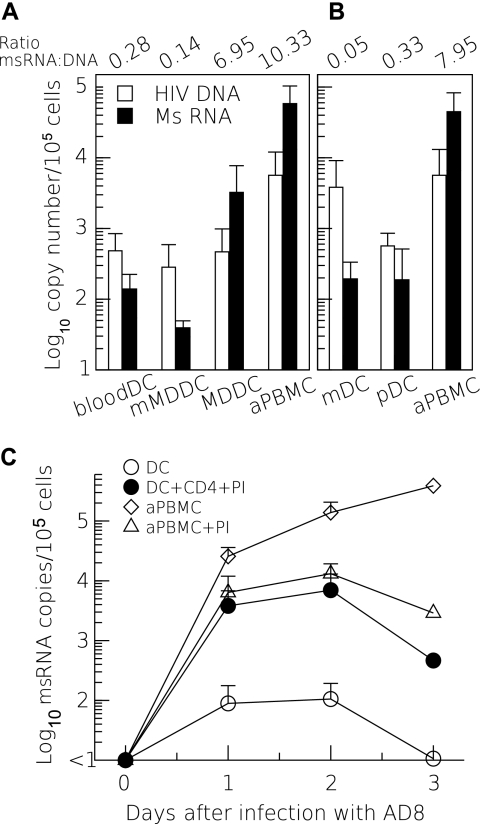
To further determine whether productive infection occurred in DCs, we infected DCs and measured HIV multiply spliced RNA (msRNA) relative to HIV-1 DNA copies. Following HIV-1 infection of isolated DCs, the level of HIV msRNA was low compared to the level of HIV DNA, suggesting lack of integration or nonproductive infection (Fig. 5A and B). Mature MDDCs were similar to the freshly isolated blood DCs in that they contained a low msRNA-to-DNA ratio (Fig. 5A). Infection of immature MDDCs with R5 virus, however, resulted in a high msRNA-to-DNA ratio similar to the infection of activated PBMCs consistent with previous reports (7, 24). Since interactions between DCs and T cells lead to activation of DCs by CD40-CD154 interactions (17, 51), we infected DCs (in the absence of T cells) immediately after isolation from PBMCs and quantitated msRNA over time. We found low levels of msRNA in DCs compared to the levels in activated PBMCs (Fig. 5C). Coculture of infected DCs with activated CD4+ T cells and PI (at a concentration that completely blocked spreading infection in the PBMCs) resulted in a 2-log-unit increase in the levels of msRNA production, consistent with upregulation of viral expression in the DCs, possibly induced by CD40-CD154 interactions (17, 51).
FIG. 5.
Low levels of msRNA in infected DCs is increased during cognate interactions with CD4 T cells. (A and B) Sorted DCs and SEB-activated PBMCs (aPBMC) were infected with R5 virus HIV-1AD8 (A) or HIV-1Bal (B). HIV-1 viral DNA was measured by real-time PCR, and multiple spliced RNA (MsRNA) was measured by reverse transcription and quantitative real-time PCR. (A) The mean (plus standard deviation [error bar]) numbers of copies of HIV-1 DNA and msRNA per 105 cells following 24 to 48 h in culture are shown with the ratios of copies of msRNA to HIV-1 DNA shown above the histograms (n = 3). mMDDC, MDDCs matured with tumor necrosis factor for 48 h; MDDC, monocyte-derived DCs. (C) Sorted cells were infected with HIV-1AD8, and msRNA was quantified over 3 days postinfection. DCs were cultured alone or were treated with the protease inhibitor indinavir (PI) immediately after infection and during culture with CD4+ T cells activated with superantigen SEB. PI was added to prevent spreading infection in the DC-T-cell cocultures. Spreading infection was blocked by the addition of PI to HIV-1 infected PBMCs, as shown by the reduced level of msRNA after 24 h. The addition of CD4+ T cells resulted in increased levels of msRNA in the DCs at all time points from 24 h.
DISCUSSION
In this study we demonstrated that entry of HIV into blood DCs ex vivo occurs much more frequently than in any other blood leukocyte population. Although both DCs and resting CD4+ T cells could be infected at low frequencies with R5 and X4 viruses, there was increased permissiveness to infection in DCs by R5 isolates. Infection of DCs with R5 virus was associated with the ability of these DCs to transfer infection to T cells. There was a strong correlation between the ability of an HIV-1 isolate to use CCR5 and the ability to infect DCs. Multiply spliced RNA was found at low level in the uncultured DCs infected ex vivo but increased during cognate interactions with CD4+ T cells and superantigen. Taken together, these observations suggest that R5 HIV-1 and not X4 HIV-1 is able to infect resting DCs in situ and may contribute to selective transmission of R5 viruses following parenteral infection.
These observations differ significantly from the MDDC model of DC-T-cell interactions where DC-SIGN and C-type lectin receptors have been considered critical for HIV-1 uptake and transfer from an intracellular endosomal compartment to CD4+ T cells during a cognate interaction (20, 38). A pivotal role of DC-SIGN in both the uptake of HIV-1 and formation of the immunological synapse into which the carried virion is exocytosed (45) has been proposed, but DC-SIGN expression is not found on primary blood DCs (69) or LCs (64) but is restricted to dermal DCs (71) in skin and occurs predominantly on macrophages in lymph nodes (22). Further, DC-SIGN is not required for DC-T-cell interactions (22).
We have shown that HIV-1 transfer in MDDCs occurs in two phases with a second infectious transfer phase; the first phase of transinfection decays rapidly and is followed by a second phase consistent with infection. The infectious pathway of transfer is the dominant process after 24 h (70). Although transinfection occurs with both R5 and X4 viruses, only R5 virus replicates in immature MDDCs (24). During MDDC maturation, however, there is increased susceptibility to both X4 and R5 viruses (24), and like macrophages, prolonged virus production can occur (55). Preferential infection with R5 virus also occurs in immature DCs generated from CD34+ cells (1, 7). DCs develop from CD34+ cells via two pathways; the CD1a+ precursors of LCs and a CD14+ precursor of interstitial DCs (8). The CD14+ DC precursors are permissive for infection with R5 virus, but reduced production occurs following differentiation into immature DCs (37). Restriction to R5 infection has been found in CD34-derived LCs (62).
Blood DCs are a heterogeneous population of cells circulating from bone marrow to tissue. They include cells that will give rise to LCs and interstitial DCs. Up to five populations have been identified (43), but the two predominant subpopulations found using the protocol we have used to isolate DCs are CD11c− CD123+ pDCs and CD11c+ mDCs. Other populations include CD14+ monocytes, CD16+ DCs, and CD34+ precursors which may include CD34+ CLA+ precursors of LCs. As shown in this study, sorted CD14+ and CD16+ monocytes were less frequently infected with HIV-1 than were the mDCs and pDCs. In this study we have used lin− DCs that will exclude the CD16+ DCs but include most of the myeloid DC subpopulations defined by BDCA1 and BDCA3 and different levels of HLA-DR expression (25). We have also sorted for individual pDC and mDC subsets. However, these subsets may also be heterogeneous, as has recently been shown for the CD1c-expressing mDCs (25).
Primary blood pDCs and mDCs, in contrast to immature MDDCs or tissue DCs, do not express DC-SIGN or other CLR-binding HIV (69), so transfer of HIV-1 will depend on direct infection or uptake and transfer by non-CLR-mediated mechanisms. Early work on blood DCs sorted after culture found that both X4 and R5 viruses could infect DCs and were transferred to T cells (6, 16). The phenotype of these cells (18) will be similar to the phenotype of the cells obtained after prolonged culture in the presence of virus. In this study we found such prolonged culture associated with transmission of both X4 and R5 virus. More recently, blood DCs have been isolated directly from PBMCs with minimal culture (42, 50, 63) and were permissive for infection with both X4 and R5 viruses. These studies differ from the present study, as DCs were cultured with virus for up to 18 h (50) or used a short 12-h preincubation in cytokines followed by HIV-1 infection for 6 to 72 h (42, 63). These culture conditions would lead to significant changes in blood DCs, although an immature CD1c+ mDC subpopulation may be preferentially infected and remain immature (25). We found higher entry into the CD11c− pDCs after prolonged HIV-1 exposure, in keeping with previous work (50), but found similar frequency of infection with R5 into the pDCs and mDCs after a short virus pulse. We have interpreted this difference in viral selection following infection for 2 and 18 h as a reflection of the predominance of CCR5 on the immature cells (39). In the context of immature DCs that have efficient antigen-processing pathways, we propose that there is a rapid loss of cell-associated infectious virions in primary DCs as we have demonstrated in MDDCs (70). This will result in a preferential survival of HIV-1 that can use CCR5. In cultured cells where CXCR4 is also expressed, both X4 and R5 viruses will be able to escape from the less efficient degradation in the DCs via an infectious pathway or by sequestration in specific endosomal compartments.
Our study is the first to quantitate entry into different blood leukocytes and unfractionated PBMCs under conditions of minimal culture and short virus exposure that would mimic the exposure in intravenous drug use or blood products. The highest frequency of viral DNA was found in DCs, but later, viral DNA was also found in DC-T-cell clusters. More mature DCs that are susceptible to HIV-1 infection may cluster directly (73), but we found that the overall frequency of infection in individual sorted pDCs and mDCs was higher than in the CD3+ DR+ DC-T-cell clusters. Further, we found that incubation with a PI did not alter the frequency of HIV-1 in clustering cells,suggesting that at least up to 36 h, there was no transfer or productive infection in the clustered cells. Cell clustering is an important function of DCs and at later time points may significantly contribute to DC-mediated HIV-1 transmission and replication as shown with skin emigrants (53) and mature blood DC cultures (5, 42).
Our data reinforce the critical role of DC maturation in the process of HIV-1 selection and productive infection. The uncultured blood pDCs and mDCs are selectively infected with R5 virus similar to the immature DCs in epidermis (56) and uncultured tonsil and thymic DCs (4). Neither tonsil nor blood DC subpopulations bind gp120 by CLR, so binding of gp120 and virus is completely dependent on CD4 (69).
Separate pathways of carriage and infection have been shown in LCs (2) that can bind gp120 via a range of C-type lectin receptors (71). However, the ability of CLR pathways to mediate virus carriage appears to be limited to DCs in skin and mucosal sites, but even here strong selection for R5 virus is seen with skin explants (32, 34, 56), arguing for a predominance of the second phase transfer (70). The high antigen-processing capacity of immature DCs and the changes during maturation have been well defined (46). Without CLR to facilitate viral sequestration in nonproteolytic compartments (38), the infectious pathway is the only mechanism for viral escape from uptake into potent degradative pathways present in immature DCs (57, 58). Preferential R5 infection may also depend on the efficiency of using CCR5, the predominant chemokine receptor on immature DCs (39). In contrast, cultured DCs will have a wider repertoire of chemokine receptors (23, 74) as well as reduced endosomal uptake and antigen processing.
Our data support infection of immature blood DCs and not transfer of virus by transinfection as the predominant method of selection of R5 virus during parenteral transmission. It is likely that the pathway of preferential infection of DCs in situ provides both for selection for R5 virus and for dissemination of infection to peripheral tissue and lymphoid tissue early in HIV infection.
Acknowledgments
This work was supported by project research grant 9935677 from the National Health and Medical Research Council and equipment grants from the Wellcome Trust and the ANZ trustees. P.U.C. was supported by a C. R. Roper fellowship from the University of Melbourne. S.R.L. was supported by the NHMRC, the Ian Potter Foundation, and the Alfred Foundation.
P.U.C., S.R.L., and D.F.J.P. designed the research. P.U.C., A.J.H., A.E.S., D.C.B., and N.B. performed the research. P.U.C., A.J.H., and A.E.S. analyzed the data. P.U.C. and S.R.L. wrote the manuscript.
Footnotes
Published ahead of print on 13 December 2006.
REFERENCES
- 1.Bakri, Y., C. Schiffer, V. Zennou, P. Charneau, E. Kahn, A. Benjouad, J. C. Gluckman, and B. Canque. 2001. The maturation of dendritic cells results in postintegration inhibition of HIV-1 replication. J. Immunol. 166:3780-3788. [DOI] [PubMed] [Google Scholar]
- 2.Blauvelt, A., H. Asada, M. W. Saville, V. Klaus-Kovtun, D. J. Altman, R. Yarchoan, and S. I. Katz. 1997. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J. Clin. Investig. 100:2043-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron, P. U., M. G. Lowe, F. Sotzik, A. F. Coughlan, S. M. Crowe, and K. Shortman. 1996. The interaction of macrophage and non-macrophage tropic isolates of HIV-1 with thymic and tonsillar dendritic cells in vitro. J. Exp. Med. 183:1851-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron, P. U., M. Pope, S. Gezelter, and R. M. Steinman. 1994. Infection and apoptotic cell death of CD4+ T cells during an immune response to HIV-1-pulsed dendritic cells. AIDS Res. Hum. Retrovir. 10:61-71. [DOI] [PubMed] [Google Scholar]
- 6.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383-387. [DOI] [PubMed] [Google Scholar]
- 7.Canque, B., Y. Bakri, S. Camus, M. Yagello, A. Benjouad, and J. C. Gluckman. 1999. The susceptibility to X4 and R5 human immunodeficiency virus-1 strains of dendritic cells derived in vitro from CD34(+) hematopoietic progenitor cells is primarily determined by their maturation stage. Blood 93:3866-3875. [PubMed] [Google Scholar]
- 8.Caux, C., B. Vanbervliet, C. Massacrier, C. Dezutter-Dambuyant, B. de Saint-Vis, C. Jacquet, K. Yoneda, S. Imamura, D. Schmitt, and J. Banchereau. 1996. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J. Exp. Med. 184:695-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, J., H. M. Naif, S. Li, J. S. Sullivan, C. M. Randle, and A. L. Cunningham. 1996. Twin studies demonstrate a host cell genetic effect on productive human immunodeficiency virus infection of human monocytes and macrophages in vitro. J. Virol. 70:7792-7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow, A., D. Toomre, W. Garrett, and I. Mellman. 2002. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature 418:988-994. [DOI] [PubMed] [Google Scholar]
- 11.Dean, M., M. Carrington, C. Winkler, G. Huttley, M. Smith, R. Allikmets, J. Goedert, S. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, and S. O'Brien. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE. Science 273:1856-1862. [DOI] [PubMed] [Google Scholar]
- 12.de Baey, A., I. Mende, G. Riethmueller, and P. A. Baeuerle. 2001. Phenotype and function of human dendritic cells derived from M-DC8(+) monocytes. Eur. J. Immunol. 31:1646-1655. [DOI] [PubMed] [Google Scholar]
- 13.Donaghy, H., B. Gazzard, F. Gotch, and S. Patterson. 2003. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood 101:4505-4511. [DOI] [PubMed] [Google Scholar]
- 14.Embretson, J., M. Zupancic, J. L. Ribas, A. Burke, P. Racz, K. Tenner-Racz, and A. T. Haase. 1993. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature 362:359-362. [DOI] [PubMed] [Google Scholar]
- 15.Engering, A., S. J. Van Vliet, T. B. H. Geijtenbeek, and Y. Van Kooyk. 2002. Subset of DC-SIGN(+) dendritic cells in human blood transmits HIV-1 to T lymphocytes. Blood 100:1780-1786. [DOI] [PubMed] [Google Scholar]
- 16.Ferbas, J. J., J. F. Toso, A. J. Logar, J. S. Navratil, and C. R. J. Rinaldo. 1994. CD4+ blood dendritic cells are potent producers of IFN-alpha in response to in vitro HIV-1 infection. J. Immunol. 152:4649-4662. [PubMed] [Google Scholar]
- 17.Fong, L., M. Mengozzi, N. W. Abbey, B. G. Herndier, and E. G. Engleman. 2002. Productive infection of plasmacytoid dendritic cells with human immunodeficiency virus type 1 is triggered by CD40 ligation. J. Virol. 76:11033-11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freudenthal, P. S., and R. M. Steinman. 1990. The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc. Natl. Acad. Sci. USA 87:7698-7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 20.Geijtenbeek, T. B. H., D. J. E. B. Krooshoop, D. A. Bleijs, S. J. van Vliet, G. C. F. van Duijnhoven, V. Grabovsky, R. Alon, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat. Immunol. 1:353-357. [DOI] [PubMed] [Google Scholar]
- 21.Geissmann, F., M. C. Dieu-Nosjean, C. Dezutter, J. Valladeau, S. Kayal, M. Leborgne, N. Brousse, S. Saeland, and J. Davoust. 2002. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J. Exp. Med. 196:417-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granelli-Piperno, A., A. Pritsker, M. Pack, I. Shimeliovich, J. Arrighi, C. G. Park, C. Trumpfheller, V. Piguet, T. M. Moran, and R. M. Steinman. 2005. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J. Immunol. 175:4265-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granelli-Piperno, A., B. Moser, M. Pope, D. Chen, Y. Wei, F. Isdell, U. O'Doherty, W. Paxton, R. Koup, S. Mojsov, N. Bhardwaj, I. Clark-Lewis, M. Baggiolini, and R. M. Steinman. 1996. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J. Exp. Med. 184:2433-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R. M. Steinman. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72:2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granelli-Piperno, A., I. Shimeliovich, M. Pack, C. Trumpfheller, and R. M. Steinman. 2006. HIV-1 selectively infects a subset of nonmaturing BDCA1-positive dendritic cells in human blood. J. Immunol. 176:991-998. [DOI] [PubMed] [Google Scholar]
- 26.Grouard, G., M. Rissoan, L. Filgueira, I. Durand, J. Banchereau, and Y. Liu. 1997. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 185:1101-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hladik, F., G. Lentz, E. Delpit, A. McElroy, and M. McElrath. 1999. Coexpression of CCR5 and IL-2 in human genital but not blood T cells: implications for the ontogeny of the CCR5+ Th1 phenotype. J. Immunol. 163:2306-2313. [PubMed] [Google Scholar]
- 28.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito, T., M. Inaba, K. Inaba, J. Toki, S. Sogo, T. Iguchi, Y. Adachi, K. Yamaguchi, R. Amakawa, J. Valladeau, S. Saeland, S. Fukuhara, and S. Ikehara. 1999. A CD1a+/CD11c+ subset of human blood dendritic cells is a direct precursor of Langerhans cells. J. Immunol. 163:1409-1419. [PubMed] [Google Scholar]
- 30.Ito, T., R. Amakawa, M. Inaba, S. Ikehara, K. Inaba, and S. Fukuhara. 2001. Differential regulation of human blood dendritic cell subsets by IFNs. J. Immunol. 166:2961-2969. [DOI] [PubMed] [Google Scholar]
- 31.Kadowaki, N., S. Antonenko, J. Lau, and Y. Liu. 2000. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J. Exp. Med. 192:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawamura, T., F. O. Gulden, M. Sugaya, D. T. McNamara, D. L. Borris, M. M. Lederman, J. M. Orenstein, P. A. Zimmerman, and A. Blauvelt. 2003. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc. Natl. Acad. Sci. USA 100:8401-8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawamura, T., M. Qualbani, E. K. Thomas, J. M. Orenstein, and A. Blauvelt. 2001. Low levels of productive HIV infection in Langerhans cell-like dendritic cells differentiated in the presence of TGF-beta1 and increased viral replication with CD40 ligand-induced maturation. Eur. J. Immunol. 31:360-368. [DOI] [PubMed] [Google Scholar]
- 34.Kawamura, T., S. S. Cohen, D. L. Borris, E. A. Aquilino, S. Glushakova, L. B. Margolis, J. M. Orenstein, R. E. Offord, A. R. Neurath, and A. Blauvelt. 2000. Candidate microbicides block HIV-1 infection of human immature Langerhans cells within epithelial tissue explants. J. Exp. Med. 192:1491-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohrgruber, N., N. Halanek, M. Groger, D. Winter, K. Rappersberger, M. Schmitt-Egenolf, G. Stingl, and D. Maurer. 1999. Survival, maturation, and function of CD11c− and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines. J. Immunol. 163:3250-3259. [PubMed] [Google Scholar]
- 36.Krzysiek, R., A. Rudent, L. Bouchet-Delbos, A. Foussat, C. Boutillon, A. Portier, D. Ingrand, D. Sereni, P. Galanaud, L. Grangeot-Keros, and D. Emilie. 2001. Preferential and persistent depletion of CCR5+ T-helper lymphocytes with nonlymphoid homing potential despite early treatment of primary HIV infection. Blood 98:3169-3171. [DOI] [PubMed] [Google Scholar]
- 37.Kwan, W., A. Helt, C. Maranon, J. Barbaroux, A. Hosmalin, E. Harris, W. H. Fridman, and C. G. F. Mueller. 2005. Dendritic cell precursors are permissive to dengue virus and human immunodeficiency virus infection. J. Virol. 79:7291-7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 39.Lee, B., M. Sharron, L. J. Montaner, D. Weissman, and R. W. Doms. 1999. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc. Natl. Acad. Sci. USA 96:5215-5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewin, S., M. Vesanen, L. Kostrikis, A. Hurley, M. Duran, L. Zhang, D. Ho, and M. Markowitz. 1999. Use of real-time PCR and molecular beacons to detect virus replication in human immunodeficiency virus type 1-infected individuals on prolonged effective antiretroviral therapy. J. Virol. 73:6099-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, Q., L. Duan, J. D. Estes, Z. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 42.Lore, K., A. Smed-Sorensen, J. Vasudevan, J. R. Mascola, and R. A. Koup. 2005. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J. Exp. Med. 201:2023-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacDonald, K. P. A., D. J. Munster, G. J. Clark, A. Dzionek, J. Schmitz, and D. N. J. Hart. 2002. Characterization of human blood dendritic cell subsets. Blood 100:4512-4520. [DOI] [PubMed] [Google Scholar]
- 44.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 45.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295-1297. [DOI] [PubMed] [Google Scholar]
- 46.Mellman, I., and R. M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255-258. [DOI] [PubMed] [Google Scholar]
- 47.Meng, G., X. Wei, X. Wu, M. Sellers, J. Decker, Z. Moldoveanu, J. Orenstein, M. Graham, J. Kappes, J. Mestecky, G. Shaw, and P. Smith. 2002. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat. Med. 8:150-156. [DOI] [PubMed] [Google Scholar]
- 48.O'Doherty, U., M. Peng, S. Gezelter, W. J. Swiggard, M. Betjes, N. Bhardwaj, and R. M. Steinman. 1994. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology 82:487-493. [PMC free article] [PubMed] [Google Scholar]
- 49.Owen, S., S. Masciotra, F. Novembre, J. Yee, W. Switzer, M. Ostyula, and R. Lal. 2000. Simian immunodeficiency viruses of diverse origin can use CXCR4 as a coreceptor for entry into human cells. J. Virol. 74:5702-5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patterson, S., A. Rae, N. Hockey, J. Gilmour, and F. Gotch. 2001. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J. Virol. 75:6710-6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinchuk, L. M., P. S. Polacino, M. B. Agy, S. J. Klaus, and E. A. Clark. 1994. The role of CD40 and CD80 accessory cell molecules in dendritic cell-dependent HIV-1 infection. Immunity 1:317-325. [DOI] [PubMed] [Google Scholar]
- 52.Pöhlmann, S., F. Baribaud, B. Lee, G. J. Leslie, M. D. Sanchez, K. Hiebenthal-Millow, J. Münch, F. Kirchhoff, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J. Virol. 75:4664-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pope, M., M. G. Betjes, N. Romani, H. Hirmand, P. U. Cameron, L. Hoffman, S. Gezelter, G. Schuler, and R. M. Steinman. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78:389-398. [DOI] [PubMed] [Google Scholar]
- 54.Pope, M., S. Gezelter, N. Gallo, L. Hoffman, and R. M. Steinman. 1995. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J. Exp. Med. 182:2045-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Popov, S., A. Chenine, A. Gruber, P. Li, and R. M. Ruprecht. 2005. Long-term productive human immunodeficiency virus infection of CD1a-sorted myeloid dendritic cells. J. Virol. 79:602-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reece, J. C., A. J. Handley, E. J. Anstee, W. A. Morrison, S. M. Crowe, and P. U. Cameron. 1998. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J. Exp. Med. 187:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romani, N., S. Koide, M. Crowley, M. Witmer-Pack, A. M. Livingstone, C. G. Fathman, K. Inaba, and R. M. Steinman. 1989. Presentation of exogenous protein antigens by dendritic cells to T cell clones. Intact protein is presented best by immature, epidermal Langerhans cells. J. Exp. Med. 169:1169-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, and R. G. Collman. 1996. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 60.Schakel, K., R. Kannagi, B. Kniep, Y. Goto, C. Mitsuoka, J. Zwirner, A. Soruri, M. von Kietzell, and E. Rieber. 2002. 6-Sulfo LacNAc, a novel carbohydrate modification of PSGL-1, defines an inflammatory type of human dendritic cells. Immunity 17:289-301. [DOI] [PubMed] [Google Scholar]
- 61.Shapiro, H. M. 1985. Practical flow cytometry. Alan R. Liss Inc., New York, NY.
- 62.Sivard, P., W. Berlier, B. Picard, O. Sabido, C. Genin, and L. Misery. 2004. HIV-1 infection of Langerhans cells in a reconstructed vaginal mucosa. J. Infect. Dis. 190:227-235. [DOI] [PubMed] [Google Scholar]
- 63.Smed-Sörensen, A., K. Loré, J. Vasudevan, M. K. Louder, J. Andersson, J. R. Mascola, A.-L. Spetz, and R. A. Koup. 2005. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J. Virol. 79:8861-8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soilleux, E., and N. Coleman. 2001. Langerhans cells and the cells of Langerhans cell histiocytosis do not express DC-SIGN. Blood 98:1987-1988. [DOI] [PubMed] [Google Scholar]
- 65.Soilleux, E. J., L. S. Morris, G. Leslie, J. Chehimi, Q. Luo, E. Levroney, J. Trowsdale, L. J. Montaner, R. W. Doms, D. Weissman, N. Coleman, and B. Lee. 2002. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J. Leukoc. Biol. 71:445-457. [PubMed] [Google Scholar]
- 66.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stahl-Hennig, C., R. M. Steinman, K. Tenner-Racz, M. Pope, N. Stolte, K. Matz-Rensing, G. Grobschupff, B. Raschdorff, G. Hunsmann, and P. Racz. 1999. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science 285:1261-1265. [DOI] [PubMed] [Google Scholar]
- 68.Sugaya, M., K. Lore, R. A. Koup, D. C. Douek, and A. Blauvelt. 2004. HIV-infected Langerhans cells preferentially transmit virus to proliferating autologous CD4+ memory T cells located within Langerhans cell-T cell clusters. J. Immunol. 172:2219-2224. [DOI] [PubMed] [Google Scholar]
- 69.Turville, S. G., J. Arthos, K. MacDonald, G. Lynch, H. Naif, G. Clark, D. Hart, and A. L. Cunningham. 2001. HIV gp120 receptors on human dendritic cells. Blood 98:2482-2488. [DOI] [PubMed] [Google Scholar]
- 70.Turville, S. G., J. J. Santos, I. Frank, P. U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. J. Piatak, J. D. Lifson, M. Pope, and A. L. Cunningham. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103:2170-2179. [DOI] [PubMed] [Google Scholar]
- 71.Turville, S. G., P. U. Cameron, A. Handley, G. Lin, S. Pohlmann, R. W. Doms, and A. L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975-983. [DOI] [PubMed] [Google Scholar]
- 72.van't Wout, A. B., N. A. Kootstra, G. A. Mulder-Kampinga, N. Albrecht-van Lent, H. J. Scherpbier, J. Veenstra, K. Boer, R. A. Coutinho, F. Miedema, and H. Schuitemaker. 1994. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J. Clin. Investig. 94:2060-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weissman, D., Y. Li, J. Orenstein, and A. Fauci. 1995. Both a precursor and a mature population of dendritic cells can bind HIV. However, only the mature population that expresses CD80 can pass infection to unstimulated CD4+ T cells. J. Immunol. 155:4111-4117. [PubMed] [Google Scholar]
- 74.Zaitseva, M., A. Blauvelt, S. Lee, C. K. Lapham, V. Klaus-Kovtun, H. Mostowski, J. Manischewitz, and H. Golding. 1997. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat. Med. 3:1369-1375. [DOI] [PubMed] [Google Scholar]
- 75.Zhang, Z., T. Schuler, M. Zupancic, S. Wietgrefe, K. Staskus, K. Reimann, T. Reinhart, M. Rogan, W. Cavert, C. Miller, R. Veazey, D. Notermans, S. Little, S. Danner, D. Richman, D. Havlir, J. Wong, H. Jordan, T. Schacker, P. Racz, K. Tenner-Racz, and N. Letvi. 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286:1353-1357. [DOI] [PubMed] [Google Scholar]