Abstract
Many viruses, including human influenza A virus, have developed strategies for counteracting the host type I interferon (IFN) response. We have explored whether avian influenza viruses were less capable of combating the type I IFN response in mammalian cells, as this might be a determinant of host range restriction. A panel of avian influenza viruses isolated between 1927 and 1997 was assembled. The selected viruses showed variation in their ability to activate the expression of a reporter gene under the control of the IFN-β promoter and in the levels of IFN induced in mammalian cells. Surprisingly, the avian NS1 proteins expressed alone or in the genetic background of a human influenza virus controlled IFN-β induction in a manner similar to the NS1 protein of human strains. There was no direct correlation between the IFN-β induction and replication of avian influenza viruses in human A549 cells. Nevertheless, human cells deficient in the type I IFN system showed enhanced replication of the avian viruses studied, implying that the human type I IFN response limits avian influenza viruses and can contribute to host range restriction.
Over the past century three major influenza A virus pandemics have taken place: the Spanish flu (H1N1) in 1918, the Asian flu (H2N2) in 1957, and the Hong Kong flu (H3N2) in 1968 (16). The natural reservoir for influenza A viruses is birds, and at least 16 different antigenic subtypes of the hemagglutinin glycoprotein and 9 subtypes of the neuraminidase glycoprotein exist in aquatic bird species. The strains that caused pandemics in humans in 1957 and 1968 arose through reassortment, a genetic mixing of segments from avian and human influenza A viruses that have infected the same host. Such reassortant viruses were readily transmitted between humans who harbored no preexisting immunity to the avian antigenic subtypes. The origin of the 1918 pandemic virus has not been fully resolved, but it has been suggested that it directly transferred from an avian reservoir without reassortment (43). Occasionally other avian influenza viruses have also directly infected humans, such as in the current outbreak of highly pathogenic H5N1 avian influenza in Southeast Asia, where to date more than 232 people have been infected and at least 134 people have died (information found at the WHO website [http://www.who.int/csr/disease/avian_influenza/country/cases_table_2006_07_26/en/index.html]). A current concern is that this H5N1 strain will acquire the ability for sustained infection in humans directly or through reassortment with circulating human strains and cause the next pandemic. It is therefore important to understand factors that limit transmission of avian viruses within humans.
It is clear that the host range restriction of avian influenza viruses is multigenic and that there are several different settings and combinations of viruses that can ultimately allow an avian virus to establish infection in humans, but two themes recur (21). The receptor binding efficiency of the viral hemagglutinin to the target sialic acid receptors, and the abundance of these receptors on target cells, can influence viral entry (12, 21, 27, 38) and, strikingly, the viral PB2 polymerase protein is also strongly implicated in the ability of the virus to replicate in human or mouse cells (1, 16, 25, 41, 49).
In host range studies in cell culture, avian viruses are not blocked at the stage of entry into cells. Indeed, for some avian influenza virus strains, typified by the highly pathogenic avian influenza (HPAI) virus Rostock, viral genes are expressed and viral RNAs are replicated during infection in mammalian cells (1, 39). However, under low-multiplicity infection conditions human and mouse cells can remain nonpermissive to multiple rounds of virus replication (49). Thus, the block to permissivity either occurs late in the replication cycle or at the stage of initiation of infection of the second cell. Under some circumstances the interferon (IFN) response of cells to infection with viruses can result in an apparently abortive infection, and blocking the IFN response results in enhanced replication, converting the infection to a productive one (3, 4, 22, 28).
Many viruses, including human influenza A virus, have developed strategies for counteracting the host type I IFN response. In the case of myxoma virus (45) and members of the Paramyxoviridae, e.g., Newcastle disease virus (NDV) (33), simian virus 5 (SV5) (7, 32), and bovine respiratory syncytial virus (5), inhibition of innate immunity is cell type specific and can account for some, if not all, of the host range restriction of these viruses.
Antagonism of the innate response by human influenza A virus has been demonstrated to be a property of nonstructural protein 1 (NS1) (9, 13, 30). It has been suggested that NS1 can prevent IFN-β induction in several ways: by sequestering double-stranded RNA (dsRNA) through its amino terminus (8, 15, 35, 42, 46, 47), by binding protein kinase R (23), and by inhibiting posttranscriptional processing of the 3′ ends of cellular antiviral mRNAs by binding cleavage and polyadenylation specificity factor and poly(A)-binding protein II (6, 11, 24, 29, 30). The predominant mechanism by which a particular influenza virus counteracts the response is strain dependent and might be determined by the amino acid sequence of NS1, which clearly has several domains that affect its anti-IFN activity (17). The NS1 genes of avian influenza viruses differ from those of viruses adapted for replication in human hosts at several species-specific positions (31, 37). NS1 genes can be placed into two different alleles, termed A and B (40). Interestingly, avian viruses have NS1 genes of allele B, whereas allele A contains members of both human and avian origin. Indeed, the NS1 gene appears to undergo positive selection following its introduction into viruses that circulate in humans (20, 40). In light of these observations, we hypothesized that one way in which avian influenza viruses might be restricted in their host range could be an inability to overcome the innate IFN-α/β response mounted by the mammalian host. We have examined the ability of a panel of avian influenza viruses to induce mammalian cells to produce IFN-β and to activate a reporter gene controlled by the IFN-β promoter. Since the virus NS1 polypeptide plays a major role in evasion of the response of a cell to infection, we further examined the ability of avian NS1 polypeptides from avian influenza viruses to block intracellular pathways of innate immunity to infection in mammalian cells.
MATERIALS AND METHODS
Cell lines and viruses.
293-T, MDCK, Vero, and A549 cells were grown in Dulbecco's modification of Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, glutamine, pyruvate, and penicillin-streptomycin. U4C and 2C4 cells were a gift from Ian Kerr, CRUK, London, United Kingdom. An A549 cell line containing a stable integrate of the firefly luciferase gene driven by the IFN-β promoter (IFN-βLuc), was maintained in the same medium supplemented with 2 mg/ml G418. Cells were incubated at 37°C and 5% CO2. Viruses were either provided by M. Zambon (Health Protection Agency, United Kingdom) or from stocks held at the Institute for Animal Health. Avian viruses, NDV (provided by J. Banks, VLA, Weybridge) and Sendai virus (SeV) vM3 were prepared in 10-day old embryonated hen's eggs. Human strains were grown in MDCK cells and quantified by plaque assay on MDCK cells as previously described (10). All experiments with HPAI strains A/Chicken/Dobson/27 (variant 4H), the genetically manipulated virus RD1 (49), and A/Chicken/Rostock/34 (Rostock variant S3) were done in an approved category 4 high-containment laboratory at the Institute for Animal Health.
Generation of NS1 expression constructs.
NS1 was amplified from a full-length cDNA of segment 8 and cloned into the pCAGGSV5 expression vector as described previously (17). The amino acid change in the NS1 gene of PR8 (D92E) was generated by standard recombinant PCR techniques.
Luciferase reporter gene assays.
Plasmids encoding the firefly luciferase gene driven by one of the following inducible promoters were used: (i) IFN-βLuc containing the IFN-β promoter; (ii) ISG54Luc containing the ISG54 promoter; (iii) NF-κBLuc containing five copies of the PRD-II region of the IFN-β promoter to serve as an NF-κB-responsive promoter. A DNA mix containing 0.25 μg of the luciferase reporter construct, 0.25 μg β-galactosidase plasmid to serve as an internal control, and 0.67 μg pCAGGSV5 NS1 or empty vector was transfected into A549 cells or Vero cells as described previously (17). At 24 h posttransfection, the IFN-βLuc reporter construct was induced by infection with a high-multiplicity of infection (MOI; >5 50% effective infectious doses [EID50]/cell) of SeV vM3 for 16 h, the ISG54Luc construct induced by treatment with 1,000 IU/ml of IFN-α (Intron A; Schering-Plough) for 6 h, and the NF-κB-responsive promoter induced by application of 10 ng/ml of tumor necrosis factor alpha (TNF-α; R&D Systems) for 3 h. Cells were then lysed in 200 μl CCLR buffer (Promega), and luciferase activity was measured and normalized to β-galactosidase activity accordingly.
Virus infections in A549 IFN Luc cells.
A549 IFN Luc cells, which contain the firefly luciferase gene under the control of the IFN-β promoter, have been described previously (17). Cells were incubated with virus diluted in serum-free DMEM for 1 h at 37°C. The inoculum was removed and replaced with 2% DMEM, and the cells were incubated for a further 8 h at 37°C and processed for luciferase activity, as described above. Intracellular staining for NP was performed in parallel for each viral infection in the A549 IFNLuc cell line. At the end of the incubation period, cells were fixed for 10 min with ice-cold methanol-acetone and washed once with phosphate-buffered saline (PBS). Cells were incubated with mouse anti-NP monoclonal antibody (Immunologics Direct) diluted 1:300 for 1 h at room temperature, washed three times in PBS, and then incubated with anti mouse β-galactosidase-conjugated antibody (Harlem Sera Labs) diluted 1:400 for 1 h. After three washes with PBS, cells were incubated with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside for 3 h at 37°C, and staining was assessed using a light microscope.
IFN-β ELISA.
A549 cells were infected at an MOI of 5 PFU/cell. At 24 h postinfection, supernatants were applied to an IFN-β enzyme-linked immunosorbent assay (ELISA) 96-well plate, and the ELISA was performed according to the manufacturer's protocol (R&D Systems).
Virus yield assay.
A549 cells were infected at an MOI of 0.01 for 1 h in serum-free DMEM at 37°C. The inoculum was removed, and cells were treated with an acid wash (PBS, adjusted to pH 5.0 with HCl) for 1 min and then washed three times with PBS before the addition of serum-free DMEM. The final trypsin concentration was 0.1 μg/ml, and fresh trypsin was added daily. Cell culture supernatants were harvested at 72 h postinfection and titrated on MDCK cells by plaque assay.
Generation of recombinant viruses.
Viral RNA (vRNA) was extracted from 140 μl of viral supernatant corresponding to approximately 106 infectious particles using a QIAmp viral RNA mini kit (QIAGEN) according to the manufacturer's instructions. cDNA was synthesized from 5 to 10 μl RNA (approximately half of that recovered) using avian myeloblastosis virus reverse transcriptase (Promega) and 50 pmol of a primer complementary to the first 12 nucleotides of the 3′ ends of all eight of the vRNA segments. The NS gene was PCR amplified from the cDNA using specific sense and antisense primers complementary to the 3′ and 5′ ends of segment 8, respectively, which contained BsmBI sites to enable cloning into the pPolIRT vector (obtained from T. Zurcher, GlaxoSmithKline) (primer sequences are available on request). The NS polymerase I (Pol I) clones were transfected with the remaining seven plasmids with cDNAs based on an influenza A/Victoria/3/75 virus genetic background or five plasmids with cDNAs based on the influenza A/PR/8/34 virus with two plasmids for the surface antigens HA and NA of influenza A/Panama/2007/99 and four helper plasmids that express PB1, PB2, PA, and the NP polypeptides of A/Victoria/3/75, into 293-T cells, as described previously (44). The cells were subsequently cocultivated with MDCK cells expressing SV5 V protein (provided by R. Randall, University of St. Andrews, United Kingdom) which are deficient in IFN-α/β signaling. The rescued viruses were plaque purified, grown, and titrated on MDCK cells. The NS gene was amplified and sequenced to confirm the correct origin of the segment.
Yield assays in A549 cells or A549 cell lines expressing bovine viral diarrhea virus (BVDV) NPro or SV5 V.
A549 cell lines constitutively expressing either the V protein of SV5 or the NPro protein of BVDV were constructed by transfecting cells with the appropriate pEF.IRES.neo derivatives (2) and selecting transfected cells with 400 μg of G418/ml. A549 cells and A549-derived cell lines were infected at a low MOI of 0.1 PFU/cell and incubated in the presence of 0.1 mg/ml trypsin in serum-free DMEM. Virus-containing supernatants were harvested 72 h postinfection, and yields were titrated on MDCK cells.
RESULTS
Avian viruses induce varying amounts of IFN-β in A549 cells.
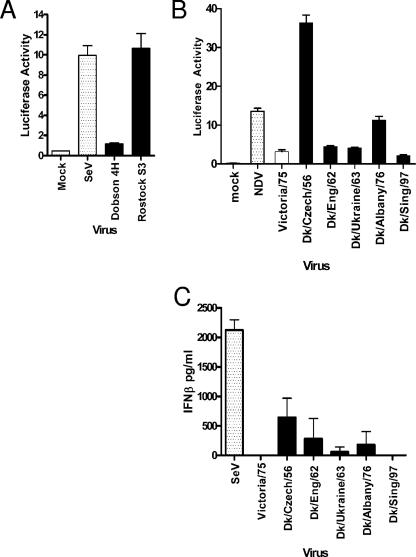
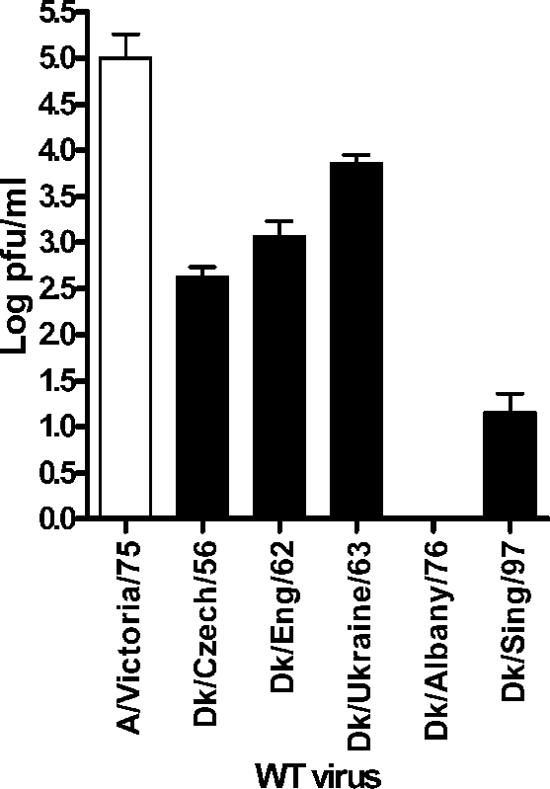
A major role of the NS1 protein of human influenza viruses is to antagonize the induction of IFNs that otherwise result from viral infection (reviewed by Haller et al. [14]). The consequence of this is that most human influenza virus strains do not induce high levels of IFN during infection of human cells in culture. To test whether avian influenza viruses activated the IFN-β promoter during infection of human cells, a human alveolar cell line carrying stably integrated luciferase under the control of the IFN-β promoter (17) was infected with a panel of avian influenza viruses representing various subtypes, none of which have been reported to infect humans (Table 1). In the experiment shown in Fig. 1A and B, infections were performed at an MOI of 5 PFU/cell, and luciferase production was measured 8 h postinfection. Production of viral NP was measured at this time by intracellular staining to confirm that every cell was infected. In other experiments a range of MOIs was used and always gave a similar pattern, as follows: interestingly, infection by some of the viruses, in particular Rostock S3, A/Dk/Czech/56, and A/Dk/Albany/76, resulted in considerable activation of the IFN-β promoter (Fig. 1A and B). This property was irrespective of the nature of the HA cleavage site, which is known to determine pathogenicity of the virus in poultry. Thus, Dobson 4H virus, an HPAI derivative that contains a highly cleavable HA protein, induced only low amounts of IFN-β promoter activity (Fig. 1A). To show that IFN was indeed synthesized and secreted from the infected cells, an IFN-β-specific ELISA was used (Fig. 1C). The induction of IFN-β was abrogated following UV treatment of the avian virus preparations, indicating that the inducer was a product of active viral replication (data not shown).
TABLE 1.
Panel of avian influenza A virus strains
| Strain | Pathogenicitya | Subtype |
|---|---|---|
| A/Chicken/Dobson/27- clone 4H | HPAI | H7N7 |
| A/Chicken/Rostock/34- clone S3 | HPAI | H7N1 |
| A/Duck/Czech/56 | LPAI | H4N6 |
| A/Duck/England/62 | LPAI | H4N6 |
| A/Duck/Ukraine/63 | LPAI | H3N8 |
| A/Duck/Albany/76 | LPAI | H12N5 |
| A/Duck/Singapore/97 | LPAI | H5N3 |
HPAI, high-pathogenicity avian influenza virus; LPAI, low-pathogenicity avian influenza virus.
FIG. 1.
Wild-type avian influenza A virus strains induce different amounts of IFN-β in A549 cells. An A549 IFN-βLuc reporter cell line was infected with high- (A) or low- (B) pathogenicity avian influenza viruses at an MOI of 5 PFU/cell. Human- or mammalian-adapted viruses were included for comparison. Luciferase activity was measured 8 h postinfection. (C) A549 cells were infected with viruses at an MOI of 5 PFU/cell. At 24 h postinfection, the amount of IFN-β released into the culture supernatant was measured by ELISA.
NS1 proteins derived from avian influenza viruses block IFN-β induction.
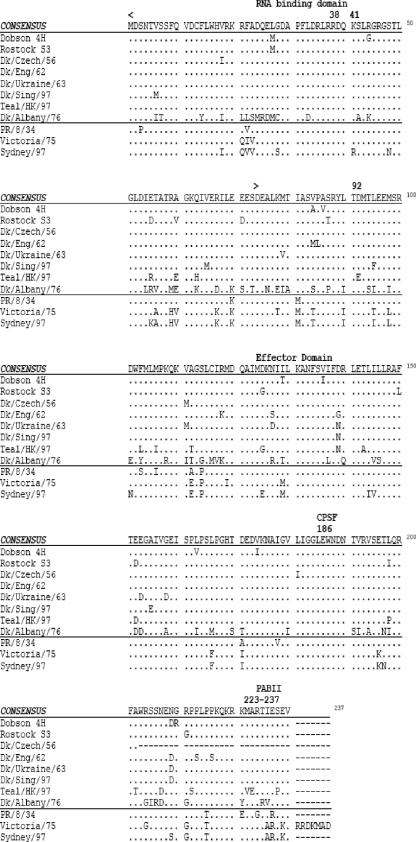
Based on the observations in Fig. 1, we hypothesized that some avian influenza A viruses, such as A/Dk/Czech/56 and A/Dk/Albany/76, might have NS1 proteins that are unable to counter the induction of human IFN-β. Sequence analysis of the NS1 genes from each of the avian influenza viruses demonstrated extensive variation among the NS1 proteins (Fig. 2). The NS1 derived from strain A/Dk/Albany/76 was of allele B; the remaining ones were of allele A, with the NS1 protein of A/Dk/Czech/56 truncated at residue 202, whereas all the other avian NS1 proteins were of equal length at 230 amino acids. In positions 22, 81, 112, 114, 171, and 215 and 227 there was complete amino acid replacement between the avian sequences in comparison with the human consensus.
FIG. 2.
Sequence alignment of the NS1 proteins of human and avian influenza viruses used in this study.
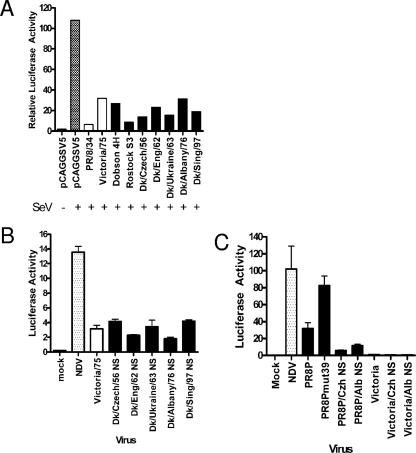
We therefore assessed the ability of NS1 proteins derived from the avian viruses to block IFN-β induction in A549 cells or to alter the induced response to IFN or other cytokines. The NS1 genes were cloned into the pCAGGSV5 expression vector, and their ability to block SeV-stimulated IFN-β induction in A549 cells was tested (Fig. 3A). For comparison, the NS1 proteins from two human viruses, A/PR/8/34 and A/Victoria/3/75, were included. Unexpectedly, all of the NS1 proteins from avian viruses blocked induction to a similar extent and with equal efficiency as the human ones. Furthermore, no differences were observed between the different NS1 constructs over a wide range of concentrations of expression plasmid (data not shown).
FIG. 3.
NS1 proteins derived from avian influenza viruses have the ability to block IFN-β induction. (A) A549 cells were transfected with pCAGG-NS1, a β-galactosidase expression plasmid, and an IFN-β Luc construct. At 24 h posttransfection, cells were infected with SeV for 16 h. Luciferase activity was measured and normalized to β-galactosidase activity for each transfection to account for differences in translation and transfection efficiencies. NS1 proteins from human viruses are represented with white bars, and NS1 proteins from avian viruses are shown with black bars. (B) The A549 IFN-βLuc reporter cell line was infected with recombinant viruses containing the avian NS gene in a stable human genetic background (A/Victoria/3/75) at an MOI of 5 PFU/cell, and luciferase activity was measured after 8 h. (C) The A549 IFN-βLuc reporter cell line was infected with recombinant viruses containing the avian NS gene in a stable human genetic background (PR8P is the 6 + 2 recombinant of A/PR/8/34 and A/Panama/2007/99) at an MOI of 5 PFU/cell, and luciferase activity was measured after 8 h.
It was important to demonstrate whether the avian NS1 proteins could act to block IFN induction in the context of an influenza virus infection. Reverse genetics techniques were used to generate recombinant influenza viruses that differed only in the origin of segment 8 RNAs, which encode the NS1 and NEP genes. The first set of viruses produced contained segment 8 from each of the avian viruses in the genetic background of the human strain A/Victoria/3/75. The A549 IFN-β reporter cell line was infected with the recombinant viruses, and IFN-β induction was measured 8 h postinfection (Fig. 3B). All of the recombinant viruses induced only low amounts of IFN-β, similar to that induced by the human parental A/Victoria/3/75 virus. To exclude the possibility that an additional gene(s) of A/Victoria/3/75 was acting to suppress IFN induction and thus masking a defect in NS1 function, we generated additional recombinant viruses containing the NS1 genes of either A/Dk/Czech/56 or A/Dk/Albany/76 in a different genetic background. PR8/Pan (PR8P) is a “6 + 2” recombinant virus with six internal genes of A/PR/8/34 and surface antigens HA and NA of A/Panama/2007/99. In this genetic background, deficiencies in NS1 gene function were evident. To show this, we made a mutant virus with a lesion in a domain of NS1 that is associated with deficiency in IFN control. This mutant has a coding change of aspartic acid to glycine at residue 39 in the NS1 protein and was less able to control induction of IFN than the isogenic wild-type virus (Fig. 3C, compare PR8Pmut 39 with PR8P). In contrast, the two recombinant viruses based on PR8P that contained the NS genes of A/Dk/Czech/56 or A/Dk/Albany/76 were able to control induction of IFN as well as the virus with the wild-type A/PR8 NS gene (Fig. 3C). Thus, the high induction of IFN in human cells by wild-type avian influenza viruses A/Dk/Czech/56 and A/Dk/Albany/76 was not genetically accounted for by a deficiency in their NS genes.
Avian NS1 proteins inhibit expression of newly induced genes.
We recently demonstrated that human influenza A virus strains can use different mechanisms to counteract the IFN response: significantly, we found that A/PR/8/34 NS1 was much less able than other human virus NS1 proteins to block the expression of newly synthesized mRNAs, as typified by the induction of gene expression in response to either IFN itself or to TNF-α stimulation of cells (17). We therefore explored the ability of the avian virus NS1 proteins to block interferon-induced gene expression from an interferon-stimulated response element (ISRE)-containing promoter in A549 cells (Fig. 4). All of the NS1 proteins limited expression of the reporter gene, although the NS1 proteins derived from A/Dk/Ukraine/63 and A/Teal/HongKong/317/97 strains were less efficient than other avian virus NS1 proteins and behaved like the A/PR/8/34 NS1 protein in this assay. The inability of NS1 from A/Teal/HongKong/317/97 virus to block IFN-induced gene expression was surprising because viruses that share this NS gene have been shown to be resistant to IFN treatment, a property conferred by the natural substitution of glutamic acid at position 92 of NS1 in place of the usual aspartate residue (36). To investigate the significance of the D92E change further, a mutant of A/PR/8/34 NS1 bearing the D92E mutation was engineered and compared with wild-type A/PR/8/34 NS1 for its ability to block the IFN-α-induced expression of luciferase controlled by the ISG54 promoter (Fig. 4). It can be seen that expression of either the wild-type A/PR/8/34 NS1 or the D92E mutant did not reduce the IFN-induced expression from the ISRE: levels of expression were as high as when a plasmid with the PR8 NS1 gene cloned in reverse was transfected. Hence, the D92E change did not confer resistance to the induction and expression of interferon-induced genes. Overall, the same pattern of control of induced gene expression was evident when TNF-α treatment of cells was used to induce transcription of an NF-κB-responsive promoter in the presence of expressed NS1 proteins (data not shown).
FIG. 4.
Avian NS1 proteins, like human NS1 proteins, block expression from an interferon-inducible promoter. A549 cells were transfected with ISG54 Luc and β-galactosidase reporter constructs together with pCAGG-NS1 constructs for expression of avian influenza virus NS1 proteins or NS1 proteins from PR/8 wt, PR/8 NS1 with residue 92 as aspartic acid, or a control plasmid with the wt PR/8 NS1 gene in reverse orientation, PR/8 Rev. Cells were incubated with 1,000 IU IFN-α/ml for 6 h, and luciferase activity was measured. For each transfection, the β-galactosidase activity was measured and luciferase activity was normalized accordingly.
Lack of correlation between induction of IFN response and replication in human cells.
We examined the possibility that the failure to undergo fully productive replication in mammalian cells for at least some avian influenza viruses is associated with the induction of IFN in infected cells. For example, the well-studied HPAI virus Rostock S3 induced a relatively high level of IFN-β in A549 cells, where it is unable to form plaques, in contrast to the host range mutant Dobson 4H (Fig. 1A). Previously, we described a series of Rostock S3-based recombinant viruses that differ only in segment 1 RNA and have an extended host range. These mutants encode PB2 proteins that contain sequences derived from the C-terminal region of the PB2 gene of the 4H mutant and are able to undergo multiple rounds of replication in mouse L cells and in A549 cells (49; Y. Yao, J. W. McCauley, and W. S. Barclay, unpublished data). We tested the level of IFN-β induction by one such recombinant virus that displays a broad host range, RD1, and found the level to be similar to that induced by Rostock S3 virus itself and unlike the low levels induced in cells infected with Dobson 4H (Fig. 5). Thus, it did not follow that a virus that induced high IFN-β resulted in a restricted host range in mammalian cells.
FIG. 5.
IFN induction does not correlate with host range restriction of the HPAI virus Rostock S3 and related recombinant virus. A549 IFN-β Luc cells were infected with avian influenza viruses at an MOI of 5 PFU/cell, and luciferase activity was measured 16 h after infection.
To examine further any possible correlation between the replication characteristics of the avian virus strains in A549 cells and the extent of IFN induction, cells were infected with each virus at low MOI and the virus produced over the following 72 h was measured by an assay on MDCK cells (Fig. 6). The yields of all of the avian viruses 72 h postinfection were lower than of the human strain, A/Victoria/3/75. A/Dk/Singapore/97 and A/Dk/Albany/76 replicated only to low levels in the human cells. However, the level of replication of these viruses in A549 cells showed no correlation with any of the IFN-inducing or -controlling properties of the NS1 genes of these viruses. We also measured the ability of recombinant viruses that have an avian RNA segment 8 in a human influenza virus background, such as Dk/Albany/76/NS and Dk/Sing/97 NS, to replicate in A549 cells. These recombinant viruses replicated to lower titers than the parental human virus, Victoria/3/75 WT (see Fig. 8, below), even though the amounts of IFN they induced were no higher (Fig. 3B). Thus, for these recombinant viruses there was also no correlation between levels of IFN induced and replication efficiency in human cells, although it is still possible that the viruses differ in their sensitivity to the IFN that is induced.
FIG. 6.
Replication of avian influenza viruses shows no correlation with the IFN-modulating properties of the NS1 proteins. A549 cells were infected with wild-type avian influenza A viruses at an MOI of 0.01 PFU/cell. Culture supernatants were then harvested at 72 h postinfection and titrated on MDCK cells.
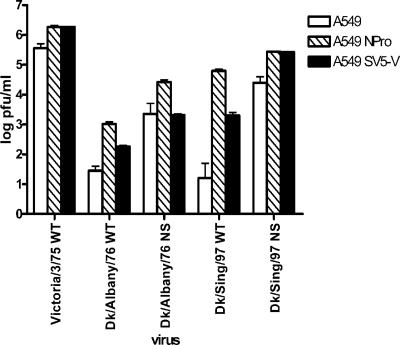
FIG. 8.
Replication by avian influenza virus in human cells is enabled by viral antagonists of the IFN response, such as BVDV NPro and the SV5 V protein. A549 cells were generated that expressed BVDV NPro or SV5 V proteins as indicated. Cells were infected with influenza viruses at an MOI of 0.01 and incubated with serum-free medium containing 0.1 μg/ml trypsin. Trypsin was replenished daily. Yields of virus in cell supernatants were titrated at 72 h postinfection by plaque assay in MDCK cells.
Replication of avian influenza virus in human cells is enhanced in the absence of a functional IFN system.
The present data indicate that induction of type I IFN in human cells is controlled by both avian and human influenza virus NS1 proteins. However, it was still possible that the replication of avian influenza viruses was restricted by the low levels of IFN that are produced upon infection with these viruses. We therefore assessed whether abrogating the IFN-α/β system would allow an avian influenza virus to undergo multiple cycles of replication, as indicated by the formation of plaques in human cells. We used two different examples of cells defective in the IFN system. Firstly, we utilized a human cell line (U4C) deficient in the Jak1 gene, leading to a failure to induce an antiviral response in IFN-treated cells (48). The parental wild-type cell line, 2C4, and the U4C cells were infected with the avian virus Rostock S3 or with the mammalian-adapted virus Dobson 4H, and plaque formation was assessed after 3 days (Fig. 7). As expected, Dobson 4H formed plaques in both cell lines. Interestingly, Rostock S3 was able to form plaques in the Jak1-deficient cells but did not form plaques in the parental cell line.
FIG. 7.
Plaque formation by avian influenza virus in human cells is enabled in the absence of a functional IFN system. Human cell lines 2C4 (Jak1+/+) and U4C (Jak1-/-) were infected with Dobson 4H and Rostock S3, and plaque formation was assessed 3 days postinfection following fixation in formal saline and staining with toluidine blue. The virus dilutions were 10−6, 10−7, and 10−8, as indicated.
Secondly, we generated two A549 cell lines that constitutively expressed virus-derived antagonists of the IFN response: the V protein from SV5 (that blocks type I IFN signaling through the targeted degradation of STAT1 and limits the induction of IFN-β in response to dsRNA [7, 34]), and the NPro protein from BVDV (that completely abolishes IFN-β induction through destablizing IRF-3 [18]). In these cell lines, the yield of a human influenza virus strain A/Victoria/3/75 was enhanced by approximately 10-fold. Likewise, the yields of two recombinant viruses carrying the avian RNA segment 8 in the human virus genetic background were also enhanced by between 10- and 20-fold in the cells that expressed NPro (Fig. 8). Remarkably, the yields of wild-type avian influenza virus strains, which were very low in unmodified human cells, were considerably enhanced in the modified cell lines. Strikingly, the yield of A/Dk/Singapore/97 increased more than 10,000-fold, and that of Dk/Albany/76 increased by over 100-fold in the cell lines expressing NPro. These results suggest a role for an IRF-3-dependent response of cells to infection in controlling the host ranges of avian influenza viruses.
DISCUSSION
Human influenza A viruses have evolved to counteract the type I IFN response through their NS1 protein. We postulated that avian influenza viral NS1 proteins might be less well adapted to the human host and, therefore, less capable of combating such a response: this might contribute to host range restriction. Indeed, a significant amount of sequence heterogeneity among NS1 proteins derived from a panel of avian influenza A viruses was observed. In the manuscript we have demonstrated that human cells infected with avian influenza viruses showed variations in their levels of IFN-β induction. This does not appear to be due to limitations of the NS1 protein, since despite the sequence heterogeneity, the avian NS1 proteins were capable of controlling the induction of the IFN-β response in a manner similar to human strains. Each individual avian NS1 protein expressed in A549 cells prevented the induction of an IFN-β promoter by SeV. Although the levels and timing of NS1 protein expression in this type of transfection assay might not represent the situation in the infected cell, nevertheless, the conclusions were supported by results from infections performed with recombinant viruses. In the context of a human virus genetic background (A/Victoria/3/75), all of the recombinant viruses containing heterologous NS genes encoding NS1 proteins derived from avian viruses induced a low amount of IFN-β similar to the human wild-type strain. Since we have shown a good correlation between the nuclear localization of IRF-3 and the level of induction of the IFN-β promoter (17), we suggest that both human and avian influenza virus NS1 proteins block these IFN induction pathways by preventing the detection of an inducer or the transmission of the signal that results in IRF-3 nuclear translocation.
Interestingly, three of the avian wild-type viruses still induced some IFN-β during virus infection despite producing amounts of NS1 protein similar to the human virus A/Victoria/3/75 NS1 at this time point and despite NS1 being present in the same subcellular location during infection (data not shown). These particular avian viruses did not contain sequence differences in their NS1 proteins at sites previously assigned to specific functions for dsRNA or cleavage and polyadenylation specificity factor binding, although one of them, A/Dk/Czech/56, had a truncation that ablated the poly(A)-binding protein II binding site. It may be that, for these viruses, induction occurred through a mechanism that the NS1 protein does not successfully block, an observation we have previously described for the human A/Sydney/97 strain (17). This pathway appears to be dependent on virus replication, since IFN induction by both A/Sydney/97 and by the avian viruses is abrogated following UV inactivation of the virus preparation.
Although the avian NS1 proteins were capable of preventing IFN-β induction, we found that substituting the NS gene of a human virus (A/Victoria/3/75) with one derived from an avian strain attenuated the human virus considerably in A549 cells. This implies that one of the gene products encoded by the avian NS genes, NS1 or NEP, affected virus replication, or that either of these genes functioned suboptimally within the A/Victoria/3/75 genetic background. There were several positions in NS1 where the amino acid sequence differed between the avian and human influenza virus strains; hence, one of these might define this property. In addition, there are two differences in the sequence of NEP, at position 14 (human Leu, avian Met/Gln) and 70 (human Gly, avian Ser) that could account for the attenuation of replication of these recombinant viruses.
We have previously reported that A/PR/8/34 differs from the majority of other human influenza virus strains in that it only poorly controls the expression of induced mRNAs (17). Here we show that two avian NS1 proteins, from influenza virus A/Dk/Ukraine/63 and from A/Teal/HK/97, share this phenotype. It is noticeable that two out of three of these NS1 proteins (A/PR/8/34 and A/Teal/HK/97) differ from the consensus at residue 103 (Fig. 2), although we have no evidence at present to ascertain whether this change accounts for their poor control over expression of induced mRNAs. It has been reported that the HK/97 viruses, which carry the same NS1 gene sequence as A/Teal/HK/97, are resistant to pretreatment of porcine cells with IFN. This phenotype was conferred upon an A/PR/8/34 NS1 protein by the mutation D92E (36). Here we have shown that expression of neither wild-type A/Teal/HK/97 NS1 protein nor of the PR8D92E mutant NS1 protein blocked ISRE signaling or ISRE expression compared with NS1 from A/PR/8/34 with aspartic acid at position 92. Thus, the mechanism for IFN resistance conferred on the virus by this mutation remains elusive.
Although we found no absolute correlation between IFN induction and the ability of avian influenza viruses to replicate in human cell culture, it is feasible, based on our results examining the enhanced replication of avian influenza viruses in cells with compromised IFN responses, that the IFN response plays an important role in limiting influenza virus host range: the dramatic enhancement of avian influenza virus yield in human cells that express the BVDV NPro gene underscores this. Interestingly, the enhancement of yield is not so marked for the recombinant viruses that have only segment 8 RNA derived from the avian viral source. Therefore, the sensitivity of avian influenza viruses to human IFN might map to genes other than NS1. It seems possible that the sensitivity of different strains of influenza viruses to the IFN-stimulated genes could vary markedly and that this could contribute to host range (26). Indeed, mechanisms have already been described by which influenza viral gene products counter the activity of individual IFN-stimulated genes (19, 23, 50).
Taken together, our studies suggest that abrogating the type I IFN-α/β response may enable an avian virus to establish infection in human cells more readily, not necessarily because its own NS1 cannot counteract the normal innate response but because it is one less factor for the virus to overcome during suboptimal infection in the new host to which it has not yet adapted. The gene products of RNA segment 8 can influence multicycle replication, but it still remains unclear as to exactly how this may be linked to the IFN-α/β response. In vivo, other cells would also be important for the outcome of infection, such as macrophages and dendritic cells, which might produce a much higher level of antiviral cytokines than A549 cells, thus mounting a stronger response against the virus.
Acknowledgments
We thank M. Zambon (Health Protection Agency, United Kingdom) for providing the human and some of the avian influenza viruses, J. Banks (VLA Weybridge, United Kingdom) for providing NDV, and T. Zurcher (GlaxoSmithKline, United Kingdom) for providing plasmids for rescue of A/Victoria/3/75 virus. We also thank R. Randall (St. Andrews University, United Kingdom) for providing the MDCK cell line expressing SV5 V protein and Diane Watling and Ian Kerr for 2C4 and U4C cells.
This work was funded by The Wellcome Trust and through a BBSRC core strategic grant to I.A.H.
Footnotes
Published ahead of print on 20 December 2006.
REFERENCES
- 1.Almond, J. W. 1977. A single gene determines the host range of influenza virus. Nature 270:617-618. [DOI] [PubMed] [Google Scholar]
- 2.Andrejeva, J., D. F. Young, S. Goodbourn, and R. E. Randall. 2002. Degradation of STAT1 and STAT2 by the V proteins of simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons. J. Virol. 76:2159-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baigent, S. J., G. Zhang, M. D. Fray, H. Flick-Smith, S. Goodbourn, and J. W. McCauley. 2002. Inhibition of beta interferon transcription by noncytopathogenic bovine viral diarrhea virus is through an interferon regulatory factor 3-dependent mechanism. J. Virol. 76:8979-8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barreca, C., and P. O'Hare. 2004. Suppression of herpes simplex virus 1 in MDBK cells via the interferon pathway. J. Virol. 78:8641-8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bossert, B., and K. K. Conzelmann. 2002. Respiratory syncytial virus (RSV) nonstructural (NS) proteins as host range determinants: a chimeric bovine RSV with NS genes from human RSV is attenuated in interferon-competent bovine cells. J. Virol. 76:4287-4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Z., Y. Li, and R. M. Krug. 1999. Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3′-end processing machinery. EMBO J. 18:2273-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 73:9928-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donelan, N. R., C. F. Basler, and A. Garcia-Sastre. 2003. A recombinant influenza A virus expressing an RNA-binding-defective NS1 protein induces high levels of beta interferon and is attenuated in mice. J. Virol. 77:13257-13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egorov, A., S. Brandt, S. Sereinig, J. Romanova, B. Ferko, D. Katinger, A. Grassauer, G. Alexandrova, H. Katinger, and T. Muster. 1998. Transfectant influenza A viruses with long deletions in the NS1 protein grow efficiently in Vero cells. J. Virol. 72:6437-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elleman, C. J., and W. S. Barclay. 2004. The M1 matrix protein controls the filamentous phenotype of influenza A virus. Virology 321:144-153. [DOI] [PubMed] [Google Scholar]
- 11.Fortes, P., A. Beloso, and J. Ortin. 1994. Influenza virus NS1 protein inhibits pre-mRNA splicing and blocks mRNA nucleocytoplasmic transport. EMBO J. 13:704-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagneux, P., M. Cheriyan, N. Hurtado-Ziola, E. C. van der Linden, D. Anderson, H. McClure, A. Varki, and N. M. Varki. 2003. Human-specific regulation of alpha 2-6-linked sialic acids. J. Biol. Chem. 278:48245-48250. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Sastre, A., A. Egorov, D. Matassov, S. Brandt, D. E. Levy, J. E. Durbin, P. Palese, and T. Muster. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324-330. [DOI] [PubMed] [Google Scholar]
- 14.Haller, O., G. Kochs, and F. Weber. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344:119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatada, E., and R. Fukuda. 1992. Binding of influenza A virus NS1 protein to dsRNA in vitro. J. Gen. Virol. 73:3325-3329. [DOI] [PubMed] [Google Scholar]
- 16.Hatta, M., P. Gao, P. Halfmann, and Y. Kawaoka. 2001. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293:1840-1842. [DOI] [PubMed] [Google Scholar]
- 17.Hayman, A., S. Comely, A. Lackenby, S. Murphy, J. McCauley, S. Goodbourn, and W. Barclay. 2006. Variation in the ability of human influenza A viruses to induce and inhibit the IFN-beta pathway. Virology 347:52-64. [DOI] [PubMed] [Google Scholar]
- 18.Hilton, L., K. Moganeradj, G. Zhang, Y. H. Chen, R. E. Randall, J. W. McCauley, and S. Goodbourn. 2006. The NPro product of bovine viral diarrhea virus inhibits DNA binding by interferon regulatory factor 3 and targets it for proteasomal degradation. J. Virol. 80:11723-11732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, T., J. Pavlovic, P. Staeheli, and M. Krystal. 1992. Overexpression of the influenza virus polymerase can titrate out inhibition by the murine Mx1 protein. J. Virol. 66:4154-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawaoka, Y., O. T. Gorman, T. Ito, K. Wells, R. O. Donis, M. R. Castrucci, I. Donatelli, and R. G. Webster. 1998. Influence of host species on the evolution of the nonstructural (NS) gene of influenza A viruses. Virus Res. 55:143-156. [DOI] [PubMed] [Google Scholar]
- 21.Kuiken, T., E. C. Holmes, J. McCauley, G. F. Rimmelzwaan, C. S. Williams, and B. T. Grenfell. 2006. Host species barriers to influenza virus infections. Science 312:394-397. [DOI] [PubMed] [Google Scholar]
- 22.Kumagai, T., T. Shimizu, and M. Matumoto. 1958. Detection of hog cholera virus by its effect on Newcastle disease virus in swine tissue culture. Science 128:366. [DOI] [PubMed] [Google Scholar]
- 23.Li, S., J. Y. Min, R. M. Krug, and G. C. Sen. 2006. Binding of the influenza A virus NS1 protein to PKR mediates the inhibition of its activation by either PACT or double-stranded RNA. Virology 349:13-21. [DOI] [PubMed] [Google Scholar]
- 24.Li, Y., Z. Y. Chen, W. Wang, C. C. Baker, and R. M. Krug. 2001. The 3′-end-processing factor CPSF is required for the splicing of single-intron pre-mRNAs in vivo. RNA 7:920-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, Z., H. Chen, P. Jiao, G. Deng, G. Tian, Y. Li, E. Hoffmann, R. G. Webster, Y. Matsuoka, and K. Yu. 2005. Molecular basis of replication of duck H5N1 influenza viruses in a mammalian mouse model. J. Virol. 79:12058-12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcus, P. I., J. M. Rojek, and M. J. Sekellick. 2005. Interferon induction and/or production and its suppression by influenza A viruses. J. Virol. 79:2880-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matrosovich, M., A. Tuzikov, N. Bovin, A. Gambaryan, A. Klimov, M. R. Castrucci, I. Donatelli, and Y. Kawaoka. 2000. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J. Virol. 74:8502-8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura, S., T. Shimazaki, K. Sakamoto, A. Fukusho, Y. Inoue, and N. Ogawa. 1995. Enhanced replication of orbiviruses in bovine testicle cells infected with bovine viral diarrhoea virus. J. Vet. Med. Sci. 57:677-681. [DOI] [PubMed] [Google Scholar]
- 29.Nemeroff, M. E., S. M. Barabino, Y. Li, W. Keller, and R. M. Krug. 1998. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′end formation of cellular pre-mRNAs. Mol. Cell 1:991-1000. [DOI] [PubMed] [Google Scholar]
- 30.Noah, D. L., K. Y. Twu, and R. M. Krug. 2003. Cellular antiviral responses against influenza A virus are countered at the posttranscriptional level by the viral NS1A protein via its binding to a cellular protein required for the 3′ end processing of cellular pre-mRNAS. Virology 307:386-395. [DOI] [PubMed] [Google Scholar]
- 31.Obenauer, J. C., J. Denson, P. K. Mehta, X. Su, S. Mukatira, D. B. Finkelstein, X. Xu, J. Wang, J. Ma, Y. Fan, K. M. Rakestraw, R. G. Webster, E. Hoffmann, S. Krauss, J. Zheng, Z. Zhang, and C. W. Naeve. 2006. Large-scale sequence analysis of avian influenza isolates. Science 311:1576-1580. [DOI] [PubMed] [Google Scholar]
- 32.Parisien, J. P., J. F. Lau, and C. M. Horvath. 2002. STAT2 acts as a host range determinant for species-specific paramyxovirus interferon antagonism and simian virus 5 replication. J. Virol. 76:6435-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park, M. S., A. Garcia-Sastre, J. F. Cros, C. F. Basler, and P. Palese. 2003. Newcastle disease virus V protein is a determinant of host range restriction. J. Virol. 77:9522-9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poole, E., B. He, R. A. Lamb, R. E. Randall, and S. Goodbourn. 2002. The V proteins of simian virus 5 and other paramyxoviruses inhibit induction of interferon-beta. Virology 303:33-46. [DOI] [PubMed] [Google Scholar]
- 35.Qian, X. Y., F. Alonso-Caplen, and R. M. Krug. 1994. Two functional domains of the influenza virus NS1 protein are required for regulation of nuclear export of mRNA. J. Virol. 68:2433-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo, S. H., E. Hoffmann, and R. G. Webster. 2002. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat. Med. 8:950-954. [DOI] [PubMed] [Google Scholar]
- 37.Shaw, M., L. Cooper, X. Xu, W. Thompson, S. Krauss, Y. Guan, N. Zhou, A. Klimov, N. Cox, R. Webster, W. Lim, K. Shortridge, and K. Subbarao. 2002. Molecular changes associated with the transmission of avian influenza a H5N1 and H9N2 viruses to humans. J. Med. Virol. 66:107-114. [DOI] [PubMed] [Google Scholar]
- 38.Shinya, K., M. Ebina, S. Yamada, M. Ono, N. Kasai, and Y. Kawaoka. 2006. Avian flu: influenza virus receptors in the human airway. Nature 440:435-436. [DOI] [PubMed] [Google Scholar]
- 39.Smith, G. L., and A. J. Hay. 1982. Replication of the influenza virus genome. Virology 118:96-108. [DOI] [PubMed] [Google Scholar]
- 40.Suarez, D. L., and M. L. Perdue. 1998. Multiple alignment comparison of the non-structural genes of influenza A viruses. Virus Res. 54:59-69. [DOI] [PubMed] [Google Scholar]
- 41.Subbarao, E. K., W. London, and B. R. Murphy. 1993. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J. Virol. 67:1761-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, and A. Garcia-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 74:7989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taubenberger, J. K., A. H. Reid, R. M. Lourens, R. Wang, G. Jin, and T. G. Fanning. 2005. Characterization of the 1918 influenza virus polymerase genes. Nature 437:889-893. [DOI] [PubMed] [Google Scholar]
- 44.Thompson, C. I., W. S. Barclay, and M. C. Zambon. 2004. Changes in in vitro susceptibility of influenza A H3N2 viruses to a neuraminidase inhibitor drug during evolution in the human host. J. Antimicrob. Chemother. 53:759-765. [DOI] [PubMed] [Google Scholar]
- 45.Wang, F., Y. Ma, J. W. Barrett, X. Gao, J. Loh, E. Barton, H. W. Virgin, and G. McFadden. 2004. Disruption of Erk-dependent type I interferon induction breaks the myxoma virus species barrier. Nat. Immunol. 5:1266-1274. [DOI] [PubMed] [Google Scholar]
- 46.Wang, W., K. Riedel, P. Lynch, C. Y. Chien, G. T. Montelione, and R. M. Krug. 1999. RNA binding by the novel helical domain of the influenza virus NS1 protein requires its dimer structure and a small number of specific basic amino acids. RNA 5:195-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, X., M. Li, H. Zheng, T. Muster, P. Palese, A. A. Beg, and A. Garcia-Sastre. 2000. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 74:11566-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watling, D., D. Guschin, M. Muller, O. Silvennoinen, B. A. Witthuhn, F. W. Quelle, N. C. Rogers, C. Schindler, G. R. Stark, J. N. Ihle, et al. 1993. Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway. Nature 366:166-170. [DOI] [PubMed] [Google Scholar]
- 49.Yao, Y., L. J. Mingay, J. W. McCauley, and W. S. Barclay. 2001. Sequences in influenza A virus PB2 protein that determine productive infection for an avian influenza virus in mouse and human cell lines. J. Virol. 75:5410-5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan, W., and R. M. Krug. 2001. Influenza B virus NS1 protein inhibits conjugation of the interferon (IFN)-induced ubiquitin-like ISG15 protein. EMBO J. 20:362-371. [DOI] [PMC free article] [PubMed] [Google Scholar]