The hepatitis B virus nucleocapsid is a very immunogenic structure (7) that activates mouse B cells in a T-cell-independent manner (3). We reported a similar observation with human B cells: nucleocapsids induced capsid-binding immunoglobulin M molecules when purified B cells of unprimed humans were transferred into the spleens of NOD/Scid mice (2). However, recent results which we report in this letter suggest that an immunostimulatory contaminant in the HBcAg preparation rather than the nucleocapsids themselves were likely responsible for the observed T-cell-independent B-cell activation. This emerged when four different nucleocapsid preparations were used to study the stimulatory potential of nucleocapsids for purified human B cells. HBcAg-c1 (Diasorin), HBcAg-c2 (Biodesign), and HBcAg-c3 (Biodesign) were produced in Escherichia coli. HBcAg-y was produced in Saccharomyces cerevisiae (GlaxoSmithKline Biologicals, Belgium). HBcAg-c1 nucleocapsids lack the first 2 amino acids and contain 11 foreign amino acids, 8 of which are derived from β-galactosidase (4). HBcAg-c1, HBcAg-y, and HBcAg-c2 contain encapsidated RNA, while HBcAg-c3 nucleocapsids do not, as these lack amino acids 145 to 183. The lipopolysaccharide (LPS) contents of HBcAg-c1, -y, -c2, and -c3 were 136, 0.422, 9.48, and 0.17 endotoxin units LPS/μg nucleocapsid, respectively.
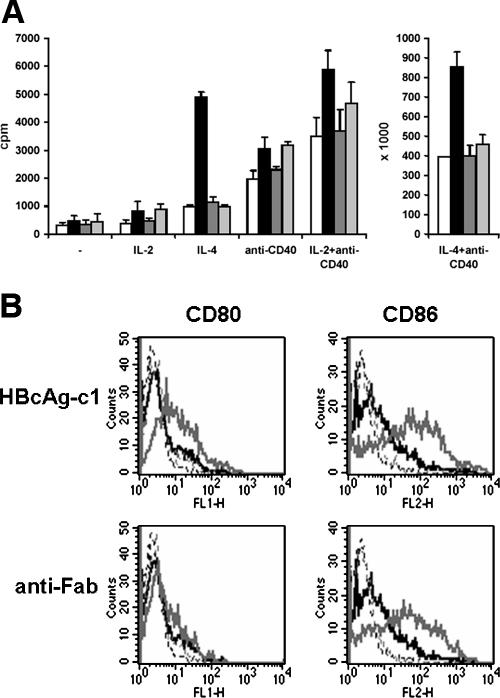
We first investigated whether nucleocapsids induced or enhanced the proliferation of purified B cells. Neither HBcAg-c1, HBcAg-y, nor HBcAg-c2 induced thymidine (TdR) incorporation (Fig. 1A). The addition of HBcAg-c1 to B cells stimulated with interleukin-4 (IL-4), anti-CD40 plus IL-2, and anti-CD40 plus IL-4 had a clear synergistic effect on incorporation of TdR. This was not observed with HBcAg-y and HBcAg-c2. Next, we observed that HBcAg-c1 caused a clear up-regulation of CD86 and CD80 (Fig. 1B). This was never observed with HBcAg-y, HBcAg-c2, and HBcAg-c3 (data not shown). A goat anti-human Fab-specific F(ab)2 fragment induced only the expression of CD86, indicating that the intracellular pathways triggered by HBcAg-c1 and the anti-Fab were different. Because only HBcAg-c1 stimulated B cells, we concluded that a contaminant was present. Total purified human circulating B cells do not respond to different toll-like receptor 2 (TLR2), TLR4, TLR2/TLR6, and TLR7/8 ligands (1), suggesting that HBcAg-c1-encapsidated RNA and contaminating LPS and TLR2 ligands (5, 6) were not responsible for the activation of B cells. The capacity of HBcAg-c1 to induce CD80 and CD86 was not destroyed by boiling for 30 min (data not shown), demonstrating indeed that HBcAg-c1 nucleocapsids themselves were not responsible for the stimulation of B cells. Unluckily, the use of HBcAg-c1 in our experiments suggested that nucleocapsids behaved as T-cell-independent B-cell antigens (2). We suggested previously that the stimulatory capacities of nucleocapsids for monocytes and dendritic cells might be attributed to contaminating TLR4 and TLR2 ligands (5, 6). Our data again highlight that caution is needed when studying the stimulatory capacities of nucleocapsids, especially when produced in a bacterium. Proteins produced in E. coli inherently contain products like LPS, bacterial DNA, porins, lipid A-associated proteins, fimbrial proteins, protein A, and lipoproteins. All these can activate cells (8).
FIG. 1.
(A) HBcAg-c1 enhances B-cell proliferation induced by IL-4 and anti-CD40 plus IL-2 or IL-4. Purified B cells were incubated for 3 days with 1 μg/ml of HBcAg-c1 (black), HBcAg-y (dark gray), or HBcAg-c2 (light gray) or without nucleocapsids (white), together with 10 ng/ml IL-2, 20 ng/ml IL-4, 250 ng/ml anti-CD40, or 250 ng/ml anti-CD40 plus 10 ng/ml IL-2 or 20 ng/ml IL-4. [3H]Thymidine was added for 18 h. Error bars represent standard deviations (n = 3). cpm, counts per minute. (B) Only HBcAg-c1 induces the costimulatory molecules CD80 and CD86 on purified B cells. Purified B cells were mock treated or incubated for 3 days with 10 μg/ml of HBcAg-c1 or anti-Fab. Cells were immunostained for CD80 and CD86, and the fluorescence intensity was measured. Black lines represent mock-treated cells, gray lines represent stimulated cells, and dotted lines represent isotypic controls. (The HBcAg-c1-induced expression of CD86 and CD80 occurred with B cells from at least 3 other donors [data not shown]). Human peripheral blood mononuclear cells were isolated from buffy coats by using Ficoll-Hypaque (density = 1.077 g/ml; Nycomed Pharma, Oslo, Norway) centrifugation. Cells were stored in liquid nitrogen. All experiments were performed with CD19+ cells (B cells) positively selected with anti-CD19 microbeads (Miltenyi Biotec) from thawed peripheral blood mononuclear cells.
Footnotes
Published ahead of print on 6 December 2006.
REFERENCES
- 1.Bekeredjian-Ding, I. B., M. Wagner, V. Hornung, T. Giese, M. Schnurr, S. Endres, and G. Hartmann. 2005. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J. Immunol. 174:4043-4050. [DOI] [PubMed] [Google Scholar]
- 2.Cao, T., U. Lazdina, I. Desombere, P. Vanlandschoot, D. R. Milich, M. Sällberg, and G. Leroux-Roels. 2001. Hepatitis B virus core antigen binds and activates naive human B cells in vivo: studies with a human PBL-NOD/SCID mouse model. J. Virol. 75:6359-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milich, D. R., M. Chen, F. Schodel, D. L. Peterson, J. E. Jones, and J. L. Hughes. 1997. Role of B cells in antigen presentation of the hepatitis B core. Proc. Natl. Acad. Sci. USA 94:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stahl, S., P. MacKay, M. Magazin, S. A. Bruce, and K. Murray. 1982. Hepatitis B virus core antigen: synthesis in Escherichia coli and application in diagnosis. Proc. Natl. Acad. Sci. USA 79:1606-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanlandschoot, P., and G. Leroux-Roels. 2005. The role of heparan sulfate and TLR2 in cytokine induction by hepatitis B virus capsids. J. Immunol. 175:6253-6255. [DOI] [PubMed] [Google Scholar]
- 6.Vanlandschoot, P., F., Van Houtte, P. Ulrichts, J. Tavernier, and G. Leroux-Roels. 2005. Immunostimulatory potential of hepatitis B nucleocapsid preparations: lipopolysaccharide contamination should not be overlooked. J. Gen. Virol. 86:323-331. [DOI] [PubMed] [Google Scholar]
- 7.Vanlandschoot, P., T. Cao, and G. Leroux-Roels. 2003. The nucleocapsid of the hepatitis B virus: a remarkable immunogenic structure. Antiviral Res. 60:67-74. [DOI] [PubMed] [Google Scholar]
- 8.Wakelin, S. J., I. Sabroe, C. D. Gregory, I. R. Poxton, J. L. R. Forsythe, O. J. Garden, and S. E. M. Howie. 2006. “Dirty little secrets”—endotoxin contamination of recombinant proteins. Immunol. Lett. 106:1-7. [DOI] [PubMed] [Google Scholar]