Abstract
Dendritic cells (DCs) potently stimulate the cell-cell transmission of human immunodeficiency virus type 1 (HIV-1). However, the mechanisms that underlie DC transmission of HIV-1 to CD4+ T cells are not fully understood. DC-SIGN, a C-type lectin, efficiently promotes HIV-1 trans infection. DC-SIGN is expressed in monocyte-derived DCs (MDDCs), macrophage subsets, activated B lymphocytes, and various mucosal tissues. MDDC-mediated HIV-1 transmission to CD4+ T cells involves DC-SIGN-dependent and -independent mechanisms. DC-SIGN transmission of HIV-1 depends on the donor cell type. HIV-1 Nef can upregulate DC-SIGN expression and promote DC-T-cell clustering and HIV-1 spread. Nef also downregulates CD4 expression; however, the effect of the CD4 downmodulation on DC-mediated HIV-1 transmission has not been examined. Here, we report that CD4 expression levels correlate with inefficient HIV-1 transmission by monocytic cells expressing DC-SIGN. Expression of CD4 on Raji B cells strongly impaired DC-SIGN-mediated HIV-1 transmission to T cells. By contrast, enhanced HIV-1 transmission was observed when CD4 molecules on MDDCs and DC-SIGN-CD4-expressing cell lines were blocked with specific antibodies. Coexpression of CD4 and DC-SIGN in Raji cells promoted the internalization and intracellular retention of HIV-1. Interestingly, internalized HIV-1 particles were sorted and confined to late endosomal compartments that were positive for CD63 and CD81. Furthermore, in HIV-1-infected MDDCs, significant downregulation of CD4 by Nef expression correlated with enhanced viral transmission. These results suggest that CD4, which is present at various levels in DC-SIGN-positive primary cells, is a key regulator of HIV-1 transmission.
Understanding human immunodeficiency virus (HIV)-host cell interactions and defining the mechanisms of cell-mediated virus transmission are essential for developing effective strategies to combat HIV-1 infection (60). Dendritic cells (DCs) perform a pivotal role in the induction and regulation of adaptive immune responses (3). DCs are proposed to be among the first cells that encounter HIV-1 at the mucosa and play an important and multifaceted role in HIV-1 infection (7, 39, 60). Coculture of HIV-1-pulsed DCs with CD4+ T cells dramatically enhances the infection of the T cells (7, 39, 40). DC-captured HIV-1 is directed to synaptic junctions or infectious synapses that form between DCs and CD4+ T cells, which facilitate HIV-1 trans infection (32). However, the mechanisms underlying DC-enhanced HIV-1 trans infection are not fully understood.
A C-type lectin, DC-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN, also known as CD209), functions as an attachment factor of HIV-1 and facilitates DC-mediated viral transmission (18, 19). DC-SIGN-expressing DCs from human rectal mucosa efficiently bind and transfer HIV-1 to CD4+ T cells (23). A recent study indicated that DC-SIGN induced on activated primary B-lymphocytes potentiates HIV-1 transmission to CD4+ T cells (41). Moreover, the suppression of DC-SIGN expression can impair the formation of the infectious synapse between DCs and T cells, which inhibits the transmission of X4 HIV-1 to T cells (1, 2). Those studies implicate DC-SIGN in the pathogenesis of HIV.
DC-SIGN-independent mechanisms are also involved in DC-mediated HIV-1 trans infection of CD4+ T cells (4, 21, 22, 53, 59, 63). The mechanisms or alternative molecule(s) that accounts for the DC-SIGN-independent HIV-1 transmission by DCs has not currently been elucidated. Despite the detection of DC-SIGN in monocyte-derived DCs (MDDCs), macrophage subpopulations, activated B cells, and other human tissues (13, 19, 21, 23, 24, 31, 41, 49, 50), major DC subsets in vivo, including myeloid DCs, plasmacytoid DCs, and Langerhans cells, do not express DC-SIGN (54, 55), suggesting that these cells utilize DC-SIGN-independent mechanisms of HIV-1 transmission. Nonetheless, DC-SIGN-dependent and -independent transmission seems to rely on the access of pathways that direct virus synaptic junctions between cells, suggesting that common underlying mechanisms may be utilized.
Interestingly, Nef can upregulate cell surface expression of DC-SIGN and significantly increase the clustering of HIV-1-infected MDDCs with T lymphocytes (51). The Nef protein of HIV-1 and simian immunodeficiency virus (SIV) is required for efficient viral replication and AIDS pathogenicity in HIV-1-infected humans or SIV-infected macaques (10, 11, 26, 27). The mechanisms by which the Nef protein acts as a pathogenic factor in vivo are not fully understood, although a recent finding suggests that the inability of lentivirus Nef to suppress CD4+ T-cell activation correlates with viral pathogenesis (45). It has been reported that HIV-1 Nef expression is required for efficient viral replication in cocultures of MDDCs and T cells (37). Notably, the Nef protein downregulates the cell surface expression of the HIV-1 receptors CD4 and CCR5, which protects the infected cells from superinfection (34, 38). Nef-mediated downregulation of major histocompatibility complex class I facilitates the immune evasion of HIV-1-infected cells from recognition by cytotoxic T lymphocytes (8, 46). HIV-1 and SIV Nef proteins can downmodulate CD28 and disrupt T-cell activation (52). However, it is unknown whether the downregulation of any of these molecules affects DC-mediated HIV-1 transmission to target cells, particularly the downmodulation of CD4, the primary HIV-1 receptor.
Here, we report that the cis expression of CD4 strongly impairs DC-SIGN-mediated HIV-1 transmission to T cells and alters intracellular viral trafficking. Rescue of HIV-1 transmission to T cells was observed when CD4 molecules on donor cell surfaces were blocked with specific antibodies known to interrupt the binding of the HIV-1 envelope protein (Env). Interestingly, in HIV-1-infected MDDCs, significant downregulation of CD4 by Nef expression correlates with enhanced viral transmission. Our results suggest that CD4 levels may modulate DC-mediated HIV-1 transmission, which provides a new insight into HIV-1 pathogenesis.
MATERIALS AND METHODS
Plasmids.
The following plasmids have been previously described: the HIV-1 proviral vector pNL-Luc-E−R− (HIV-Luc), which contains a firefly luciferase reporter gene (9), HIV-1 Env expression plasmids pJRFL and pHXB2, DC-SIGN expression vector pMX-DC-SIGN (59, 63), and the vesicular stomatitis virus G protein-expressing construct pL-VSV-G (57). The human CD4-expressing construct pCEN-CD4 with a neomycin-resistant gene was obtained from Dan Littman (New York University Medical Center). Wild-type (WT) HIV-1 proviral vectors pNLAD8 (R5 tropic) and pNL4-3 (X4 tropic) (14) were kind gifts from Eric Freed (National Cancer Institute, Frederick, MD). HIV-1 nef-inactivated proviral vector pNLAD8Δnef (37) was a kind gift from Olivier Schwartz (Pasteur Institute, France). pNL4-3Δnef was generated by inserting a frameshift mutation at the unique XhoI site of the viral genome as described previously (37). Nef expression by HIV-1 proviral vectors was confirmed by Western blotting.
Primary cells.
Human peripheral blood mononuclear cells were separated from buffy coat units of healthy donors (provided by the Blood Center of Wisconsin, Milwaukee, WI) using Histopaque (Sigma-Aldrich) gradient centrifugation. CD14+ monocytes and CD4+ T cells were isolated separately from peripheral blood mononuclear cells using anti-human CD14- and anti-human CD4-coated magnetic particles according to the manufacturer's instructions (BD Biosciences). Immature MDDCs were generated from purified monocytes treated with 50 ng/ml of granulocyte-macrophage colony-stimulating factor and interleukin 4 (IL-4) (R&D Systems) for 5 days as previously described (59, 63). CD4+ T cells were activated using phytohemagglutinin (Sigma-Aldrich) as described previously (57) and cultured in RPMI 1640 medium with 10% fetal bovine serum (FBS) (Atlanta Biologicals) in the presence of recombinant IL-2 (NIH AIDS Research and Reference Reagent Program).
Cell lines.
The following cell lines have been previously described (59, 63): the human B-cell line Raji, monocytic cell line THP-1ATCC, and their derived stable DC-SIGN-expressing cell lines, human embryonic kidney cell line HEK293T, human T-cell line Hut/CCR5, and HIV-1 indicator cell line GHOST/X4/R5. The human monocytic cell line U937 (ATCC CRL-1593.2) was obtained from the American Type Culture Collection (ATCC). The stable DC-SIGN-expressing U937 cell line was generated by transduction of the parental cells with the pMX-DC-SIGN retroviral vector, which was then followed by fluorescence-activated cell sorting to obtain high levels of DC-SIGN expression as described previously (63). U937/DC-SIGN cells were cultured in RPMI 1640 medium with 10% FBS. The stable CD4-expressing cell lines Raji/CD4 and Raji/DC-SIGN/CD4 were generated separately by electroporation of Raji cells and Raji/DC-SIGN cells with pCEN-CD4, followed by neomycin selection and fluorescence-activated cell sorting as described previously (62). CD4-expressing cells were cultured in RPMI 1640 medium (Invitrogen) with 10% FBS in the presence of neomycin (0.5 mg/ml).
Flow cytometry and antibodies.
To assess expression levels of cell surface CD4, DC-SIGN, CCR5, or CXCR4, various cells (1 × 105 cells) were stained with specific monoclonal antibodies (mAbs) or isotype-matched immunoglobulin G (IgG) controls as previously described (63). Phycoerythrin (PE)-conjugated DC-SIGN mAb (clone 120507) was purchased from R&D Systems. Purified CXCR4 mAb (clone 44717; R&D Systems) was obtained through the NIH AIDS Research and Reference Reagent Program. PE- or fluorescein isothiocyanate-conjugated mouse anti-human CD4 (clone S3.5), PE- or fluorescein isothiocyanate-conjugated goat anti-mouse IgG, and purified normal human and mouse IgG were purchased from Caltag Laboratories. PE-conjugated CCR5 mAb (clone 2D7/CCR5) and mouse IgG isotype control mAbs were purchased from the BD Biosciences. Stained cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson).
HIV stocks.
Single-cycle, infectious HIV-1 stocks were generated by calcium phosphate cotransfections of HEK293T cells with pNL-Luc-E−R− and expression plasmids for Env of HIV-1JRFL or HIV-1HXB2 as described previously (63). The infectivities of the virus stocks were evaluated by limiting dilution on GHOST/X4/R5 cells as described previously (63). Aldrithiol-2 (AT-2)-inactivated HIV-1 Bal/Supt1-CCR5 cl30 (16, 42) was a kind gift from Jeffery Lifson (AIDS Vaccine Program, SAIC, Frederick, MD).
HIV-1 binding and internalization assay.
Raji cells and their derivatives (3 × 105 cells) were incubated separately with AT-2-inactivated HIV-1 (20 ng of p24-equivalent viruses) for 2 h at 37°C or 4°C. Cells were then washed thoroughly and lysed with 1% Triton X-100 buffer for Gag p24 quantification using an enzyme-linked immunosorbent assay (anti-p24-coated plates were purchased from the AIDS Vaccine Program, SAIC, Frederick, MD) as previously described (62). To test the protease sensitivity of cell-associated HIV-1, virus-pulsed cells were treated with 0.25% trypsin (Invitrogen) at room temperature for 4 min, and cells were subsequently neutralized with culture medium containing 10% FBS and washed prior to cell lysis.
HIV-1 transmission and infection assays.
HIV-1 transmission and direct infection assays using luciferase viruses were performed as described previously (63). For the antibody-blocking experiments, DCs or cell lines were preincubated with 10 μg/ml of either anti-CD4 (clone M-T441; Ancell Corporation), anti-human CD4 complex mAb B4 (NIH AIDS Research and Reference Reagent Program), or anti-DC-SIGN (clone 120526; R&D Systems) at room temperature for 30 min prior to HIV-1 incubation. Cell lysates were obtained 2 days after infection and analyzed for luciferase activity with a commercially available kit (Promega).
For DC infection and transmission assays using replication-competent HIV-1, immature MDDCs (3 × 105 cells) were incubated separately with WT and ΔNef R5 or X4 HIV-1 (50 ng p24 equivalent) for 16 h; cells were then washed thoroughly and cultured for the indicated times. Cell-free supernatants from the HIV-1-infected DCs were harvested for Gag p24 quantification at 3, 5, and 7 days postinfection (dpi). In parallel, the infected DCs (1.5 × 105 cells) were washed intensively and then cocultured with Hut/CCR5 cells (1.5 × 105 cells) for an additional 3 days. Cell-free supernatants were harvested for p24 quantification using an enzyme-linked immunosorbent assay. As a control, Hut/CCR5 cells (1 × 105 cells) were pulsed with the replication-competent viruses (5 ng p24) for 2 h, washed thoroughly, and cultured for 3 days before harvesting supernatants for p24 quantification.
Electron microscopy.
Raji/DC-SIGN and Raji/DC-SIGN/CD4 cells were incubated separately with AT-2-inactivated HIV-1 (2 μg of p24-equivalent viruses per 106 cells) at 37°C for 2 h, cells were washed and immediately fixed with 2% glutaraldehyde (Sigma-Aldrich) in phosphate-buffered saline (PBS) for 30 min on ice, and the samples were then processed for conventional transmission electron microscopy as previously described (12). To observe the intracellular viral retention, AT-2-inactivated HIV-1-pulsed cells were cultured for an additional 24 h prior to the fixation and sample preparation. Thin sections were examined with a Hitachi H-600 transmission electron microscope operating at 75 kV.
Confocal microscopy.
Raji/DC-SIGN cells or Raji/DC-SIGN/CD4 cells (6 × 105 cells) were incubated with AT-2-inactivated HIV-1 (40 ng of p24-equivalent viruses) at 37°C for 2 h and washed twice with 1 ml PBS containing 2% FBS. The following immunostaining was performed at room temperature unless specified. Washed cells were attached to poly-l-lysine-coated microscope slides (Polysciences) at 37°C for 20 min and fixed with 4% paraformaldehyde (Sigma-Aldrich) in PBS for 20 min, and cells were permeabilized with 0.1% Triton X-100 in PBS for 2 min and then incubated with 0.1 M glycine and PBS containing 3% bovine serum albumin (Sigma-Aldrich) for 20 min. Cells were first stained with mouse anti-human CD63 (a kind gift from Amy Hudson, Medical College of Wisconsin) or anti-CD81 (2 μg/ml) (clone JS-81; BD-Pharmingen) for 60 min and washed three times with PBS containing 3% bovine serum albumin. Cells were then stained with Alexa Fluor 568-labeled goat anti-mouse IgG (2 μg/ml; Molecular Probes) for 60 min and washed as described above. Cells were stained subsequently with Alexa Fluor 488-labeled mAb against HIV-1 p24 (2 μg/ml) (clone 24-2; NIH AIDS Research and Reference Reagent Program) for 60 min. The p24 mAbs were fluorescently labeled with the Zenon One Alexa Fluor 488 labeling kit according to the manufacturer's protocol (Molecular Probes). Following immunostaining, cells were fixed, washed, and mounted with Fluoromount G (Electron Microscopy Sciences); cells were then examined using a laser scanning confocal microscope (Leica TCS SP2).
Statistical analyses.
Statistical analyses were performed using Wilcoxon's paired t test or Mann-Whitney's unpaired t test with Prism software.
RESULTS
CD4 expression levels correlate with monocytic cell restriction of DC-SIGN-mediated HIV-1 transmission.
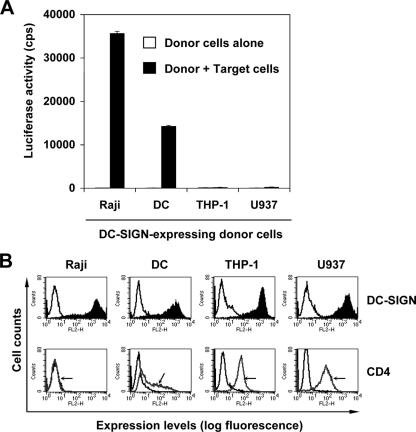
Previous studies reported that DC-SIGN-mediated HIV-1 transmission is cell type dependent (53, 61, 62). The cell type restriction of the DC-SIGN function in HIV-1 transmission provides a valuable means to explore the mechanisms of DC-mediated HIV-1 transmission and cellular features that regulate the viral transfer. To better understand the mechanism of cell type restriction of DC-SIGN-mediated HIV-1 transmission, a screen for cell types that are permissive or restrictive for DC-SIGN-mediated HIV-1 transmission was performed with more than 20 different human cell lines and MDDCs (61, 62; L. Wu et al., unpublished data). The screen revealed that cell surface CD4 expression levels correlate with the restriction phenotype of monocytic cell lines (Fig. 1). After pulsing with single-cycle luciferase HIV-1, Raji/DC-SIGN cells and immature MDDCs efficiently transferred HIV-1 to cocultured CD4+ CCR5+ human T cells (Hut/CCR5) (Fig. 1A). By contrast, the monocytic cell lines THP-1 and U937 did not support HIV-1 trans infection despite high levels of exogenous DC-SIGN expression (Fig. 1A). These two monocytic cell lines that expressed high levels of endogenous CD4 were restrictive for HIV-1 trans infection, whereas permissive Raji/DC-SIGN cells were negative for CD4 as measured by flow cytometry (Fig. 1B). Compared with Raji/DC-SIGN cells, immature MDDCs that express moderate levels of CD4, but similar levels of DC-SIGN, supported less efficient HIV-1 transmission (Fig. 1). Because HIV-1 can also interact with CD4, we hypothesized that CD4 expression might regulate DC-SIGN-mediated HIV-1 trans infection.
FIG. 1.
CD4 expression levels correlate with monocytic cell restriction of DC-SIGN-mediated HIV-1 transmission. (A) Monocytic cell lines restrict DC-SIGN-mediated HIV-1 transmission. The HIV-1 transmission assay was performed as described previously (59). Immature MDDCs and DC-SIGN-expressing donor cell lines were pulsed separately with single-cycle luciferase HIV-1 (R5 EnvJRFL) and cocultured with Hut/CCR5 target cells. Donor cells alone were used as controls. The data show the means ± standard deviations (SD) of triplicate wells of cell samples. One representative experiment out of four is shown. cps, counts per second. (B) Surface expression levels of DC-SIGN and CD4 of immature MDDCs and DC-SIGN-expressing cell lines. (Top) Staining of DC-SIGN with mAbs against DC-SIGN (solid peaks) or isotypic controls (open peaks). (Bottom) Staining of CD4 with mAbs against CD4 (gray peaks, arrowheads) or isotypic controls (black peaks). Antibody staining (FL2-H) is depicted by the histogram plots along the x axis.
CD4 expression impairs DC-SIGN-mediated HIV transmission.
To test whether CD4 expression impairs DC-SIGN-mediated HIV-1 transmission, CD4-negative Raji cells and Raji/DC-SIGN cells were used to generate Raji/CD4 and Raji/DC-SIGN/CD4 stable cell lines, respectively. Comparable levels of cell surface expression of DC-SIGN and CD4 were detected in the appropriate cells by flow cytometry (Fig. 2A). These Raji-derived cells were then used as donor cells and examined for R5 HIV-1 transmission to Hut/CCR5 cells or activated human primary CD4+ T cells. Similar to the negative control Raji cells, Raji/CD4 cells did not support HIV-1 transmission (Fig. 2B), whereas Raji/DC-SIGN cells efficiently transferred single-cycle R5 HIV-1 to various target T cells (Fig. 2B). Hut/CCR5 target cells were more susceptible to viral infection than primary T-cell targets, which is most likely due to higher constitutive expression of viral receptors by the stable cell line (Wu et al., unpublished). Thus, the expression of CD4 in Raji/DC-SIGN cells dramatically impairs their capacity to transfer R5 HIV-1 to cocultured T cells.
FIG. 2.
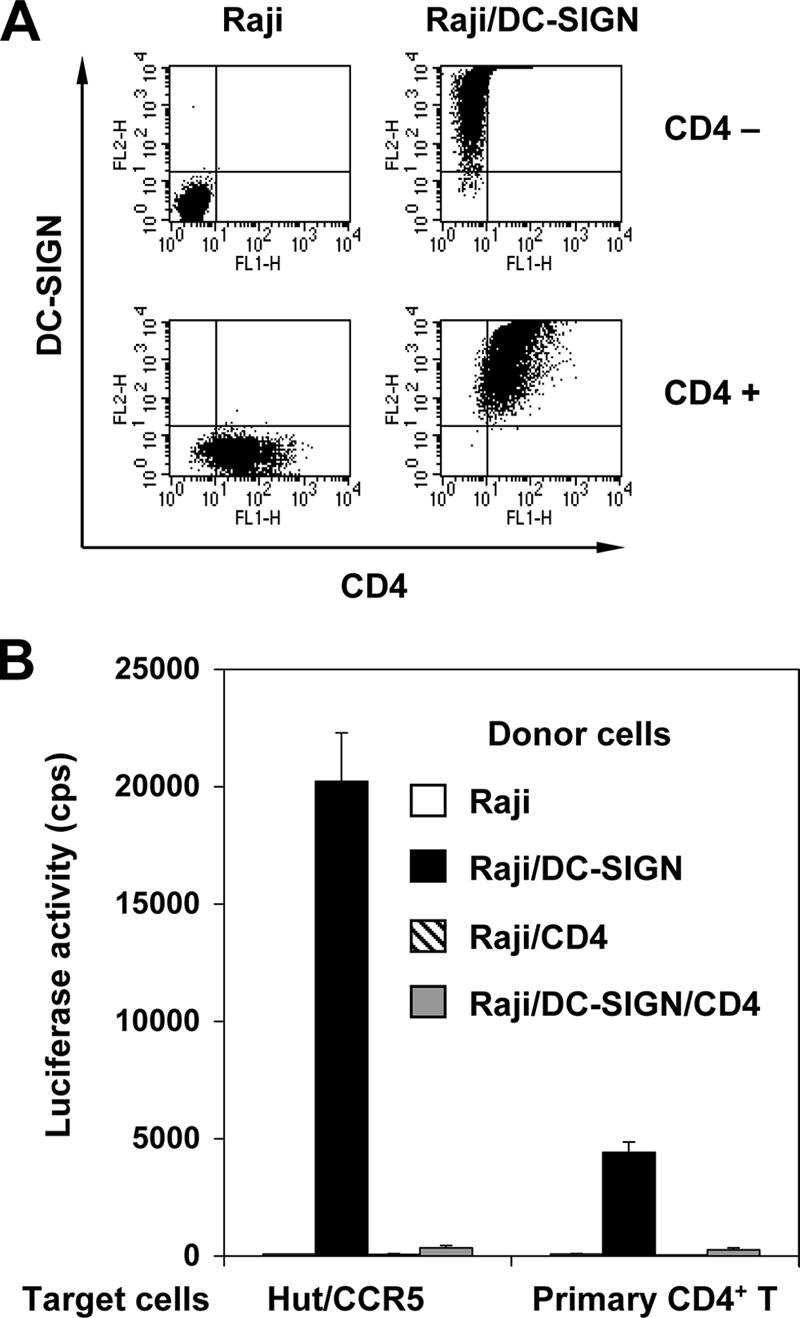
CD4 expression impairs DC-SIGN-mediated HIV-1 transmission. (A) Cell surface expression of DC-SIGN and CD4 of Raji-derived cells. Cells were double stained with DC-SIGN and CD4 mAbs and then analyzed by flow cytometry. Antibody staining (FL1-H and FL2-H) is depicted by the dot plots along the x and y axes. (B) Coexpression of CD4 and DC-SIGN impairs HIV-1 transmission. The HIV-1 transmission assay was performed using single-cycle luciferase HIV-1 (R5 EnvJRFL) as previously described (59). The Hut/CCR5 T-cell line or CD4+ primary T cells were used as target cells. The data show the means ± SD of triplicate wells of infected cells. One representative experiment out of four is shown. cps, counts per second.
Coexpression of CD4 and DC-SIGN in Raji cells enhances X4 HIV-1 susceptibility.
It has been shown that cis expression of DC-SIGN allows for a more efficient entry of HIV-1 through viral receptors (29). To determine whether the coexpression of CD4 and DC-SIGN on Raji cells targets HIV-1 to productive cis infection, these cells were challenged with single-cycle HIV-1. Parental Raji cells and CD4- and DC-SIGN-expressing derivatives were separately pulsed with R5 or X4 HIV-1 and cultured 3 days before detection of viral infection. Hut/CCR5 cells were used as positive controls for R5 and X4 HIV-1 infection. Cell surface expression of CCR5 and CXCR4 on these cells was examined by immunostaining and flow cytometry. Similar levels of CXCR4 expression were observed on Raji cells and Raji derivatives. CCR5 was negative on these B-cell lines. High levels of CCR5 and CXCR4 expression were detected on Hut/CCR5 cells (Fig. 3A). As expected, CD4-negative Raji cells and Raji/DC-SIGN cells were not permissive to HIV-1 infection, and none of the Raji cell lines were susceptible to R5 HIV-1 due to the absence of the coreceptor CCR5 (Fig. 3A and B). By contrast, ectopic CD4 expression and endogenous CXCR4 expression rendered Raji cells highly susceptible to X4 HIV-1 infection. Compared with Raji/CD4 cells, the coexpression of CD4 and DC-SIGN enhanced X4 HIV-1 infection about 10-fold (P < 0.001) (Fig. 3B).
FIG. 3.
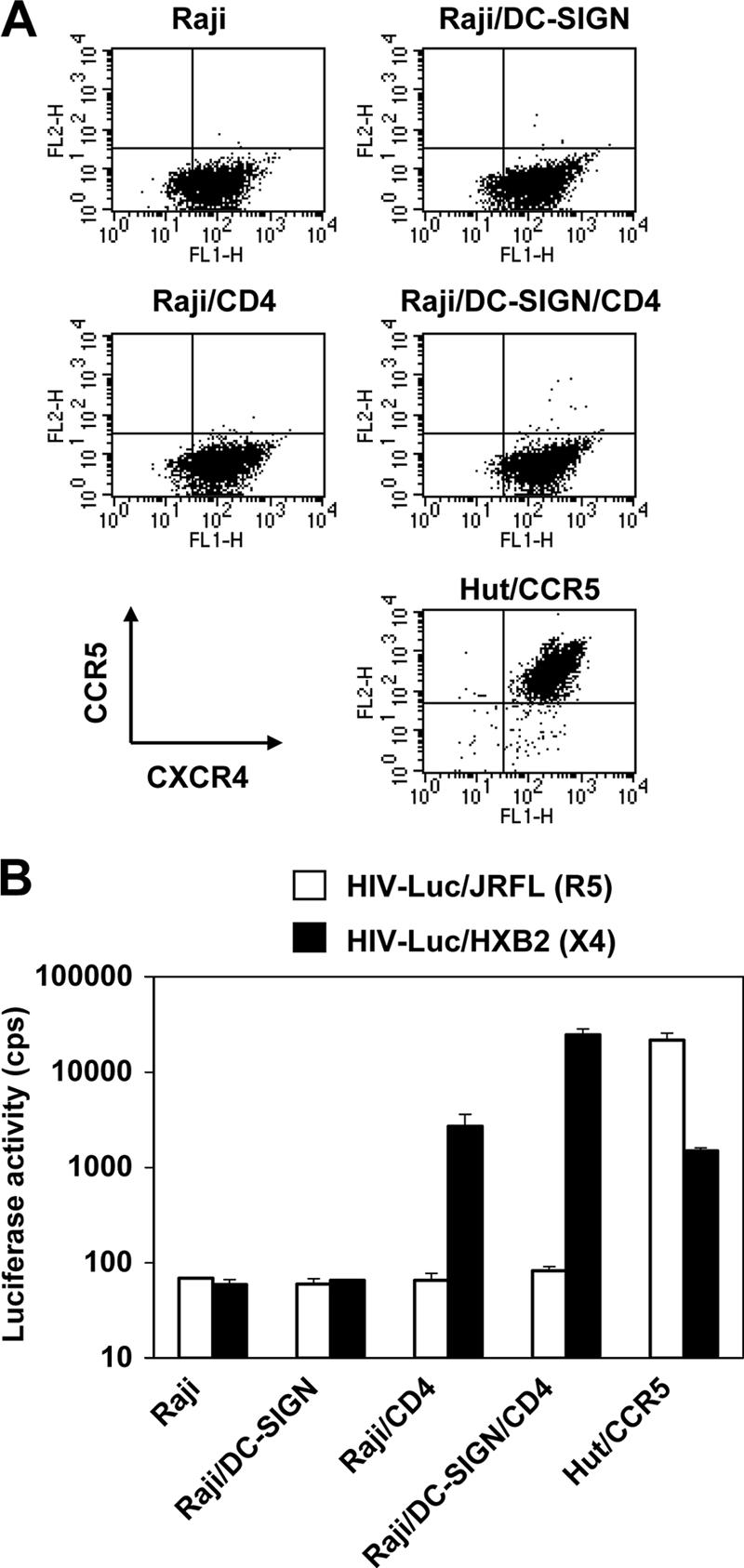
Coexpression of CD4 and DC-SIGN promotes X4 HIV-1 infection. (A) Cell surface expression of CCR5 and CXCR4. Cells were double stained with mAbs against CCR5 and CXCR4 and then analyzed by flow cytometry. Antibody staining (FL1-H and FL2-H) is depicted by the dot plots along the x and y axes. (B) HIV-1 direct infection. R5- and X4-tropic single-cycle luciferase (Luc) HIV-1 was used separately to infect Raji cells and derivatives. Cells were washed after viral exposure and cultured for 3 days before the detection. Hut/CCR5 cells were used as positive controls. The data show the means ± SD of duplicate wells of infected cells. One representative experiment out of three is shown. cps, counts per second.
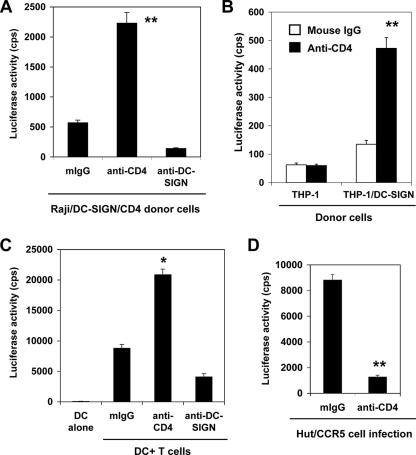
Blockade of cell surface CD4 with specific mAbs promotes HIV-1 trans infection.
As described above, the introduction of CD4 impaired HIV-1 transmission mediated by Raji/DC-SIGN cells, suggesting that CD4 coexpression regulates HIV-1 transmission versus direct infection after capture by DC-SIGN. To determine whether CD4 binding of HIV-1 competed with DC-SIGN-mediated transmission to cells in trans, mAbs against CD4 were used to block CD4 molecules on the donor cell surface, and HIV-1 transmission to target T cells was subsequently quantified. Preincubation of CD4 mAbs with Raji/DC-SIGN/CD4 cells and THP-1/DC-SIGN cells increased R5 HIV-1 transmission fourfold (P < 0.01) (Fig. 4A and B). Interestingly, viral transmission by immature MDDCs was also enhanced 2.4-fold (P < 0.05) when cells were preincubated with CD4 mAbs before HIV-1 incubation (Fig. 4C). Consistent with previous results (4, 22, 53, 63), HIV-1 transmission decreased by 53% when a DC-SIGN mAb was incubated with MDDCs prior to virus binding (Fig. 4C). Notably, these data show that CD4 engagement of HIV-1 in MDDCs has a greater effect on viral transmission than DC-SIGN interactions with the virus. As a positive control for the CD4 mAbs blocking HIV-1 Env interactions, viral infection of Hut/CCR5 cells decreased by 86% (P < 0.01) when the cells were preincubated with the CD4 mAbs before virus incubation (Fig. 4D).
FIG. 4.
Blockade of cell surface CD4 with specific mAbs promotes HIV-1 transmission. (A) Raji/DC-SIGN/CD4 cells, (B) THP-1/DC-SIGN cells, or (C) immature MDDCs were preincubated separately with the CD4 mAbs or anti-DC-SIGN mAbs before HIV-1 incubation. Mouse IgG (mIgG) was used as a control. The HIV-1 transmission assay was performed as described previously (59). Hut/CCR5 cells were used as target T cells. HIV-1 trans infection was determined 2 days postinfection by measuring the luciferase activity of cell lysates. (D) Preincubation with purified mouse anti-human CD4 mAbs blocked HIV-1 infection of Hut/CCR5 cells. The data show the means ± SD of triplicate cell samples. One representative experiment out of four is shown. cps, counts per second. Asterisks indicate significant differences compared with mouse IgG controls (*, P < 0.05; **, P < 0.01).
Coexpression of CD4 and DC-SIGN facilitates HIV-1 internalization and alters viral trafficking.
To elucidate the mechanism by which the coexpression of CD4 and DC-SIGN impairs HIV-1 transmission in trans, virus binding, internalization, and trafficking of Raji/DC-SIGN cells were compared with those of Raji/DC-SIGN/CD4 cells. To examine the cell-virus interactions in the absence of potentially confounding effects of productive viral infection, we used AT-2-inactivated HIV-1 in these experiments. Previous studies showed that AT-2-inactivated HIV-1 is conformationally authentic and interacts with MDDCs similarly to infectious viruses (16, 56).
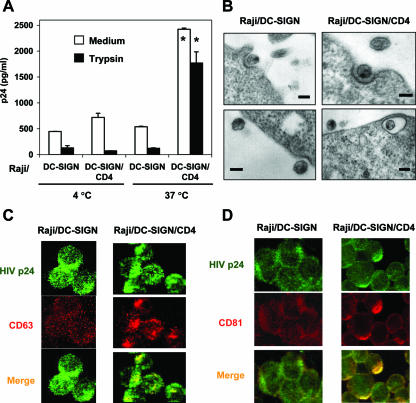
To measure virus binding and internalization, Raji/DC-SIGN cells and Raji/DC-SIGN/CD4 cells were pulsed separately with AT-2-inactivated R5 HIV-1 at 4°C or 37°C for 2 h, and after rigorous washing, cells were lysed, and HIV-1 Gag p24 was quantified. To test the proteolysis sensitivity of cell-associated HIV-1, cells were treated with trypsin after virus incubation. At 4°C, HIV-1 binding to Raji/DC-SIGN/CD4 cells was nearly twofold higher than that to Raji/DC-SIGN cells (Fig. 5A). Trypsin treatment greatly reduced cell-associated HIV-1 to background levels of Raji cell controls (Fig. 5A and data not shown), indicating that virus remained on the cell surface upon binding at 4°C. By contrast, HIV-1 internalization by Raji/DC-SIGN/CD4 cells at 37°C was fivefold greater (P < 0.01) than that by Raji/DC-SIGN cells. At 37°C, 80% of Raji/DC-SIGN/CD4 cell-associated HIV-1 was resistant to proteolysis, whereas the Raji/DC-SIGN cell-associated HIV-1 was largely sensitive to trypsin (Fig. 5A).
FIG. 5.
Coexpression of DC-SIGN and CD4 facilitates HIV-1 internalization and alters viral trafficking. (A) Coexpression of DC-SIGN and CD4 enhances HIV-1 binding and internalization. Raji/DC-SIGN cells or Raji/DC-SIGN/CD4 cells were separately incubated with AT-2-inactivated R5 HIV-1 for 2 h. Cells were washed and treated with trypsin or medium and lysed for HIV-1 p24 quantification. The data represent the means ± SD of triplicate cell samples. One representative experiment out of 10 is shown. *, significant differences compared with Raji/DC-SIGN cells (P < 0.01). (B) Raji/DC-SIGN/CD4 cells internalize HIV-1 in intracellular vesicles. Cells were washed after exposure to AT-2-inactivated HIV-1 and were prepared immediately for electron microscopy. Scale bars, 100 nm. (C and D) HIV-1 colocalized with the late endosomal markers CD63 (C) and CD81 (D) in Raji/DC-SIGN/CD4 cells. Cells were pulsed with AT-2-inactivated HIV-1, coimmunostained, and analyzed by confocal microscopy. Green, HIV-1 p24; red, CD63 or CD81. Magnification, ×60.
Next, HIV-1 trafficking in these cells was visualized using electron and confocal microscopy. After a 2-h HIV-1 exposure to cells at 37°C, only cell surface-bound HIV-1 particles were found on Raji/DC-SIGN cells, in apparent association with clathrin-coated pits (Fig. 5B, left); however, in addition to the cell surface-bound HIV-1 particles (Fig. 5B, upper right), virus particles were observed within intracellular compartments of Raji/DC-SIGN/CD4 cells, which were close to cell membranes (Fig. 5B, lower right). Immunostaining and confocal microscopy indicated that HIV-1 internalized within Raji/DC-SIGN/CD4 cells colocalized with the late endosomal markers CD63 and CD81 (Fig. 5C and D), whereas HIV-1 bound to Raji/DC-SIGN cell surfaces did not colocalize with these markers. In addition, compared with Raji/DC-SIGN cells, the late endosomal compartments were concentrated and polarized within Raji/DC-SIGN/CD4 cells after exposure to HIV-1 (Fig. 5C and D).
Collectively, these data indicate that the coexpression of CD4 and DC-SIGN in Raji cells significantly enhances HIV-1 internalization within late endosomal compartments, suggesting that the altered viral trafficking contributes to the CD4 inhibition of DC-SIGN-mediated HIV-1 trans infection.
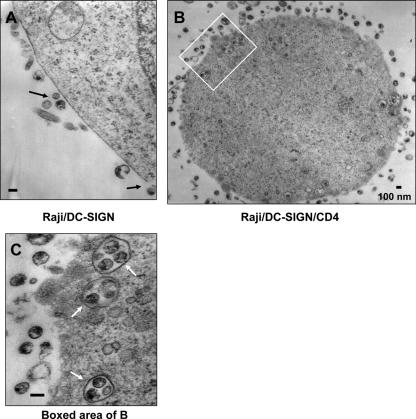
HIV-1 retention in Raji cells coexpressing CD4 and DC-SIGN.
To examine the stability of HIV-1 internalized in Raji/DC-SIGN/CD4 cells, cells were cultured for an additional 24 h after 2 h of exposure of AT-2-inactivated HIV-1 and then analyzed by electron microscopy. No viral particles were observed in mock control cells without incubation of AT-2-inactivated HIV-1 (data not shown). Only cell surface-associated virus particles were observed from Raji/DC-SIGN cells (Fig. 6A). By contrast, in addition to abundant HIV-1 particles bound on the cell surfaces of Raji/DC-SIGN/CD4 cells, intact virus particles were still confined to intracellular compartments in these cells after a 24-h culture (Fig. 6B and C). A high magnification of the boxed area in Fig. 6B indicated that intact HIV-1 particles were trapped in the endosomal compartments (Fig. 6C). Thus, HIV-1 internalized by Raji/DC-SIGN/CD4 cells can be retained in the cells for at least 24 h.
FIG. 6.
Raji/DC-SIGN/CD4 cells retain intracellular HIV-1 particles for at least 24 h. Cells were incubated with AT-2-inactivated R5 HIV-1 at 37°C for 2 h, washed, and cultured for an additional 24 h. Cells were then analyzed by electron microscopy. (A) Raji/DC-SIGN cells. Arrows indicated cell surface-associated HIV-1 particles. (B) Raji/DC-SIGN/CD4 cells. (C) Higher-magnification image of the boxed area from panel B. Arrows indicate the intracellular compartments that trapped intact HIV-1 particles. Scale bars, 100 nm.
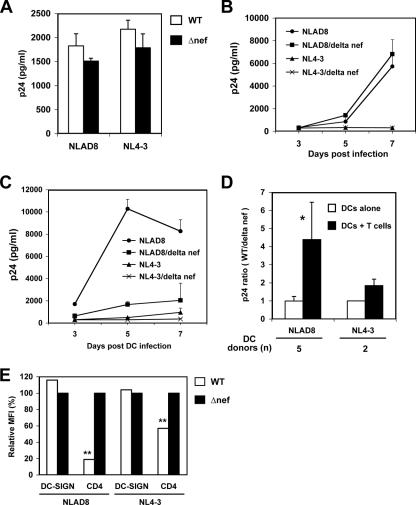
Downregulation of CD4 by Nef expression in DCs correlates with enhanced viral transmission.
We next wished to examine the effect of CD4 levels on DC-mediated HIV-1 transmission. In addition to DC-mediated HIV-1 trans infection, long-term viral transfer from DCs to T cells depends on HIV-1 infection of DCs (6, 30, 35, 56). MDDCs express both CD4 and DC-SIGN and are susceptible to HIV-1 infection, albeit at reduced replication levels (35). HIV-1 Nef downregulates cell surface expression of CD4 (38) but upregulates DC-SIGN expression (51). We thus hypothesized that HIV-1 Nef expression upon viral infection may facilitate DC-mediated HIV-1 transmission through the modulation of CD4 and/or DC-SIGN expression. To test this model, WT and nef-inactivated (Δnef) HIV-1 infection upon viral transmission by MDDCs was examined.
First, levels of viral replication of R5 (NLAD8) or X4 (NL4-3) WT and Δnef HIV-1 in Hut/CCR5 target cells were compared by using equivalent inoculums. By quantification of Gag p24 in cell-free supernatants, comparable levels of virus replication were detected between WT and Δnef HIV-1-infected Hut/CCR5 cells at 3 dpi (Fig. 7A). HIV-1 production by infected immature MDDCs was then measured. WT and Δnef HIV-1-infected MDDCs showed similar virus replication kinetics (Fig. 7B). In MDDCs, viral replication of X4 HIV-1 was significantly lower than that of R5 viruses (Fig. 7B), which is consistent with previous studies showing that susceptibility of DCs to R5 HIV is generally higher than that to X4 HIV due to the higher expression of CCR5 or the lack of CXCR4 expression on DC surfaces (20, 25, 35, 36, 48, 64).
FIG. 7.
Nef-induced CD4 downregulation in HIV-1-infected DCs promotes viral transfer to CD4+ T cells. (A) WT and nef-inactivated (Δnef) HIV-1 replicate similarly in Hut/CCR5 cells. Cells were pulsed with R5 (NLAD8) or X4 (NL4-3) HIV-1 (5 ng p24 each), and cell-free supernatants were harvested 3 dpi for p24 quantification. (B) Comparable WT and Δnef HIV-1 infections of DCs. Immature MDDCs were pulsed with HIV-1 (50 ng p24 per inoculum) and cultured. Cell-free supernatants were measured for p24 at the indicated times. (C) Nef expression facilitates DC-mediated HIV-1 transmission. At 3, 5, and 7 dpi, MDDCs were washed and cocultured with Hut/CCR5 cells for an additional 3 days. Cell-free supernatants of cocultures were measured for p24. The data show the means ± SD of triplicate cell samples. One representative experiment out of five is shown. (D) Comparison of infection and transmission between WT and Δnef HIV-1-infected MDDCs at 5 dpi. The average ratio of p24 production of DCs alone and DC-T-cell cocultures is shown. Data are the means ± SD of results of DCs derived from different donors in independent experiments. (E) Nef modulates DC-SIGN and CD4 expression of HIV-1-infected MDDCs. The cell surface expression of DC-SIGN and CD4 was measured by flow cytometry at 5 dpi. MFI, mean fluorescence index. Relative expression levels versus Δnef HIV-1-infected MDDCs are shown. One representative experiment out of five is shown. Asterisks indicate a significant difference (*, P < 0.05 compared with DC alone; **, P < 0.01 compared with the WT).
At 3, 5, and 7 dpi, DCs were washed thoroughly and then cocultured with Hut/CCR5 cells for an additional 3 days to quantify viral transmission. Cell-free supernatants of the cocultures were then measured for p24 production. Interestingly, compared with Δnef HIV-1-infected MDDCs, Nef-expressing, WT NLAD8 HIV-1 infection significantly enhanced DC-mediated viral transmission twofold, sixfold (P < 0.01), and fourfold (P < 0.01) at 3, 5, and 7 days after DC infection, respectively. As expected, X4-tropic NL4-3 replicated more weakly in the cocultures using preinfected MDDCs, but the presence of Nef increased virus production twofold and threefold (P < 0.05) at 5 and 7 days after DC infection, respectively (Fig. 7C).
Repeat experiments using DCs derived from five different donors indicated that efficient HIV-1 transmission occurred when cocultures were initiated at 5 days after DC infection (Fig. 7C and data not shown). These conditions likely allow for virus spread in the MDDCs and modulation of cell surface receptors. Therefore, we compared the results of HIV-1 replication and those of viral transmission that were obtained at 5 days after DC infection. For both R5 and X4 viruses, the average ratio of p24 production between WT and Δnef HIV-1-infected DCs alone was 1 (Fig. 7D), indicating similar levels of viral replication. By contrast, the ratio of p24 production of NLAD8-infected DC-T-cell cocultures was 4.4 ± 2.1 (P < 0.05; average results from five donors), whereas the ratio of NL4-3-infected DC-T-cell cocultures was 1.9 ± 0.4 (average results from two donors). Thus, Nef expression by WT HIV-1 infection efficiently facilitates viral dissemination in DC-T-cell cocultures. In parallel, at 5 dpi, the cell surface expression of DC-SIGN and CD4 of infected DCs was also measured. Compared with Δnef HIV-1-infected DCs, Nef-expressing NLAD8 HIV-1-infected MDDCs slightly increased DC-SIGN expression by 16% and significantly decreased CD4 expression by 81% (P < 0.01), whereas NL4-3 HIV-1-infected MDDCs showed a 4% increase in DC-SIGN expression and a 43% reduction (P < 0.01) in CD4 expression (Fig. 7E).
Together, these results indicate that Nef is required for efficient HIV-1 transmission from DCs to T cells. Nef-induced CD4 downregulation in HIV-1-infected MDDCs correlates with enhanced viral transmission.
DISCUSSION
The cell type dependency of DC-SIGN-mediated HIV-1 transmission provides a useful model system to investigate the mechanisms underlying DC-mediated viral transfer (61, 62). We previously discovered that monocytic THP-1 cells do not support DC-SIGN-mediated HIV-1 transmission, although it was widely assumed in the literature that such cells recapitulated DC transmission of HIV-1 (61). Here, we identify an underlying mechanism for the monocytic cell restriction of DC-SIGN-mediated HIV-1 transmission and present a model by which HIV-1 might exploit primary cells with similar characteristics to potentiate viral spread to CD4+ T cells.
In this report, we show that CD4 expression levels regulate DC-SIGN and MDDC transmission of HIV-1. Monocytic cell type restriction of DC-SIGN-mediated HIV-1 transmission correlates with CD4 expression, and this impairment can be blocked with CD4 antibodies that prevent interactions with HIV-1 Env. This restriction can be re-created by the expression of CD4 in Raji/DC-SIGN cells, which are otherwise permissive to DC-SIGN-mediated HIV-1 transmission. In the presence of an appropriate HIV-1 coreceptor, cells engineered to coexpress CD4 and DC-SIGN are preferentially infected. By contrast, CD4/DC-SIGN double-positive cells lacking a viral coreceptor efficiently internalize and trap HIV-1. Using primary cells expressing CD4 and DC-SIGN, we observed that HIV-1 infection changes the transmission properties of these cells. Specifically, infection by Nef-positive HIV-1 efficiently promotes DC-mediated HIV-1 transmission, which correlates with CD4 downregulation in these cells. Collectively, these data suggest that modulations of CD4 levels may play an important regulatory role in DC-mediated HIV-1 transmission.
DC-mediated HIV-1 transmission to CD4+ T cells involves at least two pathways (60), namely, HIV-1 trans infection without viral infection in DCs, and long-term viral transfer depended on the HIV-1 replication in infected DCs (6, 30, 35, 56). It has been reported that certain DC subsets and macrophages in vivo express DC-SIGN, CD4, and HIV-1 coreceptors (24, 29, 50), and both cell types were proposed to facilitate mucosal transmission of HIV-1 (47). Thus, it is conceivable that these types of cells are more susceptible to HIV-1 infection due to the coexpression of CD4, DC-SIGN, and viral coreceptors, which may augment viral dissemination by DCs and macrophages using the cis infection pathway. Upon HIV-1 infection, the Nef protein may convert these cells into more potent HIV-1 transmitters through a significant downregulation of CD4. CD4 and DC-SIGN molecules have dileucine-based internalization motifs in the cytoplasmic domains (5, 19), and the dileucine motifs of CD4 and DC-SIGN are critical for endocytosis function and regulation by Nef (51). Although MDDCs have provided a convenient tool to study DC-HIV interactions, further examinations of bona fide DC subsets ex vivo for viral infection and transmission would be beneficial for understanding the contribution of DCs to AIDS pathogenesis.
Internalization of HIV-1 and distinct viral trafficking have been suggested to be important for DC-mediated HIV-1 trans infection (28, 53); however, it is unclear how internalized virions remain infectious, recycle back to the cell surface, and are transferred to the T cells. By contrast, our viral binding and electron microscopy results suggest that HIV-1 internalization in Raji/DC-SIGN cells is not required for efficient HIV-1 transmission. Consistent with our observations, Burleigh and colleagues recently reported that DC-SIGN-mediated HIV-1 internalization is dispensable for both trans infection of T cells and the retention of viral infectivity (6). We found that the coexpression of CD4 and DC-SIGN in Raji cells significantly promoted viral internalization and altered viral trafficking to late endosomal compartments (also known as multivesicular bodies [MVBs]). Although endocytosis-mediated HIV-1 entry can lead to productive viral infection (15, 44), only X4 HIV-1, but not R5 HIV-1, replicated in CD4-expressing Raji cells. These results suggest that the fusion-mediated entry of X4 HIV-1 leads to productive infection in these cells; however, endocytosed R5 HIV-1 is trapped or inactivated in acidified endosomes and is eventually degraded in lysosomes. Indeed, compared with Raji/DC-SIGN cells, we have observed significant colocalization of the HIV-1 p24 protein with lysosome-associated membrane protein 1 in Raji/DC-SIGN/CD4 cells (data not shown), suggesting enhanced HIV-1 degradation within lysosomes in CD4-DC-SIGN-expressing cells.
Interestingly, it was shown that HIV-1 captured by immature MDDCs is also internalized to MVBs, and infectious HIV-1 is constitutively released into the extracellular milieu in association with the exosomes to initiate viral transfer to T cells (58). Similarly, HIV-1 particles internalized by mature MDDCs are also sequestered into MVBs (17). In contrast to the Raji/DC-SIGN/CD4 cells, it remains unclear how internalized HIV-1 circumvents the intracellular degradation machinery in DCs and recycles back to the DC surface to be transferred to the T cells. Further investigations of HIV-1 trafficking in DCs in comparison to Raji/DC-SIGN/CD4 cells may help us to understand this unique feature of DC-mediated viral transmission.
The blocking of exposed CD4 on DCs with specific mAbs promoted HIV-1 trans infection, most likely by impairing virus internalization and intracellular degradation. Similar results were confirmed using DCs generated from different donors and using different CD4 mAbs in independent experiments (data not shown). However, the enhancement of HIV-1 transmission by CD4 mAb blocking appears to be limited, with 2.4-fold and 4-fold increases in MDDCs and various cell lines, respectively. It is conceivable that other regions of CD4 outside the gp120 binding domain contribute to the inhibition of DC-SIGN-mediated HIV-1 transmission. In addition, during the 2-day coculture with T-cell targets, the newly synthesized CD4 proteins on the donor cell surfaces diminished the blockade effect of CD4 mAb. In DCs, CD4 mAb alone may not completely block HIV-1 internalization due to the coexpression of DC-SIGN or other C-type lectins. These results also suggest that CD4 expression is not the sole cellular determinant that controls the efficiency of DC-SIGN-mediated HIV-1 trans infection. For example, we previously reported that CD4-negative human erythroleukemic K562 cells do not efficiently transmit HIV-1 despite high levels of exogenous DC-SIGN expression (62). Presumably, another unidentified cellular factor(s) can also influence DC-SIGN-mediated HIV-1 transmission, given its cell type dependence in CD4-negative cells (61, 62).
Nef-expressing HIV-1-infected DCs promoted viral transmission to cocultured T cells. Indeed, Nef modulation of DC-SIGN and CD4 expression was observed despite the limited levels of DC-SIGN upregulation. These results provide an additional context to consider previous observations of Nef facilitating DC-mediated HIV-1 spread to T cells (37). It has been reported that HIV-1 transmission efficiency can be enhanced by the maturation of DCs (32, 43). To examine the possibility that Nef-promoted HIV-1 transmission results from Nef-induced DC maturation, maturation markers (such as HLA-DR, CD83, and CD86) of WT and Δnef HIV-1-infected DCs were compared, but no Nef-induced upregulation of the maturation markers was observed (unpublished data). It has also been reported that Nef-expressing immature MDDCs stimulate T-cell activation but without upregulating DC maturation markers (33). We confined our analyses to a transformed CD4+ T cell that is constitutively activated. With resting, primary CD4+ T cells, it is likely that Nef may also facilitate DC-mediated HIV-1 transmission by promoting DC-T-cell interactions or by enhancing T-cell clustering (51).
In summary, we find that CD4 coexpression with DC-SIGN enhances HIV-1 internalization and retention but strongly impairs HIV-1 transmission to T cells. The blocking of CD4 on the DC surfaces promotes HIV-1 trans infection. Significantly, Nef facilitates DC-mediated HIV-1 transmission, which correlates with Nef-induced downregulation of CD4. These data provide a novel insight into cellular characteristics that influence DC-mediated HIV-1 dissemination and highlight Nef's role as a multifunctional pathogenic factor capable of regulating these processes.
Acknowledgments
We thank Mark McNally for critical reading of the manuscript. We thank Eric Freed, Amy Hudson, Jeffery Lifson, and Olivier Schwartz for the kind gift of reagents. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: recombinant IL-2 from Maurice Gately, Hoffmann-La Roche Inc.; CXCR4 mAb (44717.111); CD4 complex mAb B4 from United Biomedical, Inc.; and p24 mAb from Michael Malim.
This work was supported by a grant (R01-AI068493) to L.W. from the National Institutes of Health (NIH). V.N.K. is supported by intramural research funds from the National Cancer Institute, NIH.
A.M.J. and W.J.O. contributed equally to this study.
Footnotes
Published ahead of print on 6 December 2006.
REFERENCES
- 1.Arrighi, J. F., M. Pion, E. Garcia, J. M. Escola, Y. van Kooyk, T. B. Geijtenbeek, and V. Piguet. 2004. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J. Exp. Med. 200:1279-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrighi, J. F., M. Pion, M. Wiznerowicz, T. B. Geijtenbeek, E. Garcia, S. Abraham, F. Leuba, V. Dutoit, O. Ducrey-Rundquist, Y. van Kooyk, D. Trono, and V. Piguet. 2004. Lentivirus-mediated RNA interference of DC-SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells. J. Virol. 78:10848-10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 4.Baribaud, F., S. Pohlmann, G. Leslie, F. Mortari, and R. W. Doms. 2002. Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells. J. Virol. 76:9135-9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentham, M., S. Mazaleyrat, and M. Harris. 2003. The di-leucine motif in the cytoplasmic tail of CD4 is not required for binding to human immunodeficiency virus type 1 Nef, but is critical for CD4 down-modulation. J. Gen. Virol. 84:2705-2713. [DOI] [PubMed] [Google Scholar]
- 6.Burleigh, L., P.-Y. Lozach, C. Schiffer, I. Staropoli, V. Pezo, F. Porrot, B. Canque, J.-L. Virelizier, F. Arenzana-Seisdedos, and A. Amara. 2006. Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells. J. Virol. 80:2949-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383-387. [DOI] [PubMed] [Google Scholar]
- 8.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 9.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 10.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938-1941. [DOI] [PubMed] [Google Scholar]
- 11.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, V. A. Lawson, S. Crowe, A. Maerz, S. Sonza, J. Learmont, J. S. Sullivan, A. Cunningham, D. Dwyer, D. Dowton, and J. Mills. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 12.DeMasi, J., and P. Traktman. 2000. Clustered charge-to-alanine mutagenesis of the vaccinia virus H5 gene: isolation of a dominant, temperature-sensitive mutant with a profound defect in morphogenesis. J. Virol. 74:2393-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engering, A., S. J. van Vliet, K. Hebeda, D. G. Jackson, R. Prevo, S. K. Singh, T. B. Geijtenbeek, H. van Krieken, and Y. van Kooyk. 2004. Dynamic populations of dendritic cell-specific ICAM-3 grabbing nonintegrin-positive immature dendritic cells and liver/lymph node-specific ICAM-3 grabbing nonintegrin-positive endothelial cells in the outer zones of the paracortex of human lymph nodes. Am. J. Pathol. 164:1587-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Englund, G., T. S. Theodore, E. O. Freed, A. Engelman, and M. A. Martin. 1995. Integration is required for productive infection of monocyte-derived macrophages by human immunodeficiency virus type 1. J. Virol. 69:3216-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fackler, O. T., and B. M. Peterlin. 2000. Endocytic entry of HIV-1. Curr. Biol. 10:1005-1008. [DOI] [PubMed] [Google Scholar]
- 16.Frank, I., M. Piatak, Jr., H. Stoessel, N. Romani, D. Bonnyay, J. D. Lifson, and M. Pope. 2002. Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): differential intracellular fate of virions in mature and immature DCs. J. Virol. 76:2936-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia, E., M. Pion, A. Pelchen-Matthews, L. Collinson, J. F. Arrighi, G. Blot, F. Leuba, J. M. Escola, N. Demaurex, M. Marsh, and V. Piguet. 2005. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic 6:488-501. [DOI] [PubMed] [Google Scholar]
- 18.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 19.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 20.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R. M. Steinman. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72:2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Granelli-Piperno, A., A. Pritsker, M. Pack, I. Shimeliovich, J. F. Arrighi, C. G. Park, C. Trumpfheller, V. Piguet, T. M. Moran, and R. M. Steinman. 2005. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J. Immunol. 175:4265-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gummuluru, S., M. Rogel, L. Stamatatos, and M. Emerman. 2003. Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors via a cholesterol-dependent pathway. J. Virol. 77:12865-12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurney, K. B., J. Elliott, H. Nassanian, C. Song, E. Soilleux, I. McGowan, P. A. Anton, and B. Lee. 2005. Binding and transfer of human immunodeficiency virus by DC-SIGN+ cells in human rectal mucosa. J. Virol. 79:5762-5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jameson, B., F. Baribaud, S. Pohlmann, D. Ghavimi, F. Mortari, R. W. Doms, and A. Iwasaki. 2002. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J. Virol. 76:1866-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawamura, T., F. O. Gulden, M. Sugaya, D. T. McNamara, D. L. Borris, M. M. Lederman, J. M. Orenstein, P. A. Zimmerman, and A. Blauvelt. 2003. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc. Natl. Acad. Sci. USA 100:8401-8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 27.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 28.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 29.Lee, B., G. Leslie, E. Soilleux, U. O'Doherty, S. Baik, E. Levroney, K. Flummerfelt, W. Swiggard, N. Coleman, M. Malim, and R. W. Doms. 2001. cis expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J. Virol. 75:12028-12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lore, K., A. Smed-Sorensen, J. Vasudevan, J. R. Mascola, and R. A. Koup. 2005. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J. Exp. Med. 201:2023-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lore, K., A. Sonnerborg, C. Brostrom, L. E. Goh, L. Perrin, H. McDade, H. J. Stellbrink, B. Gazzard, R. Weber, L. A. Napolitano, Y. van Kooyk, and J. Andersson. 2002. Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. AIDS 16:683-692. [DOI] [PubMed] [Google Scholar]
- 32.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295-1297. [DOI] [PubMed] [Google Scholar]
- 33.Messmer, D., J. M. Jacque, C. Santisteban, C. Bristow, S. Y. Han, L. Villamide-Herrera, E. Mehlhop, P. A. Marx, R. M. Steinman, A. Gettie, and M. Pope. 2002. Endogenously expressed nef uncouples cytokine and chemokine production from membrane phenotypic maturation in dendritic cells. J. Immunol. 169:4172-4182. [DOI] [PubMed] [Google Scholar]
- 34.Michel, N., I. Allespach, S. Venzke, O. T. Fackler, and O. T. Keppler. 2005. The Nef protein of human immunodeficiency virus establishes superinfection immunity by a dual strategy to downregulate cell-surface CCR5 and CD4. Curr. Biol. 15:714-723. [DOI] [PubMed] [Google Scholar]
- 35.Nobile, C., C. Petit, A. Moris, K. Skrabal, J. P. Abastado, F. Mammano, and O. Schwartz. 2005. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J. Virol. 79:5386-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson, S., A. Rae, N. Hockey, J. Gilmour, and F. Gotch. 2001. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J. Virol. 75:6710-6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petit, C., F. Buseyne, C. Boccaccio, J. P. Abastado, J. M. Heard, and O. Schwartz. 2001. Nef is required for efficient HIV-1 replication in cocultures of dendritic cells and lymphocytes. Virology 286:225-236. [DOI] [PubMed] [Google Scholar]
- 38.Piguet, V., O. Schwartz, S. Le Gall, and D. Trono. 1999. The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol. Rev. 168:51-63. [DOI] [PubMed] [Google Scholar]
- 39.Pope, M., M. G. Betjes, N. Romani, H. Hirmand, P. U. Cameron, L. Hoffman, S. Gezelter, G. Schuler, and R. M. Steinman. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78:389-398. [DOI] [PubMed] [Google Scholar]
- 40.Pope, M., S. Gezelter, N. Gallo, L. Hoffman, and R. M. Steinman. 1995. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J. Exp. Med. 182:2045-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rappocciolo, G., F. J. Jenkins, H. R. Hensler, P. Piazza, M. Jais, L. Borowski, S. C. Watkins, and C. R. Rinaldo, Jr. 2006. DC-SIGN is a receptor for human herpesvirus 8 on dendritic dells and macrophages. J. Immunol. 176:1741-1749. [DOI] [PubMed] [Google Scholar]
- 42.Rossio, J. L., M. T. Esser, K. Suryanarayana, D. K. Schneider, J. W. Bess, Jr., G. M. Vasquez, T. A. Wiltrout, E. Chertova, M. K. Grimes, Q. Sattentau, L. O. Arthur, L. E. Henderson, and J. D. Lifson. 1998. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J. Virol. 72:7992-8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanders, R. W., E. C. de Jong, C. E. Baldwin, J. H. Schuitemaker, M. L. Kapsenberg, and B. Berkhout. 2002. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J. Virol. 76:7812-7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaeffer, E., V. B. Soros, and W. C. Greene. 2004. Compensatory link between fusion and endocytosis of human immunodeficiency virus type 1 in human CD4 T lymphocytes. J. Virol. 78:1375-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schindler, M., J. Munch, O. Kutsch, H. Li, M. L. Santiago, F. Bibollet-Ruche, M. C. Muller-Trutwin, F. J. Novembre, M. Peeters, V. Courgnaud, E. Bailes, P. Roques, D. L. Sodora, G. Silvestri, P. M. Sharp, B. H. Hahn, and F. Kirchhoff. 2006. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell 125:1055-1067. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 47.Shattock, R. J., and J. P. Moore. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 1:25-34. [DOI] [PubMed] [Google Scholar]
- 48.Smed-Sorensen, A., K. Lore, J. Vasudevan, M. K. Louder, J. Andersson, J. R. Mascola, A. L. Spetz, and R. A. Koup. 2005. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J. Virol. 79:8861-8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soilleux, E. J., L. S. Morris, B. Lee, S. Pohlmann, J. Trowsdale, R. W. Doms, and N. Coleman. 2001. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J. Pathol. 195:586-592. [DOI] [PubMed] [Google Scholar]
- 50.Soilleux, E. J., L. S. Morris, G. Leslie, J. Chehimi, Q. Luo, E. Levroney, J. Trowsdale, L. J. Montaner, R. W. Doms, D. Weissman, N. Coleman, and B. Lee. 2002. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J. Leukoc. Biol. 71:445-457. [PubMed] [Google Scholar]
- 51.Sol-Foulon, N., A. Moris, C. Nobile, C. Boccaccio, A. Engering, J. P. Abastado, J. M. Heard, Y. van Kooyk, and O. Schwartz. 2002. HIV-1 Nef-induced upregulation of DC-SIGN in dendritic cells promotes lymphocyte clustering and viral spread. Immunity 16:145-155. [DOI] [PubMed] [Google Scholar]
- 52.Swigut, T., N. Shohdy, and J. Skowronski. 2001. Mechanism for down-regulation of CD28 by Nef. EMBO J. 20:1593-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trumpfheller, C., C. G. Park, J. Finke, R. M. Steinman, and A. Granelli-Piperno. 2003. Cell type-dependent retention and transmission of HIV-1 by DC-SIGN. Int. Immunol. 15:289-298. [DOI] [PubMed] [Google Scholar]
- 54.Turville, S. G., J. Arthos, K. Mac Donald, G. Lynch, H. Naif, G. Clark, D. Hart, and A. L. Cunningham. 2001. HIV gp120 receptors on human dendritic cells. Blood 98:2482-2488. [DOI] [PubMed] [Google Scholar]
- 55.Turville, S. G., P. U. Cameron, A. Handley, G. Lin, S. Pohlmann, R. W. Doms, and A. L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975-983. [DOI] [PubMed] [Google Scholar]
- 56.Turville, S. G., J. J. Santos, I. Frank, P. U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. Piatak, Jr., J. D. Lifson, M. Pope, and A. L. Cunningham. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103:2170-2179. [DOI] [PubMed] [Google Scholar]
- 57.Unutmaz, D., V. N. KewalRamani, S. Marmon, and D. R. Littman. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 189:1735-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiley, R. D., and S. Gummuluru. 2006. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc. Natl. Acad. Sci. USA 103:738-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu, L., A. A. Bashirova, T. D. Martin, L. Villamide, E. Mehlhop, A. O. Chertov, D. Unutmaz, M. Pope, M. Carrington, and V. N. KewalRamani. 2002. Rhesus macaque dendritic cells efficiently transmit primate lentiviruses independently of DC-SIGN. Proc. Natl. Acad. Sci. USA 99:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu, L., and V. N. KewalRamani. 2006. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat. Rev. Immunol. 6:859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu, L., T. D. Martin, M. Carrington, and V. N. KewalRamani. 2004. Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology 318:17-23. [DOI] [PubMed] [Google Scholar]
- 62.Wu, L., T. D. Martin, Y. C. Han, S. K. Breun, and V. N. KewalRamani. 2004. Trans-dominant cellular inhibition of DC-SIGN-mediated HIV-1 transmission. Retrovirology 1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu, L., T. D. Martin, R. Vazeux, D. Unutmaz, and V. N. KewalRamani. 2002. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J. Virol. 76:5905-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zaitseva, M., A. Blauvelt, S. Lee, C. K. Lapham, V. Klaus-Kovtun, H. Mostowski, J. Manischewitz, and H. Golding. 1997. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat. Med. 3:1369-1375. [DOI] [PubMed] [Google Scholar]