Abstract
The myxoma virus (MV) ankyrin repeat, host range factor M-T5 has the ability to bind and activate cellular Akt, leading to permissive MV replication in a variety of diverse human cancer cell lines (G. Wang, J. W. Barrett, M. Stanford, S. J. Werden, J. B. Johnston, X. Gao, M. Sun, J. Q. Cheng, and G. McFadden, Proc. Natl. Acad. Sci. USA 103:4640-4645, 2006). The susceptibility of permissive human cancer cells to MV infection is directly correlated with the basal or induced levels of phosphorylated Akt. When M-T5 is deleted from MV, the knockout virus, vMyxT5KO, can no longer productively infect a subset of human cancer cells (designated type II) that exhibit little or no endogenous phosphorylated Akt. In searching for a host counterpart of M-T5, we noted sequence similarity of M-T5 to a recently identified ankyrin repeat cellular binding protein of Akt called PIKE-A. PIKE-A binds and activates the kinase activity of Akt in a GTP-dependent manner and promotes the invasiveness of human cancer cell lines. Here, we demonstrate that transfected PIKE-A is able to rescue the ability of vMyxT5KO to productively infect type II human cancer cells that were previously resistant to infection. Also, cancer cells that were completely nonpermissive for both wild-type and vMyxT5KO infection (called type III) were rendered fully permissive following ectopic expression of PIKE-A. We conclude that the MV M-T5 host range protein is functionally interchangeable with the host PIKE-A protein and that the activation of host Akt by either M-T5 or PIKE-A is critical for the permissiveness of human cancer cells for MV.
Myxoma virus (MV) is a rabbit-specific poxvirus pathogen that is being developed as an oncolytic therapeutic because it is nonpathogenic in humans but nevertheless can infect and kill a wide spectrum of human cancer cells (10, 18). MV permissiveness in a subset of human cancer cells is critically dependent upon the expression of a viral host range protein called M-T5. M-T5 has no significant sequence homology to nonviral proteins in the sequence database but is categorized as a member of a larger poxvirus superfamily designated the ankyrin repeat (ANK) host range proteins (11). M-T5 was first identified as a virulence factor that is critical for productive myxomatosis in infected rabbits, based on its ability to inhibit apoptosis in rabbit T lymphocytes (13). More recently, M-T5 was shown to regulate cell cycle progression at the G0/G1 checkpoint through interactions with cellular cullin 1, which directly enhances ubiquitination of p27 (Kip-1) and subsequent degradation through the 26S proteasome pathway (7). MV exhibits strict species specificity for rabbits, causing a lethal disease called myxomatosis in European rabbits (Oryctolagus cuniculus) and localized fibromas that resolve in rabbits (Sylvilagus sp.) of the Americas (5). MV is nonpathogenic for all other tested vertebrates; however, MV can productively infect a variety of human cancer cells that either possess activated Akt or can permit Akt activation following MV infection (21).
In virus-infected human tumor cells, M-T5 forms a complex with cellular Akt and upregulates its kinase activity (21). Given this ability to regulate Akt activation, we initiated a search for a host analogue of M-T5 that could bind and upregulate Akt activation. A recently identified cellular protein, PIKE-A (PI3-kinase enhancer activating AKT), has also been shown to bind directly to activated Akt in a guanine nucleotide-dependent manner, stimulating the kinase activity of Akt and promoting the invasiveness of cancer cell lines (2). PIKE-A exhibits broad tissue specificity and contains an N-terminal GTPase domain and a C-terminal ankyrin repeat motif, both of which associate with the regulatory and partial catalytic domains of Akt (3). Overexpression of PIKE-A in human cancer cells inhibits apoptosis by enhancing the kinase activity of Akt, whereas rapid apoptosis and a loss of Akt activity is observed when PIKE-A is knocked down by small interfering RNA (2).
Our analysis of the permissiveness of human cancer cells for MV indicated that the cells fell into three categories: (i) type I cells possessed endogenous activated Akt and were permissive for MV and vMyxT5KO, (ii) type II cells had low levels of Akt activation and were permissive for MV but not for vMyxT5KO, and (iii) type III cells had no activated Akt and were nonpermissive for both MV and vMyxT5KO. Thus, if Akt was preactivated or could be activated by MV infection via M-T5, the cancer cells were permissive, but if Akt remained unactivated, the cells were nonpermissive for MV infection.
We were therefore prompted to look for cellular proteins capable of binding and activating Akt in a fashion similar to that of M-T5 (21). In type II human cancer cells, for which MV deficient in M-T5 expression (vMyxT5KO) is nonpermissive, we demonstrate here that ectopic overexpression of PIKE-A rescues vMyxT5KO replication. In addition, type III cancer cells, which did not support the replication of either vMyxlac or vMyxT5KO, were rendered permissive for MV replication when these cells were transiently transfected to express PIKE-A before infection. Elevated levels of phosphorylated Akt were observed when PIKE-A-transfected cancer cells were infected with either vMyxlac or vMyxT5KO virus. Finally, virus-induced apoptosis in infected type II and type III cancer cells was blocked by transfected PIKE-A prior to MV infection. The implications of these results for the development of MV as an oncolytic agent to treat human cancer will be discussed further below.
MATERIALS AND METHODS
Cell culture.
Established cell lines used in this study included baby green monkey kidney (BGMK) cells, human embryo kidney (HEK) 293 cells, and the human tumor cell lines HOS, 786-0, and MDA-MB435, which were obtained from the NCI (Canada) reference collection. All cells were propagated in 5% CO2 at 37°C in Dulbecco's modified Eagle medium completed with 10% fetal bovine serum (FBS) (Sigma), 100 units penicillin/ml, and 100 μg streptomycin/ml (Invitrogen).
Viruses and infection.
The recombinant viruses used in this study have been described previously and include vMyxlac, a control MV (strain Lausanne) that expresses β-galactosidase and wild-type M-T5 (16), and vMyxT5KO, which also expresses β-galactosidase but fails to express M-T5 due to targeted disruption of both copies of the M-T5 open reading frame (M005R/L) (13). All viruses were propagated and titrated by focus formation on BGMK cells as described previously (16). For infection studies, cells were incubated at the indicated multiplicity of infection (MOI) with either virus for 1 h at 37°C; infected cells were then washed three times with phosphate-buffered saline to remove excess virus and cultured in normal medium until they were used in subsequent experiments. β-Galactosidase staining has been described previously (16).
Transfection of PIKE-A.
Cells were seeded in six-well plates at a density of 5 × 105 cells per well in complete growth medium with 10% FBS. Transfections were performed with Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer's instructions. HOS, 786-0, or MDA-MB435 and HEK 293 cells were transfected with the plasmid myc-PIKE-A, which has been described previously and was the kind gift of K. Ye (1, 2), or the control vector pcDNA3.1 (5 μg). The cells were collected at various time points, and lysate was used for detection with appropriate antibodies.
Viral growth curves.
For single-step growth analysis, HOS, 786-0, and MDA-MB435 cells (5 × 105) were either mock transfected or transfected with the myc-PIKE-A plasmid. The following day, the cells were infected with vMyxlac or vMyxT5KO at an MOI of 5 for 1 h. Unadsorbed virus was removed by washing the cells with serum-free medium three times, and the cells were grown in complete growth medium supplemented with 10% FBS. Cells were harvested following infection at the indicated time points: 0, 4, 8, 12, 24, and 48 h. Virus titers were determined by serial dilution and infection of BGMK cells, followed by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining of fixed monolayers, as outlined previously (16). All growth analyses were performed in triplicate, and data were expressed as log10 focus-forming units per 106 cells.
Immunoblot analysis.
Cultured cells were collected, and cell lysis was prepared as previously described (7). Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoreactive proteins were detected by chemiluminescence (Perkin-Elmer, Boston, MA). The antibodies used included mouse monoclonal anti-Serp1 and rabbit polyclonal anti-T7, which have been described previously (7, 8, 14); rabbit polyclonal phospho-Akt (Thr-308) antibody; mouse monoclonal phospho-Akt (Ser-473; 587F11) antibody; and polyclonal Akt antibody that detects total levels of endogenous Akt1, Akt2, and Akt3 proteins (Cell Signaling Technology). An anti-Myc monoclonal antibody from Invitrogen was also used to detect the expression of PIKE-A. Horseradish peroxidase-conjugated goat anti-mouse and goat anti-rabbit secondary antibodies were obtained from Jackson ImmunoResearch Laboratories. Densitometry levels of Akt phosphorylation were detected with Molecular Imaging software (Kodak) and compared to the protein level of Akt. Variability between films was normalized.
RESULTS
The M-T5 sequence exhibits similarity to cellular PIKE-A.
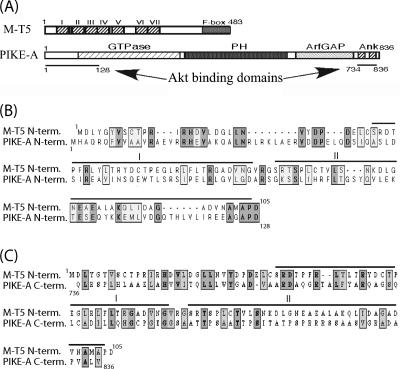
Recent work in our laboratory has demonstrated that the basal level of endogenous phosphorylated Akt in human cancer cells is related to their susceptibility to infection and killing by MV. In MV-infected cells, M-T5 forms a complex with cellular Akt and, as a result, upregulates its kinase activity (21). A database search for protein binding partners of Akt identified a particular cellular protein with functions similar to those of M-T5. PIKE-A, a physiological mediator of Akt, functions to stimulate Akt activation through two domains that contribute to Akt binding (2). The 128 residues of the PIKE-A amino terminus and the two ANK repeats of the carboxyl terminus both independently interact with Akt to upregulate kinase activity (Fig. 1A). Sequence alignment between MV M-T5 and PIKE-A identified some similarity between the N terminus of M-T5 and the N-terminal 128 residues of PIKE-A (18% identity; 33% similarity) (Fig. 1B). In addition, sequence similarity was observed between the C terminus of PIKE-A and the N terminus of M-T5 (18% identity; 36% similarity) (Fig. 1C). That PIKE-A contains ANK repeats, binds to Akt, and blocks apoptosis in cancer cells are all functional features we had previously observed for MV M-T5 (21). Based on the present literature, PIKE-A is the only cellular protein that has been shown to bind to Akt and upregulate its kinase activity. Therefore, we speculate that the sequence homology and functional similarities shared by these two proteins could be of particular importance. To further investigate whether M-T5 represented a viral molecule that had adopted functions similar to those of cellular PIKE-A, we performed a series of experiments to examine this functional similarity.
FIG. 1.
M-T5 exhibits sequence similarity to PIKE-A. (A) M-T5 features, including the predicted ankyrin repeats (I to VII) and the F box located at the C terminus, compared to PIKE-A structure. The underlined sections indicate the regions of PIKE-A sufficient to independently bind Akt; the left line matches the amino acid sequence alignment in panel B, and the right line matches panel C. (B and C) The N-terminal sequence (amino acids 1 to 128) (B) and the C terminal sequence (amino acids 734 to 836) (C) of PIKE-A were aligned with the N terminus of MV M-T5. Conserved residues are boxed. Dark shading indicates identical residues, and light shading indicates similar residues. The bars above the M-T5 sequence define the predicted ankyrin repeats I and II.
Transient expression of PIKE-A rescues MV replication in restrictive human tumor cells.
Virus permissivity was observed to be dependent on the basal level of endogenous phosphorylated Akt in a wide spectrum of human tumor cell lines when they were screened for the ability to support MV replication (18, 21). Since PIKE-A has been demonstrated to bind and upregulate the kinase activity of Akt, we wanted to determine if PIKE-A was able to influence the permissiveness of MV in various cancer cell types. For this study, the following human cancer cells lines were used as representatives of the three cell types: HOS, human osteosarcarcoma (type I); 786-0, renal cancer (type II); and MDA-MB435, breast cancer (type III).
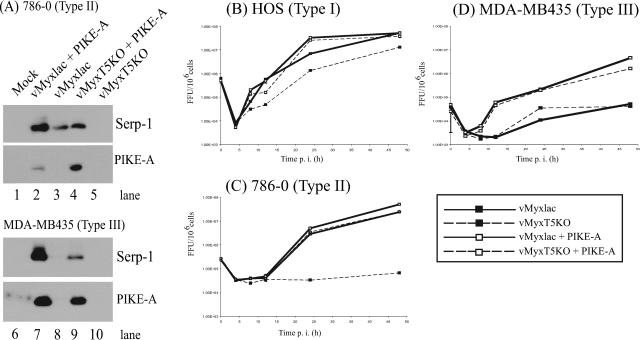
The cell lines 786-0 and MDA-MB435 were mock treated or transfected with a plasmid containing PIKE-A for 8 h and then mock infected or infected with either vMyxlac or vMyxT5KO at an MOI of 5. Cell lysates were collected 48 h postinfection (p.i.), and expression levels of the Serp-1 MV late gene were assessed by Western blotting. Because poxvirus late genes require active virus replication to undergo expression, our laboratory uses the Serp-1 MV late gene as a marker to represent successful virus replication. Based on the presence of the Serp-1 gene, 786-0 cells supported replication of vMyxlac (Fig. 2A, lane 3); however, they were nonpermissive for vMyxT5KO, because expression of Serp-1 was not detected by Western blotting (Fig. 2A, lane 5). Similarly, MDA-MB435 cells did not support replication of either virus (Fig. 2A, lanes 8 and 10), as confirmed by the absence of Serp-1 expression. When both cell lines, 786-0 (Fig. 2A, lanes 2 and 4) and MDA-MB435 (Fig. 2A, lanes 7 and 9), were transfected with PIKE-A 8 h prior to being infected with either vMyxlac or vMyxT5KO, expression of Serp-1 was detected in all cases (Fig. 2A, lanes 2, 4, 7, and 9). No Serp-1 expression was detected in either mock-infected 786-0 or MDA-MB435 cells (Fig. 2A, lanes 1 and 6, respectively). Samples were probed with an antibody against Myc to demonstrate expression of the transfected Myc-tagged PIKE-A protein. Expression of the Myc epitope was detected only in cells transfected with the PIKE-A plasmid (Fig. 2A, lanes 2, 4, 7 and 9). Increased expression of Serp-1 does not correlate with the level of PIKE-A expression in transfected cells but is dependent upon infection by vMyxlac, suggesting that in the absence of M-T5, the knockout virus requires increased expression of PIKE-A to promote virus replication. Virus replication, as confirmed by the expression of MV Serp-1 protein, was observed in human cancer cells that were previously nonpermissive for MV when they were transfected with PIKE-A prior to infection. This suggested that overexpression of PIKE-A preceding MV infection was able to rescue previously nonproductive infection and allow virus replication.
FIG. 2.
Expression of cellular PIKE-A rescues MV infection in restrictive tumor cells. (A) MV-restrictive type II human renal cancer cells (786-0) and MV-nonpermissive type III abortive breast cancer cells (MDA-MB435) were transfected with a Myc-tagged PIKE-A-expressing plasmid (lanes 2, 4, 7, and 9) or mock transfected (lanes 1, 3, 5, 6, 8, and 10) for 8 h and then mock infected (lane 1) or infected with either vMyxlac (lanes 2,3, 7, and 8) or vMyxT5KO (lanes 4, 5, 9, and 10) at an MOI of 5. Cell samples were collected at 48 h p.i., and the cell lysates were examined by immunoblotting them with anti-Serp-1 (late viral gene) and anti-Myc (PIKE-A). In single-step growth analysis, HOS (B), 786-0 (C), and MDA-MB435 (D) cells were transfected with PIKE-A (□) for 12 h or mock transfected (▪) and then infected with either vMyxlac (solid line) or vMyxT5KO (dashed line) at an MOI of 5. Cells were harvested at the indicated times postinfection, and infectious-virus titers were determined on BGMK cells. Each viral growth analysis was performed in triplicate.
To quantitatively assess the ability of the virus to replicate, single-step growth curves on representative type I, II, and III cells were performed. Each cell type was mock treated or transfected with PIKE-A and was infected 8 h later with either vMyxlac or vMyxT5KO at an MOI of 5. Samples were harvested for infectious virus particles at 0, 4, 8, 12, 24, and 48 h p.i., and all time point samples were titrated on BGMK cells by serial dilution and stained with X-Gal at 48 h p.i. to visualize foci. Infection of HOS cells (type I) with vMyxlac and vMyxT5KO produced growth curves characteristic of classical poxvirus replication kinetics. A minimum virus titer was reached at approximately 4 h p.i., followed by a continuous increase up to 48 h p.i., at which point the virus yield reached maximal levels. Identical replication curves were generated for vMyxlac infection of HOS cells, regardless of the expression of PIKE-A. However, HOS cells infected with the knockout virus (vMyxT5KO) produced a slightly lower yield. Nevertheless, transfection of PIKE-A before infection restored the virus titer to a level similar to that of cells infected with vMyxlac (Fig. 2B). Type II cells (786-0) completely supported vMyxlac infection but were nonpermissive for vMyxT5KO. When 786-0 cells were transfected with PIKE-A and then infected with vMyxT5KO, the viral titers indicated that transfection of PIKE-A before infection was able to rescue vMyxT5KO replication to levels similar to those of cells infected with vMyxlac (Fig. 2C). Replication of either vMyxlac or vMyxT5KO virus was not supported in the type III cells (MDA-MB435), and as a result, little or no viral amplification was observed over time. The viral titers increased considerably when the cells were transfected with PIKE-A prior to infection, indicating rescue of virus replication (Fig. 2D). The ability of PIKE-A to rescue viral replication in previously nonpermissive human cancer cell lines was further demonstrated when cells were transfected with PIKE-A and infected with viruses that express green fluorescent protein (vMyxgfp or vMyxT5KOgfp). No foci were observed when type II cells were infected with vMyxT5KOgfp; however, endogenous PIKE-A expression prior to infection with vMyxT5KOgfp rescued focus formation. Virus replication of either vMyxgfp or vMyxT5KOgfp in type III cells was supported only when the cells were transfected with PIKE-A 8 h before infection with either virus (data not shown). These data demonstrate that overexpression of exogenous PIKE-A has the ability to render previously restricted human cancer cells fully permissive for MV infection.
Among viruses, poxviruses are unique in that they have the ability to effectively and efficiently enter almost any cell type. However, virus replication is often restricted because the virus is unable to complete its replicative cycle within the infected cell (12). Earlier studies have shown that in nonpermissive cancer cells, MV can successfully bind, uncoat, and begin early-gene expression. The block to a productive infection, however, lies in the inability of M-T5 to bind and activate Akt (21). In cell lines previously nonpermissive for MV replication, overexpression of PIKE-A is predicted to specifically upregulate the normally low kinase activity of Akt. PIKE-A, therefore, directly modulates the PI-K3/Akt signal pathway, promoting virus permissivity by releasing the block prior to virus replication and virus late-gene expression, but does not alter virus entry into the cell.
Transient expression of PIKE-A upregulates the kinase activity of Akt in type II and type III human cancer cells.
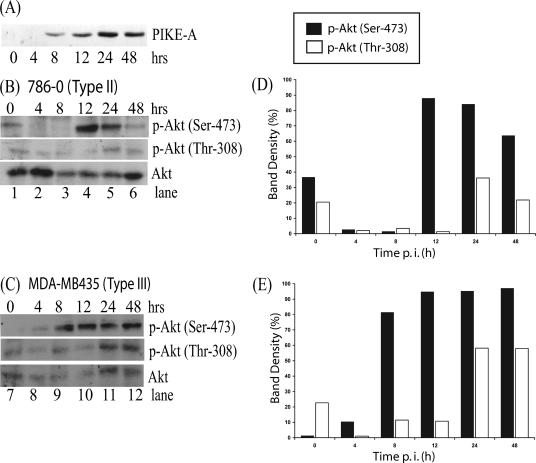
Amplification of PIKE-A has been observed in a variety of human glioblastoma cells and coincidently results in the upregulation of Akt kinase activity (1). A plasmid containing PIKE-A was transfected into HEK 293 cells, and lysates were collected at various time points and resolved by Western blotting. The blot was then probed with an antibody against the Myc epitope, which was fused to PIKE-A protein. Expression of PIKE-A was detected 8 h after transfection, and expression continued to increase over time (Fig. 3A). To determine if overexpression of PIKE-A would have the ability to increase the kinase activity of Akt, type II cells (786-0) and type III cells (MDA-MB435) were transfected with PIKE-A. Cell lysates were collected at various time points following transfection of PIKE-A, and Akt phosphorylation was assessed by Western blotting. Low levels of endogenous Akt phosphorylation were detected at both Ser-473 and Thr-308 sites in the 786-0 cells at 0 h (Fig. 3B, lane 1). Following transfection of PIKE-A, phosphorylation of Akt at Ser-473 was dramatically increased as early as 12 h (Fig. 3B, lane 4) and phosphorylation of site Thr-308 was detected at 24 h (Fig. 3B, lane 5). In the MBA-MB435 cells, very low levels of Akt phosphorylation were detected at 0 h (Fig. 3C, lane 7); however, at 8 h, overexpression of PIKE-A considerably induced Akt phosphorylation at Ser-473, and increased phosphorylation levels of Thr-308 were detected at 24 h (Fig. 3C, lane 11). The levels of total Akt protein remained relatively constant (Fig. 3B and C). As predicted, the overexpression of exogenous PIKE-A induces the phosphorylation of Akt at Ser-473 and Thr-308 sites in type II and III cell lines. In both cell lines, the phosphorylation of Ser-473 occurred earlier and the band intensity was more intense, in contrast to phosphorylation of Thr-308 (Fig. 3D and E). However, trivial differences in the pattern of Akt phosphorylation were observed, suggesting that the response to the overexpression of PIKE-A may be unique for each cell line. Therefore, in human cancer cells, which express little or no detectable endogenous phosphorylated Akt, Akt kinase activity can be induced through the overexpression of its physiological regulator, PIKE-A.
FIG. 3.
Induction of endogenous Akt phosphorylation following transfection of PIKE-A in human cancer cells. (A) HEK 293 cells were transfected with the PIKE-A plasmid, and expression was detected at various time points by Western blotting with an anti-Myc antibody. PIKE-A plasmid was transfected into (B) 786-0 and (C) MDA-MB435 cells, and Akt phosphorylation at both p-Akt Ser-473 and p-Akt Thr-308 sites was detected in cell lysates (50 μg per lane) by Western blotting at various times following transfection. The levels of Akt phosphorylation at Ser-473 and Thr-308 were determined with Molecular Imaging software (Kodak) and compared to the protein level of Akt to quantify the stimulation induced by overexpression of PIKE-A in (D) 786-0 and (E) MDA-MB435 cells. Immunoblot signal variability between films was normalized.
Induction of Akt kinase activity following transfection of PIKE-A in vMyxT5KO virus-infected type II human cancer cells.
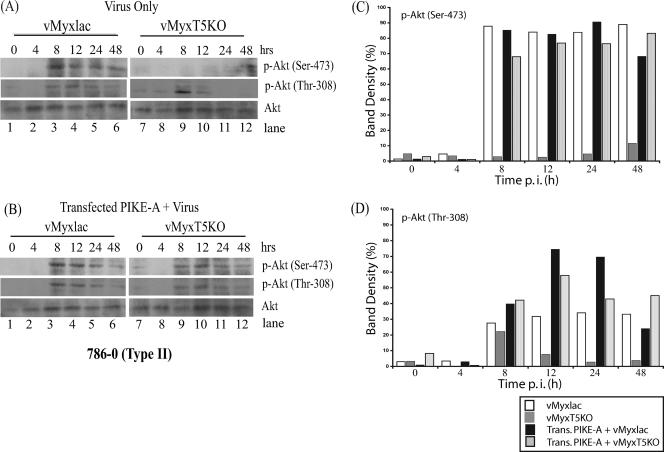
The susceptibility of human cancer cells to MV infection is dependent upon the basal level of endogenous phosphorylated Akt (21). In the 786-0 cells (type II), the level of endogenous phosphorylated Akt was shown to be very low; however, infection with vMyxlac dramatically induced Akt phosphorylation. The viral protein M-T5 was a critical determinant of MV tropism in human cancer cells, and in its absence, endogenous levels of phosphorylated Akt remained relatively unchanged in vMyxT5KO-infected 786-0 cells (21). As overexpression of PIKE-A increases Akt kinase activity, we wanted to examine the levels of phosphorylated Akt following vMyxlac or vMyxT5KO infection in the presence or absence of exogenous PIKE-A. Cells were mock treated or transfected with the PIKE-A plasmid for 8 h and then infected with either vMyxlac or vMyxT5KO. Cell lysates were collected at various time points, and phosphorylation of Akt was assessed by Western blotting. Increased levels of endogenous phosphorylated Akt at both Ser-473 and Thr-308 sites were detected at 8 h p.i. in 786-0 cells infected with vMyxlac either in the presence or absence of endogenous expression of PIKE-A (Fig. 4A and B, lanes 1 to 6). In contrast, 786-0 cells infected with vMyxT5KO exhibited very little Akt phosphorylation (Fig. 4A, lanes 7 to 12), yet transfection of PIKE-A prior to infection by vMyxT5KO considerably induced phosphorylation of Akt at both Ser-473 and Thr-308 sites compared to the levels observed in the absence of exogenous PIKE-A (Fig. 4B, lanes 7 to 12). The levels of total Akt protein remained relatively constant following vMxylac and vMyxT5KO infection (Fig. 4A and B). Densitometry levels of Akt phosphorylation at both Ser-473 (Fig. 4C) and Thr-308 (Fig. 4D) sites were detected with Molecular Imaging software (Kodak) and compared to total Akt to quantitate phosphorylation induction following transfection of PIKE-A. Taken together, the data in Fig. 2 to 4 confirm that overexpression of PIKE-A, in type II cells (786-0), was able to upregulate the kinase activity of Akt, which is critical for the replication of MV even in the absence of M-T5.
FIG. 4.
PIKE-A upregulates Akt phosphorylation in type II (786-0) cells infected with vMyxT5KO. Human 786-0 (type II) cancer cells were either mock treated (A) or transfected with PIKE-A plasmid (B) and 8 h later were infected with either vMyxlac (lanes 1 to 6) or vMyxT5KO (lanes 7 to 12) at an MOI of 5. Cells were harvested at the indicated times postinfection, and Akt phosphorylation, at both p-Akt Ser-473 and p-Akt Thr-308 sites, was detected in cell lysates (50 μg per lane). Total Akt protein levels are shown in the bottom rows. Densitometry was used to measure induction of Akt phosphorylation at both Ser-473 (C) and Thr-308 (D), as described in the legend to Fig. 3.
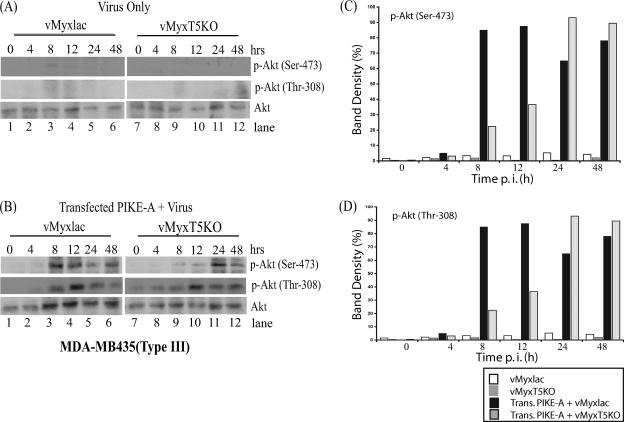
Transfection of PIKE-A stimulates Akt phosphorylation in type III cells infected with either vMyxlac or vMyxT5KO.
The basal level of endogenous phosphorylated Akt was undetectable in type III cells (MDA-MB435) (Fig. 5A, lanes 1 and 7), which do not support replication of either vMyxlac or vMyxT5KO. Levels of Akt phosphorylation remained undetectable following vMyxlac and vMyxT5KO infection (Fig. 5A, lanes 1 to 12), suggesting that M-T5 is unable to activate Akt kinase activity in type III cells. Transfection of PIKE-A in MDA-MB435 cells prior to infection with either vMyxlac (Fig. 5B, lanes 1 to 6) or vMyxT5KO (Fig. 5B, lanes 7 to 12) dramatically induced phosphorylation of endogenous Akt at Ser-473 and Thr-308 sites at 8 h p.i. The total Akt protein levels remained relatively unchanged (Fig. 5A and B), as observed in the 786-0 cells (Fig. 4A and B). Induction of Akt phosphorylation was determined by measuring the levels of Akt phosphorylation at both Ser-473 (Fig. 5C) and Thr-308 (Fig. 5D) sites with Molecular Imaging software (Kodak) and was compared to the expression levels of the Akt protein. Together, these data indicate that type III cells, which do not support replication of MV, are unable to activate Akt kinase activity when infected with MV in the presence or absence of M-T5. However, ectopic expression of PIKE-A induces Akt phosphorylation at Ser-473 and Thr-308 sites and rescues the replication of both vMyxlac and vMyxT5KO. These results help to explain the mechanism by which overexpression of PIKE-A contributes to rescuing virus replication in the type III cells, as observed previously (Fig. 2B and D).
FIG. 5.
Overexpression of PIKE-A stimulates Akt phosphorylation in type III (MDA-MB435) cells infected with either vMyxlac or vMyxT5KO. Human MDA-MB435 (type III) breast cancer cells were either mock treated (A) or transfected with PIKE-A plasmid (B). Eight hours after transfection, the cells were infected with either vMyxlac (A and B, lanes 1 to 6) or vMyxT5KO (A and B, lanes 7 to 12) at an MOI of 5. Cells were harvested at the indicated times postinfection, and Akt phosphorylation, at both p-Akt Ser-473 and p-Akt Thr-308 sites, was detected in the cell lysates (50 μg per lane). Total Akt protein levels are shown in the bottom rows. Densitometry was used to measure induction of Akt phosphorylation at both Ser-473 (C) and Thr-308 (D), as described in the legend to Fig. 3.
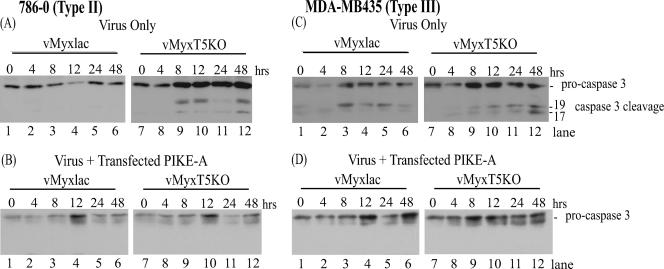
PIKE-A inhibition of apoptosis in MV-infected human cancer cells.
Following MV infection of human cancer cells, a cascade of events that induce apoptosis is initiated. When M-T5 is present and expressed, it plays a critical role in preventing apoptosis through manipulation of the cell cycle (7). PIKE-A, a physiological regulator of Akt activation (6), is often amplified in human cancer cells and, coincidently, has been shown to promote cellular proliferation by inhibiting apoptosis through stimulation of Akt (1). Since transient expression of PIKE-A in type II and type III human cancer cells stimulates phosphorylation of Akt (Fig. 4 and 5), we wanted to determine if overexpression of PIKE-A also functioned to inhibit apoptosis induction following vMyxT5KO infection of type II cells and MV infection of type III cells. To investigate this possibility, type II (786-0) and type III (MDA-MB435) cells were infected with either vMyxlac or vMyxT5KO, and cleavage of procaspase 3 to its active form was assessed by Western blotting. As expected from our previous studies, infection of 786-0 cells with MV did not induce apoptosis (7), and no cleavage of procaspase 3 was observed (Fig. 6A, lanes 1 to 6). Type II cells (786-0) infected with vMyxT5KO induced activation of caspase 3, observed as early as 8 h p.i. (Fig. 6A, lanes 7 to 12). However, when type II cells were transfected with PIKE-A 8 h prior to infection with vMyxT5KO, cleavage of caspase 3 was not detected, indicating that inhibition of caspase-mediated apoptosis was observed even in the absence of M-T5 (Fig. 6B, lanes 7 to 12).
FIG. 6.
PIKE-A inhibits activation of apoptosis in type II (786-0) cells infected with vMyxT5KO and type III (MDA-MB435) cells infected with vMyxlac and vMyxT5KO. Human renal cancer 786-0 (type II) cells (A and B) and breast cancer MDA-MB435 (type III) cells (C and D) were transfected with PIKE-A plasmid (B and D) or mock transfected (A and C) and 8 h later were infected with either vMyxlac (A to D, lanes 1 to 6) or vMyxT5KO (A to D, lanes 7 to 12) at an MOI of 5. Cells were harvested at the indicated times postinfection, and the cell lysates (50 μg per lane) were probed by immunoblotting for procaspase 3 degradation.
Induction of apoptosis, as indicated by caspase 3 cleavage in type III cells (MDA-MB435), was observed when the cells were infected with either vMyxlac (Fig. 6C, lanes 1 to 6) or vMyxT5KO (Fig. 6C, lanes 7 to 12). Exogenous expression of PIKE-A was successful at inhibiting apoptosis following infection with either vMyxlac (Fig. 6D, lanes 1 to 6) or vMyxT5KO (Fig. 6D, lanes 7 to 12) by preventing the cleavage of procaspase 3 into its active form. Therefore, transfection of PIKE-A in type II cells was able to inhibit vMyxT5KO-induced apoptosis in type II cells. Virus-induced apoptosis was also inhibited in type III cells infected with either vMyxlac or vMyxT5KO when the cells had previously been transfected with PIKE-A. Stimulation of Akt kinase activity, in response to overexpression of PIKE-A, promoted the inhibition of the apoptotic signaling cascade, which would otherwise be activated in MV-infected cells (type III), especially in the absence of M-T5 (type II and type III cells).
DISCUSSION
A number of cellular pathways, frequently mutated in cancer, were examined in MV-permissive human cancer cells, and the Akt pathway was identified as a key restriction determinant for virus replication (21). The oncogene Akt is a critical regulator of diverse cellular processes and has been demonstrated to contribute to cancer progression through stimulation of proliferation and inhibition of apoptosis (15, 19). The critical role of Akt in the regulation of multiple cellular functions makes it a central manipulator of cellular signaling, and therefore, it is not surprising that a number of viruses have developed sophisticated strategies to manipulate the activation of Akt (9). For example, respiratory syncytial virus induces activation of the PI-3K/Akt signaling pathway during early viral infection (20), thereby increasing cell survival and ensuring that the virus has sufficient time to complete its replicative cycle (4). MV also manipulates the Akt pathway through the actions of the protein M-T5, which has been shown to bind and upregulate the kinase activity of Akt during MV infection (21). Akt kinase activity is upregulated through direct binding to a recently identified cellular protein, PIKE-A. Overexpression of PIKE-A stimulates Akt activity, promoting cellular transformation leading to the development of cancer, while knockdown of PIKE-A diminishes Akt activity and increases apoptosis. Amplified PIKE-A has been identified in a number of human glioma cancer cells that express increased levels of Akt phosphorylation and reduced activation of apoptosis (1).
Here, we demonstrated that restrictive type II human cancer cells switch from resistant to susceptible to vMyxT5KO infection following transient expression of PIKE-A. In the absence of M-T5, MV is unable to stimulate the kinase activity of Akt; however, overexpression of exogenous PIKE-A in type II cells considerably increased the levels of Akt phosphorylation at both Ser-473 and Thr-308 sites (Fig. 4). Type III human cancer cells are nonpermissive for both vMyxlac and vMyxT5KO infection, and type III cells do not express basal levels of detectable, endogenous phosphorylated Akt (Fig. 5). Similar to the observation in type II cells, expression of PIKE-A renders nonpermissive type III cells susceptible to both vMyxlac and vMyxT5KO infection and upregulates Akt kinase activity (Fig. 2, 4, and 5). The fact that exogenous PIKE-A rescues MV replication in previously nonpermissive human cancer cells only strengthens the argument that the Akt pathway is a key restriction determinant for permissiveness of human cancer cells for MV. In addition to stimulating Akt kinase activity, transfection of PIKE-A was responsible for inhibiting the activation of virus-induced apoptosis following MV infection in type II and III human cancer cells. M-T5 also inhibits MV-induced apoptosis by protecting MV-infected cells from cell cycle arrest, which otherwise would promote the activation of the apoptotic cascade (7). Sequence similarity between MV M-T5 and cellular PIKE-A is limited to the previously identified region of PIKE-A that is necessary to bind Akt. However, both M-T5 and PIKE-A contain ANK repeats and share the ability to upregulate the Akt pathway, block apoptosis, and interact with Akt. Functionally, M-T5 and PIKE-A are viral and cellular molecules evolved to control Akt activation.
A number of virus-encoded proteins, several of which are encoded by host-related immunomodulatory genes, share sequence and functional similarity with cellular proteins and are categorized as viral homologs. Many of these viral homologs are gene products that have been hijacked from the host, increasing the replicative ability of the virus (17). Although M-T5 and PIKE-A share similar functions, based on this preliminary study, we predict that M-T5 represents a viral strategy evolved to mimic the cellular activity of PIKE-A. We demonstrated that exogenous PIKE-A is able to upregulate Akt kinase activity and rescue MV replication in type III human cancer cells. Interaction of type III cells with MV does not produce a productive infection, even though M-T5 is expressed. We suspect that M-T5 expression and localization are altered during infection of type III cells (21). Therefore, the mechanism by which PIKE-A activates Akt may exhibit some differences from the method employed by M-T5.
PIKE-A provides an alternative model for studying the importance of Akt phosphorylation during a productive MV infection in human cancer cells. Understanding the mechanism by which PIKE-A rescues MV replication in previously nonpermissive human cancer cells may provide additional clues to how M-T5 functions during MV infection. We predict that cells with a high level of PIKE-A expression will be naturally more susceptible to MV infection. Additionally, the M-T5 protein possesses seven ankyrin repeat domains, which are thought to mediate specific protein-protein interactions. Therefore, we speculate that M-T5 acts as a molecular scaffold, bringing together proteins that might otherwise be spatially and temporally isolated, thus stimulating signaling pathways critical for successful MV replication. Studying the functional role of PIKE-A may give us further insight into additional proteins that may interact with M-T5 and counteract MV replication in human cancer cells. The results in this study suggest that manipulation of the Akt pathway through the actions of PIKE-A may allow the oncolytic capacity of this virus to extend to an even broader spectrum of human cancer cells. In conclusion, this knowledge may have significant implications for the rational design of the next generation of oncolytic viruses, as the development of new and improved cancer therapies continues.
Acknowledgments
We thank K. Ye (Emory University School of Medicine, Atlanta, GA) for sending us the PIKE-A plasmid.
This work was supported by the National Cancer Institute of Canada and Canadian Institutes of Health Research grants (to G.M.). G.M. holds a Canada Research Chair in Molecular Virology and is an International Scholar of The Howard Hughes Medical Institute.
Footnotes
Published ahead of print on 6 December 2006.
REFERENCES
- 1.Ahn, J. Y., Y. Hu, T. G. Kroll, P. Allard, and K. Ye. 2004. PIKE-A is amplified in human cancers and prevents apoptosis by up-regulating Akt. Proc. Natl. Acad. Sci. USA 101:6993-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn, J. Y., R. Rong, T. G. Kroll, E. G. Van Meir, S. H. Snyder, and K. Ye. 2004. PIKE (phosphatidylinositol 3-kinase enhancer)-A GTPase stimulates Akt activity and mediates cellular invasion. J. Biol. Chem. 279:16441-16451. [DOI] [PubMed] [Google Scholar]
- 3.Ahn, J. Y., R. Rong, X. Liu, and K. Ye. 2004. PIKE/nuclear PI 3-kinase signaling mediates the antiapoptotic actions of NGF in the nucleus. EMBO J. 23:3995-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooray, S. 2004. The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J. Gen. Virol. 85:1065-1076. [DOI] [PubMed] [Google Scholar]
- 5.Fenner, F., and F. Ratcliffe. 1965. Myxomatosis. University Press, Cambridge, England.
- 6.Hu, Y., Z. Liu, and K. Ye. 2005. Phosphoinositol lipids bind to phosphatidylinositol 3 (PI3)-kinase enhancer GTPase and mediate its stimulatory effect on PI3-kinase and Akt signalings. Proc. Natl. Acad. Sci. USA 102:16853-16858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston, J. B., G. Wang, J. W. Barrett, S. H. Nazarian, K. Colwill, M. Moran, and G. McFadden. 2005. Myxoma virus M-T5 protects infected cells from the stress of cell cycle arrest through its interaction with host cell cullin-1. J. Virol. 79:10750-10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lalani, A. S., K. Graham, K. Mossman, K. Rajarathnam, I. Clark-Lewis, D. Kelvin, and G. McFadden. 1997. The purified myxoma virus gamma interferon receptor homolog M-T7 interacts with the heparin-binding domains of chemokines. J. Virol. 71:4356-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawlor, M. A., and D. R. Alessi. 2001. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 114:2903-2910. [DOI] [PubMed] [Google Scholar]
- 10.Lun, X., W. Yang, T. Alain, Z. Q. Shi, H. Muzik, J. W. Barrett, G. McFadden, J. Bell, M. G. Hamilton, D. L. Senger, and P. A. Forsyth. 2005. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 65:9982-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFadden, G. 2005. Poxvirus tropism. Nat. Rev. Microbiol. 3:201-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moss, B. 2006. Poxvirus entry and membrane fusion. Virology 344:48-54. [DOI] [PubMed] [Google Scholar]
- 13.Mossman, K., S. F. Lee, M. Barry, L. Boshkov, and G. McFadden. 1996. Disruption of M-T5, a novel myxoma virus gene member of poxvirus host range superfamily, results in dramatic attenuation of myxomatosis in infected European rabbits. J. Virol. 70:4394-4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nash, P., A. Whitty, J. Handwerker, J. Macen, and G. McFadden. 1998. Inhibitory specificity of the anti-inflammatory myxoma virus serpin, SERP-1. J. Biol. Chem. 273:20982-20991. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson, K. M., and N. G. Anderson. 2002. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 14:381-395. [DOI] [PubMed] [Google Scholar]
- 16.Opgenorth, A., K. Graham, N. Nation, D. Strayer, and G. McFadden. 1992. Deletion analysis of two tandemly arranged virulence genes in myxoma virus, M11L, and myxoma growth factor. J. Virol. 66:4720-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seet, B. T., J. B. Johnston, C. R. Brunetti, J. W. Barrett, H. Everett, C. Cameron, J. Sypula, S. H. Nazarian, A. Lucas, and G. McFadden. 2003. Poxviruses and immune evasion. Annu. Rev. Immunol. 21:377-423. [DOI] [PubMed] [Google Scholar]
- 18.Sypula, J., F. Wang, Y. Ma, J. Bell, and G. McFadden. 2004. Myxoma virus tropism in human tumor cells. Gene Ther. Mol. Biol. 8:103-114. [Google Scholar]
- 19.Testa, J. R., and A. Bellacosa. 2001. AKT plays a central role in tumorigenesis. Proc. Natl. Acad. Sci. USA 98:10983-10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas, K. W., M. M. Monick, J. M. Staber, T. Yarovinsky, A. B. Carter, and G. W. Hunninghake. 2002. Respiratory syncytial virus inhibits apoptosis and induces NF-kappa B activity through a phosphatidylinositol 3-kinase-dependent pathway. J. Biol. Chem. 277:492-501. [DOI] [PubMed] [Google Scholar]
- 21.Wang, G., J. W. Barrett, M. Stanford, S. J. Werden, J. B. Johnston, X. Gao, M. Sun, J. Q. Cheng, and G. McFadden. 2006. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc. Natl. Acad. Sci. USA 103:4640-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]