Abstract
Crimean-Congo hemorrhagic fever virus (CCHFV) is an etiological agent of a disease with mortality rates in patients averaging 30%. The disease is characterized by fever, myalgia, and hemorrhage. Mechanisms underlying the hemorrhage have to our knowledge not been elucidated for CCHFV. Possibly, a direct or indirect viral effect on tight junctions (TJ) could cause the hemorrhage observed in patients, as TJ play a crucial role in vascular homeostasis and can cause leakage upon deregulation. Moreover, there is no knowledge regarding the site of entry and release of CCHFV in polarized epithelial cells. Such cells represent a barrier to virus dissemination within the host, and as a site of viral entry and release, they could play a key role in further spread. For the first time, we have shown preferential basolateral entry of CCHFV in Madin-Darby canine kidney 1 (MDCK-1) epithelial cells. Furthermore, we demonstrated basolateral release of CCHFV in polarized epithelial cells. Interestingly, by measuring transepithelial electrical resistance, we found no effect of CCHFV replication on the function of TJ in this study. Neither did we observe any difference in the localization of the TJ proteins ZO-1 and occludin in CCHFV-infected cells compared to mock-infected cells.
Crimean-Congo hemorrhagic fever virus (CCHFV) is a member of the Nairovirus genus within the Bunyaviridae family. Bunyaviridae virions are enveloped and contain a trisegmented single-strand RNA genome of negative sense orientation. The large segment encodes an RNA-dependent RNA polymerase, the medium segment a precursor of glycoproteins, and the smallest segment a nucleocapsid protein (NP) (29). Replication takes place in the cytoplasm followed by association of nucleocapsid protein with viral RNA to yield ribonucleoparticles (29). The glycoproteins are synthesized as polyprotein precursors and become posttranslationally processed within the Golgi apparatus, producing the mature structural proteins GN and GC and a newly discovered glycoprotein, GP38, whose role has yet to be determined (3, 28). It has been suggested that nucleocapsid protein targets the viral ribonucleoparticle to the Golgi apparatus, where assembly of the progeny virion occurs by association with the glycoproteins (22). Budding into cisternae of the Golgi apparatus is thought to be initiated by the interaction of the ribonucleoparticle with the glycoproteins, which leads to release of the progeny virion within vesicles similar to those seen in the secretory pathway (29).
CCHFV is a causative agent of a human disease called Crimean-Congo hemorrhagic fever with symptoms such as fever, prostration, and severe hemorrhages, in severe cases leading to death (35). Transmission of the virus occurs by either tick bite or contact with infected tissues or blood (36). The factors determining the molecular mechanisms underlying viral uptake/entry into target cells and pathogenicity of CCHFV remain largely unexplored. Consequently, efficient disease control strategies can be designed only when a clearer picture of virus host-cell interactions is obtained.
Among the factors that remain to be elucidated are the host cell entry and release of CCHFV in polarized epithelial cells and whether tight junctions (TJ) in epithelial cells are disturbed.
Epithelial cells have asymmetric plasma membranes containing distinct apical and basolateral parts (41). The TJ enable maintenance of polarity by functioning as a barrier by keeping membrane proteins segregated (30). Another function of TJ is to regulate paracellular permeability controlling, for instance, the vascular homeostasis of the organism (2). Disturbance of TJ can lead to leakage between adjacent cells (37), which could also cause the hemorrhages observed in CCHF patients. One of the most obvious symptoms in viral hemorrhagic fever patients is bleeding in different organs. This could be explained by an increased permeability of the endothelium, which is a subtype of epithelia (2), or by cellular detachment for underlying tissues. Endothelial dysfunction could further be caused by either direct viral infection or activation of immunological and inflammatory pathways (31), but until now, there has been no knowledge regarding the mechanism behind these phenomena for CCHFV.
Due to the lethal nature of CCHFV, any handling of this virus has been restricted to biosafety level 4 laboratories, limiting research efforts to clarify the virus-epithelial cell interaction and studies of the mechanisms underlying pathogenesis. Here, we report for the first time polarized entry and release of CCHFV in epithelial cells. We also found that CCHFV, per se, neither causes a redistribution of the TJ proteins occludin and ZO-1 nor does disturbs the functional integrity of TJ.
MATERIALS AND METHODS
Cells and antibodies.
Madin-Darby canine kidney 1 (MDCK-1) cells and Vero E6 cells were all grown in Dulbecco's modified Eagle's minimal essential medium (Gibco, Invitrogen, Carlsbad, CA) supplemented with 2% or 10% fetal bovine serum, antibiotics (10 U/ml penicillin and 10 μg/ml streptomycin), and 2% HEPES (Gibco, Invitrogen, Carlsbad, CA). Antibodies used in this study included a rabbit polyclonal anti-CCHFV NP antibody (1), a mouse anti-occludin antibody (Zymed Laboratories), and a rat monoclonal anti-ZO-1 antibody (Chemicon International, Inc., Temecula, CA).
Indirect immunofluorescence.
MDCK-1 cells were grown on Lab-Tek II chamber slides (Nunc) and infected with CCHFV strain Ibar 10200, initially isolated from Hyalomma excavatum ticks in Nigeria in 1966 (6). After infection, cells were fixed with cold 95% ethanol, permeabilized with ice-cold acetone, and blocked in 2% bovine serum albumin (Sigma, St. Louis, MO) in phosphate-buffered saline (PBS). The cells were incubated with primary rabbit CCHFV NP (diluted 1:100 in dilution buffer, 0.2% bovine serum albumin, and 0.1% Triton-X in PBS) in a combination with either mouse occludin (1:100 in dilution buffer) or rat ZO-1 (1:100 in dilution buffer) antibodies for 1 h at 37°C. After rinsing with PBS, the cells were incubated with anti-rabbit/rat fluorescein isothiocyanate-conjugated (Dako-Cytomation, Copenhagen, Denmark) or anti-rabbit/mouse tetramethyl rhodamine isothiocyanate-conjugated antibodies (Jackson, Baltimore, MD) for 1 h at 37°C. After three washes, the slides were mounted using mounting medium (Dako-Cytomation) and analyzed by immunofluorescence microscopy (Nikon Eclipse TE300, Tokyo, Japan). DAPI (4′,6′-diamidino-2-phenylindole) (Sigma, St. Louis, MO) was applied to indicate the cell nucleus. Pictures were obtained with a Hamamatsu digital camera (type Wasabi 1,4 Hamamatsu; Photonics, GmbH, Germany). All handling of the virus occurred in the biosafety level 4 laboratory.
TER measurements.
Establishment of confluence was determined by measuring transepithelial electrical resistance (TER) over the cell layer, using a Millicell-ERS volt-ohmmeter (Millipore, Billerica, MA). Values were obtained by subtraction of a background value (i.e., TER of filters without cell growth) and by multiplication by the area of the filter. Once TER values reached 1,500 Ω · cm2, the cell layer was considered confluent.
The TER of both infected and mock-infected cell layers was measured daily to evaluate integrity of TJ.
Phorbol 12-myristate 13-acetate (PMA) (Sigma, St. Louis, MO) was added apically to confluent MDCK-1 cell layers on Transwell 3.0-μm-pore filters at concentrations of 100 and 1000 nM, respectively, and TER values obtained at the indicated time points were used as positive controls. Mock-infected cell layers were used to indicate a state of normality.
Entry and release assay.
MDCK-1 cells were grown to confluence on 3.0-μm-pore-size Transwell filters (Corning Inc., Corning, NY) with a diameter of 4.7 cm2 and incubated at 37°C in a 5% CO2 humidified atmosphere. The CCHFV suspension was added to either the apical or basolateral compartment, incubated for 1 h at 37°C, and washed three times with PBS, followed by addition of 10% fetal bovine serum medium (described above) and incubation at 37°C. Cells were harvested in lysis buffer 72 h postinfection (hpi), and CCHFV NP was detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). To investigate the route of release of CCHFV, supernatants were collected 24, 48, and 72 hpi and exchanged for 10% fetal bovine serum medium. Supernatants were titrated for analysis of progeny virions as with the immunofluorescence focus unit assay.
A 15 mM concentration of ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) (Sigma, St. Louis, MO), which disrupts the junctional complex between cells (25, 39), was applied to confluent monolayers on Transwell 3.0-μm-pore filters preinfection and incubated for 1 h at 37°C in a 5% CO2 humidified atmosphere. Cells were then infected as described above. Mock-treated cells were incubated with medium.
SDS-PAGE and Western blotting.
Infected cells were harvested in lysis buffer (10 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% SDS) containing Complete mini protease inhibitor (Roche, Basel Switzerland) and boiled in reducing sample buffer for 10 min. Separation of polypeptides was performed by reducing SDS-PAGE, using a 5% concentration gel and a 10% separation gel (Bio-Rad). Electrophoresis was carried out at a constant current of 30 A. Proteins were transferred to nitrocellulose membranes using a transfer buffer containing 25 mM Tris, 192 mM glycine, and 20% (vol/vol) methanol at 100 V for 1 h, and membranes were blocked in 5% nonfat dry milk in 0.1% Tween (PBS-T) overnight at 4°C. The membranes were incubated in rabbit anti-CCHFV NP antibody and rabbit anti-calnexin, to ensure equal loading of samples in wells, in blocking buffer for 1 h at room temperature. After being washed three times with PBS-T, the membranes were incubated with goat anti-rabbit horseradish peroxidase-conjugated antibody for 1 h at room temperature. The membranes were then washed three times with PBS-T and developed with ECL Plus Western blotting detection reagents (Amersham, Pharmacia, Buckinghamshire, United Kingdom), according to the manufacturer's protocol.
Assay for determination of FFU.
For determination of fluorescence focus units (FFU), Vero cells grown in 96-well plates (Sarstedt, Nümbrecht, Germany) were infected with serially diluted (5- and 10-fold) harvested supernatants. After absorption for 1 h at 37°C, the supernatants were removed and the cells were washed with PBS. Cells were fixed in ice-cold 80% acetone at 24 hpi, rabbit anti-CCHFV NP was added for 1 h at 37°C, and the cells were washed twice in PBS and further incubated with swine anti-rabbit fluorescein isothiocyanate-conjugated antibody (Dako-Cytomation, Copenhagen, Denmark) for 1 h at 37°C. Fluorescent foci were counted in the fluorescence microscope, thereby enabling calculation of progeny virus titers.
RESULTS
CCHFV entry into polarized MDCK-1 cells occurs preferentially at the basolateral surface.
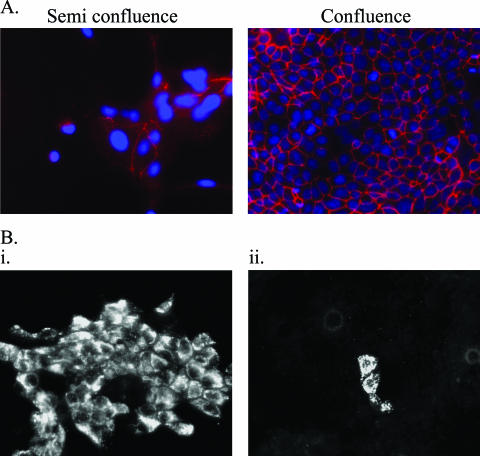
In order to study the polarity of CCHFV entry, confluent and semiconfluent cells were infected. To verify semiconfluent cells had not established tight junctions at the time of infection, semiconfluent and confluent cells were stained for occludin (Fig. 1A). It was clear that semiconfluent cells have a limited presence of occludin in cell-cell boundaries, indicating these cells have not established tight junctions. CCHFV would therefore have access to viral attachment proteins in semiconfluent cells as polarization has not occurred yet.
FIG. 1.
(A) Semiconfluent cells are not polarized as indicated by TJ staining. Semiconfluent and confluent cell layers were stained for occludin (red) in order to indicate the level of cell polarization upon infection with CCHFV. Cell nucleus is indicated by DAPI staining (blue). (B) Subconfluent cell layers are more susceptible to infection with CCHFV than confluent cell layers. MDCK-1 cell layers were grown to either semiconfluence (i) or confluence (ii) and inoculated with CCHFV at an MOI of 0.02. Cell layers were fixed and stained for CCHFV NP 48 hpi and examined by immunofluorescence microscopy.
Mock-infected or CCHFV-infected cells grown on chamber slides were fixed at 48 hpi. Inoculation of confluent cells (1B.ii) revealed very few infected cells, in contrast to inoculation of semiconfluent cells (1B.i), where more than 50% of the cell layer was infected (Fig. 1B). This indicates a segregation of the viral attachment proteins to the basolateral surface upon establishment of tight junctions and polarization of the epithelial cell.
To further study the route of entry of CCHFV in polarized cells, MDCK-1 cells were grown to confluence on 3.0-μm-pore permeable Transwell filters and infected with CCHFV at a multiplicity of infection (MOI) of 1 either apically or basolaterally. The integrity of the cell layer was monitored throughout the infections by measuring TER. Cell layers were lysed 72 hpi, and viral nucleocapsids were detected by Western blotting, as shown in Fig. 2A. Cells infected from the basolateral compartment showed a high expression of viral nucleocapsids. In contrast, CCHFV NP could not be detected in apically infected cells. These observations and the results shown in Fig. 1B indicate a preference for basolateral entry in polarized cells.
FIG. 2.
CCHFV has basolateral entry. (A) MDCK-1 cells were grown to confluence on Costar membranes, and a viral suspension was added to either the basolateral or the apical compartment at an MOI of 1. At 72 hpi, the cells were harvested and analyzed for intracellular viral nucleocapsids by Western blotting using calnexin as a loading reference. (B) MDCK-1 cells were infected apically at semiconfluence and confluence as indicated by TER and thereafter infected with CCHFV at an MOI of 1. At 48 hpi, cells were harvested and analyzed for infectivity by detecting viral nucleocapsids by Western blotting. (C) Confluent cell layers were treated or mock treated with 15 mM EGTA for 1 h and thereafter apically infected with CCHFV at an MOI of 1. At 48 hpi, the cells were harvested and CCHFV NP was detected by Western blotting.
To investigate the effect of increasing TER on CCHFV's ability to infect MDCK-1 cells, cells at different confluence levels (measured by TER) were apically infected with CCHFV at an MOI of 1 and harvested by lysis 48 hpi. As indicated in Fig. 2B, the infectivity decreases with increase of TER, utilized as a marker of confluence, which supports the obtained results in Fig. 1B.
Accessibility to the basolateral plasma membrane seems to be the limiting factor to CCHFV infectivity. To verify this, the junctional complex was disrupted by 15 mM EGTA treatment in confluent cell layers and infected with the virus suspension at an MOI of 1. At 48 hpi, cells were harvested and CCHFV NP was detected by Western blotting. As illustrated in Fig. 2C, the infectivity of CCHFV increases when virus has access to the basolateral membrane in comparison to mock-treated infected cells.
Release of CCHFV from MDCK-1 cells occurs preferentially at the basolateral surface.
To determine the preferred site of release of CCHFV, confluent cell layers grown on 3.0-μm permeable Transwell filters were inoculated basolaterally with CCHFV at an MOI of 1. Once again, measurement of electrical resistance across the cell layer monitored the integrity of the cell layer. Supernatants were collected at 24, 48, and 72 hpi from both the apical and basolateral compartments and assessed for progeny virions by fluorescence focus assay as described in Materials and Methods. These results demonstrate a preference for basolateral release as shown in Fig. 3. It should also be mentioned that we found a minimal amount of progeny virions at 24 and 72 hpi in apical supernatants.
FIG. 3.
CCHFV progeny virion release is primarily basolateral. Confluent monolayers on semipermeable membranes were inoculated basolaterally with CCHFV at an MOI of 1. Infected supernatants were retrieved daily and exchanged for fresh medium. The titers of infectious virions in the basolateral and apical supernatants (sup) were determined by fluorescence focus unit assay as described in Materials and Methods. Values from TER are given to indicate monolayer integrity for both mock-infected cells and infected cells.
CCHFV has no effect on the distribution of ZO-1 and occludin in MDCK-1 cells and no effect on the integrity of TJ.
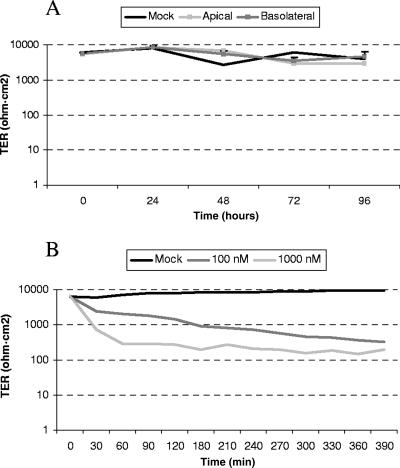
TER is primarily believed to reflect the resistance over TJ (20), and this was employed to investigate the general impact of CCHFV on TJ. Confluent cell layers on semipermeable 3.0-μm-pore Transwell filters were inoculated either apically or basolaterally with CCHFV at an MOI of 1. The TER was measured daily for 4 days to observe any effects compared to a mock-infected cell layer. As shown in Fig. 4, there was no noteworthy deviation from TER measured over the mock-infected cell layer, indicating that CCHFV does not have a direct impact on TJ.
FIG. 4.
The integrity of TJ is not disturbed by CCHFV. (A) Confluent cell layers grown on 3.0-μm semipermeable membranes were inoculated with an MOI of 1 of CCHFV either apically or basolaterally. TER was measured daily, and results are mean values of triplicates. (B) PMA at 100 and 1,000 nM was added to confluent cell layers on 3.0-μm-pore membranes, and TER was measured every 30 min. The results are mean values of triplicates.
PMA has been shown to disrupt TJ in MDCK-1 cells and was therefore used as a positive control (33). PMA at 100 or 1,000 nM was applied apically to confluent cell layers grown on 3.0-μm semipermeable membranes, and TER was measured every 30 min. Already after 30 min, a 10-fold decrease in TER was observed for the cell layer inoculated with 1,000 nM PMA and the same phenomenon was observed after 3.5 h for cell layers inoculated with 100 nM (Fig. 4).
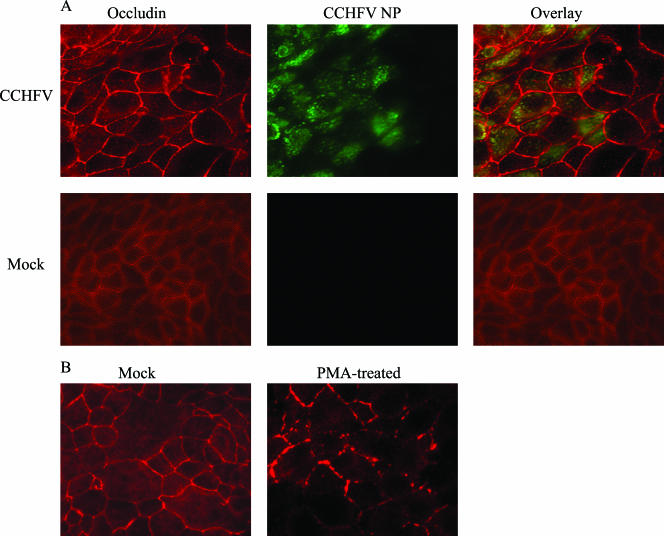
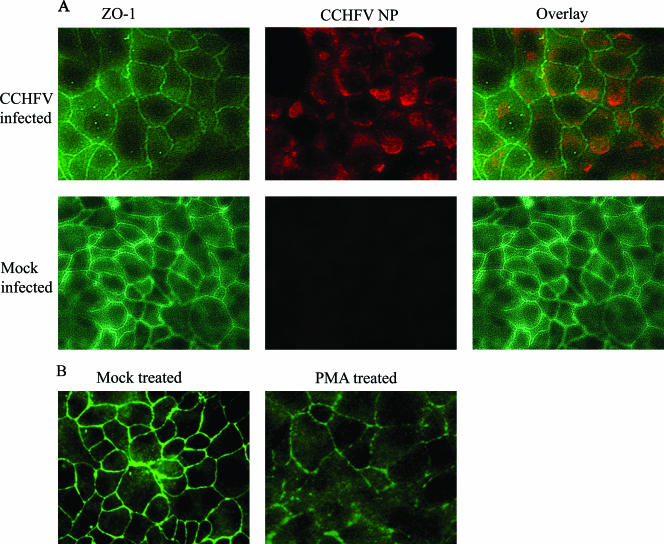
In order to detect whether CCHFV hinders the localization of occludin and ZO-1 to TJ upon infection, semiconfluent (approximately 50%) cell layers grown on chamber slides were inoculated or mock inoculated with CCHFV suspension at an MOI of 0.02. Cells were fixed at different time points, analyzed for occludin (Fig. 5) and ZO-1 (Fig. 6) structure, and costained for CCHFV NP by immunofluorescence. Both occludin and ZO-1 were localized to cell-cell boundaries in infected as well as mock-infected cells as shown in Fig. 5 (occludin) and 6 (ZO-1); no effect of CCHFV replication could therefore be displayed on these two TJ proteins.
FIG. 5.
Occludin localizes to cell-cell boundaries in infected MDCK-1 cells. (A) Subconfluent cells grown in chamber slides were either infected with CCHFV (top row) or mock infected (bottom row) and fixed 48 hpi. CCHFV NP is shown in green, and occludin is shown in red. (B) Confluent cells were mock treated or treated with PMA (100 nM) for 1 h. After fixation, cells were stained for occludin as described in Materials and Methods.
FIG. 6.
ZO-1 localizes to cell-cell boundaries in infected MDCK-1 cells. (A) Subconfluent cell layers of MDCK-1 in chamber slides were either infected with CCHFV (top row) or mock infected (bottom row) and fixed 48 hpi. CCHFV NP is shown in red, and ZO-1 is shown in green. (B) Confluent cells were mock treated or treated with PMA (100 nM) for 1 h. After fixation, cells were stained for ZO-1 as described in Materials and Methods.
PMA was used as a positive control for perturbance of TJ. Confluent cell layers were exposed to 100 nM PMA for 1 h and thereafter analyzed for altered distribution of tight junction proteins by immunofluorescence for both occludin and ZO-1. Both occludin and ZO-1 lost their localization at cell-cell boundaries when exposed to PMA, as seen in Fig. 5B and 6B.
DISCUSSION
For the first time, we here show polarized entry for a virus belonging to the Nairovirus genus, with entry occurring from the basolateral compartment. Examples of other viruses known to have basolateral entry are vaccinia virus (25), vesicular stomatitis virus (14), and human cytomegalovirus (12). The presence or absence of receptors or coreceptors essential for entry is one of the factors determining whether an infection can be established (40). Moreover, for polarized epithelial cells, the segregation of viral receptors determines the restrictiveness of attachment to either membrane. Based on the present studies, we hypothesize the receptors utilized by CCHFV must be primarily localized to the basolateral domain, as infection occurs primarily when inoculating epithelial cells with virus suspension basolaterally or when both cell membranes are accessible. Further studies are, however, needed to clarify which receptors are utilized by CCHFV and also the cellular distribution. The specific receptors used for viruses within the Bunyaviridae remain mostly unknown; however, β3 and β1 integrins have been shown to be used as receptors for hantaviruses (15, 16, 23). Within the Bunyaviridae family itself, little is known about polarity of infection. What has been clarified so far indicates an inconsistency of preferred entry site with apical entry for Black Creek Canal virus (Hantavirus) (24) and bidirectional entry for both Rift Valley Fever virus (Phlebovirus) (19) and Andes virus (Hantavirus) (26).
We have also found that CCHFV was primarily released from the basolateral compartment in the epithelial MDCK-1 cells. The maturation of Bunyaviridae particles is thought to occur by budding into Golgi cisternae, transported to the cell membrane in vesicles along the secretory pathway, and consequent fusion with the cell membrane releasing the infectious virion to the extracellular environment (11, 27, 34). The retention of viral glycoproteins in the Golgi membrane is believed to determine the assembly site; however, what determines the fate of the resulting vesicles has not been clarified yet. Targeting of CCHFV vesicles to the basolateral membrane could depend on an interaction with host cell proteins, which are secreted in a polarized manner.
We have previously demonstrated an essential role of the cytoskeleton in maturation of CCHFV. Disruption of actin filaments caused a drastic decrease in the release of infectious virions (1). In what aspect CCHFV release is dependent on the cytoskeleton is currently being studied, and this may help clarify the mechanisms behind polarization of release.
The distribution of viral receptor(s) determines the tropism of the virus in the organism. If receptors are segregated to the basolateral membrane in epithelial cells, the virus must first traverse the epithelial linings before it can interact with the receptor(s) (10). Systemic spread of CCHFV could result from an early infection of blood-borne monocytes, followed by extravasation into parenchymal tissue, enabling the virus to interact with basolateral receptors, as has been speculated for Ebola virus (5). Secondary replication in these organs followed by basolateral release would further systemic spread of the virus. Indirect support of this hypothesis comes from postmortem studies of CCHF patients in which Kupffer cells in the liver were shown to be major targets of the virus (7). The development of an animal model would benefit studies of viral dissemination, as has been performed for Ebola virus (17).
Infection of CCHFV did not cause any cytopathic effect in MDCK-1 cells, and no effect on TJ could be either visualized or measured by TER, demonstrating there is no direct viral effect on TJ in the MDCK-1 epithelial cells. Studies of other etiological agents of viral hemorrhagic fever give an indication of what could occur during CCHFV-mediated hemorrhage. Infection with dengue virus leads to redistribution of the tight junction protein occludin and an increase in paracellular permeability (38). However, this effect seems to be mediated by the release of cytokines, as virus-free supernatants from infected cells and even acute-phase sera from infected patients cause activation and leakage (4, 8, 9, 38). Similar observations have been made for Ebola viruses (13, 17, 18, 21, 32). As we find no direct effect on TJ, the bleeding observed in patients must be due to other mechanisms, possibly immune mediated.
Acknowledgments
This project received financial support from the Swedish Medicine Council (K2007-56X-20349-01-3).
Footnotes
Published ahead of print on 13 December 2006.
REFERENCES
- 1.Andersson, I., M. Simon, A. Lundkvist, M. Nilsson, A. Holmstrom, F. Elgh, and A. Mirazimi. 2004. Role of actin filaments in targeting of Crimean Congo hemorrhagic fever virus nucleocapsid protein to perinuclear regions of mammalian cells. J. Med. Virol. 72:83-93. [DOI] [PubMed] [Google Scholar]
- 2.Bazzoni, G., and E. Dejana. 2004. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol. Rev. 84:869-901. [DOI] [PubMed] [Google Scholar]
- 3.Bertolotti-Ciarlet, A., J. Smith, K. Strecker, J. Paragas, L. A. Altamura, J. M. McFalls, N. Frias-Staheli, A. Garcia-Sastre, C. S. Schmaljohn, and R. W. Doms. 2005. Cellular localization and antigenic characterization of Crimean-Congo hemorrhagic fever virus glycoproteins. J. Virol. 79:6152-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch, I., K. Xhaja, L. Estevez, G. Raines, H. Melichar, R. V. Warke, M. V. Fournier, F. A. Ennis, and A. L. Rothman. 2002. Increased production of interleukin-8 in primary human monocytes and in human epithelial and endothelial cell lines after dengue virus challenge. J. Virol. 76:5588-5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray, M., and T. W. Geisbert. 2005. Ebola virus: the role of macrophages and dendritic cells in the pathogenesis of Ebola hemorrhagic fever. Int. J. Biochem. Cell Biol. 37:1560-1566. [DOI] [PubMed] [Google Scholar]
- 6.Burt, F. J., and R. Swanepoel. 2005. Molecular epidemiology of African and Asian Crimean-Congo haemorrhagic fever isolates. Epidemiol. Infect. 133:659-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burt, F. J., R. Swanepoel, W. J. Shieh, J. F. Smith, P. A. Leman, P. W. Greer, L. M. Coffield, P. E. Rollin, T. G. Ksiazek, C. J. Peters, and S. R. Zaki. 1997. Immunohistochemical and in situ localization of Crimean-Congo hemorrhagic fever (CCHF) virus in human tissues and implications for CCHF pathogenesis. Arch. Pathol. Lab. Med. 121:839-846. [PubMed] [Google Scholar]
- 8.Cardier, J. E., E. Marino, E. Romano, P. Taylor, F. Liprandi, N. Bosch, and A. L. Rothman. 2005. Proinflammatory factors present in sera from patients with acute dengue infection induce activation and apoptosis of human microvascular endothelial cells: possible role of TNF-alpha in endothelial cell damage in dengue. Cytokine 30:359-365. [DOI] [PubMed] [Google Scholar]
- 9.Carr, J. M., H. Hocking, K. Bunting, P. J. Wright, A. Davidson, J. Gamble, C. J. Burrell, and P. Li. 2003. Supernatants from dengue virus type-2 infected macrophages induce permeability changes in endothelial cell monolayers. J. Med. Virol. 69:521-528. [DOI] [PubMed] [Google Scholar]
- 10.Compans, R. W. 1995. Virus entry and release in polarized epithelial cells. Curr. Top. Microbiol. Immunol. 202:209-219. [DOI] [PubMed] [Google Scholar]
- 11.Donets, M. A., M. P. Chumakov, M. B. Korolev, and S. G. Rubin. 1977. Physicochemical characteristics, morphology and morphogenesis of virions of the causative agent of Crimean hemorrhagic fever. Intervirology 8:294-308. [DOI] [PubMed] [Google Scholar]
- 12.Esclatine, A., M. Lemullois, A. L. Servin, A.-M. Quero, and M. Geniteau-Legendre. 2000. Human cytomegalovirus infects Caco-2 intestinal epithelial cells basolaterally regardless of the differentiation state. J. Virol. 74:513-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldmann, H., H. Bugany, F. Mahner, H.-D. Klenk, D. Drenckhahn, and H.-J. Schnittler. 1996. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J. Virol. 70:2208-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuller, S., C. H. von Bonsdorff, and K. Simons. 1984. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell 38:65-77. [DOI] [PubMed] [Google Scholar]
- 15.Gavrilovskaya, I. N., E. J. Brown, M. H. Ginsberg, and E. R. Mackow. 1999. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by β3 integrins. J. Virol. 73:3951-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavrilovskaya, I. N., M. Shepley, R. Shaw, M. H. Ginsberg, and E. R. Mackow. 1998. beta3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. USA 95:7074-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geisbert, T. W., L. E. Hensley, T. Larsen, H. A. Young, D. S. Reed, J. B. Geisbert, D. P. Scott, E. Kagan, P. B. Jahrling, and K. J. Davis. 2003. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am. J. Pathol. 163:2347-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geisbert, T. W., H. A. Young, P. B. Jahrling, K. J. Davis, T. Larsen, E. Kagan, and L. E. Hensley. 2003. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am. J. Pathol. 163:2371-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerrard, S. R., P. E. Rollin, and S. T. Nichol. 2002. Bidirectional infection and release of Rift Valley fever virus in polarized epithelial cells. Virology 301:226-235. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Mariscal, L., A. Avila, and A. Betanzos. 2001. The relationship between structure and function of tight junctions, 2nd ed. CRC Press, Boca Raton, FL.
- 21.Hensley, L. E., H. A. Young, P. B. Jahrling, and T. W. Geisbert. 2002. Proinflammatory response during Ebola virus infection of primate models: possible involvement of the tumor necrosis factor receptor superfamily. Immunol. Lett. 80:169-179. [DOI] [PubMed] [Google Scholar]
- 22.Kuismanen, E., K. Hedman, J. Saraste, and R. F. Pettersson. 1982. Uukuniemi virus maturation: accumulation of virus particles and viral antigens in the Golgi complex. Mol. Cell. Biol. 2:1444-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mou, D. L., Y. P. Wang, C. X. Huang, G. Y. Li, L. Pan, W. S. Yang, and X. F. Bai. 2006. Cellular entry of Hantaan virus A9 strain: specific interactions with beta3 integrins and a novel 70kDa protein. Biochem. Biophys. Res. Commun. 339:611-617. [DOI] [PubMed] [Google Scholar]
- 24.Ravkov, E. V., S. T. Nichol, and R. W. Compans. 1997. Polarized entry and release in epithelial cells of Black Creek Canal virus, a New World hantavirus. J. Virol. 71:1147-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez, D., J.-R. Rodriguez, G. K. Ojakian, and M. Esteban. 1991. Vaccinia virus preferentially enters polarized epithelial cells through the basolateral surface. J. Virol. 65:494-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowe, R. K., and A. Pekosz. 2006. Bidirectional virus secretion and nonciliated cell tropism following Andes virus infection of primary airway epithelial cell cultures. J. Virol. 80:1087-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rwambo, P. M., M. K. Shaw, F. R. Rurangirwa, and J. C. DeMartini. 1996. Ultrastructural studies on the replication and morphogenesis of Nairobi sheep disease virus, a Nairovirus. Arch. Virol. 141:1479-1492. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez, A. J., M. J. Vincent, B. R. Erickson, and S. T. Nichol. 2006. Crimean-Congo hemorrhagic fever virus glycoprotein precursor is cleaved by furin-like and SKI-1 proteases to generate a novel 38-kilodalton glycoprotein. J. Virol. 80:514-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmaljohn, C., and J. W. Hooper. 2001. Bunyaviridae: the viruses and their replication, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 30.Schneeberger, E. E., and R. D. Lynch. 2004. The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 286:C1213-C1228. [DOI] [PubMed] [Google Scholar]
- 31.Schnittler, H. J., and H. Feldmann. 2003. Viral hemorrhagic fever—a vascular disease? Thromb. Haemostasis 89:967-972. [PubMed] [Google Scholar]
- 32.Schnittler, H. J., F. Mahner, D. Drenckhahn, H. D. Klenk, and H. Feldmann. 1993. Replication of Marburg virus in human endothelial cells. A possible mechanism for the development of viral hemorrhagic disease. J. Clin. Investig. 91:1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sjo, A., K. E. Magnusson, and K. H. Peterson. 2003. Distinct effects of protein kinase C on the barrier function at different developmental stages. Biosci. Rep. 23:87-102. [DOI] [PubMed] [Google Scholar]
- 34.Smith, J. F., and D. Y. Pifat. 1982. Morphogenesis of sandfly viruses (Bunyaviridae family). Virology 121:61-81. [DOI] [PubMed] [Google Scholar]
- 35.Swanepoel, R., D. E. Gill, A. J. Shepherd, P. A. Leman, J. H. Mynhardt, and S. Harvey. 1989. The clinical pathology of Crimean-Congo hemorrhagic fever. Rev. Infect. Dis. 11(Suppl. 4):S794-S800. [DOI] [PubMed] [Google Scholar]
- 36.Swanepoel, R., A. J. Shepherd, P. A. Leman, S. P. Shepherd, G. M. McGillivray, M. J. Erasmus, L. A. Searle, and D. E. Gill. 1987. Epidemiologic and clinical features of Crimean-Congo hemorrhagic fever in southern Africa. Am. J. Trop. Med. Hyg. 36:120-132. [DOI] [PubMed] [Google Scholar]
- 37.Tafazoli, F., C. Q. Zeng, M. K. Estes, K.-E. Magnusson, and L. Svensson. 2001. NSP4 enterotoxin of rotavirus induces paracellular leakage in polarized epithelial cells. J. Virol. 75:1540-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talavera, D., A. M. Castillo, M. C. Dominguez, A. E. Gutierrez, and I. Meza. 2004. IL8 release, tight junction and cytoskeleton dynamic reorganization conducive to permeability increase are induced by dengue virus infection of microvascular endothelial monolayers. J. Gen. Virol. 85:1801-1813. [DOI] [PubMed] [Google Scholar]
- 39.Topp, K. S., A. L. Rothman, and J. H. Lavail. 1997. Herpes virus infection of RPE and MDCK cells: polarity of infection. Exp. Eye Res. 64:343-354. [DOI] [PubMed] [Google Scholar]
- 40.Tucker, S. P., and R. W. Compans. 1993. Virus infection of polarized epithelial cells. Adv. Virus Res. 42:187-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Wouden, J. M., O. Maier, S. C. van Ijzendoorn, and D. Hoekstra. 2003. Membrane dynamics and the regulation of epithelial cell polarity. Int. Rev. Cytol. 226:127-164. [DOI] [PubMed] [Google Scholar]