Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) is the infectious cause of Kaposi's sarcoma, primary effusion lymphoma, and plasmablastic multicentric Castleman's disease. STAT3 has been shown to be important for the maintenance of primary effusion lymphoma cells in culture and is chronically activated in many tumor cell lines. However, little is known about the role of KSHV in the activation of STAT3 or the role of STAT3 in KS tumors. We demonstrate that STAT3 is activated by KSHV infection of endothelial cells, the KS tumor cell type, in a biphasic fashion. Viral binding and entry activate STAT3 in the first 2 h after infection, but this activation dissipates by 4 h postinfection. By 12 h after KSHV infection, concomitant with the expression of latent genes, STAT3 is once again activated, and this activation persists for as long as latent infection is maintained. Activated STAT3 translocates to the nucleus, where it can bind to STAT3-specific DNA elements and can activate STAT3-dependent promoter activity. Conditioned medium from KSHV-infected endothelial cells is able to transiently activate STAT3, indicating the involvement of a secreted factor and that a latency-associated factor in KSHV-infected cells is necessary for sustained activation. KSHV upregulates gp130 receptor expression, and both gp130 and JAK2 are required for the activation of STAT3. However, neither human nor viral interleukin-6 is required for STAT3 activation. Persistent activation of the oncogenic signal transducer, STAT3, by KSHV may play a critical role in the viral pathogenesis of Kaposi's sarcoma, as well as in primary effusion lymphomas.
Kaposi's sarcoma-associated herpesvirus (KSHV; also known as human herpesvirus 8) is the infectious cause of Kaposi's sarcoma (KS), the most common tumor in AIDS patients worldwide. KSHV is also associated with two lymphoproliferative disorders: primary effusion lymphoma (PEL), a pleural cavity B-cell lymphoma, and plasmablastic multicentric Castleman's disease, a B-cell disorder of lymph nodes (14, 46). In KS tumors, KSHV is present in spindle cells, the predominant tumor cell (1, 6, 47). Spindle cells are endothelial in origin and express markers of lymphatic endothelium (28, 45). Nearly all spindle cells support latent KSHV infection, although a low percentage of cells undergoing lytic reactivation are always present (47).
There have been several reports outlining the transcriptional changes that can be observed during KSHV latent infection of endothelial cells (12, 38, 42). For example, recent studies in primary blood endothelial cells and tert-immortalized microvascular endothelial cells (TIME cells) have found that KSHV can reprogram blood endothelial cells to differentiate into lymphatic endothelial cells (12, 27, 50). Array experiments have identified oncogenic signaling pathways activated by KSHV, including c-KIT in endothelial cells immortalized by papillomavirus E6 and E7 genes (38). Array experiments have also led to the discovery of viral activation of the hypoxia response in endothelial cells (13). Although extremely informative, microarray experiments do not directly detect alterations in cellular signaling pathways that are characterized by changes in the phosphorylation state of proteins.
KSHV encodes many genes that activate cellular signaling and can act as oncogenes. For example, expression of the viral G-protein-coupled receptor, a viral interferon regulatory factor and an ITAM-containing protein can immortalize mouse or human cells in culture (4, 22, 32, 51). In addition, many secreted KSHV genes, such as the viral interleukin-6 (IL-6) homolog and multiple macrophage inflammatory proteins, are capable of activating various cellular signaling pathways (36, 41). Although these genes are reported to act as oncogenes or modulators of cellular signaling, they have predominantly been characterized in overexpression studies in the absence of viral infection. Less is known about how KSHV infection alters cellular signaling pathways.
In this report, we look at changes in the phosphorylation state of cellular signaling proteins upon KSHV infection. Among the mammalian signaling pathways altered upon KSHV infection is the JAK/STAT pathway. The signal transducer and activator of transcription (STAT) proteins are a family transcription factors that are found in many cell types and play different roles in normal cellular signaling (9, 20). They are activated by phosphorylation on a single tyrosine residue, allowing the STAT proteins to dimerize and translocate into the nucleus where they activate transcription of target genes (44). Maximum transcriptional activation is also dependent on a secondary serine phosphorylation event (52). STAT3 is activated by a number of cytokines, including the IL-6 family of ligands. hIL-6 signals through a heterodimer of gp130 and gp80 (also known as the IL-6Rα), which in turn activates JAK2, leading to phosphorylation of STAT3 (26). The KSHV IL-6 homolog activates STAT3 through the same pathway but can also activate STAT3 in a gp130-dependent, gp80-independent fashion (35, 49).
There are extensive data indicating that aberrant activation of STAT3 and STAT5 proteins is correlated with oncogenesis (8, 24). A number of recent studies have also indicated that a diverse array of oncoproteins can activate STAT proteins, particularly STAT3 and STAT5 (7). Many primary tumors and tumor cell lines have chronically activated STAT3 (7, 24). Targeted deletion of STAT3 in epidermal precursor cells has been shown to prevent epithelial cancer (15). STAT3 is also required for the formation of B-cell lymphomas in a transgenic mouse model where STAT3 is specifically deleted in B and T cells (18). In addition, STAT3 and/or STAT5 are also commonly observed to be activated by viral oncoproteins in transforming viruses, such as Epstein-Barr virus and hepatitis C virus, and in the closely related KSHV homologue herpesvirus saimiri (HVS) (16, 19, 23, 34). HVS oncoprotein TIP-C484 was shown to activate STAT3 through the tyrosine kinase Lck, and HVS STP-A11 was also shown to interact with and activate STAT3 (19, 34). Interestingly, STAT3 is constitutively activated in some KSHV-positive PEL cell lines and expression of a dominant-negative form of STAT3 caused apoptosis in PEL cells (3). These data point to a potentially important role for the activation of STAT3 in KSHV pathogenesis and oncogenic transformation. However, whether KSHV directly activates STAT3 or the activation is due to a selected cellular mutation in the genesis of the lymphoma is unknown.
We demonstrate here that STAT3 is phosphorylated in response to KSHV infection of endothelial cells, the predominant cell type of KS tumors, within 30 min of infection, and this response disappears by 4 h postinfection. Interestingly, STAT3 is phosphorylated and activated again by 12 h, concomitant with the establishment of latency, and this second phase requires viral gene expression, whereas the early phase does not. The sustained activation is present for as long as viral latency is maintained. Viral activation of STAT3 can be mediated by a soluble factor and requires the gp130 receptor and the JAK2 kinase, but persistence requires a factor present in the latently infected cell since conditioned medium only induces a transient activation. Interestingly, STAT3 activation does not depend on hIL-6 signaling since neutralizing antibodies to hIL-6 and gp80 do not block activation. In addition, whereas KSHV encodes a homolog of hIL-6 (vIL-6), this viral gene is not required for persistent activation of STAT3 in KSHV-infected endothelial cells. In this report, we identify a potentially oncogenic signaling pathway activated by KSHV that is likely to be important for the pathogenesis of KS tumors.
MATERIALS AND METHODS
Reagents and antibodies.
Phospho-STAT3 (Y705) and pan-STAT3 antibodies (Cell Signaling Technologies) were used as specified by the manufacturer. Antibodies to β-actin (Sigma), ORF59 (Advanced Biotechnologies, Inc.), LANA (Advanced Biotechnologies, Inc., and a kind gift from D. Ganem), and gp130 (Santa Cruz Biotechnologies) were used in immunoblot and immunofluorescence analysis as outlined below. Antibodies to hIL-6, gp80, and gp130 (R&D Systems) were used in neutralization experiments as described herein. Kinase inhibitors AG490, SU6656, and WHI-P131 (Calbiochem) were reconstituted in dimethyl sulfoxide (DMSO) at 100 mM and used at the concentrations specified in the text.
Cells.
TIME cells (48) were maintained as monolayer cultures in EBM-2 medium (Cambrex) supplemented with a bullet kit containing 5% fetal bovine serum, vascular endothelial growth factor, basic fibroblast growth factor, insulin-like growth factor 3, epidermal growth factor, and hydrocortisone (EGM-2 media). Primary human dermal microvascular endothelial cells (hDMVEC) (Clonetics) were also maintained in EGM-2. BCBL-1 cells (43) and BJAB cells (17) were maintained in RPMI 1640 medium (Celgro; Mediatech, Inc.) supplemented with 10% fetal bovine serum, penicillin, streptomycin, glutamine, and β-mercaptoethanol. vIL-6+ and vIL-6− KSHV BJAB cells (harboring vIL-6+/− recombinant KSHV (17, 17a) were maintained in the same media with the addition of 10 μg of puromycin/ml.
Viruses and infection.
KSHV inoculum was obtained from BCBL-1 cells (5 × 105 cells/ml) induced with 20 ng of TPA (12-O-tetradecanoylphorbol-13-acetate; Sigma)/ml. After 6 days, cells were pelleted, and the supernatant was run through a 0.45-μm-pore-size filter (Whatman). Virions were pelleted at 15,000 rpm for 2 h in a JA-14 rotor, Avanti-J-25 centrifuge (Beckman Coulter). The viral pellet was resuspended in EGM-2 and used as viral inoculum in subsequent experiments. KSHV infections of TIME and primary hDMVEC were performed in serum-free EBM-2 for 3 h, after which the medium was replaced with complete EGM-2. Mock infections were performed identically except that concentrated virus was omitted. Infection rates were assessed by immunofluorescence for all experiments, using antibodies against the latency-associated nuclear antigen (LANA) and the lytic protein ORF59. In all infections performed with wild-type (wt) KSHV >85% of the cells were LANA positive and <1% were ORF59 positive. UV inactivation of KSHV viral stocks (5 × 1,200 μJ) was performed in a UV Stratalinker 1800 (Stratagene). vIL-6+/− virus was induced in BJAB cells with 4,000 particles per cell of Ad50 (adenovirus expressing the lytic switch protein ORF50; kindly provided by D. Ganem) and isolated as previously described (17).
Immunofluorescence.
Prior to harvesting cells for immunoblot analysis, an aliquot of mock or KSHV-infected TIME cells was seeded on LabTek Permanox four-well chamber slides (Intermountain Sci) and fixed with 4% (vol/vol) paraformaldehyde in phosphate-buffered saline or 100% methanol. Immunofluorescence was performed as described previously (31) or as specified by the manufacturer (pSTAT3). Briefly, cells were incubated with primary antisera at a dilution of 1:100 (α-pSTAT3) or 1:1,000 (rabbit or rat α-LANA; mouse α-ORF59). Cells were then incubated with fluor-conjugated secondary antibodies (goat anti-rabbit Alexa Fluor 488, goat anti-mouse Alexa Fluor 594, or goat anti-rat Alexa Fluor 488; Molecular Probes/Invitrogen). Cells were mounted in medium containing DAPI (4′,6′-diamidino-2-phenylindole) before being viewed under a Nikon Eclipse E400 fluorescence microscope (Nikon, Inc.). Images were captured with the Micropublisher RTV camera using QCapture Suite (QImaging) and analyzed by using Adobe Photoshop (Adobe Systems, Inc.).
Immunoblot analysis.
Cells were harvested by using a cell scraper and pelleted using the Sorvall RT7 Plus centrifuge at 4°C. An aliquot of the cells was seeded onto chamber slides for immunofluorescence analysis. Cell pellets were washed once in cold phosphate-buffered saline and then resuspended in lysis buffer (20 mM Tris [pH 7.0], 2 mM EGTA, 5 mM EDTA, phosphatase inhibitors, protease inhibitors, and 1% Triton X-100). Samples were subjected to three cycles of freeze-thawing, sonicated for 10 min, and then spun at 6,000 × g at 4°C. Cell extracts were fractionated on an sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, and the proteins were transferred electrophoretically to Immobilon P polyvinylidene difluoride membranes (Millipore) in Tris-glycine buffer (25 mM Tris, 192 mM glycine, 20% methanol). Blots were incubated with the indicated antibody (dilutions: 1:1,000 for α-pSTAT3, α-STAT3, and α-gp130; 1:20,000 for α-actin) and subsequently with horseradish peroxidase-conjugated goat anti-mouse or rabbit immunoglobulin G (IgG) (Molecular Probes/Invitrogen). Immunoreactive proteins were visualized by chemiluminescence using the Amersham ECL Plus Western blotting detection reagents (GE Healthcare).
DNA-binding analysis.
Whole-cell extracts were prepared using a BD TransFactor whole-cell extraction kit (BD Biosciences Clontech) as specified by the manufacturer. Extract concentrations were determined by using the BCA protein assay kit (Pierce). Equivalent concentrations of extracts were diluted in 1× TransFactor blocking buffer and 10 μg of poly(dI-dC)/ml. For competition studies, wt STAT3 competitor oligonucleotides (250 to 500 ng) were added to the extracts. The DNA-binding activity in these extracts was assessed by using a BD TransFactor STAT3 chemiluminescent kit (BD Biosciences Clontech) as specified by the manufacturer. Luminescence was measured by using a Tecan GeniosPro 96-well microplate reader, and data were collected by using XFluor GeniosPro software.
Luciferase assays.
TIME cells were transfected with 1 to 3 μg of pTATA-Tkluc and pTATA-TK-luc 4XM67 constructs (5) and 1 ng of the control Renilla luciferase expression vector pRL-SV40 (Promega) using TransIT Jurkat reagent (Mirus). At 24 h posttransfection, cells were either mock infected or infected with wt KSHV. Cells were harvested at 24 h postinfection (hpi), i.e., 48 h posttransfection, and processed as specified by the Promega dual luciferase assay system. Luminescence was measured on a manual luminometer (Berthold). Transactivation of the STAT3-dependent promoter was presented as firefly luminescence normalized to Renilla luminescence.
Conditioned media and neutralization experiments.
Conditioned media from wt KSHV-infected cells at 24 hpi were clarified to remove virus by high-speed centrifugation. Uninfected TIME cells were serum starved for 1 h before treatment with clarified conditioned media. For neutralization experiments, conditioned media and/or TIME cells were preincubated with the antibody for 1 h. Treatments were performed for 30 min unless otherwise specified. Cells were harvested and processed for immunoblot analysis as described.
RESULTS
Biphasic activation of STAT3 by KSHV infection of endothelial cells.
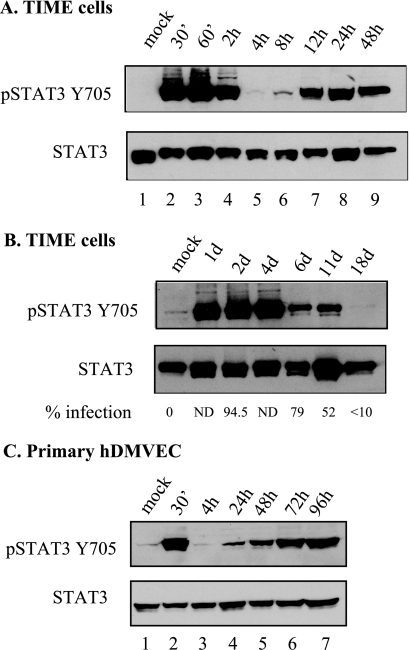
To characterize changes in signal transduction that can be observed during KSHV infection of TIME cells, whole-cell extracts from mock- and KSHV-infected TIME cells at 48 hpi were examined by a phosphosite screen (Kinexus Bioinformatics Corp., Vancouver, British Columbia, Canada). The STAT3 transcription factor was identified as strongly phosphorylated at the serine 727 residue 48 h after infection compared to mock-infected TIME cells. To confirm this result, an immunoblot analysis using an antibody against the phosphorylated form of STAT3 (STAT3Y705) in mock-infected and KSHV-infected TIME cells at different time points postinfection was performed. Figure 1A shows that STAT3 is phosphorylated in infected but not uninfected TIME cells. The two bands recognized by the STAT antibody have been previously described (10, 49) but are not always resolved in every gel. There are two separate peaks of activation: one at early time points (30 min to 2 h, lanes 2 to 4) and another at late time points (12 to 48 h, lanes 7 and 8), coinciding with latent gene expression and the establishment of latency. STAT3 activation can be detected in KSHV-infected TIME cells past 10 days postinfection (Fig. 1B), until infection rates drop below 10% (∼18 days postinfection), indicating that KSHV infection leads to persistent STAT3 activation. The same biphasic and persistent activation of STAT3 is also seen after infection of primary hDMVEC (Fig. 1C), indicating that activation is a general property of endothelial cells and not unique to TIME cells.
FIG. 1.
STAT3 is persistently activated in KSHV-infected TIME cells and primary hDMVEC. (A) Immunoblot analysis of extracts from mock and KSHV-infected TIME cells harvested at the indicated hours postinfection probed with antibodies to phosphorylated STAT3 (Y705) and total STAT3. There are two peaks of activation: at 30 min to 2 hpi and at 12 to 48 hpi. (B) STAT3 activation persists to 11 days postinfection in TIME cells. Infection rates at the time of harvest as determined by immunofluorescence analysis are indicated below the corresponding time point. (C) Extracts from mock- and KSHV-infected primary hDMVECs show a bimodal mode of activation similar to that seen in TIME cells.
Persistent STAT3 activation requires viral gene expression.
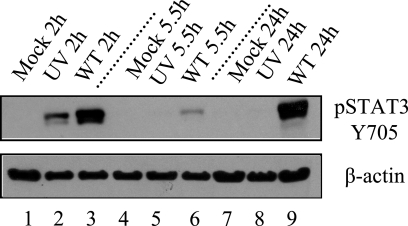
To distinguish whether this activation can be attributed to binding and entry of the virus alone or whether gene expression is necessary for activation, cells were infected with either UV-inactivated virus or wt virus and screened for STAT3 activation using immunoblot analysis with the pSTAT3 antibody. Although wt virus resulted in STAT3 activation at both the early and late peaks of STAT3 activation (compare lanes 3 and 9, Fig. 2), UV-inactivated virus only resulted in activation during early time points (lanes 2 and 8). This demonstrates that viral gene expression is a necessary component of the second sustained peak of STAT3 activation. The appearance of this second peak coincides with the appearance of LANA. In separate infections, LANA was undetectable at 2 h and could be detected in less than 3% of the cells at 5 h, 48% of the cells at 12 h, and in 85% of the cells by 24 h. This timing is similar to the large increase in LANA RNA expression between 12 and 24 h seen previously by others (30). These results demonstrate that whereas KSHV binding and entry activates STAT3 transiently, establishment of long-term KSHV infection leads to sustained long-term activation of STAT3, as seen in many cancer cell lines and tumors.
FIG. 2.
Persistent STAT3 activation but not the early peak of activation requires viral gene expression. Extracts from mock (Mock), UV-inactivated KSHV (UV), or KSHV (WT) infections of TIME cells at 2, 5.5, and 24 hpi were analyzed via immunoblot analysis with the pSTAT3 Y705 antibody and antibody to β-actin as a loading control. KSHV infections showed STAT3 activation at both early and late peaks in contrast to UV-inactivated infections, where STAT3 was activated only at the early peak.
STAT3 translocates to the nucleus of infected cells and binds STAT3 response elements.
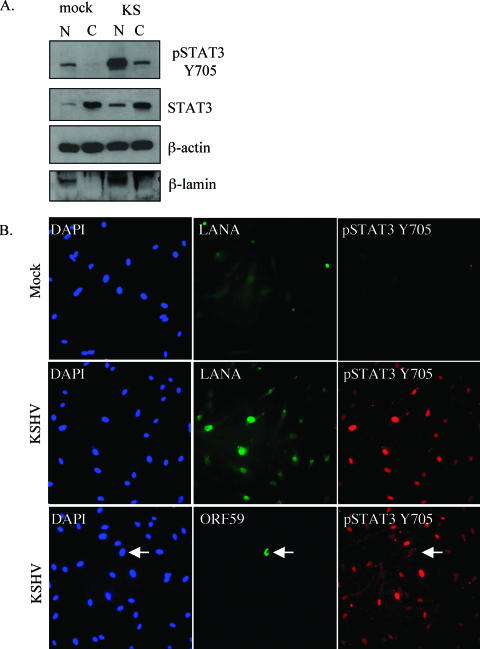
Since tyrosine phosphorylation is the initial event in the STAT3 signaling cascade, we also used a variety of methods to confirm downstream events associated with activation. The Kinexus screen illustrates that the second phosphorylation event at Ser727 occurs in KSHV-infected TIME cells. To determine whether pSTAT3 translocates to the nucleus after infection, we separated the nuclear and cytoplasmic fractions of mock- and KSHV-infected cells. At 48 h postinfection, phosphorylated STAT3 translocates to the nucleus (Fig. 3A) as determined by immunoblot analysis of nuclear and cytoplasmic fractions with the pSTAT3 Y705 antibody. We used immunoblot analysis with antibodies against β-lamin to verify the proper fractionation of extracts.
FIG. 3.
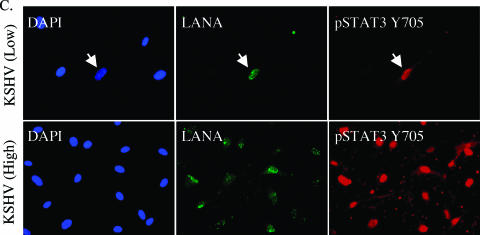
The phosphorylated form of STAT3 translocates to the nucleus in latently infected TIME cells. (A) Immunoblot analysis of nuclear and cytoplasmic fractions from mock- and KSHV-infected TIME cells (48 hpi) probed with antibodies to pSTAT3 Y705, STAT3, β-actin, and β-lamin. Phospho-STAT3 is increased and localizes to the nucleus upon KSHV infection. (B) Immunofluorescent detection of mock- and KSHV-infected TIME cells at 48 hpi stained with DAPI and reacted with antibodies to pSTAT3 Y705, KSHV LANA, or KSHV ORF59 as indicated. Phospho-STAT3 is detected in KSHV-infected (middle and bottom rows) but not in mock-infected (top row) TIME cells and colocalizes with the KSHV LANA (middle row) and not solely with the KSHV lytic protein, ORF59 (arrow, bottom row). (C) Immunofluorescence analysis of low (top row)- and high (bottom row)-level KSHV infections using the same antibodies outlined in panel B. Phospho-STAT3 is detected only in cells expressing latent antigens regardless of whether there is high- or low-level infection. The arrow in the low infection panels points to the only infected cell in the field.
The increase in phosphorylated STAT3 and its translocation to the nucleus is also seen in immunofluorescence analysis of mock- versus KSHV-infected cells as 48 hpi (Fig. 3B). When we obtained greater than 90% infection rates, as determined by LANA staining, all LANA-positive cells also showed an intense nuclear pSTAT3 staining (Fig. 3B and Fig. 3C [High]), indicating that STAT3 activation correlates with latent KSHV infection. In addition, when we performed immunofluorescence on cells that were only 50% infected, only the low percentage of cells that expressed LANA also expressed pSTAT3 (Fig. 3C [Low]). The same percentage of cells exhibited nuclear punctate LANA staining when either the rat monoclonal or the rabbit polyclonal anti-LANA antibody was used. Shown in Fig. 3 is the rat antibody to allow costaining with the rabbit polyclonal anti-phospho-STAT3-Y705 antibody. The rat anti-LANA antibody exhibits higher background staining with the methanol fixation used to optimize the STAT3 staining. This background can also be seen in the mock-infected cells, although the nuclear punctate staining can still be clearly seen only in the KSHV-infected cells in the higher-magnification panels.
pSTAT3 nuclear expression can be detected in greater than 90% of the highly infected cells, whereas ORF59 is only detected in less than 1% of the cells (Fig. 3B, lower panels). This indicates that the vast majority of cells with nuclear pSTAT3 expression are latently infected and that lytic replication is unlikely to play a primary role in the activation of STAT3 during KSHV infection.
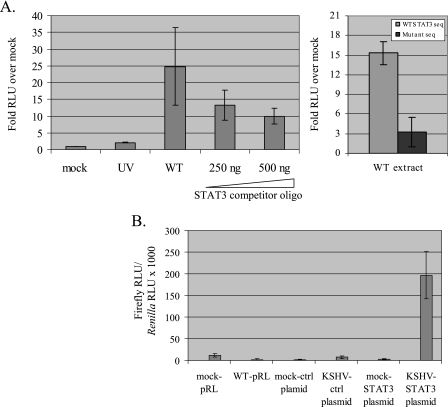
To determine whether extracts from KSHV-infected cells show specific STAT3 DNA-binding activity, we used a BD TransFactor STAT3 chemiluminescent kit (BD Biosciences/Clontech), where transcription factors in whole-cell lysates are tested for binding to specific DNA elements in an enzyme-linked immunosorbent assay based format. As seen in Fig. 4A, extracts from wt KSHV infections but not from mock or UV-inactivated virus infections were able to specifically bind STAT3-specific DNA elements (left panel). The specificity of this binding is demonstrated by the ability of STAT3-specific oligonucleotides to reduce DNA binding (Fig. 4A, STAT3 competitor oligo), as well as by the decreased binding of wt extracts to mutant STAT3 DNA elements (Fig. 4A, right panel).
FIG. 4.
Extracts from KSHV-infected TIME cells bind STAT3-specific DNA elements and activate a STAT3-dependent promoter driving luciferase expression. (A) For the left panel, extracts (20 μg) from mock-infected (Mock), UV-inactivated (UV), and KSHV infections (WT) were assayed for DNA-binding activity to STAT3-specific sequences using the BD TransFactor STAT3 chemiluminescent assay. Extracts from wild-type infections show a >20-fold increase in STAT3 DNA binding activity compared to mock infections or infections with UV-inactivated virus. The addition of STAT3 competitor oligonucleotide to wild-type extracts decreases the observed DNA binding activity by up to threefold. The data represent the average of two separate experiments. For the right panel, extracts (20 μg) from wild-type infections show a fivefold decrease in binding to mutant versus wild-type STAT3 sequences. The data represent the average of two separate experiments. (B) Luciferase assays of TIME cells transfected with the indicated plasmid and mock or KSHV infected for 24 h. Transactivation of a STAT3-dependent luciferase construct (pTATA-4XM67TKluc) was observed in KSHV but not mock infections of TIME cells. The data represent the average of two separate experiments.
STAT3-dependent transcriptional activation also occurs after KSHV infection. To ascertain whether transcriptional activation can be observed in lysates from KSHV-infected cells versus mock-infected cells, we used a construct (pTATA-TKluc-4XM67) where consensus binding sites for STAT3 are upstream of the luciferase gene under the control of the HSV-TK promoter (5). As seen in Fig. 4B, there was a >150-fold boost in luciferase activity in KSHV-infected cells versus mock-infected cells transfected with the STAT3-dependent luciferase construct. There is minimal difference in luciferase activation between the mock and KSHV infections in cells transfected with the control plasmid (pTATA-TKluc) where the STAT3-specific elements are absent.
KSHV activates STAT3 through a secreted factor.
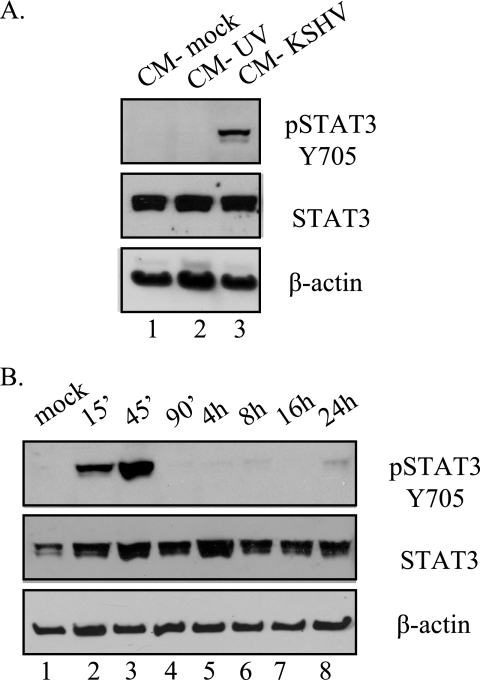
Activation of STAT3 during infection can be mediated by either direct activation via a viral protein, as seen in infections with the KSHV homolog HVS where activation of the STAT pathway is mediated by the viral protein STP-A11. Another possibility is that viral infection upregulates a soluble factor released during infection that can then activate KSHV-infected cells in a paracrine fashion. To determine whether KSHV infection induces a secreted factor that leads to the activation of STAT3, we took conditioned media from mock-infected cells and cells infected with either UV-inactivated or wt KSHV at 24 h postinfection when STAT3 is chronically activated. Virus that might be present in the media was removed by high-speed centrifugation. Uninfected TIME cells were treated with this conditioned media for 30 min, and STAT3 activation was determined. As seen in Fig. 5A, treatment with KSHV-conditioned media (at 24 hpi), but not with conditioned media from mock or UV-inactivated virus infections, results in STAT3 activation. This indicates that a soluble factor released during KSHV infection at late times is able to activate STAT3. When we added KSHV conditioned medium to uninfected TIME cells and allowed the treatment to proceed for different times (15 min to 24 h), we see that the activation of STAT3 is transient, persisting for only 45 min, and does not recur after 16 to 24 h of treatment (Fig. 5B). This is in contrast to activation seen in infected cells, where phosphorylation persists beyond 1 week postinfection (Fig. 1B). Together with the immunofluorescence data showing that only LANA-positive cells showed intense nuclear phospho-STAT3 staining, this indicates that persistent STAT3 activation in TIME cells requires latent KSHV gene expression in the cell where STAT3 is activated in addition to a secreted factor.
FIG. 5.
Conditioned media from TIME cells infected with KSHV for 24 h activates STAT3. (A) Immunoblot analysis of extracts from uninfected TIME cells treated with 24 hpi conditioned media from mock-infected, UV-inactivated KSHV, and KSHV-infected TIME cells. Only conditioned media from wild-type KSHV infections were able to activate STAT3 in uninfected TIME cells. (B) Conditioned medium from KSHV-infected TIME cells was harvested 24 hpi and used to treat fresh TIME cells over a time course. Immunoblot analysis for pSTAT3 Y705 shows that conditioned medium from TIME cells at 24 hpi induces only transient STAT3 activation (lasting only 90 min) in the absence of KSHV infection.
KSHV activates STAT3 through JAK2.
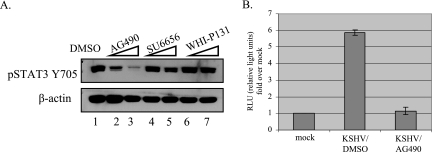
To help in the identification of the ligand(s) responsible for the activation of STAT3 in KSHV-infected TIME cells, we employed the use of various kinase inhibitors to identify which kinase phosphorylates STAT3, leading to its activation. There are various families of kinases that can activate STAT3 (7). These include nonreceptor tyrosine kinases such as src and abl, and the Janus kinases (JAK), which mediate activation by cytokines (29). TIME cells were infected in the presence of specific kinase inhibitors for each of these potential activators. As seen in Fig. 6A, cells infected in the presence of the JAK2 inhibitor, AG490, showed a dose-dependent decrease in the phosphorylation of STAT3 compared to DMSO-treated infections (lanes 1 to 3). There was no significant decrease in STAT3 phosphorylation in cells infected in the presence of the src family inhibitor SU6656 or the JAK3 inhibitor WH1-P131. Infection rates in the presence or absence of the different kinase inhibitors at the concentrations used all remained above 90%, indicating that there is no significant change in infection rates due to the inhibitors. When we used the TransFactor assay to look at DNA-binding activity, extracts from AG490-treated infections showed about a fivefold reduction in DNA-binding activity compared to extracts from DMSO-treated infections at 24 h (Fig. 6B). This indicates that STAT3 is activated in a JAK2-dependent manner during persistent KSHV infection.
FIG. 6.
The JAK2 inhibitor AG490 reduces STAT3 activation in KSHV infections at 24 hpi. (A) Immunoblot of extracts from KSHV-infected TIME cells (24 hpi) treated with the indicated kinase inhibitors were probed with pSTAT3 Y705 or β-actin antibodies. Only the JAK2 inhibitor AG490 reduced phospho-STAT3 levels in a dose-dependent fashion. The JAK3 inhibitor WHI-PI3I and the src family kinase inhibitor SU6656 had no effect on STAT3 phosphorylation. (B) Extracts from wild-type KSHV infections performed in the presence of AG490 or DMSO were subjected to DNA-binding assays for STAT3-specific sequences as described in Fig. 4. The DNA-binding activity of STAT3 in KSHV-infected TIME cells was inhibited by the JAK2 inhibitor AG490.
gp130 but not gp80 is required for and is upregulated by KSHV infection.
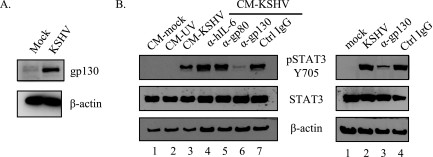
Since conditioned media from KSHV infections activates STAT3 in uninfected cells, a soluble factor(s) such as a growth factor(s) or cytokine(s) is implicated as the ligand responsible for STAT3 activation. We previously showed that the activation is mediated by the JAK2 kinase. One of the main ligands that signal through JAK2 is the gp130 family of ligands. We have previously reported that gp130 and hIL-6 are transcriptionally upregulated during latent infection (12). We also showed by cytokine array that concentrations of hIL-6 in the medium increase upon KSHV infection. Both hIL-6 and the viral homolog vIL-6 are known to activate STAT3 in transient-transfection systems in other cell types. Here we show that the gp130 protein is also upregulated in KSHV-infected cells at 48 hpi (Fig. 7A). To determine whether the activating ligand for STAT3 signals through the gp130 receptor or hIL-6, we performed antibody neutralization experiments. STAT3 activation was assessed in uninfected TIME cells treated with 24-hpi conditioned media that had been incubated with neutralizing antibodies to hIL-6, gp80, gp130, or control IgG. Only antibodies to the gp130 receptor were able to neutralize the activation of STAT3 in conditioned media (Fig. 7b, lane 6). Antibodies to hIL-6 or to gp80, the hIL-6R α-subunit, did not neutralize the activation of STAT3. We confirmed that the antibodies to hIL-6, gp80, and gp130 were indeed neutralizing by their ability to block transient STAT3 activation in TIME cells treated with recombinant hIL-6 (data not shown). Neutralizing antibodies to gp130 are also able to reduce STAT3 activation seen during infection when added during the last 3 h of a 24 h infection, although this reduction is not complete (Fig. 7B, right panel, lane 3). This indicates that the activating ligand for STAT3 during KSHV infection in endothelial cells is not hIL-6.
FIG. 7.
Neutralizing antibodies to the gp130 receptor block the activation of STAT3. (A) Immunoblot analysis of KSHV-infected TIME cells with antibodies to gp130 or β-actin. At 48 hpi, the expression of the gp130 receptor is increased in extracts of KSHV-infected TIME cells compared to mock-infected cells. (B) For the left panel, extracts from TIME cells treated with KSHV-conditioned media in the presence of the indicated neutralizing antibody were subjected to immunoblot analysis with antibodies to pSTAT3 Y705, STAT3, or β-actin. The conditioned media were clarified from KSHV infections at 24 hpi, and conditioned media from mock and UV-irradiated virus infections were used as controls. Only the gp130 antibody neutralized STAT3 activation upon conditioned media treatment. For the right panel, immunoblot analysis (with the indicated antibodies) of extracts from KSHV-infected TIME cells incubated with neutralizing antibodies to either gp130 or control IgG for the last 3 h of a 24-h infection was performed. The gp130 neutralizing antibody decreased STAT3 phosphorylation during infection.
STAT3 activation by KSHV is independent of vIL-6.
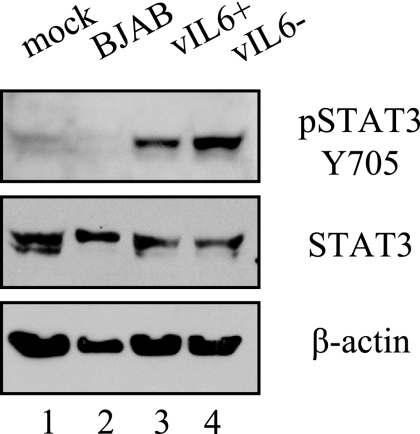
KSHV encodes a homolog of hIL-6, vIL-6. To determine whether vIL-6 is involved in the activation of STAT3 after infection, we utilized a recombinant virus previously characterized by our lab where the ORFK2 gene encoding vIL6 is deleted (17a). BJAB cells harboring the vIL-6 deletion recombinant or a control vIL-6 containing recombinant were both induced to produce virus. TIME cells were then infected with the virus stocks from these BJAB cell inductions. STAT3 is phosphorylated 48 h after infection of TIME cells with both the vIL-6 deletion and the control virus (Fig. 8, lanes 3 to 4), indicating that vIL-6 is not responsible for the observed activation of STAT3. As an additional control, we induced uninfected BJAB cells, and supernatants were harvested identically to the supernatants from recombinant KSHV-infected BJAB cells. The inoculum from uninfected BJAB inductions did not activate the phosphorylation of STAT3 (lane 2), indicating that this was a KSHV-specific phenomenon and not due to the induction conditions.
FIG. 8.
STAT3 activation by KSHV does not require vIL-6. Extracts from TIME cells infected with either vIL-6+ or vIL-6− recombinant KSHV isolates were probed with antibodies to pSTAT3 Y705, STAT3, or β-actin. STAT3 was phosphorylated under both conditions (lanes 3 and 4) but not in mock and control BJAB infections (lanes 1 and 2). BJAB cells harboring these green fluorescent protein/puromycin-expressing KSHV recombinants, with either vIL-6 intact (+) or deleted (−), were induced to lytic replication and vIL-6+ or vIL-6− recombinant virus was isolated from the supernatant of these induced cells as described in Materials and Methods. Parallel inductions were performed on BJAB cells, and the supernatants were processed and used as a control (BJAB, lane2).
DISCUSSION
STAT3 is persistently activated in a diverse group of primary tumors and in many cancer cell lines. Known viral oncogenes have also been shown to activate STAT3 (7). Oncogenic strains of HVS, a close homolog of KSHV, encode a protein capable of directly activating STAT3 (33), although this activation may not be critical for the viral oncogenic potential in cell culture (25). In addition, STAT3 is also persistently activated in transforming viruses such as hepatitis C virus and Epstein-Barr virus (16, 23). Aberrant activation of the STAT3 transcription factor leads to the deregulation of important oncogenic signaling pathways such as those involved in cell cycle progression, antiapoptosis, and tumor angiogenesis (24). In a number of tumor cell lines, the inhibition of chronic STAT3 activation leads to apoptosis (3, 37). In addition, knockout mice with targeted deletions of STAT3 in epidermal cells do not develop skin cancer at the levels of wt litter mates (15). In similar experiments, the formation of B-cell lymphomas was shown to be dependent on STAT3 in transgenic mice with STAT3 knocked out specifically in B and T cells (18). Thus, when persistently activated, STAT3 is a legitimate oncogene, and various strategies targeting STAT3 activation are now being evaluated as valid anticancer therapies (24).
In this report we demonstrate that KSHV infection of endothelial cells upregulates gp130, leading to phosphorylation and activation of STAT3 though JAK2. The activation of STAT3 initially occurs as a result of binding and entry, but this activation is suppressed by 4 h postinfection. However, by 12 h postinfection, STAT3 is again phosphorylated and remains persistently phosphorylated for over a week postinfection. This later phase of persistent STAT3 phosphorylation requires KSHV gene expression and is concomitant with the establishment of latency in endothelial cells, as determined by the expression of LANA. The persistent tyrosine phosphorylation of STAT3 leads to true activation, as demonstrated by relocalization of the STAT3 phosphoprotein to the nucleus, DNA binding, and promoter activation.
The early phase of STAT3 activation is associated with viral binding and entry and is likely related to the innate immune response since it is quickly turned off in the infected cell. This initial activation event is inhibited by the JAK3 inhibitor WHI-P131 (A. S. Punjabi and M. Lagunoff, unpublished data), indicating a separate activation pathway from the late phase that is strongly inhibited by the JAK2 inhibitor AG490. Characterization of the factors necessary for the early phase activation of STAT3 is currently under way.
STAT3 is activated when endothelial cells are treated with conditioned media from KSHV infections (at 24 hpi), indicating that a secreted factor is involved in this activation. To begin characterizing the secreted factor involved, we used neutralizing antibodies to hIL-6 and the IL-6 receptor alpha (gp80) and also to gp130, the common receptor for the IL-6 family of cytokines. Although we have previously demonstrated that hIL-6 expression is upregulated upon KSHV infection of endothelial cells (12), neutralizing antibodies to hIL-6 or to gp80 were not able to block STAT3 activation induced by conditioned media. This indicates that hIL-6 is not required for KSHV conditioned medium-induced activation of STAT3. In contrast, a neutralizing antibody to gp130, the common subunit of the hIL-6 receptor and a number of other cytokines, strongly blocked the activation of STAT3. When infected cells were incubated with neutralizing antibodies to gp130 for the last 3 h of infection, STAT3 activation was decreased, although not as strongly as in conditioned media. This could be due to problems with the antibody during infection or that the activation of STAT3 during infection does not solely go through gp130. Since the viral IL-6 homolog, vIL-6, can signal through gp130 but does not require gp80 for signaling (35), it was the most obvious candidate for the soluble factor activating STAT3. However, a recombinant KSHV isolate with vIL-6 deleted activates STAT3 similarly to the control recombinant, indicating that vIL-6 is not required for this activation. Taken together, persistent activation of STAT3 by KSHV requires a secreted factor that signals through gp130 but is not human or viral IL-6. We also looked at oncostatin M, an IL-6 family cytokine that utilizes gp130, since it was shown to be a growth factor for KS spindle cells ex vivo (11, 40). However, neutralization experiments demonstrate that oncostatin M is also not required for STAT3 activation by KSHV (Punjabi and Lagunoff, unpublished). Although we have ruled out the most obvious candidates, a number of other cytokines signal through gp130, and further work to identify the activating ligand and/or cytokine involved is under way.
The activation of STAT3 by conditioned media in uninfected endothelial cells was transient, an effect observed when recombinant cytokines such as hIL-6 are exogenously added to endothelial cells. This indicates that the secreted factor induced by KSHV infection is not sufficient for sustained STAT3 activation and that a KSHV-associated factor is necessary for the persistent activation of STAT3 in infected cells. Viral infection upregulates gp130, a receptor utilized by a number of cytokines that signal through the JAK/STAT pathway and activate STAT3. Although it is conceivable that increased expression of the receptor could facilitate this sustained activation, at this point, it is not clear whether this is sufficient. It is more likely that another virally associated factor is involved. This viral factor would be expressed during latency, as shown by immunofluorescence assay for activated STAT3 and LANA (Fig. 3B and C), where all latently infected cells contain activated STAT3 in the nucleus, but uninfected cells in the same culture do not show the same levels of activated STAT3 in the nucleus. A recent report demonstrated that LANA can interact with STAT3 (39). However, a LANA-expressing TIME cell line does not have constitutively active STAT3 (A. S. Punjabi, V. Morris, and M. Lagunoff, unpublished results). This indicates that LANA alone does not induce the secreted factor that activates and maintains persistent activation of STAT3. However, this does not rule out the scenario wherein another latent protein induces the secreted factor that activates STAT3, and that LANA's potential interaction with STAT3 would help in the maintenance of this activation. Work to examine this scenario is ongoing with LANA and other KSHV latent proteins.
The question remains as to what role persistently activated STAT3 plays in KSHV pathogenesis. Three different cell culture systems have shown that KSHV can aid in the immortalization or transformation of endothelial cells. The first demonstrated that KSHV infection of bone marrow microvascular endothelial cells caused immortalization (21). Interestingly, only a low percentage of cells maintained KSHV infection and, although it is likely that KSHV played a role in the immortalization, other cellular genes were also probably altered since similar attempts to immortalize primary dermal microvascular endothelial cells have failed. Another study demonstrated that dermal microvascular endothelial cells immortalized with the E6 and E7 genes of human papillomavirus were transformed by long-term infection with KSHV (38). Again, while KSHV certainly plays a role, the E6 and E7 genes are also required for this transformation as other immortalized cell lines are not transformed by KSHV (31). Finally, a recent study showed that primary human umbilical vein endothelial cells could be fully transformed by KSHV (2). Again, KSHV probably plays a role, but a secondary cellular event is required since this transformation event is rare. The involvement of these unidentified and random secondary cellular events makes it difficult to determine the role of activated STAT3 in KSHV-induced cell growth of endothelial cells in culture. The activation of STAT3 could play a critical role in the growth of KSHV-infected endothelial cells but by itself may not be sufficient to induce immortalization or transformation. However, this does not rule out that, together with other cellular events, persistent STAT3 activation by KSHV leads to the activation and/or maintenance of endothelial cell growth. Interestingly, in some PEL cell lines, STAT3 is required for continued growth, demonstrating that STAT3 can play a direct role in a KSHV-related tumor. We demonstrate here that KSHV is capable of directly activating STAT3 in endothelial cells, and this is likely to play an important role in KS tumors as it does in primary effusion lymphomas.
Acknowledgments
We thank Sheri “Mona” Dellos-Nolan and Melaine Sarreal for technical assistance.
A.S.P. is supported by a postdoctoral training grant (T32-CA09229) from the National Cancer Institute. M.L. is supported by the PEW scholars program in the biological sciences sponsored by the PEW charitable trust, by a research scholar grant from the American Cancer Society, by a grant (5RO1CA97934) from the National Cancer Institute, and by a pilot award from the Puget Sound Partners for Global Health.
Footnotes
Published ahead of print on 6 December 2006.
REFERENCES
- 1.Aluigi, M. G., A. Albini, S. Carlone, L. Repetto, R. De Marchi, A. Icardi, M. Moro, D. Noonan, and R. Benelli. 1996. KSHV sequences in biopsies and cultured spindle cells of epidemic, iatrogenic, and Mediterranean forms of Kaposi's sarcoma. Res. Virol. 147:267-275. [DOI] [PubMed] [Google Scholar]
- 2.An, F. Q., H. M. Folarin, N. Compitello, J. Roth, S. L. Gerson, K. R. McCrae, F. D. Fakhari, D. P. Dittmer, and R. Renne. 2006. Long-term-infected telomerase-immortalized endothelial cells: a model for Kaposi's sarcoma-associated herpesvirus latency in vitro and in vivo. J. Virol. 80:4833-4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki, Y., G. M. Feldman, and G. Tosato. 2003. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 101:1535-1542. [DOI] [PubMed] [Google Scholar]
- 4.Bais, C., A. Van Geelen, P. Eroles, A. Mutlu, C. Chiozzini, S. Dias, R. L. Silverstein, S. Rafii, and E. A. Mesri. 2003. Kaposi's sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/KDR. Cancer Cell 3:131-143. [DOI] [PubMed] [Google Scholar]
- 5.Besser, D., J. F. Bromberg, J. E. Darnell, Jr., and H. Hanafusa. 1999. A single amino acid substitution in the v-Eyk intracellular domain results in activation of Stat3 and enhances cellular transformation. Mol. Cell. Biol. 19:1401-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274-1278. [DOI] [PubMed] [Google Scholar]
- 7.Bowman, T., R. Garcia, J. Turkson, and R. Jove. 2000. STATs in oncogenesis. Oncogene 19:2474-2488. [DOI] [PubMed] [Google Scholar]
- 8.Bromberg, J. 2002. Stat proteins and oncogenesis. J. Clin. Investig. 109:1139-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bromberg, J., and J. E. Darnell, Jr. 2000. The role of STATs in transcriptional control and their impact on cellular function. Oncogene 19:2468-2473. [DOI] [PubMed] [Google Scholar]
- 10.Burger, M., T. Hartmann, J. A. Burger, and I. Schraufstatter. 2005. KSHV-GPCR and CXCR2 transforming capacity and angiogenic responses are mediated through a JAK2-STAT3-dependent pathway. Oncogene 24:2067-2075. [DOI] [PubMed] [Google Scholar]
- 11.Cai, J., P. S. Gill, R. Masood, P. Chandrasoma, B. Jung, R. E. Law, and S. F. Radka. 1994. Oncostatin-M is an autocrine growth factor in Kaposi's sarcoma. Am. J. Pathol. 145:74-79. [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll, P. A., E. Brazeau, and M. Lagunoff. 2004. Kaposi's sarcoma-associated herpesvirus infection of blood endothelial cells induces lymphatic differentiation. Virology 328:7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll, P. A., H. L. Kenerson, R. S. Yeung, and M. Lagunoff. 2006. Latent Kaposi's sarcoma-associated herpesvirus infection of endothelial cells activates hypoxia-induced factors. J. Virol. 80:10802-10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 15.Chan, K. S., S. Sano, K. Kiguchi, J. Anders, N. Komazawa, J. Takeda, and J. DiGiovanni. 2004. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J. Clin. Investig. 114:720-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, H., L. Hutt-Fletcher, L. Cao, and S. D. Hayward. 2003. A positive autoregulatory loop of LMP1 expression and STAT activation in epithelial cells latently infected with Epstein-Barr virus. J. Virol. 77:4139-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, L., and M. Lagunoff. 2005. Establishment and maintenance of Kaposi's sarcoma-associated herpesvirus latency in B cells. J. Virol. 79:14383-14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Chen, L., and M. Lagunoff. 2006. The KSHV viral interleukin-6 is not essential for latency or lytic replication. Virology, in press. [DOI] [PMC free article] [PubMed]
- 18.Chiarle, R., W. J. Simmons, H. Cai, G. Dhall, A. Zamo, R. Raz, J. G. Karras, D. E. Levy, and G. Inghirami. 2005. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat. Med. 11:623-629. [DOI] [PubMed] [Google Scholar]
- 19.Chung, Y. H., N. H. Cho, M. I. Garcia, S. H. Lee, P. Feng, and J. U. Jung. 2004. Activation of Stat3 transcription factor by herpesvirus saimiri STP-A oncoprotein. J. Virol. 78:6489-6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darnell, J. E., Jr. 1997. STATs and gene regulation. Science 277:1630-1635. [DOI] [PubMed] [Google Scholar]
- 21.Flore, O., S. Rafii, S. Ely, J. J. O'Leary, E. M. Hyjek, and E. Cesarman. 1998. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature 394:588-592. [DOI] [PubMed] [Google Scholar]
- 22.Gao, S. J., C. Boshoff, S. Jayachandra, R. A. Weiss, Y. Chang, and P. S. Moore. 1997. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 15:1979-1985. [DOI] [PubMed] [Google Scholar]
- 23.Gong, G., G. Waris, R. Tanveer, and A. Siddiqui. 2001. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-κB. Proc. Natl. Acad. Sci. USA 98:9599-9604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haura, E. B., J. Turkson, and R. Jove. 2005. Mechanisms of disease: insights into the emerging role of signal transducers and activators of transcription in cancer. Nat. Clin. Pract. Oncol. 2:315-324. [DOI] [PubMed] [Google Scholar]
- 25.Heck, E., D. Lengenfelder, M. Schmidt, I. Muller-Fleckenstein, B. Fleckenstein, B. Biesinger, and A. Ensser. 2005. T-cell growth transformation by herpesvirus saimiri is independent of STAT3 activation. J. Virol. 79:5713-5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinrich, P. C., I. Behrmann, G. Muller-Newen, F. Schaper, and L. Graeve. 1998. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 334(Pt. 2):297-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong, Y. K., K. Foreman, J. W. Shin, S. Hirakawa, C. L. Curry, D. R. Sage, T. Libermann, B. J. Dezube, J. D. Fingeroth, and M. Detmar. 2004. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat. Genet. 36:683-685. [DOI] [PubMed] [Google Scholar]
- 28.Jussila, L., R. Valtola, T. A. Partanen, P. Salven, P. Heikkila, M. T. Matikainen, R. Renkonen, A. Kaipainen, M. Detmar, E. Tschachler, R. Alitalo, and K. Alitalo. 1998. Lymphatic endothelium and Kaposi's sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res. 58:1599-1604. [PubMed] [Google Scholar]
- 29.Kisseleva, T., S. Bhattacharya, J. Braunstein, and C. W. Schindler. 2002. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 285:1-24. [DOI] [PubMed] [Google Scholar]
- 30.Krishnan, H. H., P. P. Naranatt, M. S. Smith, L. Zeng, C. Bloomer, and B. Chandran. 2004. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J. Virol. 78:3601-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagunoff, M., J. Bechtel, E. Venetsanakos, A. M. Roy, N. Abbey, B. Herndier, M. McMahon, and D. Ganem. 2002. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 76:2440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, H., R. Veazey, K. Williams, M. Li, J. Guo, F. Neipel, B. Fleckenstein, A. Lackner, R. C. Desrosiers, and J. U. Jung. 1998. Deregulation of cell growth by the K1 gene of Kaposi's sarcoma-associated herpesvirus. Nat. Med. 4:435-440. [DOI] [PubMed] [Google Scholar]
- 33.Lund, T. C., R. Garcia, M. M. Medveczky, R. Jove, and P. G. Medveczky. 1997. Activation of STAT transcription factors by herpesvirus saimiri tip-484 requires p56lck. J. Virol. 71:6677-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lund, T. C., P. C. Prator, M. M. Medveczky, and P. G. Medveczky. 1999. The Lck binding domain of herpesvirus saimiri tip-484 constitutively activates Lck and STAT3 in T cells. J. Virol. 73:1689-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molden, J., Y. Chang, Y. You, P. S. Moore, and M. A. Goldsmith. 1997. A Kaposi's sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J. Biol. Chem. 272:19625-19631. [DOI] [PubMed] [Google Scholar]
- 36.Moore, P. S., C. Boshoff, R. A. Weiss, and Y. Chang. 1996. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 274:1739-1744. [DOI] [PubMed] [Google Scholar]
- 37.Mora, L. B., R. Buettner, J. Seigne, J. Diaz, N. Ahmad, R. Garcia, T. Bowman, R. Falcone, R. Fairclough, A. Cantor, C. Muro-Cacho, S. Livingston, J. Karras, J. Pow-Sang, and R. Jove. 2002. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 62:6659-6666. [PubMed] [Google Scholar]
- 38.Moses, A. V., K. N. Fish, R. Ruhl, P. P. Smith, J. G. Strussenberg, L. Zhu, B. Chandran, and J. A. Nelson. 1999. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 73:6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muromoto, R., K. Okabe, M. Fujimuro, K. Sugiyama, H. Yokosawa, T. Seya, and T. Matsuda. 2006. Physical and functional interactions between STAT3 and Kaposi's sarcoma-associated herpesvirus-encoded LANA. FEBS Lett. 580:93-98. [DOI] [PubMed] [Google Scholar]
- 40.Nair, B. C., A. L. DeVico, S. Nakamura, T. D. Copeland, Y. Chen, A. Patel, T. O'Neil, S. Oroszlan, R. C. Gallo, and M. G. Sarngadharan. 1992. Identification of a major growth factor for AIDS-Kaposi's sarcoma cells as oncostatin M. Science 255:1430-1432. [DOI] [PubMed] [Google Scholar]
- 41.Osborne, J., P. S. Moore, and Y. Chang. 1999. KSHV-encoded viral IL-6 activates multiple human IL-6 signaling pathways. Hum. Immunol. 60:921-927. [DOI] [PubMed] [Google Scholar]
- 42.Poole, L. J., Y. Yu, P. S. Kim, Q. Z. Zheng, J. Pevsner, and G. S. Hayward. 2002. Altered patterns of cellular gene expression in dermal microvascular endothelial cells infected with Kaposi's sarcoma-associated herpesvirus. J. Virol. 76:3395-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 44.Shuai, K., C. M. Horvath, L. H. Huang, S. A. Qureshi, D. Cowburn, and J. E. Darnell, Jr. 1994. Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell 76:821-828. [DOI] [PubMed] [Google Scholar]
- 45.Skobe, M., L. F. Brown, K. Tognazzi, R. K. Ganju, B. J. Dezube, K. Alitalo, and M. Detmar. 1999. Vascular endothelial growth factor-C (VEGF-C) and its receptors KDR and flt-4 are expressed in AIDS-associated Kaposi's sarcoma. J. Investig. Dermatol. 113:1047-1053. [DOI] [PubMed] [Google Scholar]
- 46.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 47.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venetsanakos, E., A. Mirza, C. Fanton, S. R. Romanov, T. Tlsty, and M. McMahon. 2002. Induction of tubulogenesis in telomerase-immortalized human microvascular endothelial cells by glioblastoma cells. Exp. Cell Res. 273:21-33. [DOI] [PubMed] [Google Scholar]
- 49.Wan, X., H. Wang, and J. Nicholas. 1999. Human herpesvirus 8 interleukin-6 (vIL-6) signals through gp130 but has structural and receptor-binding properties distinct from those of human IL-6. J. Virol. 73:8268-8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, H. W., M. W. Trotter, D. Lagos, D. Bourboulia, S. Henderson, T. Makinen, S. Elliman, A. M. Flanagan, K. Alitalo, and C. Boshoff. 2004. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 36:687-693. [DOI] [PubMed] [Google Scholar]
- 51.Wang, L., D. P. Dittmer, C. C. Tomlinson, F. D. Fakhari, and B. Damania. 2006. Immortalization of primary endothelial cells by the K1 protein of Kaposi's sarcoma-associated herpesvirus. Cancer Res. 66:3658-3666. [DOI] [PubMed] [Google Scholar]
- 52.Wen, Z., and J. E. Darnell, Jr. 1997. Mapping of Stat3 serine phosphorylation to a single residue (727) and evidence that serine phosphorylation has no influence on DNA binding of Stat1 and Stat3. Nucleic Acids Res. 25:2062-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]