Abstract
Upon viral infection, the major defense mounted by the host immune system is activation of the interferon (IFN)-mediated antiviral pathway. In order to complete their life cycles, viruses must modulate the host IFN-mediated immune response. The K3 and K5 proteins of a human tumor-inducing herpesvirus, Kaposi's sarcoma-associated herpesvirus (KSHV), have been shown to downregulate the surface expression of host immune modulatory receptors by increasing their endocytosis rates, which leads to suppression of cell-mediated immunity. In this report, we demonstrate that K3 and K5 both specifically target gamma interferon receptor 1 (IFN-γR1) and induce its ubiquitination, endocytosis, and degradation, resulting in downregulation of IFN-γR1 surface expression and, thereby, inhibition of IFN-γ action. Mutational analysis indicated that K5 appeared to downregulate IFN-γR1 more strongly than K3 and that the amino-terminal ring finger motif and the carboxyl-terminal region of K5 were necessary for IFN-γR1 downregulation. These results suggest that KSHV K3 and K5 suppress both cytokine-mediated and cell-mediated immunity, which ensures efficient viral avoidance of host immune controls.
Interferons (IFNs) are a family of cytokines that exhibit such diverse biological effects as the inhibition of cell growth and protection against viral infection. There are two major subtypes of IFNs: type I IFNs (alpha IFN [IFN-α] and IFN-β) and type II IFN (IFN-γ). Both subtypes elicit similar yet distinct biological activities. IFN-α/β are vital signals for the host immune system to initiate an antiviral response, provide the first front line of innate immune defense against virus infection, and additionally modulate adaptive immune response (29). IFN-γ, which is produced by activated T cells and natural killer cells, is primarily involved in the regulation of specific immune responses, immune surveillance, and tumor suppression (1, 4). IFNs carry out their responses through activation of the Janus kinase-signal transducer and activator of transcription (JAK-STAT) signal pathway. Activation of the STAT pathway leads to the formation of two major complexes: gamma-activated factor (GAF), which is composed of STAT1 dimers and induced by IFN-γ, and IFN-stimulated gene factor 3, which consists of STAT1 (p84/91), STAT2 (p113), and a DNA binding protein, p48 (IRF9), and is mainly induced by IFN-α/β. IRF9 and GAF complexes translocate to the nucleus, bind to the interferon-stimulated response element (ISRE) and the gamma-activated sequence (GAS), respectively, and modulate gene transcription (12, 13, 15, 47, 48).
Most viruses have evolved immune evasion strategies to protect themselves against host IFN responses, elaborating viral proteins as a counterdefense against the host IFN defenses. Recently, the IFN antagonist strategies used by many viruses have been revealed; these strategies include blocking IFN signaling by downregulation of JAK-STAT signal molecule basal levels, suppression of particular molecule modifications, and prevention of molecule translocation (19, 21, 27, 49). The Paramyxovirus family has been well demonstrated to utilize distinct molecular mechanisms to circumvent the IFN response. Sendai virus C protein and human parainfluenza virus type 3 (hPIV3) V protein inhibit STAT phosphorylation and activation (17, 20, 22). Simian virus 5 and mumps virus V proteins induce STAT1 degradation, while hPIV2 V protein induces STAT2 degradation (30). Nipah virus and hendra virus V proteins inhibit IFN signaling by preventing STAT1 and STAT2 nuclear accumulation (43, 44). Furthermore, herpesviruses have also been shown to antagonize IFN responses through numerous mechanisms. A prototype alphaherpesvirus, herpes simplex virus, encodes at least two modulators of the IFN response, US11 and ICP34.5, targeting a similar IFN response pathway, the double-stranded-RNA-dependent protein kinase PKR pathway (8). Various human cytomegalovirus proteins have been demonstrated to induce cellular IFN response genes (28). Epstein-Barr virus also blocks the antiviral IFN response through downregulation of IFN-γ receptor gene expression by BZLF1, an immediate-early gene product (37). Kaposi's sarcoma-associated herpesvirus (KSHV) has developed a unique mechanism for antagonizing cellular IFN-mediated antiviral activity by incorporating viral homologs of several cellular regulatory genes into its genome. One of the important pirated genes encoded by the open reading frame K9 is a viral homolog of the interferon regulatory factors (vIRF-1), a family of cellular transcription proteins that regulate the expression of genes involved in pathogen response, immune modulation, and cell proliferation. vIRF-1 has been shown to downregulate IFN- and IRF-mediated transcriptional activation by interacting with CBP/p300 coactivators (32, 33). This indicates that herpesviruses deregulate IFN-mediated innate immunity in various ways to establish and/or maintain persistent infection.
KSHV has been shown to contain a battery of genes whose products downregulate host immune responses at various levels. Two proteins, KSHV K3 and K5, dramatically downregulate major histocompatibility complex (MHC) class I molecules. Biochemical analyses have demonstrated that, similar to HIV Nef, expression of either K3 or K5 induces rapid endocytosis of MHC class I molecules (10, 23, 26). Interestingly, the proteins exhibit 40% amino acid identity to each other (38, 45) and contain C4HC3 RING-CH (really interesting new gene-CH) zinc finger motifs at the amino terminus, with hydrophobic transmembrane regions in the central region, but are of various sizes in the carboxyl-terminal tail (38). However, despite this similarity, K3 and K5 differ in their specificities. K3 drastically downregulates HLA-A, -B, -C, and -E, whereas K5 is active in downregulating only HLA-A and -B2 (10, 26). Furthermore, K5 also downregulates ICAM-1 and B7-2, which are ligands for NK cell-mediated cytotoxicity receptors (9, 25). As a consequence, K5 expression drastically inhibits NK cell-mediated cytotoxicity. Detailed mutational analysis shows that the RING-CH motif in the K3 amino-terminal region carries E3 ubiquitin ligase activity toward MHC class I molecules (5, 9); however, this E3 activity is not completely dependent on the cytoplasmic lysine residue of MHC class I (7). Furthermore, the C-terminal tyrosine-based endocytosis motif and diacidic clusters in the carboxyl-terminal region of K3 are also required for efficient downregulation of MHC class I surface expression (36).
In this report, we show that KSHV K3 and K5 both specifically target IFN-γR1 and induce its ubiquitination, endocytosis, and degradation, resulting in downregulation of IFN-γR1 surface expression and, thereby, inhibition of IFN-γ action. This suggests that KSHV K3 and K5 suppress both cytokine-mediated and cell-mediated immunity, which ensures a comprehensive avoidance of host immune surveillance.
MATERIALS AND METHODS
Cell culture, cytokine treatment, and transfection.
BJAB cells were grown in RPMI 1640 with 10% fetal calf serum. 293T cells were grown in Dulbecco's modified Eagle's medium with 10% fetal calf serum and 1% penicillin-streptomycin (Gibco-BRL). Treatments of cells with IFN were performed as indicated, with 1.00 U of IFN-γ per ml. For transient assays, expression plasmid DNAs were introduced by electroporation at 260 V and 975 μF in serum-free RPMI 1640 medium. To select stable cell lines, cells were selected with 2 mg/ml G418 (Sigma-Aldrich) for 5 weeks, followed by two rounds of fluorescence-activated cell sorting (FACS). For transient assays in 293T cells, each plasmid DNA was transfected using the Fugene 6 reagent (Roche) or calcium phosphate (BD Biosciences).
Plasmid construction.
Based on the KSHV genomic sequence (45), DNA containing the KSHV K3 or K5 open reading frame was amplified from BCBL-1 genomic DNA by PCR using a 5′ primer that corresponded to the amino-terminal sequence of each gene and a 3′ primer that corresponded to the carboxyl-terminal sequence of each gene. The primers used for PCR contained EcoRI and XbaI recognition sequences for subsequent cloning. Amplified DNA was ligated into the EcoRI and XbaI cloning sites of the pEpiTag vector (Invitrogen, Carlsbad, CA) for six-His tagging at the carboxyl terminus of each gene. All K5 mutant constructs used in this study were generated using oligonucleotide-directed mutagenesis and completely sequenced to verify the presence of the mutation. Each was subcloned into either the green fluorescent protein (GFP) coexpression vector pTracer-EF (Invitrogen) or the pEF1 expression vector (Invitrogen).
Flow cytometry analysis and antibodies.
Cells, 5 × 105 per sample, were washed with RPMI medium containing 10% fetal calf serum and incubated with the indicated fluorescein isothiocyanate-conjugated or phycoerythrin (PE)-conjugated monoclonal antibodies for 30 min at 4°C. After being washed, each sample was fixed with 2% paraformaldehyde solution, and flow cytometry analysis was performed with a FACS Scan (Becton Dickinson Co.).
Immunoblotting and immunoprecipitation.
Transfected cells were harvested and resuspended with lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 0.5% NP-40, and protease inhibitor cocktail [Roche]), followed by centrifugation at 12,000 rpm for 5 min. The supernatants were precleared with protein A/G-agarose (Santa Cruz Biotechnology) and incubated with specific antibody, followed by pull-down with additional protein A/G-agarose. Immune complexes were resuspended with sodium dodecyl sulfate sample buffer (Sigma), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to a polyvinylidene difluoride membrane (Roche). The membrane was blocked with phosphate-buffered saline containing 5% skim milk for 30 min, incubated with primary antibody for 1 h, and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h with appropriate wash steps between incubations. Specific signals were detected with an enhanced-chemiluminescence system (Pierce). The primary antibodies were purchased from the following sources: IFN-γR1, IFN-γR2, IFN-α/βR1, IFN-α/βR22, ICAM-1, MHC-ABC, MHC-DR, and β-tubulin antibodies from Santa Cruz Biotechnology; FLAG (M2) antibody from Sigma; and STAT1, STAT2, JAK1, JAK2, and TYK2 antibodies and phosphospecific antibodies for pSTAT1 from Cell Signaling.
Endocytosis assay.
Briefly, cells transfected with the various K5-expressing vectors were stained with PE-labeled anti-IFN-γR1 antibody at 4°C and then incubated for various periods at 37°C. They were then washed in an acidic solution to remove uninternalized antibody, fixed, and subjected to flow cytometry. The percentage of endocytosis was calculated as a ratio of the fluorescence intensity of acid-treated PE-labeled cells to that of untreated PE-labeled cells.
Luciferase reporter assay.
BJAB-EF, BJAB-K3, and BJAB-K5 cells were electroporated with a luciferase reporter plasmid and a control β-galactosidase plasmid, pGK-β-Gal. At 24 h posttransfection, the cells were incubated for 8 h in the presence or absence of IFN-α or IFN-γ. Luciferase activity was measured with a luminometer using a luciferase assay kit (Promega, Madision, WI) and normalized with β-galactosidase activity for transfection efficiency.
RESULTS
Downregulation of surface expression of MHC class I molecules by KSHV K3 and K5.
We hypothesized that KSHV K3 and K5 affected the expression of cell surface proteins other than MHC class I, B7-2, and ICAM-1. To identify additional cell surface proteins targeted by K3 and K5, we surveyed the expression of cellular surface proteins by flow cytometry analysis with numerous antibodies. This revealed that either K3 or K5 expression significantly downregulated surface expression of IFN-γR1 and that the level of IFN-γR1 downregulation by K5 was much more pronounced than that by K3 (Fig. 1). Furthermore, the downregulation of IFN-γR1 by K3 and K5 was specific; their expression did not affect the surface expression of other lymphocyte antigens, including MHC class II and immunoglobulin M, whereas it considerably downregulated MHC class I and ICAM-1 surface expression (Fig. 1 and data not shown).
FIG. 1.
Downregulation of IFN-γR1 surface expression by transient transfection of K3 and K5. BJAB cells were electroporated with GFP, GFP-K3, or GFP-K5 vector. The cell surface levels of MHC-I, IFN-γR1, ICAM-1, and MHC class II in the GFP-positive populations were assessed at 48 h posttransfection by flow cytometry. The dotted line in each histogram represents the isotype control. Data were reproduced in three independent experiments.
To further demonstrate the specific downregulation of IFN-γR1 by K3 and K5, BJAB cells were used to establish cell lines stably expressing vector or the K3 or K5 gene. Full-length K3 and K5 genes were modified to encode a V5 epitope tag at their amino termini and cloned into the expression vector pEF-IRES-puro, which contains the elongation factor 1 promoter for gene expression and the puromycin resistance gene for selection. After electroporation of empty, K3, or K5 expression vector, cells were selected by growth in medium containing 2 μg/ml of puromycin and examined for endogenous IFN-γR1 surface expression by flow cytometry. The stable expression of K3 and K5 in BJAB cells also induced a dramatic downregulation of IFN-γR1 molecules on their surfaces (Fig. 2A). Consistent with the results of the transient-expression assays (Fig. 1), K5 downregulated IFN-γR1 to a greater extent than K3 (Fig. 2). The downregulation of IFN-γR1 by K3 and K5 was specific; their expression did not affect surface levels of MHC class II, immunoglobulin M, and CD19 under the same conditions (Fig. 2A and data not shown). In addition, as previously shown, MHC class I and ICAM-1 were effectively downregulated by K3 and K5 or K5 only, respectively (Fig. 2). However, it was possible that the reduction of IFN-γR1 antibody reactivity in flow cytometry analysis was due to the alteration of IFN-γR1 structure rather than the reduction of its surface expression. To test this possibility, we generated a CD8-Flag-IFN-γR1 chimera, called F-IFN-γR1, in which the signal sequence from CD8 fused to the Flag tag was linked in frame to IFN-γR1 at amino acid 23 (immediately after the IFN-γR1 signal sequence). The expression vector pEF-IRES-puro, containing an F-IFN-γR1 gene, was transfected into BJAB cells, followed by selection with medium containing 2 μg/ml of puromycin. Flow cytometry analysis showed high levels of F-IFN-γR1 surface expression (Fig. 3). BJAB-EF vector and BJAB-K3 and BJAB-K5 cells were electroporated with pTracer vector or pTracer-F-IFN-γR1 (Fig. 3A) or BJAB-EF vector, and BJAB-F-IFN-γR1 cells were electroporated with pTracer vector, pTracer-K3 or pTracer-K5 (Fig. 3B). At 48 h posttransfection, the cell surface level of F-IFN-γR1was assessed on the GFP-positive cell population by flow cytometry with anti-Flag and anti-IFN-γR1 antibodies. Both assays showed significant reduction of F-IFN-γR1 surface expression upon K3 or K5 expression, suggesting that reduction of IFN-γR1 surface expression upon K3 and K5 expression is due to its downregulation, not to its structural alteration (Fig. 3). Finally, the amounts of surface proteins were examined by immunoblotting them with their specific antibodies. This showed that the amount of IFN-γR1 protein was markedly reduced in K3- or K5-expressing BJAB cells (Fig. 2, right). K3- or K5-expressing BJAB cells also showed a considerable reduction of MHC class I or ICAM-1 protein, respectively (Fig. 2, right). However, K3 and K5 expression showed no effect on amounts of IFN-α/βR1, IFN-α/βR2, IFN-γR2, and MHC class II proteins under the same conditions (Fig. 2, right). These results collectively demonstrate that K3 and K5 specifically downregulate IFN-γR1 protein.
FIG. 2.
Downregulation of endogenous IFN-γR1 on cells stably expressing K3 or K5. (A) Downregulation of IFN-γR1 by K3 or K5. Puromycin-resistant cells were stained with antibodies against MHC class I, IFN-γR1, ICAM-1, and MHC class II and analyzed by flow cytometry. The dotted line in each histogram represents the isotype control. (B) Expression of K3, K5, and lymphocyte surface proteins. The same amounts of BJAB-vector, BJAB-K3, and BJAB-K5 cell lysates were used for immunoblotting with various antibodies. Anti-tubulin antibody was included as a loading control.
FIG. 3.
Downregulation of exogenously expressed Flag-tagged IFN-γR1 by K3 or K5. (A) BJAB-EF, BJAB-K3, and BJAB-K5 cells were electroporated with pTracer-F-IFN-γR1 vector. The cell surface levels of F-IFN-γR1 in the GFP-positive cell population was assessed at 48 h posttransfection by staining them with IFN-γR1 or MHC class I antibody, followed by flow cytometry. The data were reproduced in three independent experiments. (B) BJAB-EF and BJAB-F-IFN-γR1 cells were electroporated with pTracer-GFP reporter, pTracer-K3, or pTracer-K5 vector. The cell surface levels of IFN-γR1 in the GFP-positive cell population were assessed at 48 h posttransfection by staining them with Flag or IFN-γR1antibody by flow cytometry. The data were reproduced in three independent experiments.
Effects of K5 mutations on IFN-γR1 downregulation.
Both K3 and K5 open reading frames contain two C4HC3 zinc finger motifs at the amino terminus, in addition to hydrophobic transmembrane domains in the central region, but are of various sizes in the carboxyl-terminal tail, with extensive sequence variation. Despite this variation, several motifs for endocytosis and signal transduction are conserved in both the K3 and K5 carboxyl-terminal regions. These include sequences containing potential tyrosine-based endocytosis motifs, Y152AAV for K3 and Y156AAN for K5, and PX2-3P potential SH3-binding motifs (36). In addition, both K3 and K5 contain conserved diacidic-cluster regions, which resemble HIV Nef (40).
Since IFN-γR1 downregulation by K5 is more pronounced than that by K3, K5 was selected for detailed mutational analysis. As depicted in Fig. 4A, a series of carboxyl-terminal deletion mutations of K5, ΔC1, ΔC2, ΔC3, and ΔC4, were constructed similarly to the K3 deletion mutations (reference 36 and unpublished results). In addition, the cysteine residues at positions 9, 12, 24, 26, 50, and 53 in the putative zinc finger motifs were replaced with serine residues to generate the mZn mutant (Fig. 4A). BJAB cells were electroporated with pTracer vector alone or vector expressing wild-type (wt) or mutant K5. The effects of K5 and its mutant expression on IFN-γR1 surface expression were assessed at 48 h posttransfection in the GFP-positive cell population by flow cytometry. The ΔC1 and ΔC2 mutants were capable of downregulating IFN-γR1 at levels equivalent to wt K5, whereas the ΔC3 mutant showed a slight reduction in IFN-γR1 downregulation (Fig. 4A). In contrast, the ΔC4 and mZn mutations completely abolished K5-mediated downregulation of IFN-γR1 (Fig. 4A).
FIG. 4.
Mutational analysis of K5 for IFN-γR1 downregulation. (A) Point mutations of the N-terminal zinc finger motifs and deletion mutations in the C-terminal region of K5. A schematic of the locations of various mutations of the K5 protein is shown at the top. Deletion mutations in the C-terminal region of K5 were generated as follows: ΔC1, deletion of residues 233 to 256; ΔC2, deletion of residues 198 to 256; ΔC3, deletion of residues 194 to 256; and ΔC4, deletion of residues 178 to 256. The cysteine residues at 15, 18, 30, 32, 56, and 59 in the putative zinc finger motifs in the N-terminal region of K5 were replaced with serine residues to generate the mZn mutant. The abilities of wild-type K5 and each mutant to downregulate IFN-γR1, as assessed by flow cytometry, were given relative scores. ++++, ++, and − indicate very strong, strong, and no activity of the K5 mutant in IFN-γR1 downregulation, respectively. To examine the abilities of K5 and its mutants to downregulate IFN-γR1, BJAB cells were electroporated with pTracer-GFP-K5 (WT), GFP-K5 mZn (mZn), or one of the deletion mutants. Cell surface levels of IFN-γR1 in the GFP-positive populations were assessed 48 h posttransfection by staining them with an IFN-γR1-specific antibody. The data were reproduced in at least two independent experiments. (B) Mutations in sequence-specific motifs of K5 and IFN-γR1 downregulation. The schematic at the top shows the locations of various mutations of the K5 protein. Six mutations, Y156F (Y/F), Y156/A (Y/A), P/A, DE1/QQ, DE2/QQ, and DE12/QQ, were introduced into K5. The locations of these mutations are described in greater detail in the text. Along the right side of the schematic, the abilities of wild-type K5 and each mutant to downregulate IFN-γR1, as shown in the below the schematic, were given relative scores. +++ and − indicate very strong and no activity of the K5 mutant in IFN-γR1 downregulation, respectively. Below are shown the differential IFN-γR1 downregulation activities of various K5 mutants. The detailed procedure is described in the legend to panel A. The data were reproduced in at least two independent experiments. (C) The NTRV sequence in the cytoplasmic region of K5 is required for IFN-γR1 downregulation. A schematic of the locations of various mutations of the K5 protein is shown at the top. Four mutations, K5 N160A (N/A), K5 T161A (T/A), K5 R162A (R/A), and K5 V163A (V/A), were introduced into K5. Each mutation is described in greater detail in the text. Along the right side, the abilities of wild-type K5 and each mutant to downregulate IFN-γR1, as shown below the schematic, were given relative scores. +++, +, and − indicate very strong, weak, and no activity of the K5 mutant in IFN-γR1 downregulation, respectively. Below are shown differential IFN-γR1 downregulation activities of various K5 mutants. The detailed procedure is described in the legend to panel A. The data were reproduced in at least two independent experiments. Cell lysates were used for anti-K5 immunoblot analysis to demonstrate the equivalent expressions of wt K5 and its mutants.
Subsequent point-mutational analysis was directed at the YAAN tyrosine-based endocytosis motif, the PX2-3P SH3-binding motif, and diacidic clusters at the carboxyl terminus of K5. The tyrosine residue Y152 in the putative tyrosine-based endocytosis motif was replaced with phenylalanine (F) or alanine (A) to generate, respectively, the Y156F and Y156A mutants (Fig. 4B). Additionally, the proline residues P167, P171, and P174 in the putative SH3-binding motif were replaced with alanines (A) to generate the P/A mutant (Fig. 4B). To define a role for the diacidic clusters of K5 in IFN-γR1 downregulation, several mutants were produced; residues E178, E179, and E182 in the first acidic cluster were replaced with glutamine (Q) residues to generate the DE1/QQ mutant; residues D194, E195, E196, D199, and E200 in the second acidic cluster were replaced with glutamine residues to generate the DE2/QQ mutant. These two mutants were combined to generate the DE12/QQ mutant (Fig. 4B). BJAB cells were electroporated with pTracer vectors containing GFP alone or GFP plus each of the various constructs. The effects of expression of K5 and its mutants on IFN-γR1 surface expression were assessed at 48 h posttransfection in the GFP-positive cell population. Mutation of Y156 to A in the putative tyrosine-based endocytosis motif of K5 abrogated its ability to downregulate IFN-γR1, whereas the Y156F mutation did not, suggesting that the YAAN sequence in the cytoplasmic region of K5 is indeed a tyrosine-based sorting motif and that sorting activity through this sequence is independent of tyrosine phosphorylation. By contrast, the P/A, DE1/QQ, DE2/QQ, and DE12/QQ mutations of K5 showed little or no change in the ability to reduce cell surface expression of IFN-γR1 compared with the wild type (Fig. 4B).
Finally, the NTRV residues downstream of the YAAV tyrosine-based endocytosis motif, which are completely conserved between K3 and K5, were mutated with alanines to generate the N16oA, T161A, R162A, and V163A mutants (Fig. 4C). The N160A and T161A mutations considerably abrogated the ability of K5 to downregulate IFN-γR1, whereas the R162A and V163A mutations had little effect on K5 IFN-γR1 downregulation activity under the same conditions (Fig. 4C). These results demonstrated that the conserved NTRV sequence is apparently engaged in efficient downregulation of IFN-γR1. All K5 mutants were expressed at levels equivalent to those of wt K5 in these cells (Fig. 4). These results indicate that the ring finger motif in the amino-terminal region, the YAAN tyrosine-based sorting motif, and NTRV sequences in the central region of K5 are involved in efficient IFN-γR1 downregulation. The diacidic-cluster region in the carboxyl-terminal region of K5 plays a role in IFN-γR1 downregulation; however, individual amino acid changes do not show detectable effects on IFN-γR1 surface expression.
Increase of IFN-γR1 endocytosis and ubiquitination by K3 and K5.
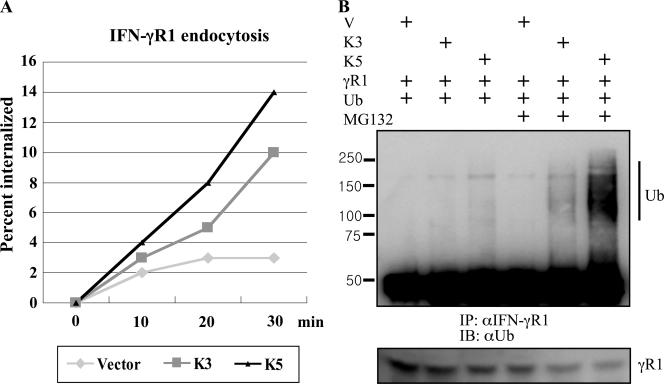
To further delineate K3 and K5 roles in IFN-γR1 downregulation, we examined their effects on the endocytosis rate of IFN-γR1 using a flow cytometry-based endocytosis assay. BJAB-EF, BJAB-K3, and BJAB-K5 cells were electroporated with pTracer containing GFP alone or GFP and IFN-γR1. The cells were labeled at 48 h posttransfection with a fluorescein isothiocyanate-conjugated IFN-γR1 antibody at 4°C, followed by several washes to eliminate unbound antibody. The cells were then incubated at 37°C for various times, washed with acidic saline solution, and analyzed for IFN-γR1 internalization in the GFP-positive population by flow cytometry. Over the time course of the analysis, the internalization of IFN-γR1 in control cells was negligible: less than 3% of IFN-γR1 was internalized after 30 min of incubation (Fig. 5A). In contrast, K3 and K5 expression significantly increased the endocytosis rate of IFN-γR1 with 10 and 14% internalized, respectively, after 30 min of incubation (Fig. 5A). These results demonstrate that K3 and K5 expression induces a rapid internalization of IFN-γR1.
FIG. 5.
Increased IFN-γR1 endocytosis and ubiquitination by K3 and K5. (A) Increased IFN-γR1 endocytosis rates. BJAB-EF, BJAB-K3, and BJAB-K5 cells were used to measure the IFN-γR1 endocytosis rate. Internalized rates of IFN-γR1 were determined using a FACS-based endocytosis assay. The results are representative of two independent experiments. (B) Increases of IFN-γR1 ubiquitination. 293T cells were transfected with K3, K5, IFN-γR1 (γR1), and ubiquitin (Ub) in various combinations and incubated with or without treatment with MG132. At 48 h posttransfection, cell lysates were immunoprecipitated (IP) with anti-IFN-γR1 antibody, followed by immunoblotting (IB) with anti-Ub antibody. Cell lysates were also used for immunoblotting with anti-IFN-γR1 antibody (bottom). Vec, vector.
KSHV K3 and K5 have been shown to mediate ubiquitinylation of MHC class I, resulting in rapid downregulation and destruction of MHC class I molecules (11). To examine whether K3 or K5 expression affected IFN-γR1 ubiquitination, 293T cells were transfected with various expression vectors, including vector, K3 or K5, IFN-γR1, and ubiquitin. At 48 h posttransfection, IFN-γR1 was immunoprecipitated with anti-IFN-γR1 antibody, followed by immunoblotting with anti-ubiquitin antibody. These showed that either K3 or K5 expression considerably increased IFN-γR1 ubiquitination and that, consistent with its downregulating activity, K5 induces IFN-γR1 ubiquitination more strongly than K3 (Fig. 5B).
Suppression of IFN-γR signal transduction by K3 and K5.
Following IFN-γ stimulation, STATs are phosphorylated by JAK, which leads to homo- or heterodimerization, nuclear localization, and transcriptional activation. To analyze the phosphorylation status of STAT1 in K3- or K5-expressing cells, BJAB cell extracts were prepared at various time points after IFN-γ stimulation, followed by immunoblotting with pY701 phosphospecific STAT1 antibody. While the total levels of STAT1 were similar in BJAB-EF, BJAB-K3, and BJAB-K5 cells, tyrosine-phosphorylated STAT1 (pY701-STAT1) was markedly reduced in BJAB-K3 and BJAB-K5 cells compared to BJAB-EF cells (Fig. 6A). In addition, STAT2, JAK1, JAK2, and TYK2 levels were not affected by K3 and K5 expression (Fig. 6B).
FIG. 6.
Suppression of IFN-γR signal transduction by K3 and K5. (A) Suppression of STAT1 phosphorylation by K3 and K5 expression. BJAB-EF, BJAB-K3, and BJAB-K5 cell extracts were prepared at various time points after IFN-γ stimulation, followed by immunoblotting with either the pY701 phosphospecific STAT1 antibody or an antibody recognizing all forms of STAT1 as a loading control. (B) JAK and STAT expression. Normalized BJAB-EF, BJAB-K3, and BJAB-K5 cell extracts were used for immunoblotting assays with antibodies to STAT1, STAT2, JAK1, JAK2, or TYK2. (C) Inhibition of IFN-γ-induced GAS promoter activation by K3 and K5. BJAB-EF, BJAB-K3, and BJAB-K5 cells were transfected with a luciferase reporter plasmid (GAS or ISRE) and a control β-galactosidase plasmid, pGK-β-Gal. At 24 h posttransfection, the cells were incubated for 8 h in the presence or absence of IFN-α or IFN-γ. Luciferase activity was then measured and normalized for transfection efficiency by β-galactosidase activity. The error bars indicate standard deviations.
To further test the roles of K3 and K5 as IFN-γ antagonists, we measured the transcriptional activities of IFN-regulated promoters using a luciferase reporter gene. Since type I IFN-α/β and type II IFN-γ act on overlapping but distinct sets of cis-acting elements (12, 13, 15, 47, 48), we employed two luciferase constructs that contained the different cis-acting elements; the ISRE of the ISG15 promoter (ISG15-ISRE) and the GAS. BJAB-EF, BJAB-K3, and BJAB-K5 cells were transfected with a luciferase reporter plasmid and a control β-galactosidase plasmid, pGK-β-Gal. At 24 h posttransfection, the cells were incubated for 8 h in the presence or absence of IFN-α or IFN-γ. Luciferase activity was normalized for transfection efficiency by β-galactosidase activity. GAS activity was strongly induced in BJAB-EF control cells upon IFN-γ stimulation, whereas its activity was drastically repressed in BJAB-K5 and BJAB-K3 cells under the same conditions (Fig. 6C). Consistent with the level of IFN-γR1 downregulation, the reduction in levels of GAS activity in K3-expressing cells was less pronounced than that in K5 cells (Fig. 6C). By striking contrast, K3 and K5 expression showed minimal or no effect on IFN-α-activated ISG15-ISRE (Fig. 6C). These results demonstrate that K3 and K5 expression leads to significant repression of type II IFN-mediated transcriptional activation without affecting type I IFN-mediated transcriptional activation.
DISCUSSION
We and others have previously reported that the KSHV K3 primarily downregulates MHC class I molecules and KSHV K5 shows broad downregulation of surface receptors, including MHC class I, ICAM-1, B7-2, and PECAM (9, 10, 23, 25, 26, 35, 36). In this report, we demonstrate that KSHV K3 and K5 specifically target IFN-γR1 for efficient downregulation, which leads to suppression of IFN-γ-mediated transcription factor activation. Thus, KSHV K3 and K5 suppress both cytokine-mediated and cell-mediated immunity, which ensures efficient viral avoidance of host immune control.
Coevolution of viruses with their hosts for millions of years has led to a host immune system of high complexity and, likewise, sophisticated viral mechanisms to antagonize host immunity (24). Early cytokines, particularly IFNs, which integrate innate and adaptive immune responses and are designed to prevent completion of the virus life cycle and the spread of infection, are essential targets for viruses. Recent studies have revealed numerous viral IFN antagonist strategies, including downregulation of JAK-STAT levels, suppression of their particular modifications, and prevention of their translocation (19, 21, 27, 49). For instance, Sendai virus C protein and hPIV3 V protein inhibit STAT phosphorylation and activation (17, 20, 22), simian virus 5 and mumps virus V proteins induce STAT1 degradation, hPIV2 V protein induces STAT2 degradation (30), and Nipah virus and hendra virus V proteins inhibit STAT1 and STAT2 nuclear accumulation (43, 44). We demonstrated that KSHV K3 and K5 target the proximal IFN signal transduction by downregulating IFN-γR1 surface expression. The reduction of IFN-γR1 surface expression by K3 and K5 led to effective suppression of IFN-γ-mediated STAT1 phosphorylation and transcriptional activation. Targeting of IFN membrane-proximal signal transduction by K3 and K5 seems to be more efficient in deregulation of IFN-mediated antiviral activity than suppressing IFN downstream signal transduction. However, in cases where K3 and K5 do not downregulate IFN-γR1 in a timely and effective fashion, KSHV may encounter IFN-γ-mediated hostile antiviral attack. To prevent this, KSHV may carry two genetically and functionally similar genes, K3 and K5, which target IFN-γR1 to different extents. Furthermore, KSHV also harbors an additional battery of IFN regulatory genes called vIRFs that suppress IFN response primarily at the transcriptional level (14, 18, 33). Thus, in combination with vIRFs, KSHV K3 and K5 comprehensively suppress host IFN-mediated antiviral activity.
We have previously shown that the amino-terminal RING-CH E3 ligase motif, the central tyrosine-based endocytosis motif, and conserved NTRV residues of K3 are involved in triggering the internalization of MHC class I molecules and redirecting them to the trans-Golgi network. Subsequently, the carboxyl-terminal diacidic-cluster region of K3 is engaged in targeting MHC class I molecules to lysosomes for degradation (36). Our biochemical analyses also demonstrated that, similar to MHC class I, B7-2, and ICAM-1, IFN-γR1 underwent ubiquitination and endocytosis upon K3 and K5 expression. Furthermore, detailed mutational analysis showed that the K5 N-terminal E3 ubiquitin ligase domain and the C-terminal tyrosine-based endocytosis motif were required for efficient downregulation of IFN-γR1 surface expression. Our comparative analysis further showed that the diacidic-cluster region was required for K5-mediated downregulation of both MHC class I and IFN-γR1 (Table 1). However, while K5 DE12/QQ point mutation demonstrated significantly reduced activity for downregulating MHC class I, it showed little or no effect on its ability to reduce IFN-γR1 surface expression (Table 1). This suggests that sequences other than acidic amino acids in the diacidic-cluster region may play roles in IFN-γR1 downregulation. Two hydrophobic amino acids, I183L184 in K3 and L186V187 in K5, which are similar to a dileucine-based sorting motif, are present in the diacidic amino acid cluster region. The dileucine-based protein-sorting motifs within the C-terminal region of CD4 and HIV-1 Nef have been shown to be important for their endocytosis and intracellular trafficking (2, 42, 46). This suggests that a dileucine-based sorting-like motif of K3 and K5 may contribute to their activities in downregulating IFN-γR1 surface expression. Furthermore, mutations at the conserved NTRV residues of K5 also showed slight differences in their effects on the downregulation of MHC class I and IFN-γR1 (Table 1). This suggests that the overall internalization schemes of MHC class I and IFN-γR1 by K3 and K5 proteins are highly conserved, using their E3 ligase domains, tyrosine-based endocytosis motifs, and diacidic regions. However, the K3/K5-mediated intracellular trafficking course and mechanism of the IFN-γR1 protein appears to be slightly different from those of the MHC class I molecule.
TABLE 1.
Summary of K5 mutant activitya
| K5 | Downregulation
|
||
|---|---|---|---|
| MHC class I | ICAM-1 | IFN-γR1 | |
| WT | +++ | +++ | +++ |
| ΔC1 | +++ | +++ | +++ |
| ΔC2 | +++ | +++ | +++ |
| ΔC3 | +++ | − | ++ |
| ΔC4 | − | − | − |
| mZn | − | − | − |
| Y/F | +++ | +++ | +++ |
| Y/A | − | − | − |
| P/A | +++ | +++ | +++ |
| DE1/QQ | ++ | +++ | +++ |
| DE2/QQ | +++ | +++ | +++ |
| DE12/QQ | + | +++ | +++ |
| N/A | − | + | ++ |
| T/A | − | +++ | ++ |
| R/A | +++ | +++ | +++ |
| V/A | +++ | +++ | +++ |
+++, very strong; ++, strong; +, weak; −, none.
IFN-α and IFN-β, type I IFNs, and IFN-γ, a type II IFN, are central in mediating antiviral response by blocking viral replication or by modulating immune responses to inhibit viral dissemination and disease (1, 3). Controlling hundreds of target genes, IFNs direct enormously complex molecular networks. IFN-α and IFN-β share a common receptor, IFN-α/βR (39). Plasmacytoid dendritic cells are the major source of IFN-α in humans, whereas IFN-β is produced in most cell types, including fibroblasts. On the other hand, IFN-γ is released primarily by activated T and natural killer cells, and IFN-γ subsequently binds to IFN-γR (1). IFN-α/βR and IFN-γR signaling involve two distinct variants of the JAK-STAT pathway and result in the expression of two different sets of genes, which are regulated by specific promoter sequences, the ISRE and GAS (31). Each set comprises great numbers of genes, which only partially overlap. We have demonstrated that K3 and K5 downregulate the surface expression and signaling activity of IFN-γR, but not IFN-α/βR. This indicates that K3 and K5 inhibit IFN-γ-mediated immune responses, the primary host immunity against viral infection, without affecting the IFN-α/β pathway. As described above, KSHV carries an additional battery of vIRF genes, vIRF1 to -4 (6, 18, 34). These vIRFs have been shown to primarily attack the IFN-α/β signaling pathway (3, 14, 16, 41). Thus, KSHV may utilize K3, K5, and vIRFs to comprehensively suppress host IFN-mediated antiviral activity, which ultimately creates a favorable milieu for the establishment and/or maintenance of persistent viral infection. Although the full significance of K3- and K5-mediated effects on IFN signal transduction needs to be studied further in vivo, our study provides insight into the viral immune evasion mechanism of KSHV. Future study of the molecular mechanisms of KSHV K3- and K5-mediated inhibition of IFN signal transduction will lead to a better understanding of viral persistency.
Acknowledgments
We thank members of our laboratory for helpful discussions and comments.
This work was partly supported by U.S. Public Health Service grants CA31363, CA082057, CA91819, CA86038, and RR00168 (to J.U.J.) and CA102535 (to R.M.).
Footnotes
Published ahead of print on 13 December 2006.
REFERENCES
- 1.Bach, E. A., M. Aguet, and R. D. Schreiber. 1997. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu. Rev. Immunol. 15:563-591. [DOI] [PubMed] [Google Scholar]
- 2.Bandres, J. C., A. S. Shaw, and L. Ratner. 1995. HIV-1 Nef protein downregulation of CD4 surface expression: relevance of the lck binding domain of CD4. Virology 207:338-341. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, B., B. Lubyova, and P. M. Pitha. 2002. On the role of IRF in host defense. J. Interferon Cytokine Res. 22:59-71. [DOI] [PubMed] [Google Scholar]
- 4.Billiau, A. 1996. Interferon-gamma: biology and role in pathogenesis. Adv. Immunol. 62:61-130. [DOI] [PubMed] [Google Scholar]
- 5.Boname, J. M., and P. G. Stevenson. 2001. MHC class I ubiquitination by a viral PHD/LAP finger protein. Immunity 15:627-636. [DOI] [PubMed] [Google Scholar]
- 6.Burysek, L., W. S. Yeow, and P. M. Pitha. 1999. Unique properties of a second human herpesvirus 8-encoded interferon regulatory factor (vIRF-2). J. Hum. Virol. 2:19-32. [PubMed] [Google Scholar]
- 7.Cadwell, K., and L. Coscoy. 2005. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science 309:127-130. [DOI] [PubMed] [Google Scholar]
- 8.Cassady, K. A., M. Gross, G. Y. Gillespie, and B. Roizman. 2002. Second-site mutation outside of the US10-12 domain of Δγ134.5 herpes simplex virus 1 recombinant blocks the shutoff of protein synthesis induced by activated protein kinase R and partially restores neurovirulence. J. Virol. 76:942-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coscoy, L., and D. Ganem. 2001. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J. Clin. Investig. 107:1599-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coscoy, L., and D. Ganem. 2000. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. USA 97:8051-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coscoy, L., D. J. Sanchez, and D. Ganem. 2001. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J. Cell Biol. 155:1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darnell, J. E., Jr. 1997. STATs and gene regulation. Science 277:1630-1635. [DOI] [PubMed] [Google Scholar]
- 13.Decker, T., D. J. Lew, J. Mirkovitch, and J. E. Darnell, Jr. 1991. Cytoplasmic activation of GAF, an IFN-gamma-regulated DNA-binding factor. EMBO J. 10:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flowers, C. C., S. P. Flowers, and G. J. Nabel. 1998. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor confers resistance to the antiproliferative effect of interferon-alpha. Mol. Med. 4:402-412. [PMC free article] [PubMed] [Google Scholar]
- 15.Fu, X. Y., C. Schindler, T. Improta, R. Aebersold, and J. E. Darnell, Jr. 1992. The proteins of ISGF-3, the interferon alpha-induced transcriptional activator, define a gene family involved in signal transduction. Proc. Natl. Acad. Sci. USA 89:7840-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuld, S., C. Cunningham, K. Klucher, A. J. Davison, and D. J. Blackbourn. 2006. Inhibition of interferon signaling by the Kaposi's sarcoma-associated herpesvirus full-length viral interferon regulatory factor 2 protein. J. Virol. 80:3092-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao, J., B. P. De, Y. Han, S. Choudhary, R. Ransohoff, and A. K. Banerjee. 2001. Human parainfluenza virus type 3 inhibits gamma interferon-induced major histocompatibility complex class II expression directly and by inducing alpha/beta interferon. J. Virol. 75:1124-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao, S. J., C. Boshoff, S. Jayachandra, R. A. Weiss, Y. Chang, and P. S. Moore. 1997. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 15:1979-1985. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Sastre, A. 2001. Inhibition of interferon-mediated antiviral responses by influenza A viruses and other negative-strand RNA viruses. Virology 279:375-384. [DOI] [PubMed] [Google Scholar]
- 20.Garcin, D., P. Latorre, and D. Kolakofsky. 1999. Sendai virus C proteins counteract the interferon-mediated induction of an antiviral state. J. Virol. 73:6559-6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 22.Gotoh, B., K. Takeuchi, T. Komatsu, and J. Yokoo. 2003. The STAT2 activation process is a crucial target of Sendai virus C protein for the blockade of alpha interferon signaling. J. Virol. 77:3360-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haque, M., K. Ueda, K. Nakano, Y. Hirata, C. Parravicini, M. Corbellino, and K. Yamanishi. 2001. Major histocompatibility complex class I molecules are down-regulated at the cell surface by the K5 protein encoded by Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8. J Gen. Virol. 82:1175-1180. [DOI] [PubMed] [Google Scholar]
- 24.Hengel, H., U. H. Koszinowski, and K. K. Conzelmann. 2005. Viruses know it all: new insights into IFN networks. Trends Immunol. 26:396-401. [DOI] [PubMed] [Google Scholar]
- 25.Ishido, S., J. K. Choi, B. S. Lee, C. Wang, M. DeMaria, R. P. Johnson, G. B. Cohen, and J. U. Jung. 2000. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi's sarcoma-associated herpesvirus K5 protein. Immunity 13:365-374. [DOI] [PubMed] [Google Scholar]
- 26.Ishido, S., C. Wang, B. S. Lee, G. B. Cohen, and J. U. Jung. 2000. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 74:5300-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerr, I. M., A. P. Costa-Pereira, B. F. Lillemeier, and B. Strobl. 2003. Of JAKs, STATs, blind watchmakers, jeeps and trains. FEBS Lett. 546:1-5. [DOI] [PubMed] [Google Scholar]
- 28.Khan, S., A. Zimmermann, M. Basler, M. Groettrup, and H. Hengel. 2004. A cytomegalovirus inhibitor of gamma interferon signaling controls immunoproteasome induction. J. Virol. 78:1831-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotenko, S. V., and S. Pestka. 2000. Jak-Stat signal transduction pathway through the eyes of cytokine class II receptor complexes. Oncogene 19:2557-2565. [DOI] [PubMed] [Google Scholar]
- 30.Kozuka, Y., Y. Yamashita, M. Kawano, M. Tsurudome, M. Ito, M. Nishio, H. Komada, and Y. Ito. 2003. Identification of amino acids essential for the human parainfluenza type 2 virus V protein to lower the intracellular levels of the STAT2. Virology 317:208-219. [DOI] [PubMed] [Google Scholar]
- 31.Leonard, W. J., and J. J. O'Shea. 1998. Jaks and STATs: biological implications. Annu. Rev. Immunol. 16:293-322. [DOI] [PubMed] [Google Scholar]
- 32.Li, M., B. Damania, X. Alvarez, V. Ogryzko, K. Ozato, and J. U. Jung. 2000. Inhibition of p300 histone acetyltransferase by viral interferon regulatory factor. Mol. Cell. Biol. 20:8254-8263.11027294 [Google Scholar]
- 33.Lin, R., P. Genin, Y. Mamane, M. Sgarbanti, A. Battistini, W. J. Harrington, Jr., G. N. Barber, and J. Hiscott. 2001. HHV-8 encoded vIRF-1 represses the interferon antiviral response by blocking IRF-3 recruitment of the CBP/p300 coactivators. Oncogene 20:800-811. [DOI] [PubMed] [Google Scholar]
- 34.Lubyova, B., and P. M. Pitha. 2000. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 74:8194-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mansouri, M., J. Douglas, P. P. Rose, K. Gouveia, G. Thomas, R. E. Means, A. V. Moses, and K. Fruh. 2006. Kaposi's sarcoma herpesvirus K5 eliminates CD31/PECAM from endothelial cells. Blood 108:1932-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Means, R. E., S. Ishido, X. Alvarez, and J. U. Jung. 2002. Multiple endocytic trafficking pathways of MHC class I molecules induced by a herpesvirus protein. EMBO J. 21:1638-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrison, T. E., A. Mauser, A. Wong, J. P. Ting, and S. C. Kenney. 2001. Inhibition of IFN-gamma signaling by an Epstein-Barr virus immediate-early protein. Immunity 15:787-799. [DOI] [PubMed] [Google Scholar]
- 38.Nicholas, J., V. Ruvolo, J. Zong, D. Ciufo, H. G. Guo, M. S. Reitz, and G. S. Hayward. 1997. A single 13-kilobase divergent locus in the Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genome contains nine open reading frames that are homologous to or related to cellular proteins. J. Virol. 71:1963-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pestka, S. 2000. The human interferon alpha species and receptors. Biopolymers 55:254-287. [DOI] [PubMed] [Google Scholar]
- 40.Piguet, V., F. Gu, M. Foti, N. Demaurex, J. Gruenberg, J. L. Carpentier, and D. Trono. 1999. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell 97:63-73. [DOI] [PubMed] [Google Scholar]
- 41.Pozharskaya, V. P., L. L. Weakland, and M. K. Offermann. 2004. Inhibition of infectious human herpesvirus 8 production by gamma interferon and alpha interferon in BCBL-1 cells. J. Gen. Virol. 85:2779-2787. [DOI] [PubMed] [Google Scholar]
- 42.Rhee, S. S., and J. W. Marsh. 1994. Human immunodeficiency virus type 1 Nef-induced down-modulation of CD4 is due to rapid internalization and degradation of surface CD4. J. Virol. 68:5156-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez, J. J., J. P. Parisien, and C. M. Horvath. 2002. Nipah virus V protein evades alpha and gamma interferons by preventing STAT1 and STAT2 activation and nuclear accumulation. J. Virol. 76:11476-11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez, J. J., L. F. Wang, and C. M. Horvath. 2003. Hendra virus V protein inhibits interferon signaling by preventing STAT1 and STAT2 nuclear accumulation. J. Virol. 77:11842-11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salghetti, S., R. Mariani, and J. Skowronski. 1995. Human immunodeficiency virus type 1 Nef and p56lck protein-tyrosine kinase interact with a common element in CD4 cytoplasmic tail. Proc. Natl. Acad. Sci. USA 92:349-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schindler, C., X. Y. Fu, T. Improta, R. Aebersold, and J. E. Darnell, Jr. 1992. Proteins of transcription factor ISGF-3: one gene encodes the 91- and 84-kDa ISGF-3 proteins that are activated by interferon alpha. Proc. Natl. Acad. Sci. USA 89:7836-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shuai, K., C. Schindler, V. R. Prezioso, and J. E. Darnell, Jr. 1992. Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science 258:1808-1812. [DOI] [PubMed] [Google Scholar]
- 49.Yokota, S., N. Yokosawa, T. Kubota, T. Suzutani, I. Yoshida, S. Miura, K. Jimbow, and N. Fujii. 2001. Herpes simplex virus type 1 suppresses the interferon signaling pathway by inhibiting phosphorylation of STATs and janus kinases during an early infection stage. Virology 286:119-124. [DOI] [PubMed] [Google Scholar]