Abstract
Virus-specific CD4+ T-cell responses are thought to be required for the induction and maintenance of many effective CD8+ T-cell and B-cell immune responses in experimental animals and humans. Although the presence of human immunodeficiency virus (HIV)-specific CD4+ T cells has been documented in patients at all stages of HIV infection, many fundamental questions regarding their frequency and function remain. A 10-color, 12-parameter flow cytometric panel was utilized to examine the frequency, memory phenotype (CD27, CCR7, and CD45RA), and cytokine production (interleukin-2 [IL-2], gamma interferon, and tumor necrosis factor alpha) of CD4+ T cells specific for HIV antigens as well as for adenovirus, Epstein-Barr virus (EBV), influenza H1N1 virus, influenza H3N2 virus, cytomegalovirus, varicella-zoster virus (VZV), and tetanus toxoid in normal controls, long-term nonprogressors (LTNP), and HIV-infected patients with progressive disease on or off therapy. The HIV-specific CD4+ T-cell responses in LTNP and patients on therapy were similar in frequency, phenotype, and cytokine production to responses directed against adenovirus, EBV, influenza virus, and VZV. HIV-specific CD4+ T cells from patients off antiretroviral therapy demonstrated a shift towards a CCR7− CD45RA− phenotype and a reduced percentage of IL-2-producing cells. The alterations in cytokine production during HIV viremia were found to be intrinsic to the HIV-specific CD4+ T cells and caused a requirement for IL-2 supplied exogenously for proliferation to occur. These observations suggest that many previously described changes in HIV-specific CD4+ T-cell function and phenotype are a consequence of high levels of antigen in viremic patients. In addition, defects in function and phenotype of HIV-specific CD4+ T cells are not readily discernible in the context of antiretroviral therapy but rather are similar to responses to other viruses.
CD4+ T cells are critical for the induction and maintenance of host immune responses to viral infections in humans and experimental animals. Viral infection in some experimental animal models that lack or have been depleted of CD4+ T cells can result in CD8+ T cells with impaired function and diminished ability to restrict viral replication (11, 26, 30, 33, 45, 47). Most viral infections of humans result in the induction of long-term CD4+ T-cell responses that can be detected by in vitro cytokine production and proliferative responses to viral antigens long after the infection has been cleared (1, 3, 10, 46, 49). In contrast, the human immunodeficiency virus (HIV)-specific CD4+ T-cell immune response is characterized by the absence of proliferative responses to HIV antigens in the vast majority of untreated individuals. However, CD4+ T-cell proliferative responses to HIV have been observed in patients with control of viral replication, including long-term nonprogressors (LTNP), patients treated with antiretroviral therapy during primary HIV infection, and a proportion of patients with chronic HIV infection on effective therapy (2, 5, 6, 24, 29, 31, 34, 35, 37, 42, 43).
It now appears clear that the absence of these responses in the majority of infected patients is not simply the result of depletion of HIV-specific CD4+ T cells. Since HIV infects CD4+ T cells, it was initially proposed that the inability to control viral replication in most patients might be due to the deletion or depletion of HIV-specific CD4+ T cells. Preferential infection of HIV-specific cells has been observed in the peripheral blood of viremic patients (9, 16). However, it is now apparent from a number of studies that patients at all stages of HIV infection have CD4+ T cells which specifically upregulate CD69 and produce gamma interferon (IFN-γ) in response to HIV antigens (21, 24, 34, 37, 39). HIV-specific CD4+ T cells have been reported to comprise between 0.1 and 2.0% of the peripheral blood CD4+ T-cell pool. Moreover, there does not appear to be a decrease in the frequency of HIV-specific CD4+ T cells in patients interrupting antiretroviral therapy despite the loss of CD4+ T-cell proliferation to HIV antigens (24, 34, 37). This discrepancy between the frequency of HIV-specific CD4+ T cells and their proliferative responses suggests that alterations of CD4+ T-cell function, rather than frequency, may account for the loss of proliferative responses in patients off antiretroviral therapy.
A number of potential explanations have been offered for the presence of HIV-specific CD4+ T cells that lack proliferative function in most HIV-infected patients. There is now general agreement that these cells persist in untreated progressors, are able to activate, and produce IFN-γ, although they have a diminished ability to proliferate and produce interleukin-2 (IL-2) (7, 17, 21, 24, 34, 38, 51). There is also now general agreement that these functions are restored in many if not most treated patients. However, controversy persists over the interpretation of these findings. Depletion of IL-2-producing subsets, anergy, and replicative senescence have been proposed as potential mechanisms for these observations. It also remains possible that diminished proliferation and IL-2 production represents skewed maturation of HIV-specific CD4+ T cells (21, 36, 38, 51). This is based upon the paradigm whereby memory CD4+ T cells are divided into several compartments based on the expression of the CCR7 or CD62L and CD45RA and that these compartments differ in proliferative capacity and cytokine production (44). Central memory (TCM) cells are defined as CCR7+ CD45RA− and are believed to circulate to lymph node organs, produce IL-2 upon encounter with antigen, and proliferate. Effector memory (TEM) cells are CCR7− CD45RA− and are thought to circulate to peripheral sites of inflammation, produce IFN-γ upon encounter with antigen, and not proliferate. Additionally, a class of CCR7− CD45RA+ “effector” cells has recently been proposed as a group of fully differentiated CD4+ T cells (21). It has been suggested that immunologic control of HIV is disrupted through the impaired development of IL-2-producing central memory cells. However, we have previously observed that interruption of therapy rapidly abrogates in vitro proliferation and IL-2 production of HIV-specific CD4+ T cells, functions that are recovered upon resumption of therapy (24, 34). In addition, maintenance of these functions was not predictive of immunologic control of HIV upon interruption of therapy (24). These findings suggested that diminished proliferation and IL-2 production is an effect, rather than a cause, of the loss of immunologic control of HIV.
A number of important questions remain regarding these observations. First, the relationship between IL-2 production, proliferation, and surface phenotype remains incompletely defined. The majority of the prior work was performed using four-color flow cytometry that did not provide the simultaneous analysis of multiple cytokines and surface markers. In addition, it remains unclear how the HIV-specific CD4+ T-cell frequency, function, and phenotype compare to the response to other viral antigens under different conditions of antigen load. Lastly, it remains unclear whether diminished proliferation during viremia is the consequence of dominant suppressive factors or is due to the lack of positive factors, such as IL-2.
In the present study, we investigated the frequency, memory phenotype, and cytokine production of HIV-specific CD4+ T cells compared with cells specific for adenovirus, cytomegalovirus (CMV), Epstein-Barr virus (EBV), influenza virus, tetanus, and varicella-zoster virus (VZV). In addition, the mechanisms responsible for the diminished proliferation of HIV-specific cells from viremic patients were examined. We observed that HIV-infected patients on therapy have HIV-specific CD4+ T cells that are remarkably similar in frequency, memory phenotype, and cytokine production to CD4+ T cells specific for adenovirus, EBV, influenza virus, and VZV, despite the inability of these patients to control HIV replication when therapy is withdrawn. Interruption of antiretroviral therapy results in changes in frequency, memory phenotype, and cytokine production of HIV-specific cells, with a greater proportion of these cells having a CCR7− phenotype and producing IFN-γ but not IL-2. These alterations in cytokine production were intrinsic to the HIV-specific T cells. These cells did not lose proliferative capacity but rather required paracrine IL-2 for proliferation in the context of high levels of antigen. This study provides insight into the nature of the HIV-specific CD4+ T-cell response in comparison with other viral infections of humans and identifies a potential mechanism responsible for the diminished CD4+ T-cell proliferative responses in HIV-infected patients with unrestricted viral replication that may extend to responses to other viruses in the context of high levels of antigen.
MATERIALS AND METHODS
Study population.
HIV type 1 (HIV-1) infection in study participants was documented by HIV-1/2 immunoassay. All subjects signed informed consent and participated in protocols approved by a National Institute of Allergy and Infectious Diseases (NIAID) investigational review board. All eight patients in the LTNP cohort were characterized by plasma HIV viral loads of <50 copies/ml in the absence of antiretroviral therapy. Four patients undergoing treatment interruption were included in both the on-therapy and off-therapy cross-sectional groups, and statistical analyses of these patients were done separately. An additional 17 HIV-1-seronegative, healthy volunteers from the National Institutes of Health donor apheresis clinic were recruited into a control cohort.
Storage of samples.
Peripheral blood mononuclear cells (PBMC) were freshly isolated from peripheral blood or apheresis donor packs by sodium diatrizoate-Ficoll density centrifugation (ICN Biomedicals, Aurora, OH). PBMC were cryopreserved in cell culture freezing medium with dimethyl sulfoxide (Invitrogen, Grand Island, NY) using a Cryomed controlled-rate freezer (ThermoForma, Waltham, MA) and were stored at −140°C.
Intracellular cytokine assays.
Cryopreserved samples were thawed, washed twice with RPMI medium containing 10% human AB serum, and aliquoted at 4 × 106 cells per stimulation tube. Anti-CD28 and anti-CD49d antibodies (PharMingen, San Diego, CA) were added at a final concentration of 1 μg/ml. To determine the frequency of antigen-specific CD4+ T cells, one of the following antigens or peptide pools was added: adenovirus type 5 (1/100 dilution), CMV antigen (5 μg/ml), or EBV viral lysate (10 μg/ml) (Advanced Biotechnologies, Columbia, MD); VZV viral lysate (10 μg/ml) (BioWhittaker, Walkersville, MD); HIV p24 antigen (1 μg/ml) (Protein Sciences Corp., Meriden, CT); influenza H1N1-New Caledonia virus (9.5 μg/ml) or influenza H3N2-Panama virus (8 μg/ml) (Advanced Immunochemicals, Long Beach, CA); or a pool containing all overlapping peptides of each HIV gene product (Gag, Pol, Nef, and Env; 2 μg/ml of each peptide; HIVHXB2) (NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, Rockville, MD). Samples were placed in an incubator at 37°C and 5% CO2, and at 2 h of incubation, brefeldin A (Sigma Aldrich, St. Louis, MO) was added to the medium at a final concentration of 10 μg/ml to inhibit cytokine secretion. At 6 h of incubation, the cells were washed twice and stained with mouse anti-CCR7 (mouse immunoglobulin M isotype; Becton-Dickinson, San Jose, CA) for 30 min at 37°C. Cells were washed and subsequently stained with anti-CD4 phycoerythrin (PE)-Texas red (Caltag Laboratories, Burlingame, CA); anti-mouse immunoglobulin M-Alexa 488, anti-CD45RA peridinin chlorophyll protein, anti-CD14, anti-CD16, anti-CD19, and anti-CD56 Alexa 700 (Becton-Dickinson); and CD27 allophycocyanin (APC)-Cy7 (eBiosciences, San Diego, CA) for 30 min at 4°C. Fixation and permeabilization were performed as previously described (19). Following permeabilization, cells were stained with anti-CD8 PE-Alexa 700, anti-CD3 Pacific Blue, anti-IL-2 PE, anti-tumor necrosis factor alpha (anti-TNF-α) PE-Cy7, and anti-IFN-γ APC (Becton Dickinson). CD4+ T-cell responses directed against antigens were considered positive if the frequency of cytokine-producing T cells was ≥3 times the frequency with medium alone.
Proliferation assays.
Cryopreserved samples were thawed and washed twice with phosphate-buffered saline, and half of the cells were labeled for 8 min in 0.625 μM 5 (and 6)-carboxyfluorescien diacetate succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR). Labeling was quenched with an equal volume of cold, heat-inactivated human AB serum (Gemini Bio-Products, Woodland, CA) for 1 min, followed by three washes in culture medium (RPMI containing 10% human AB serum with 10 mM HEPES buffer and 50 μg/ml gentamicin). For mixing experiments, labeled PBMC were mixed with unlabeled PBMC in a 1:1 ratio and plated in 96-well deep-well U-bottom plates (Nunc, Roskilde, Denmark) at a concentration of 1 × 106 cells per well. Medium, Gag peptide pools (2 μg/ml), or p24 antigen (1 μg/ml) was added into the final volume of 1 ml. For transwell experiments, unlabeled PBMC were placed into a 24-well plate (Nunc), and CFSE labeled PBMC were placed into the top chamber of a 0.4-μm transwell insert (Costar, Corning Inc., Corning, NY). The reverse configuration, with the labeled cells in the 24-well plate and the unlabeled cells in the transwell insert, was also performed. For IL-2 depletion assays, supernatant was harvested from on-therapy PBMC cultures stimulated with HIV Gag peptide pools that were spun to remove residual cells. Anti-IL-2 antibody or an isotype control antibody (Abcam, Cambridge, United Kingdom) was added at a final concentration of 10 μg/ml. The supernatant and antibody mixes were rotated continuously at 4°C for 1 h. Protein G-agarose beads (Upstate, Lake Placid, NY) were added to the mix at a final concentration of 50 μg/ml and were rotated at 4°C for 1 h. The supernatant mixes were spun to precipitate the antibody-protein G complexes, and the purified supernatant was recovered. The depletion step was repeated a second time before the supernatant was added to either the top or bottom well of a 24-well plate with a transwell insert. IL-2-depleted and control antibody-depleted supernatants were added to the proliferation cultures at days 1, 2, 3, 4, and 5. For all proliferation assays, at day 6 cells were treated with 100 units DNase I (Invitrogen) in culture medium at 37°C for 10 min, washed twice in phosphate-buffered saline with 0.01% bovine serum albumin fraction V (Sigma Aldrich), and stained with anti-CD3 peridinin chlorophyll protein and anti-CD4 APC (Becton Dickinson).
Flow cytometry.
Data were collected with a FACSAria three-laser (488, 633, and 407 nm) cytometer (Becton Dickinson). Between 70,000 and 1,000,000 CD3+ CD4+ events were collected and analyzed using FlowJo software (TreeStar, San Carlos, CA).
Statistical analysis.
Because the data were not normally distributed, independent groups were compared by the Wilcoxon two-sample test. The Wilcoxon signed rank test was used to compare paired data. The Bonferroni method was used to adjust P values for multiple testing. Medians are reported.
RESULTS
Frequency, memory phenotype, and cytokine production by HIV-specific CD4+ T cells are similar to responses to other viral infections in humans.
The consequences of HIV infection and active viral replication for the frequency, memory phenotype, and cytokine production profiles of CD4+ T cells specific for adenovirus type 5, CMV, EBV, influenza H1N1 and H3N2 viruses, VZV, tetanus toxoid, HIV p24 antigen, and HIV Gag, Nef, Pol, and Env peptide pools were examined through a 10-color flow cytometry panel. This panel (containing the gating markers CD3, CD4, CD8, CD14, CD16, CD19, and CD56; the cytokines IFN-γ, IL-2, and TNF-α; and the memory markers CD45RA, CCR7, and CD27) was used to identify antigen-specific (cytokine-producing) CD4+ T cells in normal controls (n = 17), HIV-infected patients with active viral replication off therapy (n = 12), HIV-infected patients with <50 copies of HIV RNA/ml plasma on antiretroviral therapy (n = 11), and HIV-infected patients with <50 copies of HIV RNA/ml plasma off antiretroviral therapy (patients known as LTNP) (n = 8) (Tables 1 and 2). The total antigen-specific response comprises IFN-γ-, IL-2-, or TNF-α-producing cells unless otherwise designated. Total antigen-specific responses to adenovirus, influenza H1N1 or H3N2 virus, and VZV of HIV-infected patients on therapy were nearly twofold greater than those of uninfected controls, LTNP, and HIV-infected patients off therapy. However, in absolute terms this difference was only 0.1% of CD4+ T cells. Responses to adenovirus, CMV, EBV, influenza H1N1 and H3N2 virus, VZV, and tetanus antigens were detectable in most patients, and responses against HIV peptide pools and antigens were found in all HIV-infected patients (Fig. 1A). In all patients, the CD4+ T-cell responses to adenovirus, EBV, influenza virus, and VZV were characterized by predominantly IL-2+ IFN-γ+ TNF-α+ and IL-2− IFN-γ+ TNF-α+ cells that were of a TCM (CD45RA− CCR7+) or TEM (CD45RA− CCR7−) phenotype, and the majority were CD27+(sample data are in Fig. 1A and summary data are in Fig. 2). In contrast, CD4+ T-cell responses to CMV antigen were outliers with regard to frequency and surface phenotype. Although total responses specific for most antigens or peptide pools were in the range of 0.025 to 0.2% of CD4+ T cells, the response to CMV antigen was 10- to 20-fold greater. Because the CMV genome is approximately 20-fold larger than that of HIV-1, the responses to a pp65 peptide pool were also examined. The magnitude of this total response was closer to the response to Gag, especially when corrected for the number of amino acids in the coding sequence (median of 0.52% antigen-specific CD4+ T cells/1,000 amino acids of pp65 versus median of 0.26% antigen-specific CD4+ T cells/1,000 amino acids in HIV-infected LTNP and progressors on therapy). The CMV antigen- and pp65-specific responses consisted predominantly of antigen-specific cells with an increased proportion of CCR7− cells and an increased proportion of CD27− cells. Responses to tetanus were also outliers with regard to cytokine secretion but not surface phenotype. Tetanus-specific cells were mostly IL-2+ IFN-γ− TNF-α− (Fig. 1A and 2B). These responses were reminiscent of poorly differentiated vaccine-induced CD4+ T cells previously described (15). In HIV-infected patients, including LTNP and patients with progressive disease either on or off therapy, CD4+ T-cell responses to non-HIV antigens were of comparable magnitude to those of HIV-uninfected controls and were similar in memory phenotype and cytokine production.
TABLE 1.
Patient characteristics: cross-sectional cohort
| Group and patient no. | Peripheral blood CD4+ T-cell count (cells/μl)a | Plasma HIV RNA (copy equivalents/ml)a | Therapyb |
|---|---|---|---|
| LTNP | |||
| 49 | 1,301 | <50 | |
| 53 | 825 | <50 | |
| 38 | 1,588 | <50 | |
| 34 | 1,030 | <50 | |
| 17 | 950 | <50 | |
| 4 | 1,333 | <50 | |
| 33 | 1,048 | <50 | |
| 37 | 958 | <50 | |
| On therapy | |||
| 129 | 482 | <50 | ZDV, 3TC, NEL |
| 113 | 574 | <50 | ZDV, 3TC, NEL |
| 130 | 387 | <50 | ZDV, 3TC, ABC |
| 114 | 249 | <50 | TDF, EFV, RTV, APV |
| 242 | 784 | <50 | 3TC, d4T, IND |
| 208 | 889 | <50 | ZDV, 3TC, IND, RTV |
| 128 | 1,125 | <50 | ZDV, 3TC, IND |
| Off therapy | |||
| 44 | 893 | 20,940 | |
| 15 | 738 | 540 | |
| 21 | 15 | 55,862 | |
| 147 | 811 | 27,855 | |
| 137 | 321 | 29,215 | |
| 146 | 784 | 213,759 | |
| 148 | 403 | 74,940 | |
| 108 | 249 | 61,209 |
Values shown were obtained at the time of apheresis.
ZDV, zidovudine; d4T, stavudine; NEL, nelfinavir; 3TC, lamivudine; IND, indinavir; RTV, ritonavir; EFV, efavirenz; TDF, tenofovir; ABC, abacavir; APV, amprenavir.
TABLE 2.
Patient characteristics: longitudinal cohort
| Patient no. | Time on therapy (days) | Peripheral blood CD4+ T cell count (cells/μl)a
|
Time off therapy (days) | Plasma HIV RNA (copy equivalents/ml)a
|
Therapyb | ||
|---|---|---|---|---|---|---|---|
| On therapy | Off therapy | On therapy | Off therapy | ||||
| 229 | >365 | 1,230 | 791 | 252 | <50 | 2,185 | d4T, 3TC, NEL |
| 230 | >365 | 822 | 469 | 97 | <50 | 7,515 | ZDV, 3TC, IND |
| 232 | >365 | 997 | 576 | 1,048 | <50 | 35,114 | ZDV, 3TC, NEL |
| 236 | >365 | 928 | 684 | 247 | <50 | 23,637 | d4T, ddI, NVP |
| 237 | >365 | 1,433 | 1,077 | 56 | <50 | 139,874 | ZDV, 3TC, EFV |
| 238 | >365 | 690 | 928 | 55 | 65 | 613 | ZDV, 3TC, IND |
| 239 | >365 | 759 | 579 | 63 | <50 | 136,192 | TDF, 3TC, EFV |
| 240 | >365 | 745 | 362 | 55 | <50 | 16,638 | ZDV, 3TC, ABC, TDF, LPVr |
| 241 | >365 | 720 | 609 | 56 | <50 | 9,738 | 3TC, FTC, TDF, EFV |
Values shown were obtained at the time of apheresis.
ZDV, zidovudine; d4T, stavudine; NEL, nelfinavir; 3TC, lamivudine; IND, indinavir; LPVr, lopinavir/ritonavir; NVP, nevirapine; EFV, efavirenz; ddI, didanosine; FTC, emtricitabine; TDF, tenofovir; ABC, abacavir.
FIG. 1.
Sample data from 10-color flow cytometry of CD4+ T cells specific for a panel of antigens. A. Typical responses to the indicated antigens with regard to IL-2 and IFN-γ production. In the second two rows, total IFN-γ-, TNF-α-, and IL-2-producing cells are shown (orange) on the background of all other CD4+ T cells (gray). B. Sample flow cytometry of cytokine production and surface phenotype during an interruption of therapy.
FIG. 2.
CD4+ T-cell responses to antigens in uninfected controls, LTNP, and HIV-infected patients with progressive disease who are on or off therapy. A. Frequency and subsets of CD4+ T cells producing the cytokine IFN-γ, IL-2, or TNF-α in response to viral antigens or tetanus toxoid. B. Frequency and subsets of CD4+ T cells expressing the phenotypic marker CD27, CCR7, or CD45RA. All frequencies have been adjusted for background by subtracting the corresponding subset of the medium control. All frequencies displayed represent median responses.
All patients with HIV infection demonstrated CD4+ T-cell responses to one or more of the HIV antigens tested, including overlapping peptides spanning the Env, Gag, Nef, and Pol proteins and p24 antigen. Gag peptide pools and the p24 antigen elicited the largest CD4+ T-cell responses, followed by Pol, Nef, and Env. In LTNP and in patients on therapy with control of viral replication, CD4+ T-cell responses to HIV antigens were similar in frequency and phenotype to responses against adenovirus, EBV, influenza virus, and VZV in HIV-uninfected controls. In contrast, in HIV-infected patients off therapy with active viral replication, HIV-specific CD4+ T cells undergo a significant increase in the peripheral blood with a concomitant decrease in responses to non-HIV antigens (Fig. 1B and 2A and B). In the context of viremia there was also a dramatic decrease in the proportion of HIV-specific CD4+ T cells producing IL-2, consistent with prior observations. In addition, there was an equally dramatic shift toward CCR7− cells within the HIV-specific CD4+ T-cell pool. These changes were significant both for the cross-sectional comparison of on-therapy and off-therapy patients (IL-2 producing Gag-specific cells, 78.3% on therapy and 25.4% off therapy [P < 0.001]; TEM cells, 54.4% on therapy and 78.1% off therapy [P = 0.05]) and for patients followed longitudinally during treatment interruption (IL-2+ cells, 60.3% versus 19.1% [P = 0.004]; TEM cells, 52.8% versus 80.8% [P = 0.002]). Finally, in patients undergoing treatment interruption, the frequency of CD4+ T cells responding to HIV antigens increased in most patients off therapy, while responses to non-HIV antigens declined. The reasons for this decline in non-HIV-specific responses remain unclear but may reflect impairment of T-cell responses secondary to nonspecific activation during viremia.
CD4+ T cells capable of producing IL-2, IFN-γ, and TNF-α are heterogeneous in their surface expression of CCR7 and CD45RA.
The data from the analysis of CD4+ T-cell responses to viral antigens also provided the opportunity to simultaneously analyze memory phenotype and cytokine production. Several models have been proposed to divide CD4+ T cells into various subsets based on the expression of surface markers. One common model uses the expression of CCR7 or CD62L and CD45RA to divide CD4+ T cells into the following subsets: naïve cells (CCR7+ CD45RA+), TCM cells (CCR7+ CD45RA−), TEM cells (CCR7− CD45RA−), and effector cells (CCR7− CD45RA+). TCM cells are thought to produce IL-2 but not the effector cytokine IFN-γ, while TEM cells are thought to have the reciprocal distribution.
However, this relationship between surface phenotype and cytokine production was not reliably the case. IL-2-producing CD4+ T cells were found with a TCM phenotype (tetanus toxoid and HIV Pol in some patients on therapy), a predominantly TEM phenotype (CMV), or a mixed TCM and TEM phenotype (adenovirus, EBV, influenza virus, VZV, and HIV Gag in patients on therapy) (Fig. 3). As a group, the ability of these subsets to produce IL-2 or IFN-γ was similar between antigens. Although some decrease in the mean fluorescence intensity of CD27 was noted in CCR7− memory cells compared to CCR7+ memory cells, CD27 also did not reliably define specific cytokine-producing populations(data not shown). However, individual patients had a greater tendency for responses to be skewed toward CCR7+ RA− or CCR7− RA− cells. Surprisingly, when analyzed as a group the majority of the cells producing IL-2 were found within a putative effector memory phenotype. Together, these data indicate that the cytokine production of memory CD4+ T cells is not reliably predicted on the basis of surface phenotype and is considerably more heterogeneous and patient specific than previously believed.
FIG. 3.
Cytokine production by antigen-specific CD4+ T cells does not segregate with surface phenotype. A. Cytokine production in response to CMV antigen, HIV Gag peptides, and HIV Pol peptides from one representative patient on antiretroviral therapy. B. Surface phenotype expressed as a total of cells producing either IFN-γ or IL-2 in response to the indicated antigen. Individual patient responses are shown.
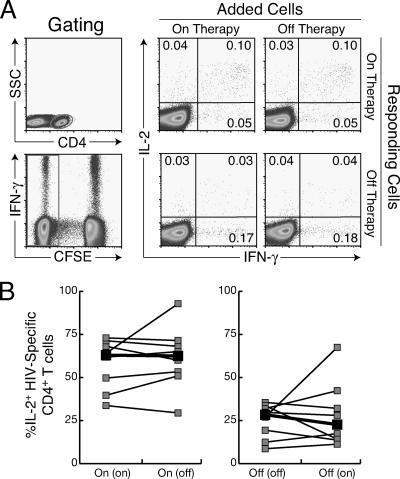
Coculture of PBMC from on-therapy and off-therapy time points rescues the diminished proliferation of HIV-specific CD4+ T cells.
The loss of proliferative responses to HIV antigens in patients off antiretroviral therapy is one of the principal changes in HIV-specific CD4+ T-cell function (24, 34, 35, 37, 42, 43). Patients on therapy with preexisting CD4+ T-cell proliferative responses rapidly lose these responses upon viral rebound during treatment interruption and regain proliferative responses once viral replication is controlled by restarting therapy (24, 34). Although these studies have provided considerable insight into the modulation of proliferative responses by HIV viremia, the mechanisms responsible for diminished proliferation to HIV antigens remain incompletely understood.
Diminished proliferation to HIV antigens in patients off therapy may be due to the absence of positive factors favoring proliferation (such as IL-2, costimulatory molecules, or proinflammatory cytokines) or due to the presence of negative factors inhibiting proliferation (for example, IFN-α, IL-10, or regulatory T cells). To systematically address these possibilities, mixing experiments were performed with on-therapy and off-therapy cells from patients undergoing treatment interruption. Cells from one time point were labeled with CFSE and cultured with unlabeled cells from the other time point for 6 days in the presence of medium or HIV antigens. A gating strategy was used to remove cells that were initially unlabeled from the analysis. Coculture of on-therapy and off-therapy PBMC resulted in a significant increase in the percentage of off-therapy CD4+ T cells that proliferated in response to p24 antigen (median of 3.79% CFSEdim CD4+ T cells when cultured alone versus 17.77% when cultured with on-therapy cells; P = 0.042) and to Gag pooled peptides (0.93% versus 5.14%; P = 0.038) (Fig. 4). This result suggested that in cultures of cells from the off-therapy time point there was an absence of factors that enhance proliferation, which were then supplied by cells from the on-therapy time point. Mixing of on-therapy and off-therapy cells did not diminish the proliferation of the on-therapy cells (P > 0.26 for both p24 and Gag antigens), suggesting that there were not inhibitory factors that were active in cultures of cells from viremic patients. Taken together, these results are consistent with an absence of a positive factor being responsible for the diminished proliferative responses in patients who were off antiretroviral therapy.
FIG. 4.
Proliferation of off-therapy CD4+ T cells to Gag peptides is rescued by coculture with autologous, on-therapy PBMC. A. Proliferation of on-therapy (left panel) and off-therapy (right panel) cells to medium and to HIV p24 antigen. CFSE-labeled cells were cultured with unlabeled cells from the same or opposite time points. A gating strategy was utilized to subtract the unlabeled cells from the culture after 6 days. B. Summary of data on proliferative responses to HIV p24 antigen and Gag peptide pools of off-therapy cells cultured with unlabeled off-therapy or on-therapy PBMC. The median response is shown in boldface.
Coculture of PBMC from on-therapy and off-therapy time points does not alter the pattern of cytokine production by HIV-specific CD4+ T cells.
We and others had previously shown that HIV viremia was associated with diminished IL-2 production by HIV-specific CD4+ T cells. In longitudinal cohorts, IL-2 production by HIV-specific CD4+ T cells was rapidly abrogated by viremia induced by an interruption of therapy. In the coculture experiments shown in Fig. 4, mixing of on- and off-therapy cells resulted in rescued proliferation of HIV-specific cells from the off-therapy time point. However, it was unclear whether mixing was changing IL-2 production by cells from the off-therapy time point or was acting in trans through other mechanisms.
In order to distinguish between these possibilities, coculture experiments were performed on cells from patients undergoing treatment interruptions. PBMC from either the on-therapy or off-therapy time point were labeled with CFSE and were cultured alone or mixed with PBMC from the opposite time point. HIV-specific CD4+ T cells from patients on therapy had similar frequencies and cytokine profiles regardless of whether they were cultured alone or mixed with off-therapy cells, and conversely, off-therapy CD4+ T cells did not have changes in frequency or cytokine production when mixed with on-therapy cells (Fig. 5). The distributions of IL-2- and IFN-γ-producing HIV-specific CD4+ T cells were significantly different between the on- and off-therapy time points (IL-2+ IFN-γ− cells, median of 18.1% on therapy versus 8.1% off therapy [P = 0.09]; IL-2+ IFN-γ+ cells, 29.1% versus 14.1% [P = 0.003]; IL-2− IFN-γ+ cells, 37.1% versus 71.6% [P < 0.001]), consistent with our prior observations, but mixing with cells from the opposite time point did not significantly change these cytokine profiles (P > 0.5). These data suggest that although mixing with cells from the on-therapy time point rescues proliferation of HIV-specific cells from the off-therapy time point, the effect is not due to rescue of IL-2 production that might act in an autocrine fashion.
FIG. 5.
Alterations in Gag-specific CD4+ T-cell cytokine production that occur during interruption of antiretroviral therapy are intrinsic to the T cells and are not rescued by culture with cells from the on-therapy time point. PBMC from on-therapy and off-therapy time points were cultured alone or mixed with autologous cells from the opposite time point. CFSE labeling of cells from one of the time points allowed the two populations of cells to be distinguished. Mixing cells from on- and off-therapy time points did not alter the frequency or proportions of cytokine-producing cells. B. Summary of data for eight patients. The median response is shown in boldface.
Autologous, on-therapy PBMC rescue proliferation of off-therapy HIV-specific CD4+ T cells via the production of IL-2.
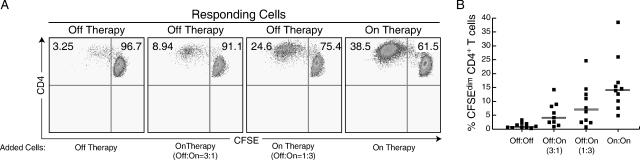
The mixing experiments demonstrated that off-therapy HIV-specific CD4+ T cells were able to proliferate in the presence of on-therapy PBMC, but whether this rescue effect was mediated via a cell contact-dependent mechanism or a soluble factor was unknown. In order to test the necessity of cell contact, proliferation experiments were performed in which the on-therapy and off-therapy cells were separated by a 0.4-μm semipermeable membrane. Proliferation of off-therapy cells to Gag pooled peptides increased from a median of 1.02% CFSEdim cells when cultured alone to 7.0% when cultured at a 3:1 ratio with on-therapy PBMC (3 off-therapy cells:1 on-therapy cell; P = 0.058) and to 11.0% when cultured at a 1:3 ratio with on-therapy PBMC (P = 0.033) (Fig. 6). These data suggested that proliferation of off-therapy CD4+ T cells to HIV antigens was rescued by a soluble factor.
FIG. 6.
Proliferation of off-therapy PBMC to HIV Gag peptides is rescued by a soluble factor produced by on-therapy cells. A. Proliferation of combinations of off-therapy and on-therapy PBMC separated by a 0.4-μm transwell insert to HIV Gag peptides. The upper chamber contained 250,000 cells and the lower chamber 750,000 cells. CD4+ T cells displayed in plots are from the time point indicated by the arrows. Proliferation was similar in upper and lower chambers when PBMC from the same time point were utilized. B. Summary of data on proliferative responses to HIV Gag peptides from eight patients undergoing treatment interruption.
Given the alterations in the cytokine secretion profile of HIV-specific CD4+ T cells in patients undergoing treatment interruption as well as our previous findings that exogenous IL-2 could induce proliferation of off-therapy CD4+ T cells, it was likely that IL-2 produced by on-therapy PBMC in response to HIV antigens might be the factor augmenting the proliferation of the off-therapy cells. In preliminary experiments, the enhancement in proliferation shown in the semipermeable-membrane experiments of Fig. 6 was recapitulated by incubating off-therapy PBMC with cell-free conditioned supernatant from on-therapy PBMC exposed to HIV antigens (data not shown). Addition of IL-2-neutralizing antibodies or CD25-blocking antibodies caused unacceptably high rates of cell death when added directly to 6-day cultures. Therefore, use of these methods would not permit an accurate determination of whether the rescue effect was attributable to the effects of IL-2. To avoid these potential problems, cell-free, conditioned supernatants from on-therapy cells were incubated in the presence of anti-IL-2 antibodies or control antibodies that were subsequently removed from the solution with protein G-agarose beads. This method was found to result in removal of >99% of the IL-2 as determined by proliferation of the IL-2-dependent CTLL-2 cell line (Fig. 7A). Adding back small amounts of exogenous IL-2 to these treated supernatants restored proliferation of the CTLL-2 cells (Fig. 7B), indicating that excess anti-IL-2 antibody was not still present in the treated supernatants. The results of IL-2 depletion experiments are shown in Fig. 7C to E. Proliferation of off-therapy CD4+ T cells to HIV antigens increased from a median of 0.8% CFSEdim cells when cultured alone to 2.96% when cultured in a 3:1 ratio (by volume) with control antibody-treated conditioned supernatant (P = 0.17) and to 3.84% when cultured in a 1:3 ratio with control antibody-treated conditioned supernatant (P = 0.02) (Fig. 7C to E). Removal of IL-2 from supernatants conditioned by on-therapy PBMC abrogated proliferation of the viremic cells from 2.96% to 0.27% (P = 0.12) at the 3:1 ratio and from 3.84% to 0.19% (P = 0.01) at the 1:3 ratio. The diminished levels of proliferation in the IL-2-depleted cultures compared with the viremic cells alone is likely due to the replacement of 25% and 75% of the medium with IL-2-depleted medium at daily intervals, diluting cytokines already present in the medium. These findings strongly suggest that IL-2 produced by on-therapy PBMC is the factor responsible for restoring proliferative responses of off-therapy cells. Together, these data suggest that in the context of viremia HIV-specific CD4+ T cells retain the ability to proliferate but simply develop the need for exogenous (paracrine) IL-2.
FIG. 7.
Conditioned supernatant from on-therapy PBMC rescues the proliferation of off-therapy PBMC, and depletion of IL-2 from the supernatant abrogates this effect. A. Proliferation of IL-2-dependent CTLL-2 cells to medium containing known concentrations of IL-2. Medium was treated with control antibody or anti-IL-2 antibody, followed by depletion of antigen-antibody complexes with protein G-agarose beads. B. Proliferation of CTLL-2 cells to untreated medium and IL-2-depleted medium that have been spiked with known amounts of IL-2. C. CD4+ T-cell proliferation to an HIV Gag peptide pool from a representative patient undergoing treatment interruption. D. Proliferation of off-therapy cells to HIV Gag peptides in the presence of conditioned supernatant by on-therapy PBMC exposed to HIV Gag peptides. Conditioned supernatants were treated with control antibody or anti-IL-2 antibody as described in the text. Low concentration, 750,000 off-therapy cells with 250 μl conditioned supernatant; high concentration, 250,000 off-therapy cells with 750 μl conditioned supernatant. E. Summary of data for off-therapy CD4+ T-cell proliferation to Gag peptides in patients undergoing treatment interruption. Off-therapy cells were cultured alone or in the presence of low or high concentrations of conditioned supernatant treated with a control antibody or anti-IL-2 antibody.
DISCUSSION
The results of the present study provide data that permit the interpretation of a number of observations of the CD4+ T-cell response to HIV in the context of responses to other viruses. The flow cytometric panel utilized in this study permitted comparisons of the frequency, phenotype, and cytokine production within subpopulations of CD4+ T cells directed against HIV with responses directed against other viral antigens. Further, the ability to alter the level of virus replication through changes in antiretroviral therapy permitted an examination of the effects of viral replication on these parameters. A number of defects within the HIV-specific memory CD4+ T-cell compartment have been proposed to explain the lack of immunologic restriction of HIV replication. Diminished HIV-specific CD4+ T-cell frequencies, skewing of CD4+ T-cell maturation, depletion of IL-2-producing or proliferating cells during HIV viremia, and replicative senescence have all been suggested as potential mechanisms. However, the results of the present study suggest that diminished IL-2 production and CCR7 expression are a consequence of the level of viremia and not a cause of the loss of restriction of viral replication. HIV-specific cells capable of proliferation were not depleted but rather developed a requirement for paracrine IL-2 in the context of viremia. More importantly, the CD4+ T-cell responses to HIV antigens in patients who are on antiretroviral therapy are remarkably similar in magnitude, memory phenotype and cytokine production to responses directed against viral infections that are well controlled in humans: adenovirus, EBV, influenza virus, and VZV. We did not detect differences in cytokine secretion patterns characteristic of cleared antigens that have been recently described (22). Rather, adenovirus-specific and influenza virus-specific responses were similar to responses to EBV, CMV, and HIV, which induce chronic viral infections. Furthermore, these results also suggest that HIV-specific CD4+ T cells from patients on therapy are indistinguishable in frequency, phenotype, cytokine production, and proliferative capacity from CD4+ T cells from long-term nonprogressors. Taken together, these observations indicate that we were unable to detect abnormalities within the HIV-specific CD4+ T-cell compartment in treated patients during the chronic phase of infection with regard to frequency, cytokine production, or surface phenotype.
A number of prior studies have proposed that the HIV-specific CD4+ T-cell compartment may contain inappropriately low frequencies of these cells. Diminished frequencies of these cells would presumably occur through preferential infection (9, 16). Many of these prior comparisons from our laboratory and others were made between HIV antigens or peptide pools and CMV antigen. In the present study, consistent with prior results, the total HIV-specific response was approximately one-half or less of the response to CMV antigen. However, this is not necessarily surprising given that the genome of human CMV is more than 20-fold larger than that of HIV. This comparison is further complicated by the fact that responses to peptide pools that do not require cleavage and processing prior to presentation commonly induce higher responses. However, we observed similar frequencies of antigen-specific CD4+ T cells in response to Gag or CMV pp65 peptide pools, which are roughly similar in size. The extraordinarily high numbers of CMV-specific cells were likely not due to the biology of the virus but rather were largely attributable to the coding sequence size. Based upon these observations in the context of controlled viral replication, the frequency of HIV-specific CD4+ T cells does not appear to be inappropriately low.
In the present study we were also unable to detect a defect in cytokine secretion or maturation based upon surface phenotype of HIV-specific CD4+ T cells during the chronic phase of infection. Consistent with our prior results and those of others, a decrease in the frequency of HIV-specific CD4+ T cells producing IL-2 was observed during an interruption of therapy. In the present study, this was also associated with a decrease in CCR7 expression. Decreases in CCR7 expression and IL-2 production have been proposed to represent an impairment of formation of central memory cells during acute infection (51). However, in the context of antiretroviral therapy cells expressing CCR7 or producing IL-2 can be found at frequencies similar to those specific for other viruses. It is difficult to conclusively demonstrate in humans that this does not represent the regeneration of new central memory cells. However, this possibility seems unlikely in the context of the reduction of antigen that would occur during antiretroviral therapy. The more likely possibility remains that these two parameters are rapidly modulated by the level of viremia.
The results of the present study also extend some previous observations regarding diminished proliferation of HIV-specific CD4+ T cells in the context of viremia. We had previously observed that HIV viremia caused a decrease in IL-2 production and proliferation of HIV-specific CD4+ T cells. Proliferation of HIV-specific CD4+ T cells could be induced by addition of IL-2, suggesting that these cells retained proliferative capacity. However, it remained unclear whether the decrease in proliferation was a consequence of a decrease in factors that might induce proliferation, such as IL-2, or dominant negative factors, such as antiproliferative cytokines or regulatory cells. The data from mixing studies indicate that decreased IL-2 production in patients with active viral replication is due to changes intrinsic to the HIV-specific CD4+ T cells and that diminished proliferation is caused by a decrease in a factor(s) that promotes proliferation. Data from the supernatant transfer studies indicate that diminished IL-2 production by CD4+ T cells from patients off antiretroviral therapy is the factor responsible for the vast majority of the decrease in proliferative responses in vitro. Although there are conflicting data regarding whether exogenous IL-2 may overcome PD-1-mediated suppression of proliferation of T cells (13, 14), these results suggest that the absence of paracrine IL-2 is the primary effect. It should also be noted that it remains unclear whether there is a need for additional IL-2 in vivo during HIV viremia. We have previously observed that patients treated with IL-2 do not have augmented HIV-specific CD4+ T-cell responses (27). These observations may extend beyond the HIV-specific response described here. It is likely that this mechanism is responsible for the decrease in in vitro proliferation of antigen-specific CD4+ T cells observed in the context of high levels of antigen in humans or experimental animals (4, 8, 12, 18, 20, 32, 40, 41, 50). Although not yet well described, it is possible that diminished CCR7 expression and IL-2 production parallels the drop in in vitro proliferation in these situations where the immune response is ultimately successful in controlling or eliminating antigen. Should this be the case, it would add further evidence to the suggestion from data in the present study that these changes do not represent a defect in the HIV-specific immune response but may be a physiologic response to high levels of antigen.
These results also may change the interpretation of prior observations regarding the relationship between CCR7 expression and cytokine production or proliferation. We were unable to confirm associations previously made between surface CCR7 expression and effector cytokine production (44). Differences in CCR7 expression of antigen-specific populations did not appear to be related to the duration of HIV infection, given that this heterogeneity was observed even within these relatively homogeneous cohorts of LTNP and progressors. Recently, a model has been proposed that divides memory CD4+ T cells into two classes: CCR7+ CD45RA− central memory (TCM) cells and CCR7− CD45RA− effector memory (TEM) cells (44). According to this model, TCM cells home to secondary lymphoid organs, produce IL-2 but not IFN-γ, and proliferate in response to an encounter with antigen. In contrast, TEM cells migrate to peripheral tissues, produce IFN-γ but not IL-2, and are not thought to proliferate upon antigen stimulation. This model has been applied to HIV-specific CD4+ T cells in the peripheral blood (17, 22, 23, 36, 38, 51). The use of the 10-color, 12-paramater flow cytometry panel in this study allowed for detailed examination of this model by simultaneously providing information on memory markers and cytokine production in CD4+ T cells. However, we did not observe a clear delineation of effector cytokine production based upon CCR7 expression, consistent with one recent paper (48). In addition, highly activated CCR7− cells retained the ability to proliferate in vitro as long as paracrine IL-2 was provided. Because prior studies used tetanus and CMV proteins as model antigens and, more importantly, sorted cells without marking and recombining them to assess proliferation, diminished proliferation of CCR7− cells was observed. However, the results of the present study suggest that these observations may be, at least in part, due to the choice of antigen, patient, and, most importantly, sorting and plating of activated cells in the absence of paracrine IL-2.
The precise role of the HIV-specific CD4+ T-cell response in the loss of immunologic control of the virus remains unclear. HIV-specific CD4+ T cells occupy a central position in HIV pathogenesis, as they are both critical elements of the immune response as well as preferred targets for the virus. We have previously found that this response in treated patients does not differ from that in LTNP and in this study found the response to a large panel of viral antigens to be similar. However, despite these similarities, there is now substantial evidence that the vast majority of patients with good control of viral replication on therapy in acute or chronic infection are not able to contain HIV replication once therapy is discontinued, even if a CD4+ T-cell proliferative response against HIV antigens is present prior to treatment interruption (24, 25, 28). Although we did not detect changes that might suggest a defect in HIV-specific CD4+ T cells during the chronic phase of infection, it remains possible that depletion of these cells during acute infection or in end-stage disease may ultimately play a role in the loss of control of HIV replication. The results of the present study help to further clarify the frequency, phenotype, and function of HIV-specific CD4+ T cells relative to those specific for other antigens and may provide a goal with regard to these parameters for vaccines or immunotherapeutic strategies aimed at boosting CD4+ T-cell responses.
Footnotes
Published ahead of print on 20 December 2006.
REFERENCES
- 1.Aihara, H., T. Takasaki, T. Matsutani, R. Suzuki, and I. Kurane. 1998. Establishment and characterization of Japanese encephalitis virus-specific, human CD4+ T-cell clones: flavivirus cross-reactivity, protein recognition, and cytotoxic activity. J. Virol. 72:8032-8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Harthi, L., J. Siegel, J. Spritzler, J. Pottage, M. Agnoli, and A. Landay. 2000. Maximum suppression of HIV replication leads to the restoration of HIV-specific responses in early HIV disease. AIDS 14:761-770. [DOI] [PubMed] [Google Scholar]
- 3.Arvin, A. M. 1992. Cell-mediated immunity to varicella-zoster virus. J. Infect. Dis. 166(Suppl. 1):S35-S41. [DOI] [PubMed] [Google Scholar]
- 4.Benigni, F., V. S. Zimmermann, S. Hugues, S. Caserta, V. Basso, L. Rivino, E. Ingulli, L. Malherbe, N. Glaichenhaus, and A. Mondino. 2005. Phenotype and homing of CD4 tumor-specific T cells is modulated by tumor bulk. J. Immunol. 175:739-748. [DOI] [PubMed] [Google Scholar]
- 5.Binley, J. M., D. S. Schiller, G. M. Ortiz, A. Hurley, D. F. Nixon, M. M. Markowitz, and J. P. Moore. 2000. The relationship between T cell proliferative responses and plasma viremia during treatment of human immunodeficiency virus type 1 infection with combination antiretroviral therapy. J. Infect. Dis. 181:1249-1263. [DOI] [PubMed] [Google Scholar]
- 6.Blankson, J. N., J. E. Gallant, and R. F. Siliciano. 2001. Proliferative responses to human immunodeficiency virus type 1 (HIV-1) antigens in HIV-1-infected patients with immune reconstitution. J. Infect. Dis. 183:657-661. [DOI] [PubMed] [Google Scholar]
- 7.Boaz, M. J., A. Waters, S. Murad, P. J. Easterbrook, and A. Vyakarnam. 2002. Presence of HIV-1 Gag-specific IFN-gamma+IL-2+ and CD28+IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. J. Immunol. 169:6376-6385. [DOI] [PubMed] [Google Scholar]
- 8.Boni, C., A. Bertoletti, A. Penna, A. Cavalli, M. Pilli, S. Urbani, P. Scognamiglio, R. Boehme, R. Panebianco, F. Fiaccadori, and C. Ferrari. 1998. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J. Clin. Investig. 102:968-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenchley, J. M., L. E. Ruff, J. P. Casazza, R. A. Koup, D. A. Price, and D. C. Douek. 2006. Preferential infection shortens the life span of human immunodeficiency virus-specific CD4+ T cells in vivo. J. Virol. 80:6801-6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callan, M. F. 2004. The immune response to Epstein-Barr virus. Microbes Infect. 6:937-945. [DOI] [PubMed] [Google Scholar]
- 11.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carney, W. P., and M. S. Hirsch. 1981. Mechanisms of immunosuppression in cytomegalovirus mononucleosis. II. Virus-monocyte interactions. J. Infect. Dis. 144:47-54. [DOI] [PubMed] [Google Scholar]
- 13.Carter, L., L. A. Fouser, J. Jussif, L. Fitz, B. Deng, C. R. Wood, M. Collins, T. Honjo, G. J. Freeman, and B. M. Carreno. 2002. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur. J. Immunol. 32:634-643. [DOI] [PubMed] [Google Scholar]
- 14.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350-354. [DOI] [PubMed] [Google Scholar]
- 15.Divekar, A. A., D. M. Zaiss, F. E. Lee, D. Liu, D. J. Topham, A. J. Sijts, and T. R. Mosmann. 2006. Protein vaccines induce uncommitted IL-2-secreting human and mouse CD4 T cells, whereas infections induce more IFN-gamma-secreting cells. J. Immunol. 176:1465-1473. [DOI] [PubMed] [Google Scholar]
- 16.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95-98. [DOI] [PubMed] [Google Scholar]
- 17.Emu, B., E. Sinclair, D. Favre, W. J. Moretto, P. Hsue, R. Hoh, J. N. Martin, D. F. Nixon, J. M. McCune, and S. G. Deeks. 2005. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J. Virol. 79:14169-14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller, M. J., and A. J. Zajac. 2003. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170:477-486. [DOI] [PubMed] [Google Scholar]
- 19.Gea-Banacloche, J. C., S. A. Migueles, L. Martino, W. L. Shupert, A. C. McNeil, L. Ehler, C. Prussin, R. Stevens, L. L., J. Altman, J. C. Lopez, and M. Connors. 2000. Maintenance of large numbers of virus specific CD8+ T cells in HIV infected progressors and long term nonprogressors. J. Immunol. 165:1082-1092. [DOI] [PubMed] [Google Scholar]
- 20.Gerlach, J. T., H. M. Diepolder, M. C. Jung, N. H. Gruener, W. W. Schraut, R. Zachoval, R. Hoffmann, C. A. Schirren, T. Santantonio, and G. R. Pape. 1999. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology 117:933-941. [DOI] [PubMed] [Google Scholar]
- 21.Harari, A., S. Petitpierre, F. Vallelian, and G. Pantaleo. 2004. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 103:966-972. [DOI] [PubMed] [Google Scholar]
- 22.Harari, A., F. Vallelian, P. R. Meylan, and G. Pantaleo. 2005. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J. Immunol. 174:1037-1045. [DOI] [PubMed] [Google Scholar]
- 23.Harari, A., F. Vallelian, and G. Pantaleo. 2004. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur. J. Immunol. 34:3525-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyasere, C., J. C. Tilton, A. J. Johnson, S. Younes, B. Yassine-Diab, R. P. Sekaly, W. W. Kwok, S. A. Migueles, A. C. Laborico, W. L. Shupert, C. W. Hallahan, R. T. Davey, Jr., M. Dybul, S. Vogel, J. Metcalf, and M. Connors. 2003. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J. Virol. 77:10900-10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen, C. A., I. M. De Cuyper, R. Steingrover, S. Jurriaans, S. U. Sankatsing, J. M. Prins, J. M. Lange, D. van Baarle, and F. Miedema. 2005. Analysis of the effect of highly active antiretroviral therapy during acute HIV-1 infection on HIV-specific CD4 T cell functions. AIDS 19:1145-1154. [DOI] [PubMed] [Google Scholar]
- 26.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852-856. [DOI] [PubMed] [Google Scholar]
- 27.Jelley-Gibbs, D. M., N. M. Lepak, M. Yen, and S. L. Swain. 2000. Two distinct stages in the transition from naive CD4 T cells to effectors, early antigen-dependent and late cytokine-driven expansion and differentiation. J. Immunol. 165:5017-5026. [DOI] [PubMed] [Google Scholar]
- 28.Kaufmann, D. E., M. Lichterfeld, M. Altfeld, M. M. Addo, M. N. Johnston, P. K. Lee, B. S. Wagner, E. T. Kalife, D. Strick, E. S. Rosenberg, and B. D. Walker. 2004. Limited durability of viral control following treated acute HIV infection. PLoS Med. 1:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lange, C. G., H. Valdez, K. Medvik, R. Asaad, and M. M. Lederman. 2002. CD4+ T-lymphocyte nadir and the effect of highly active antiretroviral therapy on phenotypic and functional immune restoration in HIV-1 infection. Clin. Immunol. 102:154-161. [DOI] [PubMed] [Google Scholar]
- 30.Leist, T. P., S. P. Cobbold, H. Waldmann, M. Aguet, and R. M. Zinkernagel. 1987. Functional analysis of T lymphocyte subsets in antiviral host defense. J. Immunol. 138:2278-2281. [PubMed] [Google Scholar]
- 31.Markowitz, M., X. Jin, A. Hurley, V. Simon, B. Ramratnam, M. Louie, G. R. Deschenes, M. Ramanathan, Jr., S. Barsoum, J. Vanderhoeven, T. He, C. Chung, J. Murray, A. S. Perelson, L. Zhang, and D. D. Ho. 2002. Discontinuation of antiretroviral therapy commenced early during the course of human immunodeficiency virus type 1 infection, with or without adjunctive vaccination. J. Infect. Dis. 186:634-643. [DOI] [PubMed] [Google Scholar]
- 32.Mathew, A., I. Kurane, S. Green, D. W. Vaughn, S. Kalayanarooj, S. Suntayakorn, F. A. Ennis, and A. L. Rothman. 1999. Impaired T cell proliferation in acute dengue infection. J. Immunol. 162:5609-5615. [PubMed] [Google Scholar]
- 33.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNeil, A. C., W. L. Shupert, C. A. Iyasere, C. W. Hallahan, J. Mican, R. T. Davey, Jr., and M. Connors. 2001. High-level HIV-1 viremia suppresses viral antigen-specific CD4+ T cell proliferation. Proc. Natl. Acad. Sci. USA 98:13878-13883. [DOI] [PMC free article] [PubMed]
- 35.Oxenius, A., D. A. Price, P. J. Easterbrook, C. A. O'Callaghan, A. D. Kelleher, J. A. Whelan, G. Sontag, A. K. Sewell, and R. E. Phillips. 2000. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA 97:3382-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer, B. E., N. Blyveis, A. P. Fontenot, and C. C. Wilson. 2005. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J. Immunol. 175:8415-8423. [DOI] [PubMed] [Google Scholar]
- 37.Palmer, B. E., E. Boritz, N. Blyveis, and C. C. Wilson. 2002. Discordance between frequency of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-producing CD4+ T cells and HIV-1-specific lymphoproliferation in HIV-1-infected subjects with active viral replication. J. Virol. 76:5925-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer, B. E., E. Boritz, and C. C. Wilson. 2004. Effects of sustained HIV-1 plasma viremia on HIV-1 Gag-specific CD4+ T cell maturation and function. J. Immunol. 172:3337-3347. [DOI] [PubMed] [Google Scholar]
- 39.Pitcher, C. J., C. Quittner, D. M. Peterson, M. Connors, R. A. Koup, V. C. Maino, and L. J. Picker. 1999. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 5:518-525. [DOI] [PubMed] [Google Scholar]
- 40.Raper, S. E., M. Yudkoff, N. Chirmule, G. P. Gao, F. Nunes, Z. J. Haskal, E. E. Furth, K. J. Propert, M. B. Robinson, S. Magosin, H. Simoes, L. Speicher, J. Hughes, J. Tazelaar, N. A. Wivel, J. M. Wilson, and M. L. Batshaw. 2002. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum. Gene Ther. 13:163-175. [DOI] [PubMed] [Google Scholar]
- 41.Rosen, H. R., D. J. Hinrichs, D. R. Gretch, M. J. Koziel, S. Chou, M. Houghton, J. Rabkin, C. L. Corless, and H. G. Bouwer. 1999. Association of multispecific CD4(+) response to hepatitis C and severity of recurrence after liver transplantation. Gastroenterology 117:926-932. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg, E. S., M. Altfeld, S. H. Poon, M. N. Phillips, B. M. Wilkes, R. L. Eldridge, G. K. Robbins, R. T. D'Aquila, P. J. Goulder, and B. D. Walker. 2000. Immune control of HIV-1 after early treatment of acute infection. Nature 407:523-526. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 44.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 45.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337-339. [DOI] [PubMed] [Google Scholar]
- 46.Shoukry, N. H., A. G. Cawthon, and C. M. Walker. 2004. Cell-mediated immunity and the outcome of hepatitis C virus infection. Annu. Rev. Microbiol. 58:391-424. [DOI] [PubMed] [Google Scholar]
- 47.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Unsoeld, H., S. Krautwald, D. Voehringer, U. Kunzendorf, and H. Pircher. 2002. Cutting edge: CCR7+ and CCR7− memory T cells do not differ in immediate effector cell function. J. Immunol. 169:638-641. [DOI] [PubMed] [Google Scholar]
- 49.Waldrop, S. L., C. J. Pitcher, D. M. Peterson, V. C. Maino, and L. J. Picker. 1997. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J. Clin. Investig. 99:1739-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whittle, H. C., J. Dossetor, A. Oduloju, A. D. Bryceson, and B. M. Greenwood. 1978. Cell-mediated immunity during natural measles infection. J. Clin. Investig. 62:678-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Younes, S. A., B. Yassine-Diab, A. R. Dumont, M. R. Boulassel, Z. Grossman, J. P. Routy, and R. P. Sekaly. 2003. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 198:1909-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]