Abstract
The majority of people infected with hepatitis C virus (HCV) fail to generate or maintain a T-cell response effective for viral clearance. Evidence from murine chronic viral infections shows that expression of the coinhibitory molecule PD-1 predicts CD8+ antiviral T-cell exhaustion and may contribute to inadequate pathogen control. To investigate whether human CD8+ T cells express PD-1 and demonstrate a dysfunctional phenotype during chronic HCV infection, peripheral and intrahepatic HCV-specific CD8+ T cells were examined. We found that in chronic HCV infection, peripheral HCV-specific T cells express high levels of PD-1 and that blockade of the PD-1/PD-L1 interaction led to an enhanced proliferative capacity. Importantly, intrahepatic HCV-specific T cells, in contrast to those in the periphery, express not only high levels of PD-1 but also decreased interleukin-7 receptor alpha (CD127), an exhausted phenotype that was HCV antigen specific and compartmentalized to the liver, the site of viral replication.
The majority of those infected with the hepatitis C virus (HCV) become chronic carriers who can progress to chronic active hepatitis, cirrhosis, and hepatocellular carcinoma (1, 2). Current estimates by the World Health Organization place the worldwide prevalence of chronic HCV infection at approximately 170 million (46, 49). These clinical sequelae of HCV infection now comprise the leading indication for liver transplantation in the United States (17). Currently, no vaccine exists to prevent HCV infection, and the only licensed therapy, alpha interferon (IFN-α), either alone or in combination with the nucleoside analog ribavirin, is expensive, associated with, at best, only a 50% clearance rate for the most prevalent genotype (genotype 1), and complicated by significant side effects (35). The paucity of efficacious anti-HCV therapeutic options highlights the need for effective interventions aimed at augmenting or supplementing the natural immune response that, alone or in concert with antiviral drug therapy, can prevent the detrimental consequences of HCV infection.
In the minority of patients who spontaneously resolve HCV infection, clearance of the virus is kinetically associated with a diverse and enduring HCV-specific CD8+ cytotoxic T-lymphocyte effector response (11, 14, 20, 29, 30, 42). However, in those that fail to control the virus and succumb to persistent infection, HCV specific CD8+ T cells either decline in magnitude and function or are lost altogether (11, 14, 20, 29, 30, 42), which is suggestive of the type of exhaustion of virus-specific T cells that was first shown during persistent lymphocytic choriomeningitis virus (LCMV) infection of mice (19, 50). Other studies of chronic HCV infection have suggested that antigen-specific CD8+ T cells may be functionally anergic or impaired (22, 45, 47) and phenotypically resemble T cells at an early stage of differentiation (3). However, the majority of phenotypic analyses of HCV-specific T cells have utilized T cells circulating in the peripheral blood. While this provides useful information, it is crucial to examine T cells infiltrating the liver, since the site of active HCV infection may differentially influence T-cell markers of differentiation, costimulation, and effector functions.
Studies of antiviral CD8+ T cells in persistent LCMV infection have identified expression of programmed death receptor-1 (PD-1) as a molecular signature of exhausted T cells (5). Importantly, interrupting the PD-1/PD-L1 receptor/ligand interaction in vivo, with blocking antibody against PD-L1 or PD-1, increased T-cell function and decreased viral load (5). PD-1 (21) is a monomeric protein whose cytoplasmic domain has two tyrosine residues, one constituting an immunoreceptor tyrosine-based inhibition motif and one an immunoreceptor tyrosine-based switch motif (38). Engagement of PD-1 by one of its ligands, PD-L1, delivers a negative signal to the T cell, preventing proliferation and interleukin-2 (IL-2) production (9, 10, 28). PD-L1 is highly expressed on mouse liver sinusoidal endothelial cells and Kupffer cells, and these cells are capable of inhibiting proliferation of PD-1-expressing effector CD8+ T cells (25). Interestingly, mice deficient in PD-1 spontaneously accumulate CD8+ T cells in the liver, and antigen-specific CD8+ T cells lacking PD-1 show impaired apoptotic levels during the contraction phase of the immune response (15).
Currently, little is known about the expression of PD-1 and its role in T-cell exhaustion in chronic HCV infection, particularly at the site of active infection, the liver. The present study was undertaken to better understand the T-cell phenotype in HCV infection by measuring expression of PD-1 on antigen-specific CD8+ T cells in both the liver and peripheral blood of patients with chronic HCV infection. We found that the HCV-specific CD8+ T cells in the liver predominantly expressed high levels of PD-1 and lacked CD127 expression in comparison with those circulating in the peripheral blood. These results suggest that the intrahepatic site of viral replication influences the local antiviral T-cell phenotype. Further, we show that blockade of the PD-1/PD-L1 interaction increases the proliferative capacity of the phenotypically exhausted HCV-specific T cells.
MATERIALS AND METHODS
Subjects.
Nineteen patients with chronic HCV infection (HCV antibody and HCV PCR positive) and negative for human immunodeficiency virus (HIV) by antibody screening were enrolled in the study from either the Emory/Crawford Long or Atlanta VA Medical Center. All patients were naïve to HCV antiviral therapies prior to enrollment. Nine of the nineteen patients were positive for HLA-A2 by fluorescence-activated cell sorter (FACS) analysis. The patient characteristics are summarized in Table 1. The protocol (IRB no. 1358-2004) was approved by the local ethics committees of Emory University and the Atlanta VA Medical Center.
TABLE 1.
Patient cohort demographic and clinical data
| Patient no. | Genderb | Age (yr) | HLA-A2 | HCV genotype | Baseline viral load (IU/ml) | ALTc (U/liter) |
|---|---|---|---|---|---|---|
| 151a | F | 45 | + | 3 | 267,000 | 49 |
| 153a | M | 43 | + | 2b | 7,340,000 | 25 |
| 178a | F | 48 | + | 2 | 18,330,000 | 62 |
| 179 | M | 54 | − | 1a | 197,000 | 197 |
| 183 | F | 56 | + | 1a | 1,170,000 | 45 |
| 190 | M | 52 | − | 1a | 5,990,000 | 27 |
| 193 | M | 66 | + | 1a | 16,120,000 | 30 |
| 601 | M | 60 | − | 1b | 4,690,000 | 25 |
| 602 | M | 48 | − | 1a | 586,000 | 80 |
| 603 | M | 58 | + | 1a | 1,820,000 | 36 |
| 604 | M | 58 | − | 1a | 2,850,000 | 57 |
| 605 | F | 30 | − | 1 | 819,000 | 57 |
| 606 | M | 50 | − | 1b | 591,000 | 18 |
| 607 | M | 59 | + | 3a | 343,000 | 31 |
| 608 | M | 57 | − | 1b | 395,000 | 16 |
| 609 | M | 55 | + | 1a | 833,000 | 67 |
| 611 | M | 53 | − | 1a | 1,220,000 | 88 |
| 613 | M | 59 | − | 1b | 6,160,000 | 40 |
| 619a | M | 53 | + | 1a | 1,450,000 | 83 |
Only a peripheral blood sample (no liver sample) was available for HCV tetramer analysis.
F, female; M, male.
ALT, alanine aminotransferase.
HCV antibody testing, viral load determination, and genotyping.
HCV antibody testing by enzyme-linked immunosorbent assay was performed at the Emory Immunology Laboratory using a kit per the manufacturer's instructions (Abbott Diagnostics, Abbott Park, IL) and at the Atlanta VA Immunology Laboratory (Bio-Rad Laboratories, Hercules, CA). HCV viral load quantification was performed at the Emory Molecular Laboratory and Atlanta VA using a real-time reverse transcription-PCR assay (Roche Molecular Systems, Alameda, CA). HCV genotyping was performed at the Emory Molecular Laboratory using a real-time reverse transcription-PCR assay (Abbott Diagnostics, Abbott Park, IL) and at the Atlanta VA using a line probe assay (Bayer Diagnostics, Research Triangle Park, NC).
DNA sequencing of HCV 1073 epitope.
RNA was extracted from 200 μl of plasma of patients chronically infected with HCV genotype 1a by using the QIAamp viral RNA minikit (QIAGEN, Valencia, CA) and was subjected to reverse transcription (SuperScript II; Invitrogen, Karlsruhe, Germany) and two rounds of PCR amplification. For reverse transcription, random primers (New England Biolabs, Ipswich, MA) were used. PCR I was run with the primers 5′-GGC YTG CCC GTC TCY GCC CG-3′ (forward) and 5′-CGG CGC ACS GGA ATG ACA TCG-3′ (reverse). PCR II was run with primers 5′-CGG CST ACK CCC ARC AGA CGM GAG GCC-3′ (forward) and 5′-CCT CGT GAC CAR GTA AAG GTC C-3′ (reverse). The amplified DNA was purified using the QIAquick PCR purification kit (QIAGEN, Valencia, CA), inserted into the pCR 2.1 TOPO vector (Invitrogen, Karlsruhe, Germany), cloned, and sequenced using M13 primers (Macrogen, Rockville, MD).
PBMCs.
EDTA- and heparin-anticoagulated blood (50 to 70 ml) was collected from each patient and used either directly for FACS staining or for peripheral blood mononuclear cell (PBMC) isolation. PBMCs were isolated using Ficoll-Paque PLUS density gradients (Amersham, Oslo, Norway), washed twice in phosphate-buffered saline, and either analyzed immediately or cryopreserved in medium containing 90% fetal calf serum (HyClone) and 10% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO).
Liver biopsy.
Liver tissue was obtained by either ultrasound-guided needle biopsy or transjugular fluoroscopic technique and immediately put into RPMI 1640 medium (Gibco) containing 10% fetal calf serum (HyClone, Logan, UT) for immunological assays.
Intrahepatic T-cell isolation.
The liver biopsy sample obtained in RPMI 1640 medium (Gibco, Carlsbad, CA) containing 10% fetal calf serum (HyClone, Logan, UT) was washed three times with the same medium to remove cell debris and red blood cells. Isolation of liver-infiltrating lymphocytes was performed using an automated, mechanical disaggregation system (Medimachine; Becton Dickinson, San Jose, CA). The sample was placed into a 50-μm Medicon, inserted into the Medimachine, and run for 15 s. Dissagregated cells were removed using a syringe in the syringe port. The Medicon was rinsed twice with RPMI medium (Gibco, Carlsbad, CA) containing 10% fetal calf serum (HyClone, Logan, UT) to ensure maximum cell recovery. Cells were used immediately for FACS staining.
Antibodies, HLA-A2 tetramers, and flow cytometry.
Cells were stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, perdinin chlorophyll protein (PerCP)-, and allophycocyanin-labeled monoclonal antibodies or tetramers according to the manufacturers' instructions, and flow cytometry was performed using a FACSCalibur (Becton Dickinson, San Jose, CA). FACS data were analyzed with FlowJo software v8.1.1 (Treestar). Anti-CD8 PerCP and anti-CD45RA allophycocyanin monoclonal antibodies were from BD PharMingen (BD Biosciences, San Jose, CA). Anti-CD62L FITC, CD3 FITC, and CD127 PE were obtained from Beckman Coulter (Fullerton, CA). Anti-PD-1 PE conjugated antibody (clone EH12) was generated as described previously (16). HLA-A2 tetramers were specific for the following CD8+ T-cell epitopes: HCV 1073, CINGVCWTV; HCV-1406, KLVALGINAV. The tetramers were generated at the National Tetramer Core Facility at Emory University School of Medicine.
CFSE labeling and antibody blockade.
PBMCs (10 × 106) were washed with phosphate-buffered saline and labeled with 3 μM carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes). Cells were adjusted to 1 × 106 cells/ml and cultured in the presence of 2 μg/ml of A2-HCV 1073 (CINGVCWTV) peptide. To increase cell yield, 10 U/ml of IL-2 was added only on day 3 poststimulation. An unstimulated control was included in each assay. Specific blocking antibodies (anti-PD-L1 clone 29E.2A3 [8] and anti-PD-1 clone EH12 [16]) were added to cell cultures at a concentration of 10 μg/ml at the time of stimulation. Cells were incubated for 6 days, harvested, stained with surface antibodies and tetramers, and analyzed by flow cytometry.
Statistical analysis.
Results were graphed and analyzed using GraphPad Prism (v4). Comparisons within the same patient were made using paired t tests. Comparisons between patients were made using unpaired t tests.
RESULTS
PD-1 expression on HCV antigen-specific CD8+ T cells.
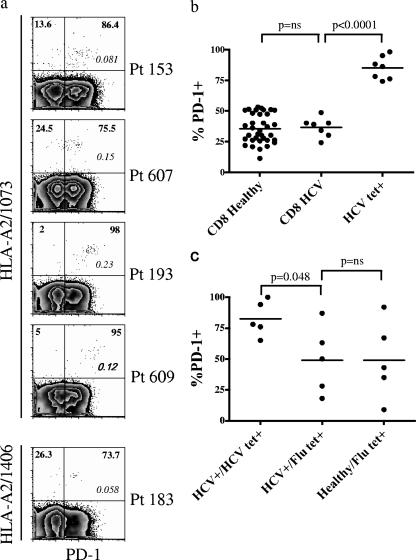
Nineteen patients with HCV infection (all HIV negative) were studied (Table 1). Fifteen patients underwent both blood and liver sampling for phenotyping by flow cytometric analysis, and all were untreated with pharmacologic antiviral therapy prior to study enrollment. Nine patients in the cohort were HLA- A2 positive and demonstrated a population of HCV-specific CD8+ T cells in the periphery by HLA tetramer staining (Table 1). These HCV-specific CD8+ T cells were evaluated for PD-1 expression (Fig. 1a). The level of PD-1 expression on total CD8+ T cells in the peripheral blood from healthy donors was not significantly different from that of the total pool of peripheral CD8+ T cells from HCV-infected patients (Fig. 1b). In contrast, the majority of HCV-specific tetramer-positive CD8+ T cells sampled from the peripheral blood were PD-1 positive (mean, 85%; standard error of the mean [SEM] 3.6) (Fig. 1a) with significantly higher expression than that of the total CD8+ T-cell population (P < 0.0001) (Fig. 1b). Expression of differentiation, costimulatory, trafficking, and effector function molecules on antigen-specific CD8+ T cells was also investigated (data not shown). The HCV-specific tetramer-positive cells exhibit a memory phenotype (high-level CD11a and low-level CD45RA expression), early differentiation markers (high-level CD27, high-level CD28, and intermediate-level of CCR7 and CD62L expression) and low levels of mediators of effector function granzyme B and perforin. Interestingly, these HCV tetramer-positive T cells in the peripheral blood expressed high levels of CD127 (IL-7 receptor α chain), a phenotypic marker that when expressed at low levels identifies impaired memory T-cell differentiation (18, 27, 48). To determine whether the phenotype of CD8+ T cells was different in the setting of nonchronic infection, we examined influenza virus-specific T cells in five healthy HLA-A2+ donors who were not infected with HCV. The percentage of peripheral influenza virus tetramer-positive CD8+ T cells that expressed PD-1 was 49% (SEM, 14.1%) (Fig. 1c). Five of the seven HLA-A2-positive chronic HCV patients were also identified by tetramer analysis to have influenza virus-specific CD8+ T cells. The percentage of influenza virus-specific T cells expressing PD-1 in these chronically infected HCV patients was not significantly different from that in the same population in healthy donors (Fig. 1c). Importantly, because five of the seven HLA-A2+ HCV patients also had detectable influenza virus-specific CD8+ T cells, we were able to directly compare, within each patient, PD-1 for T cells specific for a nonchronic (influenza virus) and a chronic (HCV) infection. The difference between influenza virus-specific and HCV-specific T-cell PD-1 expression was significant (Fig. 1c). The percentage of HCV-specific CD8+ T cells expressing PD-1 (mean, 83%; SEM, 6.4%) was greater than the percentage of PD-1+ influenza virus-specific CD8+ T cells (mean, 49%; SEM, 12.3%) (P = 0.048) (Fig. 1c).
FIG. 1.
HCV specific CD8+ T cells express PD-1 in human chronic HCV infection. (a) Representative plots from five patients with chronic HCV infection, showing the expression of PD-1 on HCV-specific CD8+ T cells. Numbers in boldface identify the frequency of PD-1 expression (x axis) on HCV-specific CD8+ T cells (y axis). Numbers in italic within the plots identify the frequency of tetramer-positive cells among total CD8+ T cells. On the y axis, 1073 and 1406 identify the HCV epitope specificity of the tetramer. Patients are identified by “Pt” followed by the patient number. Cells were gated on CD8+ lymphocytes. Plots are on a logarithmic scale. (b) Comparison of PD-1 expression on CD8+ T cells from healthy donors (CD8 Healthy) and from HCV-infected patients (CD8 HCV) and on CD8+ HCV-specific T cells (HCV tet+). (c) PD-1 expression on CD8+ T cells specific for influenza virus (Flu tet+) from HCV-infected patients (HCV+) and healthy donors (Healthy) compared with PD-1 expression on CD8+ T cells specific for HCV (HCV tet+). A paired t test was used to compare differences in expression of PD-1 within the same patient on total CD8+ T cells versus HCV-specific CD8+ T cells.
PD-1 expression on human peripheral blood and liver-infiltrating lymphocytes.
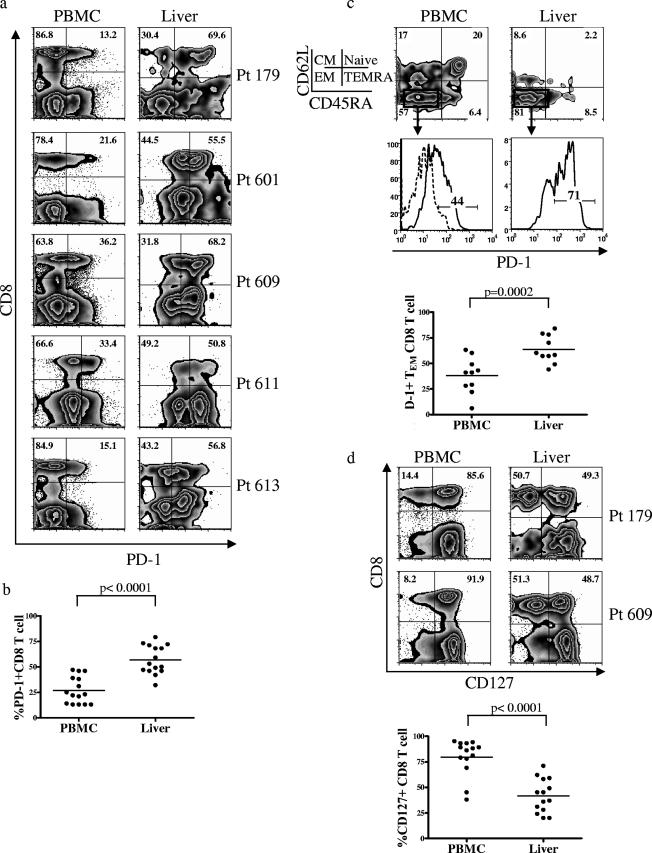
Peripheral blood and liver biopsies were analyzed for the expression of PD-1 from 15 patients chronically infected with HCV. Representative flow cytometric analysis from five patients is shown in Fig. 2a. Whereas in the peripheral blood, 27% (SEM, 3.4%) of CD8+ T cells were PD-1+, the frequency of such cells was increased twofold (mean, 57%; SEM, 3.6%) in the liver (Fig. 2b). Hence, the liver is enriched in cells expressing high levels of PD-1. While naïve cells should express high levels of both CD62L and CD45RA, in the liver the majority of CD8+ T cells were CD62L low/CD45RA low, consistent with a memory phenotype (Fig. 2c). Analysis specifically of this memory population in both the liver and the periphery showed that PD-1 expression was elevated in the liver compared with the periphery (Fig. 2c). These data suggest that the increase in the percentage of cells expressing PD-1 on the intrahepatic T cells is not merely due to the absence of the naïve population in this compartment. Rather, there is a preferential enrichment of PD-1+ CD8+ T effector memory (CD62L low/CD45RA low) cells within the liver compared to the peripheral blood (Fig. 2c).
FIG. 2.
The frequency of PD-1-expressing CD8+ T cells in the liver is greater than in the peripheral blood. (a) Representative plots from five patients with chronic HCV infection, showing the expression of PD-1 on total CD8+ T cells from the peripheral blood versus the liver. Numbers in boldface within the plots identify the frequency of cells with PD-1 expression among total CD8+ T cells in the lymphocyte gate. Plots are on a logarithmic scale. (b) Comparison of PD-1 expression on CD8+ T cells from peripheral blood versus liver in chronically HCV-infected patients. A paired t test was used to compare the difference in PD-1 expression within the same patients. (c) Comparison of PD-1 expression on the CD8+ effector memory (TEM) cells from peripheral blood versus liver. Memory subsets were identified by differential expression of CD62L and CD45RA. Boldface numbers in the top plots represent the frequency of cells in each quadrant. Cells were gated on CD8+ lymphocytes. The TEM subset was gated (boxes), and the expression of PD-1 is shown in the histogram plots below. The dotted line shows PD-1 expression on naïve CD8+ T cells (used as the negative population). The numbers in the histogram plots represent the frequency of cells expressing PD-1. A comparison of the frequency of PD-1 expression on CD8+ TEM cells for 10 patients with chronic HCV infection is summarized below the histogram plots. A paired t test was used to compare the difference in PD-1 expression on CD8+ TEM cells from the peripheral blood versus the liver within the same patient. (d) Representative plots from two patients with chronic HCV infection, showing the difference in CD127 expression on total CD8+ T cells from the peripheral blood versus the liver. Numbers in boldface identify the frequency of CD127 expression on total CD8+ T cells. Cells were gated on CD8+ lymphocytes. Plots are on a logarithmic scale. A summary of the comparison of CD127 expression on total CD8+ T cells in the peripheral blood versus the liver is shown below the FACS plots. A paired t test was used for statistical analysis.
CD127 expression on human peripheral blood and liver-infiltrating lymphocytes.
IL-7 is required for maintenance of memory CD8+ T cells (26, 36), and the alpha chain of its receptor, CD127, is downregulated on antigen-specific T cells in persistent LCMV and gammaherpesvirus infections (18, 27, 32, 48). This loss of CD127 during chronic infection correlates with impaired cytokine production, increased susceptibility to apoptosis, and a reduction in the ability of memory virus-specific CD8+ T cells to persist in the host. Accordingly, resolution of acute hepatitis B virus infection correlates with upregulation of CD127 expression and concomitant loss of PD-1 expression (6). Interestingly, in our chronic HCV patients, only 20% (SEM, 4.8%) of total peripheral CD8+ T cells were CD127 negative, but in the hepatic CD8+ T-cell infiltrates, this percentage increased significantly to 58% (SEM, 4.4%) (Fig. 2d). Hence, the liver is enriched in cells expressing an exhausted phenotype, with high PD-1 and low CD127 cells predominating. These data suggest that liver-infiltrating CD8+ T cells in chronic HCV patients do not phenotypically mirror the peripheral CD8+ T-cell population. This compartmentalization cannot simply be explained by emergence of viral variations that abrogate peripheral CD8+ T-cell recognition. Sequencing of at least five viral clones from the peripheral blood of each of three patients (eight, five, and seven clones for patients 603, 609, and 193, respectively) showed the wild-type consensus sequence at the amino acid level (sequence CINGVCWTV for NS3 1073 [genotype 1a]). Sequencing was carried out on PCR fragments spanning the 1073 NS3 epitope of the viral RNA. These patients were all infected with genotype 1a, and all expressed HLA-A2. For all three patients, the majority of peripheral blood HCV-specific CD8+ T cells expressed high levels of CD127 (Fig. 3). In the setting of HIV infection where the virus infects T cells and monocytes in the peripheral blood, low levels of CD127 are associated with functional or memory T-cell defects (7, 33). In our study, the hepatic compartmentalization of the cells showing this exhausted phenotype suggests that the phenotype is intimately tied to the site of persistent viral replication.
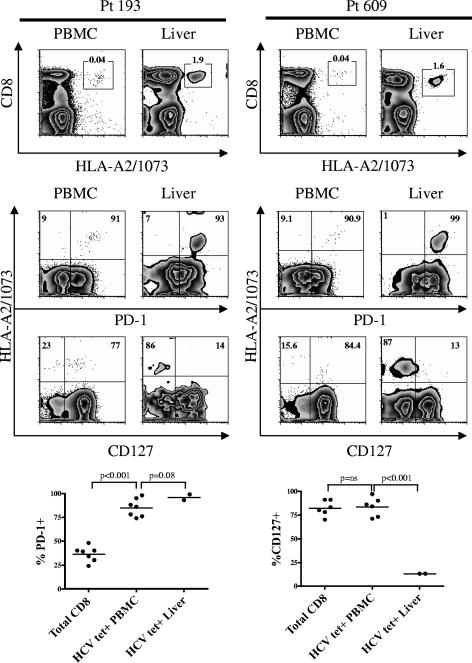
FIG. 3.
HCV-specific CD8+ T cells in the liver express an exhausted phenotype. Representative plots of PD-1 and CD127 expression on HCV-specific CD8+ T cells from the peripheral blood and the liver of two patients with chronic HCV infection are shown. The first row of plots identifies the HCV tetramer-positive population (boxes). The numbers above the boxes represent the frequency of tetramer-positive cells among CD3+ lymphocytes. The epitope specificity of the HCV tetramer is identified on the x axis (1073) for the first row of plots. The second and third rows of plots show PD-1 and CD127 expression on HCV-specific CD8+ T cells from the peripheral blood and liver of two patients with chronic HCV infection. Numbers in boldface represent the frequency of PD-1 or CD127 expression on HCV-specific CD8+ T cells. Plots are on a logarithmic scale and gated on CD3+ CD8+ lymphocytes. Below the FACS plots, a summary of the comparison of PD-1 expression (left) and CD127 expression (right) on total CD8+ T cells versus CD8+ HCV-specific T cells from the periphery (HCV tet+ PBMC) versus HCV specific CD8+ T cells from the liver (HCV tet+ Liver) is shown. Paired t tests were used to compare expression within the same patient.
PD-1 and CD127 expression on HCV antigen-specific CD8+ T cells in the liver.
Two of our HLA-A2-positive patients in the cohort also had an identifiable HCV-specific population by tetramer staining in the liver (Fig. 3). We were able to directly compare expression of PD-1 and CD127 on HCV-specific tetramer-positive CD8+ T cells in the livers versus the peripheries of these individuals. HCV-specific CD8+ T cells from the periphery were mostly PD-1 positive (mean, 85%; SEM, 3.6%) and CD127 positive (mean, 84%; SEM, 4.0%), while the hepatic HCV-specific CD8+ T cells were mostly PD-1 positive (mean, 92%) but only rarely CD127 positive (mean, 13%) (Fig. 3). At the site of viral replication, there appeared to be an expansion of CD127-negative cells expressing high levels of PD-1. Why the peripheral antigen-specific CD8+ T cells differentially express CD127 compared with the intrahepatic compartment is not presently understood; however, it may be related to the level or timing of antigen exposure needed to cause downregulation of CD127. In LCMV infection of mice, with exposure to a persistent antigen load in chronic infection, CD127 was persistently downregulated, whereas short-lived exposure to LCMV antigen using GP33 only temporarily suppressed CD127 expression and failed to induce T-cell exhaustion (27). Dependence on availability of antigen and time of exposure was also observed to affect the expression of CD62L and CD127, whereas persistent antigen led to persistent downregulation of both CD62L and CD127 (4). It is possible that in chronic HCV infection, the few HCV-specific CD8+ T cells detected in the periphery are not continuously exposed to sufficient antigen to maintain low levels of CD127. Thus, the T cells may be fooled into believing that the virus has been cleared.
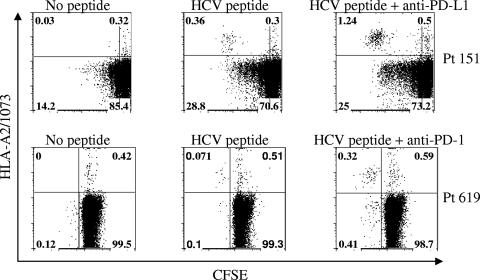
Blockade of PD-1/PD-L1 leads to increased expansion of HCV-specific tetramer-positive CD8+ T cells.
Evidence from our patient population suggests that blockade of the PD-1/PD-L1 interaction with anti-PD-L1 or anti-PD-1 antibody increases the proliferative capacity of HCV-specific T cells (Fig. 4). Addition of blocking antibodies and HCV-specific peptide resulted in a fourfold increase in expansion of the HCV-specific T cells as demonstrated by monitoring the frequency of CFSElow tetramer-labeled CD8+ T cells after stimulation with cognate peptide for 6 days.
FIG. 4.
Blockade of the PD-1/PD-L1 pathway increases the expansion of antigen-stimulated HCV-specific T cells. CFSE-labeled PBMCs from two separate HLA-A2-positive patients were stimulated using the cognate peptide antigen for 6 days in the presence of IL-2 and anti-PD-L1 antibody (top panel) or anti-PD-1 antibody (lower panel). Results for an unstimulated control are also shown. The percentages of proliferating CFSE low- and CFSE high-HCV-specific HLA-A2+ CD8+ T cells are shown in each quadrant.
DISCUSSION
There is evidence indicating that the liver may act as a specific site where activated T cells die (12). Taking this together with the observation that one ligand for PD-1, PD-L1, is highly expressed on mouse liver sinusoidal endothelial cells and Kupffer cells (25), it is possible that the PD-1/PD-L1 pathway may be involved in downregulating or impairing the function of activated intrahepatic CD8+ T cells. It is conceivable that this pathway may be involved in the apoptosis of activated T cells in the liver. Additionally, in a model of chronic hepatitis B virus infection, PD-1 expression by T cells was associated with viral replication (24). Thus, the PD-1/PD-L1 pathway may be particularly important for T-cell regulation in the liver. It would be interesting to examine whether PD-L1 expression is upregulated during chronic HCV infection in humans, but the data presented in this report suggest that the PD-1 coinhibitory pathway could be enhanced during hepatotropic infection with HCV. In support of this, a recent study has shown that PD-L1 is upregulated on liver resident cell populations by viral infection, activated T cells, and type I IFNs (31). The precise balance between negative regulators, such as the PD-1/PD-L1 pathway, and stimulatory antiviral signals may have important implications for the outcome of hepatotropic infections such as HCV.
Important studies of HIV infection show that PD-1 expression is upregulated on memory CD8+ T cells in poorly controlled chronic HIV infection (13, 34, 43). This PD-1 expression is associated with altered survival and expansion of the HIV-specific cells (13, 34, 43), with blockade of the PD-1/PD-L1 interaction yielding increased proliferative and functional capacities (13, 43). Similarly, our results show that this pathway may also be important in the T-cell dysfunction contributing to viral persistence in chronically infected HCV patients. In a recent paper by Urbani et al., the authors investigated the expression of PD-1 on peripheral antigen-specific CD8+ T cells in patients during the acute phase of HCV infection (44). Their data show that while PD-1 declined on HCV-specific CD8+ T cells during recovery, its expression remained high when HCV persisted. Blocking the PD-1/PD-L1 interaction with an anti-PD-L1 antibody improved the expansion capacity of virus-specific CD8+ T cells. Our study supports and extends these findings. First, we show that at the site of infection, the liver, the frequency of HCV-specific CD8+ T cells expressing PD-1 is high. Second, we show that the majority of HCV-specific CD8+ T cells from the peripheral blood of patients with chronic HCV infection express high levels of CD127. These data are in contrast to the findings of Urbani et al., who showed persistent downregulation of CD127 on peripheral HCV-specific CD8+ T cells from acutely infected patients failing to clear infection (44). We hypothesize that this difference is due to differences in the patient cohorts. While the cohort studied by Urbani et al. represented acutely infected patients followed up to 1 year after infection, our cohort is composed of patients infected with HCV years prior to study enrollment. It is conceivable that after an extended postinfection time and establishment of viral persistence, the CD127 levels in the peripheral blood return to high levels, whereas in the liver at the site of viral replication, persistent antigen exposure contributes to maintaining low levels of CD127. Shoukry et al. have also shown that even after resolution of experimental HCV infection in chimpanzees, intrahepatic CD8+ T cells exhibit an activated phenotype (CD69+) in the absence of continued viral replication (39). These data leave open the possibility, as also reviewed by Crispe (12), that the intrahepatic environment itself contributes to the phenotype of infiltrating T cells, and it is possible that the declining CD127 levels observed in our study are also accompanied by such a general state of activation. Additionally, CD69 upregulation can be cytokine mediated and may reflect a response to type I interferons produced by the innate immune system (37, 41). It would be interesting to address whether this phenotype is dependent on the presence of replicating virus by comparing patients with acutely resolved and chronic infections, but the difficulty in obtaining liver biopsy samples from those who have resolved infection may prohibit such analysis in human patients.
In this study, we sought to further characterize the phenotype of T cells in chronic HCV infection by studying the expression of the PD-1 molecule, which was previously linked to impaired effector function and T-cell exhaustion (5). Our results show that the majority of HCV-specific T cells in the intrahepatic compartment express PD-1 but lack CD127, a phenotype consistent with T-cell exhaustion. Few studies have directly examined HCV-specific immune cells from the livers of patients with chronic HCV infection, and most have shown only data from expanded populations in culture. We demonstrate that HCV-specific CD8+ T cells in the liver express high levels of PD-1 and low levels of CD127, despite increased CD127 expression seen in the periphery. This finding highlights the caveats in extrapolating phenotypic and functional results from antigen-specific cells found in the peripheral blood, as these cells may not accurately reflect the liver-infiltrating antigen-specific T cells. Limitation on the availability of liver biopsy material and the number of recovered liver-infiltrating cells is a major factor accounting for the lack of intrahepatic ex vivo studies. One previous study examining liver-infiltrating HCV-specific CD8+ T cells directly ex vivo showed that they all expressed the activation marker CD69 (23). Our study demonstrates that while activated, the HCV-specific CD8+ T cells are also phenotypically exhausted, with high levels of PD-1 expression and low levels of CD127 expression. Importantly, another recent study has shown that a large fraction of intrahepatic CD8+ T cells specific for HCV have an impaired ability to secrete IFN-γ (40). The reversal of T-cell exhaustion in mouse models of chronic viral infection (5) and in our own studies presented here via blockade of the PD-1/PD-L1 interaction provides promise for therapeutic immune augmentation in the setting of chronic viral infections. In this study, we have extended the finding reported for murine models that CD8+ antiviral T cells express high levels of PD-1 to an important persistent human pathogen, HCV. These results elucidate the importance of characterizing cells infiltrating the site of infection and highlight a potentially important target for therapeutic intervention.
Acknowledgments
We thank Hirotomo Nakahara, Young-Xian Xu, and Dimitri Fillos for excellent technical assistance; Francie Lasseter for patient cohort coordination; and Devon Livingston-Rosanoff for critical reading of the manuscript.
We acknowledge support from educational grant K12 RR017643 (to H.R.); the Center for AIDS Research Immunology Core (to C.C.I.), the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health (to E.J.W.); a grant from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative (to R.A. and G.J.F.); NIH grant AI56299 (to G.J.F. and R.A.); and the Cancer Research Institute Investigator Award, Woodruff Health Sciences Fund, Yerkes Research Center Base grant RR-00165, and NIH grant AI070101(to A.G.).
Footnotes
Published ahead of print on 20 December 2006.
REFERENCES
- 1.Alter, M. J., D. Kruszon-Moran, O. V. Nainan, G. M. McQuillan, F. Gao, L. A. Moyer, R. A. Kaslow, and H. S. Margolis. 1999. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N. Engl. J. Med. 341:556-562. [DOI] [PubMed] [Google Scholar]
- 2.Alter, M. J., H. S. Margolis, K. Krawczynski, F. N. Judson, A. Mares, W. J. Alexander, P. Y. Hu, J. K. Miller, M. A. Gerber, R. E. Sampliner, et al. 1992. The natural history of community-acquired hepatitis C in the United States. N. Engl. J. Med. 327:1899-1905. [DOI] [PubMed] [Google Scholar]
- 3.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann, M. F., P. Wolint, K. Schwarz, P. Jager, and A. Oxenius. 2005. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J. Immunol. 175:4686-4696. [DOI] [PubMed] [Google Scholar]
- 5.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682-687. [DOI] [PubMed] [Google Scholar]
- 6.Boettler, T., E. Panther, B. Bengsch, N. Nazarova, H. C. Spangenberg, H. E. Blum, and R. Thimme. 2006. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J. Virol. 80:3532-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutboul, F., D. Puthier, V. Appay, O. Pelle, H. Ait-Mohand, B. Combadiere, G. Carcelain, C. Katlama, S. L. Rowland-Jones, P. Debre, C. Nguyen, and B. Autran. 2005. Modulation of interleukin-7 receptor expression characterizes differentiation of CD8 T cells specific for HIV, EBV and CMV. AIDS 19:1981-1986. [DOI] [PubMed] [Google Scholar]
- 8.Brown, J. A., D. M. Dorfman, F. R. Ma, E. L. Sullivan, O. Munoz, C. R. Wood, E. A. Greenfield, and G. J. Freeman. 2003. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 170:1257-1266. [DOI] [PubMed] [Google Scholar]
- 9.Carter, L., L. A. Fouser, J. Jussif, L. Fitz, B. Deng, C. R. Wood, M. Collins, T. Honjo, G. J. Freeman, and B. M. Carreno. 2002. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur. J. Immunol. 32:634-643. [DOI] [PubMed] [Google Scholar]
- 10.Chemnitz, J. M., R. V. Parry, K. E. Nichols, C. H. June, and J. L. Riley. 2004. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J. Immunol. 173:945-954. [DOI] [PubMed] [Google Scholar]
- 11.Cooper, S., A. L. Erickson, E. J. Adams, J. Kansopon, A. J. Weiner, D. Y. Chien, M. Houghton, P. Parham, and C. M. Walker. 1999. Analysis of a successful immune response against hepatitis C virus. Immunity 10:439-449. [DOI] [PubMed] [Google Scholar]
- 12.Crispe, I. N. 2003. Hepatic T cells and liver tolerance. Nat. Rev. Immunol. 3:51-62. [DOI] [PubMed] [Google Scholar]
- 13.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. Depierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350-354. [DOI] [PubMed] [Google Scholar]
- 14.Diepolder, H. M., R. Zachoval, R. M. Hoffmann, E. A. Wierenga, T. Santantonio, M. C. Jung, D. Eichenlaub, and G. R. Pape. 1995. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet 346:1006-1007. [DOI] [PubMed] [Google Scholar]
- 15.Dong, H., G. Zhu, K. Tamada, D. B. Flies, J. M. van Deursen, and L. Chen. 2004. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity 20:327-336. [DOI] [PubMed] [Google Scholar]
- 16.Dorfman, D. M., J. A. Brown, A. Shahsafaei, and G. J. Freeman. 2006. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am. J. Surg. Pathol. 30:802-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fishman, J. A., R. H. Rubin, M. J. Koziel, and B. J. Periera. 1996. Hepatitis C virus and organ transplantation. Transplantation 62:147-154. [DOI] [PubMed] [Google Scholar]
- 18.Fuller, M. J., D. A. Hildeman, S. Sabbaj, D. E. Gaddis, A. E. Tebo, L. Shang, P. A. Goepfert, and A. J. Zajac. 2005. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J. Immunol. 174:5926-5930. [DOI] [PubMed] [Google Scholar]
- 19.Gallimore, A., A. Glithero, A. Godkin, A. C. Tissot, A. Pluckthun, T. Elliott, H. Hengartner, and R. Zinkernagel. 1998. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187:1383-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerlach, J. T., H. M. Diepolder, M. C. Jung, N. H. Gruener, W. W. Schraut, R. Zachoval, R. Hoffmann, C. A. Schirren, T. Santantonio, and G. R. Pape. 1999. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology 117:933-941. [DOI] [PubMed] [Google Scholar]
- 21.Greenwald, R. J., G. J. Freeman, and A. H. Sharpe. 2005. The B7 family revisited. Annu. Rev. Immunol. 23:515-548. [DOI] [PubMed] [Google Scholar]
- 22.Gruener, N. H., F. Lechner, M. C. Jung, H. Diepolder, T. Gerlach, G. Lauer, B. Walker, J. Sullivan, R. Phillips, G. R. Pape, and P. Klenerman. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 75:5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He, X. S., B. Rehermann, F. X. Lopez-Labrador, J. Boisvert, R. Cheung, J. Mumm, H. Wedemeyer, M. Berenguer, T. L. Wright, M. M. Davis, and H. B. Greenberg. 1999. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl. Acad. Sci. USA 96:5692-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isogawa, M., Y. Furuichi, and F. V. Chisari. 2005. Oscillating CD8(+) T cell effector functions after antigen recognition in the liver. Immunity 23:53-63. [DOI] [PubMed] [Google Scholar]
- 25.Iwai, Y., S. Terawaki, M. Ikegawa, T. Okazaki, and T. Honjo. 2003. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp. Med. 198:39-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191-1198. [DOI] [PubMed] [Google Scholar]
- 27.Lang, K. S., M. Recher, A. A. Navarini, N. L. Harris, M. Lohning, T. Junt, H. C. Probst, H. Hengartner, and R. M. Zinkernagel. 2005. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur. J. Immunol. 35:738-745. [DOI] [PubMed] [Google Scholar]
- 28.Latchman, Y., C. R. Wood, T. Chernova, D. Chaudhary, M. Borde, I. Chernova, Y. Iwai, A. J. Long, J. A. Brown, R. Nunes, E. A. Greenfield, K. Bourque, V. A. Boussiotis, L. L. Carter, B. M. Carreno, N. Malenkovich, H. Nishimura, T. Okazaki, T. Honjo, A. H. Sharpe, and G. J. Freeman. 2001. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2:261-268. [DOI] [PubMed] [Google Scholar]
- 29.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Missale, G., R. Bertoni, V. Lamonaca, A. Valli, M. Massari, C. Mori, M. G. Rumi, M. Houghton, F. Fiaccadori, and C. Ferrari. 1996. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J. Clin. Investig. 98:706-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muhlbauer, M., M. Fleck, C. Schutz, T. Weiss, M. Froh, C. Blank, J. Scholmerich, and C. Hellerbrand. 2006. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J. Hepatol. 45:520-528. [DOI] [PubMed] [Google Scholar]
- 32.Obar, J. J., S. G. Crist, D. C. Gondek, and E. J. Usherwood. 2004. Different functional capacities of latent and lytic antigen-specific CD8 T cells in murine gammaherpesvirus infection. J. Immunol. 172:1213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paiardini, M., B. Cervasi, H. Albrecht, A. Muthukumar, R. Dunham, S. Gordon, H. Radziewicz, G. Piedimonte, M. Magnani, M. Montroni, S. M. Kaech, A. Weintrob, J. D. Altman, D. L. Sodora, M. B. Feinberg, and G. Silvestri. 2005. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J. Immunol. 174:2900-2909. [DOI] [PubMed] [Google Scholar]
- 34.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203:2281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reichard, O., R. Schvarcz, and O. Weiland. 1997. Therapy of hepatitis C: alpha interferon and ribavirin. Hepatology 26:108S-111S. [DOI] [PubMed] [Google Scholar]
- 36.Schluns, K. S., W. C. Kieper, S. C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426-432. [DOI] [PubMed] [Google Scholar]
- 37.Shiow, L. R., D. B. Rosen, N. Brdickova, Y. Xu, J. An, L. L. Lanier, J. G. Cyster, and M. Matloubian. 2006. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440:540-544. [DOI] [PubMed] [Google Scholar]
- 38.Shlapatska, L. M., S. V. Mikhalap, A. G. Berdova, O. M. Zelensky, T. J. Yun, K. E. Nichols, E. A. Clark, and S. P. Sidorenko. 2001. CD150 association with either the SH2-containing inositol phosphatase or the SH2-containing protein tyrosine phosphatase is regulated by the adaptor protein SH2D1A. J. Immunol. 166:5480-5487. [DOI] [PubMed] [Google Scholar]
- 39.Shoukry, N. H., A. Grakoui, M. Houghton, D. Y. Chien, J. Ghrayeb, K. A. Reimann, and C. M. Walker. 2003. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 197:1645-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spangenberg, H. C., S. Viazov, N. Kersting, C. Neumann-Haefelin, D. McKinney, M. Roggendorf, F. von Weizsacker, H. E. Blum, and R. Thimme. 2005. Intrahepatic CD8+ T-cell failure during chronic hepatitis C virus infection. Hepatology 42:828-837. [DOI] [PubMed] [Google Scholar]
- 41.Sun, S., X. Zhang, D. F. Tough, and J. Sprent. 1998. Type I interferon-mediated stimulation of T cells by CpG DNA. J. Exp. Med. 188:2335-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trautmann, L., L. Janbazian, N. Chomont, E. A. Said, G. Wang, S. Gimmig, B. Bessette, M. R. Boulassel, E. Delwart, H. Sepulveda, R. S. Balderas, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2006. Upregulation of PD-1 expression on HIV-specific CD8 + T cells leads to reversible immune dysfunction. Nat. Med. 12:1198-1202. [DOI] [PubMed] [Google Scholar]
- 44.Urbani, S., B. Amadei, D. Tola, M. Massari, S. Schivazzappa, G. Missale, and C. Ferrari. 2006. PD-1 expression in acute hepatitis C is associated with hepatitis C virus-specific CD8 exhaustion. J. Virol. 80:11398-11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urbani, S., C. Boni, G. Missale, G. Elia, C. Cavallo, M. Massari, G. Raimondo, and C. Ferrari. 2002. Virus-specific CD8+ lymphocytes share the same effector-memory phenotype but exhibit functional differences in acute hepatitis B and C. J. Virol. 76:12423-12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wasley, A., and M. J. Alter. 2000. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin. Liver Dis. 20:1-16. [DOI] [PubMed] [Google Scholar]
- 47.Wedemeyer, H., X. S. He, M. Nascimbeni, A. R. Davis, H. B. Greenberg, J. H. Hoofnagle, T. J. Liang, H. Alter, and B. Rehermann. 2002. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J. Immunol. 169:3447-3458. [DOI] [PubMed] [Google Scholar]
- 48.Wherry, E. J., D. L. Barber, S. M. Kaech, J. N. Blattman, and R. Ahmed. 2004. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA 101:16004-16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization. 1999. Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J. Viral Hepat. 6:35-47. [PubMed] [Google Scholar]
- 50.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]