Abstract
Previously, we found that human papillomavirus type 16 (HPV-16) E5 protein is a tumor rejection antigen and can induce cytotoxic T-lymphocyte (CTL) activity. Therefore, in this study, human leukocyte antigen A*0201 (HLA-A*0201)-restricted human CTL epitopes of HPV-16 E5 protein were identified using a bioinformatics approach, and the abilities of these predicted peptides to induce an immune response in HLA-A*0201 transgenic mice were confirmed by assaying E5-specific CTLs and in vitro-generated CTLs from normal peripheral blood T lymphocytes of HLA-A2-positive human donors. Second, the CTL responses to HLA-A*0201 CTL epitopes (E5 63-71 and E7 11-20) were examined in HPV-16-infected patients with HLA-A2. Third, the effect of HLA-A-type alleles on CTL activities in response to the entire E5 and E7 proteins was examined in cervical cancer patients. E5 and E7 peptides (but not the whole proteins) stimulated E5- and E7-specific CTL recall responses in HPV-16- and HLA-A2-positive cervical cancer patients, and HPV-16 E5 and E7 proteins stimulated naïve T cells in HPV-16-negative cervical cancer patients with HLA-A11 and -A24 haplotypes. In summary, this is the first demonstration that E5 63-71 is an HLA-A*0201-restricted T-cell epitope of HPV-16 E5.
Cervical cancer accounts for almost 12% of all cancers in women and thus represents the second most frequent gynecological malignancy in the world (50). Cervical cancer is caused by specific human papillomavirus (HPV) infections. HPV type 16 (HPV-16) is the predominant etiologic agent of cervical cancer and carries three transforming oncogenes, E5, E6, and E7. E6 and E7 expression has been found in cervical cancer cell lines and cancer biopsy specimens, and the two proteins play roles in malignant transformation. They are independently able to immortalize various human cell types in tissue culture, but the efficiency of transformation is increased when they are coexpressed (50).
HPV-16 E5 protein stimulates cell growth by forming a complex with the epidermal growth factor receptor, ErbB4, platelet-derived growth factor receptor, and colony-stimulating factor 1 receptor (12, 16, 41, 50). In vivo studies have demonstrated that E5 is expressed soon after infection. E5 mRNA and protein are detectable in low-grade squamous intraepithelial lesions (2, 9), and the prevalence of E5-containing mRNA increases with the advancing severity of disease (2). However, as HPV-infected lesions progress to cervical cancer, episomal viral DNA frequently becomes integrated into the host cell DNA, and a substantial part of the genome, commonly including the E5 coding sequence, is deleted (16, 41, 50). Thus, E5 expression is not obligatory in late events of HPV-mediated carcinogenesis.
The viral oncoproteins are unique tumor antigens and can be ideally used as tumor vaccines (32, 33). HPV-16 E5, E6, and E7 have been experimentally identified as target antigens by immune intervention protocols against cervical cancer. Evidence for increased tumor incidence in T-cell-immunosuppressed patients strongly suggests that CD4 and/or CD8 T-cell responses play a vital role in controlling HPV infection (45). T-cell-mediated immunity is thought to be important in the control of HPV infection. This is supported by histological evidence of T-cell infiltration into both cutaneous and mucosal lesions during the spontaneous regression of tumors (45). Moreover, cytotoxic T-lymphocyte (CTL) responses to E6 or E7 are more commonly detected in HPV-16-positive women without cervical intraepithelial neoplasia than in HPV-16-positive women with cervical intraepithelial neoplasia (27). Until now, the CTL response to HPV-16 E5 protein has not been understood.
Previously, we identified the HPV-16 E5 protein as a tumor rejection antigen (22) and peptide E5 25-33 (VCLLIRPLL) as a Db-restricted CTL epitope that has the ability to elicit antitumor immunity in C57BL/6 mice (13). In this study, human leukocyte antigen A*0201 (HLA-A*0201)-restricted human CTL epitopes of HPV-16 E5 protein were further investigated, and the HPV-16 E5 63-71 peptide was identified as an HLA-A*0201-restricted CTL epitope. CTL responses to HPV-16 E5 63-71 and E7 11-20 in patients and healthy individuals were measured by infecting their blood lymphocytes in vitro with recombinant adenovirus (rAd) encoding HPV-16 E5 (rAd-16E5) or E7 (rAd-16E7). CTL responses to E5 and E7 T-cell epitopes (E5 63-71 and E7 11-20), as well as the whole E5 and E7 proteins, in HPV-16-infected cervical cancer patients were evaluated. Additionally, we investigated whether HLA-A-type alleles could influence the generation of E5- and E7-specific CTL clones in cervical cancer patients.
MATERIALS AND METHODS
Peptide synthesis.
To determine potential vaccine candidates, 13 potential HLA-A*0201 binding peptides of HPV-16 E5 protein were synthesized (Fig. 1A) by solid-phase strategies on an automated peptide synthesizer (Abimed AMS 422; Abimed, Langenfeld, Germany) using 9-fluorenylmethoxy carbonyl chemistry and purified by reverse-phase high-performance liquid chromatography. The peptides were lyophilized and dissolved in phosphate-buffered saline with less than 1% dimethyl sulfoxide in stock concentrations of 5 mg/ml. The peptide solutions were stored at −70°C.
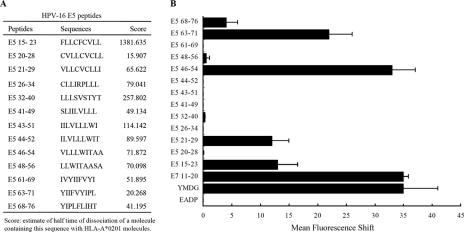
FIG. 1.
Identification of HLA-A*0201-restricted epitopes of HPV-16 E5 protein by T2 cell-binding assays. (A) Computer prediction of HPV-16 E5 peptides. (B) Stabilization of HLA-A*0201 molecules on the surfaces of T2 cells. T2 cells were incubated with peptides overnight at 26°C and then incubated at 37°C for 2 h, as described in Materials and Methods. HLA-A*0201 expression was determined by FACS staining with the monoclonal antibody PA2.1, and the mean fluorescence intensity (MFI) was calculated. The x axis indicates the mean fluorescence shift of T2 cells with tested peptide subtracted from that of T2 cells without peptide. Three experiments were conducted. The data are shown in the form of a histogram. YMDG, the HLA-A*0201 epitope of the tyrosinase-derived peptide YMDGTMSQV, which served as a positive control; E7 11-20, YMLDLQPETT, identified as an HLA-A2-specific CTL epitope of HPV-16 E7 protein, which also served as a positive control; EADP, the HLA-A1 binding peptide EADPTGHSY, which served as a negative control. The MFI values for the positive controls, YMDG and E7 11-20, were 36.8 ± 1.4 and 37.2 ± 7.6, respectively. The MFI value for the negative control, EADP, was 1.93 ± 0.7. The error bars indicate standard deviations.
T2 cell-binding assay: stabilization of HLA-A*0201 on the surfaces of T2 cells by synthetic peptides.
The T2 cell-binding assay has been described previously (8, 44). Briefly, to measure the dissociation rate of peptides from HLA-A*0201, T2 cells incubated with RPMI 1640 containing 5% fetal bovine serum were mixed with peptide (10 μg/ml RPMI containing 5% fetal bovine serum) and human β2-microglobulin (5 μg/ml) for 20 h at 26°C and subsequently shifted to 37°C for 2 h. Then, the cells were stained for HLA-A*0201 expression with fluorescein isothiocyanate (FITC)-labeled anti-human HLA-A*0201 (BD Biosciences Pharmingen, San Diego, CA), fixed with 1% paraformaldehyde, and analyzed by flow cytometry. The results were expressed as the geometric mean channel fluorescence after subtraction of the value obtained from cells that had not been incubated with peptide.
Intracytoplasmic cytokine staining and flow cytometry analysis.
HLA-A*0201 transgenic mice were purchased and imported from the Jackson Laboratory (Bar Harbor, ME) and maintained in our institute under specific-pathogen-free conditions. The transgenic mice with C57BL/6 background expressing major histocompatibility complex class I (MHC-I) molecules of human HLA-A*0201 have been described previously (7, 44). Splenocytes from peptide-vaccinated HLA-A*0201 transgenic mice or controls were incubated for 12 h with stimulator (HPV-16 E5 synthetic peptides) for the detection of E5 peptide-specific CD8+ T-cell precursors. Golgistop (PharMingen, San Diego, CA) was added, and the cells were then stained with FITC-conjugated rat anti-mouse CD8b.2 (Ly-3.2) monoclonal antibody (PharMingen). The cells were subjected to intracellular-cytokine staining using the Cytofix/Cytoperm kit according to the manufacturer's instructions (PharMingen). Phycoerythrin-conjugated rat anti-mouse gamma interferon (IFN-γ) monoclonal antibody was purchased from PharMingen. Fluorescence-activated cell sorter (FACS) analysis was performed on a Becton Dickinson FACScan with CELLQuest software (Becton Dickinson Immunocytometry Systems, Mountain View, CA).
In vitro cytolytic assay.
The in vitro cytolytic activity of T lymphocytes was measured using the recently published fluorometric assessment of T-lymphocyte antigen-specific lysis (FATAL) assay (36). The target cells (CIR-A2) were labeled with PKH-26 according to the manufacturer's instructions (Sigma, St. Louis, MO; final concentration, 2.5 × 10−6 M) (36). The PKH-26-labeled target cells were further stained with 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE) (final concentration, 2.5 × 10−6 M; Molecular Probes, Eugene, OR) and then dispensed in duplicate at 5 × 103 cells per well into 96-well U-bottom plates (Becton Dickinson). Effector cells were added at various effector-to-target (E:T) ratios and mixed with the target cells. The FATAL assay was conducted and analyzed by flow cytometry within 24 h.
Construction and generation of recombinant adenovirus containing HPV-16 E5 and E7 genes.
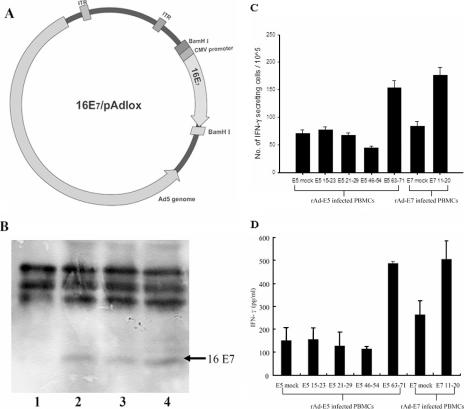
Generation of replication-deficient recombinant adenovirus carrying the HPV-16 E5 gene was described previously (22, 43). To generate replication-deficient recombinant adenoviruses carrying the HPV-16 E7 gene, we isolated a 0.3-kb BamHI fragment from HPV-16E7/pCEP4 and ligated it to pAdlox (43), after which it was named 16E7/pAdlox (see Fig. 4A). The replication-defective recombinant adenoviruses (rAd-16E7) were generated as described previously (22, 43). In rAd-16E7, E7 gene expression was driven by a minimal human cytomegalovirus early promoter. To detect the expression of the E7 protein, 293 cells were infected with rAd-16 E7 at a multiplicity of infection of 25 or 50 (see Fig. 4B, lanes 3 and 4, respectively); the positive control was CaSki cells containing about 60 to 600 copies of HPV-16 (lane 2). Forty-eight hours after infection, total cellular proteins were extracted and an immunoprecipitation assay and Western blot analysis with HPV-16 E7 antibody (ED-17; Santa Cruz Biotechnology, Inc.) was performed. We found that rAd-16E7-infected cells could express the 19-kDa E7 protein (see Fig. 4B).
FIG. 4.
Identification of an HLA-A*0201-restricted CTL epitope of HPV-16 E5 protein in normal human PBLs by in vitro vaccination of rAd-16E5 or rAd-16E7. (A) Construction and generation of recombinant adenovirus encoding HPV-16 E7. The plasmid 16E7/pAd-lox was the recombinant adenovirus vector containing the HPV-16 E7 gene. (B) Expression of the E7 protein by cells transduced with rAd16-E7. Lane 1, 293 cells infected with rAd-GFP; lane 2, CaSki cells as a positive control; lanes 3 and 4, 293 cells infected at a multiplicity of infection of 25 and 50 with rAd16-E7. (C) IFN-γ production was determined by ELISPOT assay. In vitro vaccination of HLA-A*0201 lymphocytes from healthy human donors with rAd-16E5 or rAd-16E7 is shown. Human PBLs were cocultured with autologous adherent cells that had been infected with rAd-16E5 or rAd-16E7 twice 1 week apart. Twenty-four hours after the boost vaccination, CD8+ lymphocytes were isolated from the vaccinated blood lymphocytes and stimulated with each indicated peptide. The results are expressed as means plus standard deviations, and each value represents the mean of six replicates. (D) IFN-γ production was determined by ELISA. The data represent the means and standard errors for five healthy HLA-A*0201 donors. The y axis denotes the concentration of IFN-γ produced. E5 mock and E7 mock indicate phosphate-buffered saline treatment.
Cell lines.
The cervical carcinoma cell line CaSki (CRL-1550) was obtained from the American Type Culture Collection (Manassas, VA); it is an HLA-A*0201-positive cervical carcinoma cell line expressing the HPV16 E6 and E7 proteins. CaSki cells were infected with rAd-16E5 as target cells 2 days before a 51Cr release cytotoxic T-lymphocyte assay.
Patients and healthy blood donors for HPV and HLA-A allele typing.
Sixty-three patients who presented with histologically proven cervical carcinoma at the Department of Gynecology, Mackay Memorial Hospital, Taipei, Taiwan, were enrolled. The Human Subjects Review Committee approved the protocol of this study, and written informed consent was obtained from each patient. All subjects had FIGO (International Federation of Gynecologists and Obstetricians) stage IA/IIIB and were treated by radical hysterectomy or concurrent chemoradiotherapy. Demographic information and time intervals between treatment and blood sampling are shown in Table 1. The subjects were typed for HPV-6, HPV-11, HPV-16, HPV-18, HPV-31, HPV-33, HPV-42, HPV-52, and HPV-58 using DNA isolated from cervical swab specimens, paraffin-embedded sections of biopsy specimens, or surgical resection specimens, and the DNA was amplified by PCR using consensus primers from the L1 region (11). Young women with no sexual experience were enrolled as healthy blood donors. For HLA-A typing, blood samples were collected in acid citrate dextrose tubes and transported within 24 h to the Immunohematology Reference Laboratory, Mackay Memorial Hospital. DNA typing of HLA-A loci was first performed using the Dynal RELI SSO HLA-A, -B, and -DR Typing Kit (Dynal Biotech S. A., Compiegne, France) according to the manufacturer's recommendations (10). High-resolution PCRs with sequence-specific-primer typing kits (Unitray and Pel-Freez; Dynal Biotech S. A., Compiegne, France) was performed in cases where the intermediate-resolution typing results were ambiguous.
TABLE 1.
Demographic information for cervical cancer patients
| Parameter | Value |
|---|---|
| Age (yr) | |
| Mean | 49.3 |
| Range | 30-74 |
| FIGO stage (no.) | |
| IA/IB | 6/43 |
| IIA/IIB | 10/3 |
| IIIA/IIIB | 0/1 |
| Treatment modality (no.) | |
| Radical hysterectomy | 40 |
| Concurrent chemoradiotherapy | 23 |
| Adjuvant chemotherapy (no.; n = 40) | |
| Yes | 9 |
| No | 31 |
| Postoperative radiotherapy (no.; n = 40) | |
| Yes | 10 |
| No | 30 |
| Interval of blood sampling after treatment (mo) | |
| Median | 30.8 |
| Range | 1.4-178.4 |
CTL generation and IFN-γ assay from healthy and HPV-16-negative patients.
Healthy donors and HPV-16-negative patients were assumed not to have experienced HPV-16 infection; therefore, we performed in vitro vaccination by long-term infection of rAd-16E5 or E7 to generate HPV-16 E5- or E7-specific CTLs from naïve T cells. Human peripheral blood lymphocytes (PBLs) from this population were separated by Ficoll-Hypaque density gradients and were incubated on plastic dishes (20, 49), the nonadherent lymphocytes were aspirated, and the adherent fraction was cultured in medium containing 1% pooled human AB serum, recombinant granulocyte-macrophage colony-stimulating factor (1,000 IU/ml), and recombinant human interleukin 4 (1,000 IU/ml) (R&D Systems, Minneapolis, MN). On the second day after isolation, the adherent cells were infected with rAd-16E5 (22) or rAd-16E7 at an appropriate multiplicity of infection overnight. The next day, the infected cells were treated with mitomycin C (25 μg/ml), mixed with 5 × 106 autologous PBLs (nonadherent cells) for 1 week, and then reinoculated (boosted) with the same recombinant adenovirus. Twenty-four hours after this boost, purification of CD8+ T cells was performed twice with magnetic microbeads coated with anti-CD8+ antibody in accordance with the manufacturer's recommendations (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), and the cells were separated on an Automacs device (Miltenyi Biotech GmbH) using the possel program (46). Then, the isolated CD8+ T cells were stimulated with the indicated peptide, and the following day, the supernatants were collected and analyzed by enzyme-linked immunosorbent assay (ELISA) for peptide-specific IFN-γ release by CD8+ T cells, using an ELISA kit (Quantikine, R&D Systems, Minneapolis, MN).
ELISPOT assay.
An enzyme-linked immunospot (ELISPOT) assay was performed to assess the IFN-γ production of T cells, using a human IFN-γ ELISPOT kit (BD Biosciences, San Diego, CA). The isolated CD8+ T cells, as described above, were plated at 100,000 per well with different peptides in a total volume of 200 μl/well on a BD ELISPOT plate, and the IFN-γ-secreting spots were analyzed using an automatic plate reader (Elispot Reader System; AID, Strassberg, Germany).
Chromium release assay for cytotoxicity.
Cell-mediated cytotoxicity was measured using a 51Cr release assay performed using standard protocols (22). The 51Cr-labeled target cells were added to V-bottom 96-well microtiter plates and incubated with the effector cells at various E:T ratios. The 51Cr release was counted in a TopCount microplate scintillation counter (Canberra-Packard, Pangbourne, United Kingdom). The mean percentage of specific lysis of triplicate wells was calculated as follows: percent specific lysis = [(cpm experimental release − cpm spontaneous release)/(cpm maximum, 1% Triton X-100, release − cpm spontaneous release)] × 100%.
CTL activity and IFN-γ assay for HPV-16-positive cervical cancer patients.
The recall response of PBLs from HPV-16-positive patients was measured. The PBLs were obtained and treated as described above, except infection with either rAd-E5, rAd-E7, or rAd-GFP was for only 1 day instead of 8 days (i.e., incubation overnight, treatment with mitomycin C, mixing with autologous PBLs [nonadherent cells] for 1 week, and finally reinoculation [boosting]). The isolation of CD8+ lymphocytes and assay of IFN-γ production were as indicated above.
RESULTS
Identification of HLA-A*0201-restricted epitopes of HPV-16 E5 protein by T2 cell-binding assays.
We searched the primary structure of HPV-16 E5 for sequences fitting the allele-specific motifs of the human MHC-I molecules of HLA-A*0201. Using an HLA peptide binding prediction program offered by the National Institutes of Health (http://thr.cit.nih.gov/molbio/hla_bind/), the potential CTL epitopes were scored by estimating the dissociation half-life of a molecule containing the 9-amino-acid sequences from MHC class I molecules. Thirteen potential E5-specific HLA-A*0201 binding peptides were synthesized (Fig. 1A) and were then subjected to a T2 cell-binding assay. The T2 cell line that we used is deficient in TAP1 and TAP2 transporter proteins and expresses low levels of HLA-A*0201 (23). Upon incubation with T2 cells, HLA-A*0201 binding peptides should form MHC-peptide complexes on the cell surface. HLA-A*0201 expression was quantitated by FACS staining with FITC-labeled anti-human HLA-A*0201, and the mean fluorescence intensity was calculated. The tyrosinase-derived peptide YMDGTMSQV (YMDG) (47) and the HPV-16 E7 11-20 peptide YMLDLQPETT (31) served as positive controls, and the HLA-A1 binding peptide EADPTGHSY (EADP) served as a negative control (48). The results shown in Fig. 1B revealed four peptides (E5 15-23, 21-29, 46-54, and 63-71) of HPV-16 E5 that exhibited consistently strong binding to HLA-A*0201 molecules from three independent experiments.
CTL epitopes of HPV-16 E5 mapped through vaccination of HLA-A*0201 transgenic mice.
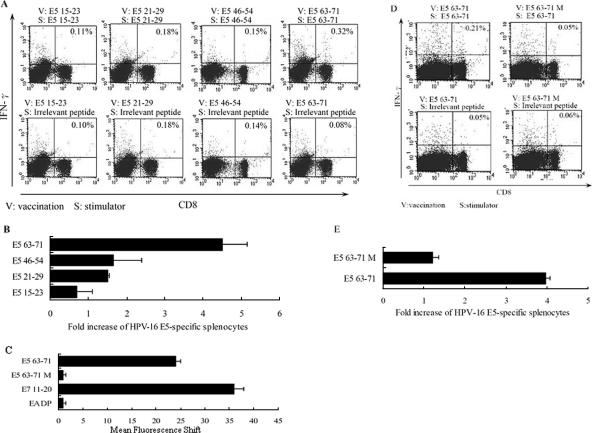
In this study, HLA-A*0201 CTL epitopes of HPV-16 E5 were mapped by inoculating HLA-A*0201 transgenic mice with the rAd-E5 gene (13, 22). Two weeks after vaccination, splenocytes were harvested and stimulated with each T2 cell-binding peptide or nonbinding peptide, and effector cells were double stained for CD8+ IFN-γ+ and assayed by flow cytometry (13, 21). The T2 cell-binding peptides E5 15-23, 21-29, 46-54, and 63-71 did not increase the number of E5-specific CD8+ IFN-γ+ T cells compared to the control group (data not shown). Therefore subsequent immunization experiments concentrated on determining if these four T2-binding peptides (Fig. 1B) could induce peptide-specific CD8+ IFN-γ+ T cells in vivo. Each of the four T2 cell-binding peptides was used as a peptide vaccine with CpG phosphorothioate oligodeoxynucleotide 1826 (CpG ODN 1826) as an adjuvant (13). Each group of five HLA-A*0201 transgenic mice was immunized with one of the epitopes plus CpG ODN 1826 via intramuscular injection three times per week. Five days after the third immunization, we measured antigen-specific CD8+ IFN-γ+ double-positive cells in HLA-A*0201 transgenic mice by flow cytometric analyses. As shown in Fig. 2A and B, the number of antigen-specific CD8+ IFN-γ+ double-positive cells induced by peptide E5 63-71 was about fourfold higher than that with the irrelevant stimulator, and the other three peptides did not induce any significant CD8+ IFN-γ+ double-positive cells. This implied that peptide E5 63-71 might be an HLA-A*0201-restricted CTL epitope of HPV-16 E5.
FIG. 2.
Identification of HLA-A*0201-restricted epitopes of HPV-16 E5 protein by vaccination of HLA-A*0201 transgenic mice with peptide plus CpG ODN 1826. Four- to 6-week-old HLA-A*0201 transgenic mice were immunized three times each with T2 cell-binding peptides plus CpG ODN 1826 (12) at 1-week intervals, with five mice in each test group. Five days after the last vaccination, splenocytes were harvested and stimulated with each indicated peptide; then, intracellular-cytokine staining with flow cytometry was performed to determine the number of CD8+ IFN-γ+ double-positive cells. (A) splenocytes from vaccinated mice were stimulated in vitro with the indicated peptide and stained with CD8 and IFN-γ antibodies. The results of one representative assay from three identical independent experiments are shown. The percentage of CD8+ and IFN-γ+ double-positive cells in the gated T-cell populations are shown in the upper corners of the plots. (B) Summary of the three independent experiments. The data represent the means and standard errors of three experiments. The x-axis values were calculated as follows: increase of E5-specific splenocytes = (number of vaccinated splenocytes stimulated with indicated peptide)/(number of vaccinated splenocytes stimulated with irrelevant peptide) × 100%. (C) The T2 cell-binding activities of the wild-type peptide E5 63-71 and the mutant E5 63-71 M. The synthesized wild-type E5 63-71 peptide sequence is YIIFVYIPL, and that of the mutant E5 63-71 is YGIFVYIPG. The measurement of T2 cell binding is described in the legend to Fig. 1B. (D) Splenocytes from vaccinated mice were stimulated in vitro with the E5 63-71 or E5 63-71 M peptide and stained with CD8 and IFN-γ antibodies as described for panel A. (E) Summary of the three independent experiments.
Moreover, to confirm E5 63-71 as a specific CTL epitope, we synthesized a mutant E5 63-71 peptide and tested its immunogenicity. Since the putative HLA-A*0201 anchor-binding motifs (8- to 11-mer) require the amino acid L, I, M, V, or A at position 2 and V, L, or A at the C terminus (14), we synthesized a mutant E5 63-71 peptide (YGIFVYIPG) in which the amino acids at the 2′ and C′ positions were changed from I and L in the wild-type E5 63-71 (YIIFVYIPL) to G and G, respectively. Subsequently, we performed T2 cell-binding assays as described previously to measure whether mutant E5 63-71 (E5 63-71 M) could bind to HLA-A*0201 molecules. As shown in Fig. 2C, E5 63-71 M had no binding affinity for HLA-A*0201 molecules. Then we vaccinated HLA-A*0201 transgenic mice with E5 63-71 M plus CpG ODN 1826 and evaluated the CD8+ IFN-γ+ double-positive cells as described above. Figure 2D and E show that E5 63-71 M could not induce an immune response, confirming that peptide E5 63-71 should be considered an HLA-A*0201-restricted CTL epitope of HPV-16 E5 protein.
In vitro cytotoxic T-lymphocyte lysis activity by peptide E5 63-71.
The recently published FATAL assay for detecting cytotoxic T-lymphocyte-mediated lysis is a nonradioactive alternative to the traditional Cr51 release assay. Here, we conducted FATAL assays to determine CTL activity. Peptide-pulsed CIR-A2 target cells were stained with the two dyes PKH-26 and CFSE and afterwards cocultured with unstained splenocytes from peptide-vaccinated transgenic mice (effector cells) as described in Materials and Methods. The double-dyed target cells incubated in the absence of effector cells (indicating spontaneous CFSE release) were used for comparative analysis. As shown in Fig. 3A, effector cells resulting from the vaccination with E5 63-71 peptide elicited the highest CTL activity compared to the other three T2 binding peptides. Moreover, Fig. 3B shows that the CTL activity of E5 63-71-vaccinated mice was proportional to the E:T ratio in the FATAL assay. In sum, peptide E5 63-71 can induce in vitro cytotoxic T-lymphocyte lysis activity in HLA-A*0201 transgenic mice.
FIG. 3.
HPV-16 E5-specific CTL responses in HLA-A*0201 transgenic mice. Four- to 6-week-old HLA-A*0201 transgenic mice were vaccinated three times with each T2 cell-binding peptide plus CpG ODN 1826 at 1-week intervals, with five mice in each test group. Five days after the last vaccination, effector splenocytes were collected and analyzed in in vitro CTL assays as described in Materials and Methods. Target CIR-A2 cells were pulsed with the indicated peptides. (A) CTL activity of each peptide induction. The ratio of effector to target cells from each group of vaccinated mice (five mice) was 100. The data are the average values for five vaccinated mice. The error bars indicate standard deviations. (B) CTL activities from various ratios of effector to target cells. The effector cells were from mice vaccinated with E5 63-71 peptide plus CpG ODN 1826. The target cells included CIR-A2 cells plus E63-71 peptide and CIR-A2 cells plus irrelevant peptide (E5 26-34).
In vitro vaccination of HLA-A2 lymphocytes from human healthy donors with rAd-16E5 or rAd-16E7.
Human PBLs from HPV-16 cervical cancer patients vaccinated with E7 peptides (31, 49), E7-containing tumor cells (17), soluble E7 proteins (27), virus-like particles carrying the E7 gene (20), or recombinant virus vectors carrying the E7 gene (6, 22, 28) have been reported by several groups. All of these results show that in vitro vaccination with various formulas of HPV-16 E7 antigen can trigger human E7-specific CTLs. Additionally; adenovirus-mediated delivery of antigenic DNA has been suggested as a feasible strategy for in vitro vaccination (29). More strikingly, one study has reported that HPV-16-E7-specific CTL clones can be generated from in vitro-vaccinated PBLs of healthy subjects (34). This observation means that mature dendritic cells can activate E7-specific CTLs from naïve precursors in vitro (19, 42). In the present study, recombinant adenovirus carrying the HPV-16 E5 or E7 gene was used to infect the adherent cells (dendritic cells and macrophages acting as antigen-presenting cells) separated from PBLs, as described in Materials and Methods. On the second day, the infected adherent cells were cocultured with autologous blood lymphocytes for 1 to 2 weeks to present E5 or E7 antigen to T cells. Then, CD8+ T cells were isolated from these blood lymphocytes. Less than 5% of these cells were CD4+ T cells and NK cells (data not shown). These isolated CD8+ T cells were stimulated with the indicated CTL peptides, and ELISPOT assays and IFN-γ ELISA were used to monitor which peptide could trigger IFN-γ production. As shown in Fig. 4C and D, one of the four HLA-A*0201 binding peptides of HPV-16 E5, peptide E5 63-71, stimulated the largest amount of IFN-γ in five HLA-A*0201 healthy donors by ELISPOT assay and ELISA, respectively. The positive control consisted of E7-vaccinated lymphocytes stimulated with the E7-specific HLA-A*0201 peptide 11-20. It indicated that human antigen-presenting cells could process endogenous E7 and E5 proteins for presentation of MHC-I complexes with E7 11-20 and with E5 63-71, respectively, to T cells. This further confirmed that E5 63-71 is an E5-specific HLA-A*0201 CTL epitope.
E5 63-71-specific T-cells exert HLA-A*0201-restricted cytolytic activity.
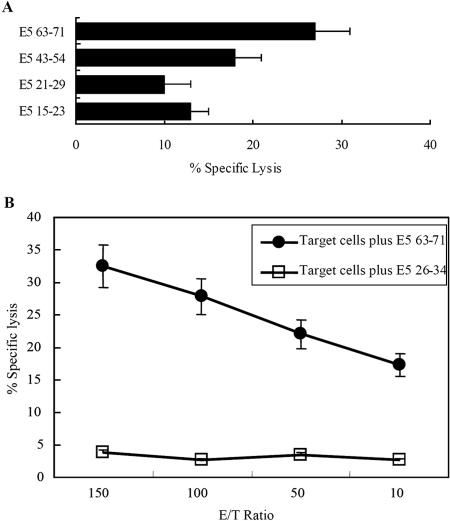
To determine whether E5 63-71- and E7 11-20-specific CTLs can kill tumor cells containing HPV-16 E5 or E7, we performed a standard 51Cr release cytotoxicity assay. Briefly, PBLs from HLA-A*0201-positive healthy human donors were stimulated with autologous antigen-presenting cells (dendritic cells and macrophages) infected with rAd-16E5 or rAd-16E7. The resulting HPV-16E5- or HPV16-E7-restricted T-cell lines were restimulated with E5 63-71 or E7 11-20 peptide and used as effector cells. As predicted, HPV-16-E7-restricted T-cell lines restimulated with E7 11-20 peptides could lyse HLA-A*0201-positive, 51Cr-loaded CaSki cell lines (Fig. 5B). IFN-γ ELISA (Fig. 4D) and ELISPOT assays (Fig. 4C) demonstrated that HPV16-E5-restricted T-cell lines (but not other control cells) restimulated with E5 63-71 peptides lysed E5-expressing CaSki cells (Fig. 5A). Assay of IFN-γ production of CD8+ cells by ELISA can thus be regarded as a measure of CTL activity, and so we used this method to assay CTL activity (below).
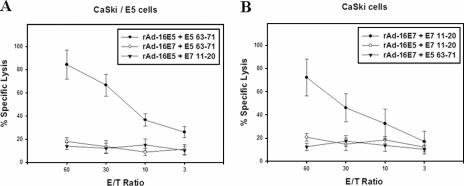
FIG. 5.
51Cr release cytotoxicity assay in normal human PBLs vaccinated in vitro with rAd-16E5 or rAd-16E7. Human PBLs from HLA-A*0201-positive healthy donors were cocultured with autologous adherent cells that had been infected with either rAd-16E5 or rAd-16E7 twice 1 week apart. Twenty-four hours after the boost vaccination, CD8+ lymphocytes were isolated from the vaccinated blood lymphocytes and stimulated with each indicated peptide. The target cells were CaSki/E5 cells (A) and CaSki cells (HPV-16 E7-expressing cells) (B). The results represent the means of triplicates; standard deviations are shown by the error bars.
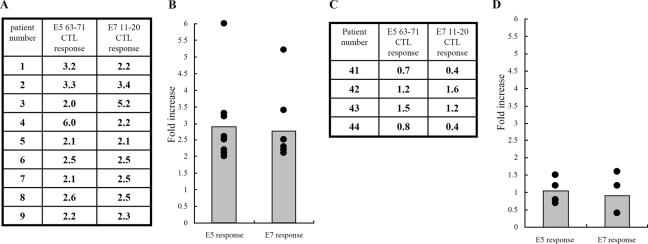
CTL responses to E5 63-71 and E7 11-20 in HPV-16-infected cervical cancer patients with HLA-A2.
To evaluate the existence of HLA-A*0201 peptide-specific recall CTL activity in HPV-16-infected cervical cancer patients, we infected blood lymphocytes isolated from cervical cancer patients with either rAd-E7, rAd-E5, or rAd-GFP (recombinant adenovirus encoding green fluorescent protein as a control) for 1 day. Then, CTL activities were measured after stimulation with either E7 11-20 or E5 63-71 peptide. Figure 6A shows the activation of CTL peptide-specific immune responses stimulated by E5 63-71 and E7 11-20 in individual HPV-16- and HLA-A2-positive cervical cancer patients. The increase is the ratio of the IFN-γ concentration resulting from peptide stimulation of rAd-E7-vaccinated CTLs to the IFN-γ concentration from mock stimulation of these cells. The summarized data (Fig. 6B) indicate that both E5 63-71 and E7 11-20 could significantly induce peptide-specific CTL responses in HPV-16-infected HLA-A2-positive cervical cancer patients, using an unpaired two-tailed t test (P = 0.017 for E5 63-71 and P = 0.05 for E7 11-20), but not in HPV-16-infected patients who were not HLA-A2 (Fig. 6C and D). By comparing Fig. 6A and C, it can be seen that stronger-than-average E7 11-20 memory responses occurred in patients 2 and 3, whereas stronger E5 63-71 memory was shown in patient 4 (Fig. 6A). Taken together, the memory response to E5 and E7 T-cell epitopes exists in HPV-16-infected HLA-A2 patients, and occasionally a strong recall response appears.
FIG. 6.
HLA-A*0201 peptide-specific CTL activity in HPV-16-positive cervical cancer patients. (A and B) E5 63-71 and E7 11-20 peptide-specific CTL responses in HPV-16-positive HLA-A2 cervical cancer patients. (A) Individual CTL responses to E5 63-71 and E7 11-20 peptides. ELISA was used to measure IFN-γ production in human CD8+ lymphocytes that were isolated from human PBLs infected in vitro with either rAd-E5, rAd-E7, or rAd-GFP for 24 h and then stimulated with either E5 63-71 peptide or E7 11-20. The increases are the IFN-γ concentrations after either E5 63-71 or E7 11-20 peptide stimulation divided by the IFN-γ concentrations after mock stimulation. (B) Summary of the data from panel A. (C and D) E5 63-71 and E7 11-20 peptide-specific CTL responses in HPV-16-positive non-HLA-A2 cervical cancer patients. The methods were the same as for panel A. (C) Individual CTL responses to E5 63-71 and E7 11-20 peptides. (D) Summary of data from panel C.
CTL responses to the entire E5 and E7 proteins in HPV-16-positive cervical cancer patients with different HLA-A haplotypes.
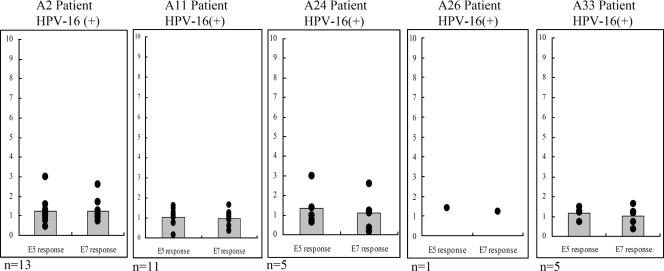
The HLA system is a major contributor to the etiology of infectious diseases and autoimmune disorders (10). To examine whether HLA-A-type alleles affect immune memory responses, E5 and E7 CTL activities were measured in HPV-16-positive cervical cancer patients with HLA-A2, -A11, -A24, -A26, and -A33 allele types. CTL responses to E5 and E7 proteins (IFN-γ production) were measured by ELISA in CD8+ lymphocytes of HPV-16-infected patients (i.e., PBLs were isolated and infected with either rAd-E5, rAd-E7, or rAd-GFP for 1 day). Increases in the ratio of the IFN-γ concentration produced by rAd-E5- or rAd-E7-infected cells to the IFN-γ concentration produced by rAd-GFP-infected cells were rare (Fig. 7). Two explanations for these weak responses to E5 and E7 are possible: (i) the existence of immune evasion in HPV-16-infected patients and (ii) low immunogenicity of HPV-16 E5 and E7 proteins.
FIG. 7.
HLA-A haplotype effects on recall CTL responses to HPV-16 E5 and E7 proteins in HPV-16-positive cervical cancer patients. Each panel shows CTL responses to the entire E5 and E7 proteins in each HLA haplotype. IFN-γ production by the isolated CD8+ lymphocytes was measured by ELISA 24 h after peripheral blood lymphocytes were infected with either rAd-E5, rAd-E7, or rAd-GFP. The increase is the IFN-γ concentration produced by either rAd-E5- or rAd-E7-infected lymphocytes divided by the IFN-γ concentration produced by rAd-GFP-infected lymphocytes. Each spot represents an individual CTL response to E5 or E7 protein. Each bar of the histogram represents the mean of all tested samples. n, the number of patients tested.
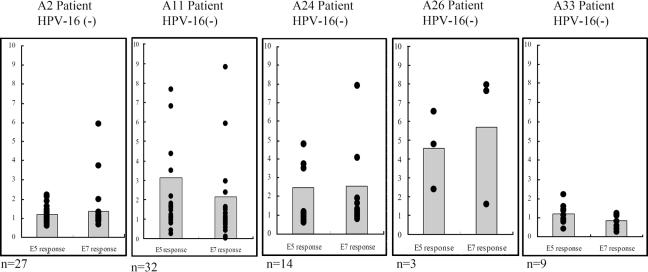
CTL response to the whole E5 and E7 proteins in HPV-16-negative cervical cancer patients with different HLA-A haplotypes.
To examine whether HLA-A haplotypes influence naïve T cells to generate E5- and E7-specific CTLs in HPV-16-negative cervical cancer patients, the experiments were performed in the same way as the above-described experiments in healthy individuals. The PBLs of HPV-16-negative patients were isolated and infected with either rAd-E5, rAd-E7, or rAd-GFP two times at a 1-week interval. CTL responses to E5 and E7 proteins (IFN-γ production) were measured as described above, and statistical analysis was performed using an unpaired two-tailed t test. As shown in Fig. 8, HLA-A11 (P = 0.009 for E5 63-71 and P = 0.048 for E7 11-20), as well as -A24 (P = 0.042 for E5 63-71 and P = 0.05 for E7 11-20), from HPV-16-negative cervical cancer patients could efficiently generate E5- and E7-specific CTL clones via in vitro vaccination compared with those from HPV-16-positive cervical cancer patients. However, HPV-16-negative cervical cancer patients with HLA-A2 (P = 0.906 for E5 63-71 and P = 0.794 for E7 11-20) and -A33 (P = 0.891 for E5 63-71 and P = 0.827 for E7 11-20) allele types could not efficiently stimulate naïve T cells. The sample size of HLA-A26 haplotypes was too small for statistical analysis. In sum, naïve T cells of HLA-A11 and -A24 haplotypes, but not HLA-A2 and -A33, can be stimulated by HPV-16 E5 and E7 proteins.
FIG. 8.
Effects of the HLA-A haplotype on HPV-16 E5 and E7 stimulation of naïve T cells to generate specific CTLs in HPV-16-negative cervical cancer patients. The generation of CTLs against HPV-16 E5 or E7 protein was examined in vitro in HPV-16-negative cervical cancer patients with HLA-A2, -A11, -A24, -A26, and -A33 allele types. Human PBLs were cocultured with autologous adherent cells that had been infected with either rAd-16E5 or rAd-16E7 for 1 week and then boosted with the same recombinant adenovirus. One day after the boost, CD8+ lymphocytes were isolated from the vaccinated blood lymphocytes and IFN-γ production was determined using an ELISA. Each spot represents an individual CTL response to E5 or E7 protein. Each bar of the histogram represents the mean of all tested samples. n, the number of patients tested. The increase is the IFN-γ concentration produced by either rAd-E5 or rAd-E7 infected lymphocytes divided by the IFN-γ concentration produced by rAd-GFP-infected lymphocytes.
DISCUSSION
Specific CTL epitopes could potentially be used for vaccination and the generation of CTL clones for adoptive transfer (5, 35). Previously, we found that HPV-16 E5 protein acts as a tumor rejection antigen and has oncogenic properties (13, 22). To avoid the oncogenic potential inherent in using E5 DNA vaccination, identification of human MHC class I CTL epitopes of HPV-16 E5 protein is very important. Here, we found that the HPV-16 E5 63-71 peptide was an HLA-A*0201-restricted CTL epitope. In this study, vaccination of HLA-A*0201 transgenic mice with the recombinant adenovirus carrying the E5 gene did not result in the presentation of HPV-16 E5 antigen to T cells (data not shown). This indicates that the antigen-presenting cells in the muscles of HLA-A*0201 transgenic mice may not be efficiently infected with the viral vector and the infected muscle cell cannot process antigen into MHC-I-peptide complexes. The cross-priming of antigen-presenting cells by infected muscle cells is important for eliciting an immune response. Similarly, it has been reported that immunization of HLA-A*0201 transgenic mice with the HPV-16 E7 11-20 epitope (but not the whole E7 protein) can elicit a CTL response (40). The previous study (40) and our present results imply that antigen-presenting cells of DNA-vaccinated mice do not efficiently present HPV-16 E5 and E7 antigens as human HLA-A*0201-restricted CTL epitopes to the appropriate precursors in the murine T-cell repertoire. This raises the question of whether human antigen-presenting cells can degrade the E5 protein into E5 CTL epitopes recognizable by human T cells. The results of our in vitro sensitization of human PBLs with E5 protein delivered by adenovirus showed that peptide E5 63-71 could stimulate E5-specific T cells (Fig. 4C and D and 5A), could induce cellular immune responses in healthy HLA-A*0201 donors, and is a naturally processed peptide in human antigen-presenting cells.
In terms of analyzing E5 and E7 CTL epitope-specific immune responses, both E5 63-71 and E7 11-20 stimulated peptide-specific CTL responses in HLA-A2- and HPV-16-positive cervical cancer patients (Fig. 6A and B), indicating T-cell memory for E5 and E7 epitopes. This is the first demonstration of an HLA-A*0201-restricted E5 63-71-specific memory response in HPV-16 and HLA-A2 patients. As to E7 CTL responses, our results are consistent with those of previous studies that demonstrated that HLA-A*0201-restricted HPV-16 E7 11-20 peptide-specific CTLs were present in peripheral blood, draining lymph nodes, and tumors of HPV-16-positive cervical cancer patients (4, 18, 25, 26, 30, 31, 49). Our report, along with the other reports, indicated that memory CTLs against E5 63-71 and E7 11-20 are present in HPV-16-infected patients. More interestingly, in HPV-16- and HLA-A2-positive cervical cancer patients, IFN-γ secretion by CD8+ cells cannot be stimulated by rAd-E5- and -E7-infected antigen-presenting cells (Fig. 7), but when E5 63-71 or E7 11-20 peptide is added, IFN-γ-production is significantly induced (Fig. 6A and B). It can be presumed that the T-cell responses in HPV-16- and HLA-A2-positive patients were induced by the presentation of these epitopes of E5 and E7 antigens on antigen-presenting cells. Hence, booster injections of these epitopes would be expected to cause E5 63-71- and E7 11-20-specific T-cell clone expansion and increased IFN-γ concentrations.
Furthermore, examination of CTL activities against the entire E5 and E7 proteins revealed that memory CTL cells were rare in all HPV-16-infected cervical cancer patients regardless of HLA haplotypes (Fig. 7), indicating that HPV-16 can evade immune recognition because of the absence of a recall response to the whole E5 and E7 proteins. Several reports have demonstrated that the levels of class I molecules are decreased (3, 15) and that local immune suppression factors (such as interleukin 10) or functional abnormalities of tumor infiltrate lymphocytes are present in neoplastic dysplasia and cervical cancers (37, 38). Additionally, HPV-16 E5 evades immune surveillance, since it can decrease MHC-I expression, retain MHC-I molecules in the Golgi apparatus, and prevent their transport to the cell surface (1). In this article, we provided clinical data to prove that though the whole E5 and E7 proteins of HPV-16 can evade host immune surveillance (Fig. 7), memory CTLs against E5 63-71 and E7 11-20 are present and can be elicited in an immune response (Fig. 6A and B). Current reports have also demonstrated that in clinical trials of E7 11-20 peptide vaccination, the majority of patients with high-grade cervical/vulvar dysplasia and cervical cancer had a detectable immune response in peripheral blood cells after injection of the peptide E7 11-20 vaccine (18, 19, 20, 24, 34, 39, 42). In cases of HPV evasion of host immune recognition, adoptive transfer of peptide-specific CTL clones may be a potential immunotherapeutic strategy to overcome host immunosuppression. Future T-cell-based immunotherapies for treatment of HPV-16-positive cervical cancer patients might include multiple-peptide vaccination (E5 and E7) or adoptive CTL (E5 and E7) transfer.
Our investigation of the effects of HLA haplotypes on the immune response found that memory T cells against HPV-16 E5 and E7 proteins were rare in all tested HLA-A allele types of HPV-16-infected patients (Fig. 7), indicating that HPV-16 infection can evade host immune surveillance regardless of HLA haplotypes. However, CTL cells (specific for the entire E5 or E7 protein) from HPV-16-negative cervical cancer patients of HLA-A11 and -A24, but not -A2 and -A33, allele types could be efficiently stimulated (Fig. 8). Here, HPV-16-negative cervical cancer patients were identified by the absence of HPV-16 DNA from cervical lesions, but not serologically, since no data were available. Because an immune response to HPV-16 E5 and/or E7 protein was induced in some HPV-16-negative patients (Fig. 8), two possibilities cannot be ruled out. First, these patients might have been previously infected with HPV-16, even though HPV-16 DNA was undetected at the time of our assay. Second, they might have been infected with other strains of HPV that had E5 and E7 antigens, which could have cross-reacted with their HPV-16 counterparts. However, when we screened the E5 and E7 amino acid sequences of other HPV types (such as HPV-6, -11, -18, -31, -33, -52, and -58) for potential CTL epitopes on the basis of predicted HLA-A*0201 peptide binding motifs as described above, no epitope had the same amino acid sequence as HPV-16 E5 63-71 and E7 11-20. Hence, a recall response to homologous amino acid sequences in other HPV types can be ruled out. On the other hand, it was interesting to find that the binding affinity of the epitope-MHC complex correlates with the epitope induction of CTL activity. A T2 cell-binding assay showed that 4 of 13 E5 peptides, predicted by bioinformatics to bind MHC molecules, bound strongly to HLA-A*0201 molecules (Fig. 1). In E5-specific cellular immunity assays in HLA-A*0201 transgenic mice (Fig. 2 and 3) and in vitro stimulation of human PBLs (Fig. 4C and D and 5A), the E5 63-71 peptide was the only HLA-A*0201-restricted CTL epitope.
In summary, this is the first demonstration that E5 63-71 is an HLA-A*0201-restricted T-cell peptide of HPV-16 E5. New strategies for immunotherapy are suggested by the existence of memory T cells specific for E5 63-71 and E7 11-20 peptides, but not the whole E5 and E7 proteins, in HPV-16-positive cervical cancer patients. Stimulation of naïve T cells may efficiently generate CTLs specific for the E5 and E7 proteins in HPV-16-negative cervical cancer patients with HLA-A11 and -A24 alleles.
Acknowledgments
We are grateful to Kernick James Deen for editing the English and Hung-Cheng Lai for discussion.
This work was supported by National Science Council grants NSC 93-3112-B-016-005 and NSC 93-2320-B-016-008; National Health Research Institute grant NHRI-EX93-9314BI; National Taiwan University grant 95 R0066-BM02-05; and Mackay Memorial Hospital grant MMH-E-95006, MMH-9501.
Footnotes
Published ahead of print on 3 January 2007.
REFERENCES
- 1.Ashrafi, G. H., M. R. Haghshenas, B. Marchetti, P. M. O'Brien, and M. S. Campo. 2005. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int. J. Cancer 113:276-283. [DOI] [PubMed] [Google Scholar]
- 2.Biswas, C., B. Kell, C. Mant, R. J. Jewers, J. Cason, P. Muir, K. S. Raju, and J. M. Best. 1997. Detection of human papillomavirus type 16 early gene transcription by reverse transcription-PCR is associated with abnormal cervical cytology. J. Clin. Microbiol. 35:1560-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bontkes, H. J., J. M. Walboomers, C. J. Meijer, T. J. Helmerhorst, and P. L. Stern. 1998. Specific MHC class I down-regulation is an early event in cervical dysplasia associated with clinical progression. Lancet 351:187-188. [DOI] [PubMed] [Google Scholar]
- 4.Bontkes, H. J., T. D. de Gruijl, A. J. van den Muysenberg, R. H. Verheijen, M. J. Stukart, C. J. Meijer, R. J. Scheper, S. N. Stacey, M. F. Duggan-Keen, P. L. Stern, S. Man, L. K. Borysiewicz, and J. M. Walboomers. 2000. Human papillomavirus type 16 E6/E7-specific cytotoxic T lymphocytes in women with cervical neoplasia. Int. J. Cancer 88:92-98. [PubMed] [Google Scholar]
- 5.Bontkes, H. J., T. D. de Gruijl, J. M. Walboomers, A. J. van den Muysenberg, A. W. Gunther, R. J. Scheper, C. J. Meijer, and J. A. Kummer. 1997. Assessment of cytotoxic T-lymphocyte phenotype using the specific markers granzyme B and TIA-1 in cervical neoplastic lesions. Br. J. Cancer 76:1353-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borysiewicz, L. K., A. Fiander, M. Nimako, S. Man, G. W. Wilkinson, D. Westmoreland, A. S. Evans, M. Adams, S. N. Stacey, M. E. Boursnell, E. Rutherford, J. K. Hickling, and S. C. Inglis. 1996. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet 347:1523-1527. [DOI] [PubMed] [Google Scholar]
- 7.Bullock, T. N., D. W. Mullins, T. A. Colella, and V. H. Engelhard. 2001. Manipulation of avidity to improve effectiveness of adoptively transferred CD8+ T cells for melanoma immunotherapy in human MHC class I-transgenic mice. J. Immunol. 167:5824-5831. [DOI] [PubMed] [Google Scholar]
- 8.Bullock, T. N., T. A. Colella, and V. H. Engelhard. 2000. The density of peptides displayed by dendritic cells affects immune responses to human tyrosinase and gp100 in HLA-A2 transgenic mice. J. Immunol. 164:2354-2361. [DOI] [PubMed] [Google Scholar]
- 9.Chang, J. L., Y. P. Tsao, D. W. Liu, S. J. Huang, W. H. Lee, and S. L. Chen. 2001. The expression of HPV-16 E5 protein in squamous neoplastic changes in the uterine cervix. J. Biomed. Sci. 8:206-213. [DOI] [PubMed] [Google Scholar]
- 10.Chen, I. H., K. L. Yang, A. Lee, H. H. Huang, P. Y. Lin, and T. D. Lee. 2002. Low frequency of HLA-B*2706 in Taiwanese patients with ankylosing spondylitis. Eur. J. Immunogenet. 29:435-438. [DOI] [PubMed] [Google Scholar]
- 11.Chen, S. L., C. P. Han, Y. P. Tsao, J. W. Lee, and C. S. Yin. 1993. Identification and typing of human papillomavirus in cervical cancers in Taiwan. Cancer 72:1939-1945. [DOI] [PubMed] [Google Scholar]
- 12.Chen, S. L., S. T. Lin, T. C. Tsai, W. C. Hsiao, and Y. P. Tsao. 3 July 2006. ErbB4 (JM-b/CYT-1) induced expression and phosphorylation of c-Jun is diminished by human papillomavirus type 16 E5 protein. Oncogene doi: 10.1038/sj.onc.1209768. [DOI] [PubMed]
- 13.Chen, Y. F., C. W. Lin, Y. P. Tsao, and S. L. Chen. 2004. Cytotoxic-T-lymphocyte human papillomavirus type 16 E5 peptide with CpG-oligodeoxynucleotide can eliminate tumor growth in C57BL/6 mice. J. Virol. 78:1333-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choppin, J., W. Cohen, A. Bianco, J. P. Briand, F. Connan, M. Dalod, and J. G. Guillet. 2001. Characteristics of HIV-1 Nef regions containing multiple CD8+ T cell epitopes: wealth of HLA-binding motifs and sensitivity to proteasome degradation. J. Immunol. 166:6164-6169. [DOI] [PubMed] [Google Scholar]
- 15.Cruz, I., C. J. Meijer, J. M. Walboomers, P. J. Snijders, and I. Van der Waal. 1999. Lack of MHC class I surface expression on neoplastic cells and poor activation of the secretory pathway of cytotoxic cells in oral squamous cell carcinomas. Br. J. Cancer 81:881-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiMaio, D., and D. Mattoon. 2001. Mechanisms of cell transformation by papillomavirus E5 proteins. Oncogene 20:7866-7873. [DOI] [PubMed] [Google Scholar]
- 17.Evans, C., S. Bauer, T. Grubert, C. Brucker, S. Baur, K. Heeg, H. Wagner, and G. B. Lipford. 1996. HLA-A2-restricted peripheral blood cytolytic T lymphocyte response to HPV type 16 proteins E6 and E7 from patients with neoplastic cervical lesions. Cancer Immunol. Immunother. 42:151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans, E. M., S. Man, A. S. Evans, and L. K. Borysiewicz. 1997. Infiltration of cervical cancer tissue with human papillomavirus-specific cytotoxic T lymphocytes. Cancer Res 57:2943-2950. [PubMed] [Google Scholar]
- 19.Fonteneau, J. F., M. Larsson, S. Somersan, C. Sanders, C. Munz, W. W. Kwok, N. Bhardwaj, and F. Jotereau. 2001. Generation of high quantities of viral and tumor-specific human CD4+ and CD8+ T-cell clones using peptide pulsed mature dendritic cells. J. Immunol. Methods 258:111-126. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann, A. M., J. Nieland, M. Schinz, M. Nonn, J. Gabelsberger, H. Meissner, R. T. Muller, I. Jochmus, L. Gissmann, A. Schneider, and M. Durst. 2001. HPV16 L1E7 chimeric virus-like particles induce specific HLA-restricted T cells in humans after in vitro vaccination. Int. J. Cancer 92:285-293. [DOI] [PubMed] [Google Scholar]
- 21.Lin, C. W., J. Y. Lee, Y. P. Tsao, C. P. Shen, H. C. Lai, and S. L. Chen. 2002. Oral vaccination with recombinant Listeria monocytogenes expressing human papillomavirus type 16 E7 can regress tumor growth in mice. Int. J. Cancer 298:805-814. [DOI] [PubMed] [Google Scholar]
- 22.Liu, D. W., Y. P. Tsao, C. H. Hsieh, J. T. Hsieh, J. T. Kung, C. L. Chiang, S. J. Huang, and S. L. Chen. 2000. Induction of CD8 T cells by vaccination with recombinant adenovirus expressing human papillomavirus type 16 E5 gene reduces tumor growth. J. Virol. 74:9083-9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luft, T., M. Rizkalla, T. Y. Tai, Q. Chen, R. I. MacFarlan, I. D. Davis, E. Maraskovsky, and J. Cebon. 2001. Exogenous peptides presented by transporter associated with antigen processing (TAP)-deficient and TAP-competent cells: intracellular loading and kinetics of presentation. J. Immunol. 167:2529-2537. [DOI] [PubMed] [Google Scholar]
- 24.Muderspach, L., S. Wilczynski, L. Roman, L. Bade, J. Felix, L. A. Small, W. M. Kast, G. Fascio, V. Marty, and J. Weber. 2000. A phase I trial of a human papillomavirus (HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clin. Cancer Res. 6:3406-3416. [PubMed] [Google Scholar]
- 25.Nakagawa, M., D. P. Stites, J. M. Palefsky, Z. Kneass, and A. B. Moscicki. 1996. CD4-positive and CD8-positive cytotoxic T lymphocytes contribute to human papillomavirus type 16 E6 and E7 responses. Clin. Diagn. Lab. Immunol. 6:494-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakagawa, M., D. P. Stites, S. Patel, S. Farhat, M. Scott, N. K. Hills, J. M. Palefsky, and A. B. Moscicki. 2000. Persistence of human papillomavirus type 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigens. J. Infect. Dis. 182:595-598. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa, M., D. P. Stites, S. Farhat, J. R. Sisler, B. Moss, F. Kong, A. B. Moscicki, and J. M. Palefsky. 1997. Cytotoxic T lymphocyte responses to E6 and E7 proteins of human papillomavirus type 16: relationship to cervical intraepithelial neoplasia. J. Infect. Dis. 175:927-931. [DOI] [PubMed] [Google Scholar]
- 28.Nimako, M., A. N. Fiander, G. W. Wilkinson, L. K. Borysiewicz, and S. Man. 1997. Human papillomavirus-specific cytotoxic T lymphocytes in patients with cervical intraepithelial neoplasia grade III. Cancer Res. 57:4855-4861. [PubMed] [Google Scholar]
- 29.Ranieri, E., W. Herr, A. Gambotto, W. Olson, D. Rowe, P. D. Robbins, L. S. Kierstead, S. C. Watkins, L. Gesualdo, and W. J. Storkus. 1999. Dendritic cells transduced with an adenovirus vector encoding Epstein-Barr virus latent membrane protein 2B: a new modality for vaccination. J. Virol. 73:10416-10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ressing, M. E., A. Sette, R. M. Brandt, J. Ruppert, P. A. Wentworth, M. Hartman, C. Oseroff, H. M. Grey, C. J. Melief, and W. M. Kast. 1995. Human CTL epitopes encoded by human papillomavirus type 16 E6 and E7 identified through in vivo and in vitro immunogenicity studies of HLA-A*0201-binding peptides. J. Immunol. 154:5934-5943. [PubMed] [Google Scholar]
- 31.Ressing, M. E., W. J. van Driel, E. Celis, A. Sette, M. P. Brandt, M. Hartman, J. D. Anholts, G. M. Schreuder, W. B. ter Harmsel, G. J. Fleuren, B. J. Trimbos, W. M. Kast, and C. J. Melief. 1996. Occasional memory cytotoxic T-cell responses of patients with human papillomavirus type 16-positive cervical lesions against a human leukocyte antigen-A*0201-restricted E7-encoded epitope. Cancer Res. 56:582-588. [PubMed] [Google Scholar]
- 32.Roden, R. B., M. Ling, and T. C. Wu. 2004. Vaccination to prevent and treat cervical cancer. Hum. Pathol. 35:971-982. [DOI] [PubMed] [Google Scholar]
- 33.Schiller, J. T., and P. Davies. 2004. Delivering on the promise: HPV vaccines and cervical cancer. Nat. Rev. Microbiol. 2:343-347. [DOI] [PubMed] [Google Scholar]
- 34.Schreurs, M. W., K. B. Scholten, E. W. Kueter, J. J. Ruizendaal, C. J. Meijer, and E. Hooijberg. 2003. In vitro generation and life span extension of human papillomavirus type 16-specific, healthy donor-derived CTL clones. J. Immunol. 171:2912-2921. [DOI] [PubMed] [Google Scholar]
- 35.Scott, M., M. Nakagawa, and A. B. Moscicki. 2001. Cell-mediated immune response to human papillomavirus infection. Clin. Diagn. Lab. Immunol. 8:209-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheehy, M. E., A. B. McDermott, S. N. Furlan, P. Klenerman, and D. F. Nixon. 2001. A novel technique for the fluorometric assessment of T lymphocyte antigen specific lysis. J. Immunol. Methods 249:99-110. [DOI] [PubMed] [Google Scholar]
- 37.Sheu, B. C., S. H. Chiou, H. H. Lin, S. N. Chow, S. C. Huang, H. N. Ho, and S. M. Hsu. 2005. Up-regulation of inhibitory natural killer receptors CD94/NKG2A with suppressed intracellular perforin expression of tumor-infiltrating CD8+ T lymphocytes in human cervical carcinoma. Cancer Res. 65:2921-2929. [DOI] [PubMed] [Google Scholar]
- 38.Sheu, B. C., S. M. Hsu, H. N. Ho, H. C. Lien, S. C. Huang, and R. H. Lin. 2001. A novel role of metalloproteinase in cancer mediated immunosuppression. Cancer Res. 61:237-242. [PubMed] [Google Scholar]
- 39.Steller, M. A., K. J. Gurski, M. Murakami, R. W. Daniel, K. V. Shah, E. Celis, A. Sette, F. L. Trimble, R. C. Park, and F. M. Marincola. 1998. Cell mediated immunologic responses in cervical and vaginal cancer patients immunized with a lipidated epitope of human papillomavirus type 16 E7. Clin. Cancer Res. 4:2103-2109. [PubMed] [Google Scholar]
- 40.Street, M. D., T. Doan, K. A. Herd, and R. W. Tindle. 2002. Limitations of HLA-transgenic mice in presentation of HLA-restricted cytotoxic T-cell epitopes from endogenously processed human papillomavirus type 16 E7 protein. Immunology 106:526-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai, T. C., and S. L. Chen. 2003. The biochemical and biological functions of human papillomavirus type 16 E5 protein. Arch. Virol. 148:1445-1453. [DOI] [PubMed] [Google Scholar]
- 42.Tsai, V., I. Kawashima, E. Keogh, K. Daly, A. Sette, and E. Celis. 1998. In vitro immunization and expansion of antigen-specific cytotoxic T lymphocytes for adoptive immunotherapy using peptide-pulsed dendritic cells. Crit. Rev. Immunol. 18:65-75. [DOI] [PubMed] [Google Scholar]
- 43.Tsao, Y. P., S. J. Huang, J. L. Chang, J. T. Hsieh, R. C. Pong, and S. L. Chen. 1999. Adenovirus-mediated p21((WAF1/SDII/CIP1)) gene transfer induces apoptosis of human cervical cancer cell lines. J. Virol. 73:4983-4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsao, Y. P., J. Y. Lin, J. T. Jan, C. H. Leng, C. C. Chu, Y. C. Yang, and S. L. Chen. 2006. HLA-A*0201 T-cell epitopes in severe acute respiratory syndrome (SARS) coronavirus nucleocapsid and spike proteins. Biochem. Biophys. Res. Commun. 344:63-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Burg, S. H., A. de Jong, M. J. Welters, R. Offringa, and C. J. Melief. 2002. The status of HPV16-specific T-cell reactivity in health and disease as a guide to HPV vaccine development. Virus Res. 89:275-284. [DOI] [PubMed] [Google Scholar]
- 46.Vigneau, S., P. S. Rohrlich, M. Brahic, and J. F. Bureau. 2003. Tmevpg1, a candidate gene for the control of Theiler's virus persistence, could be implicated in the regulation of gamma interferon. J. Virol. 77:5632-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolfel, T., A. Van Pel, V. Brichard, J. Schneider, B. Seliger, K. H. Meyer zum Buschenfelde, and T. Boon. 1994. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur. J. Immunol. 24:759-764. [DOI] [PubMed] [Google Scholar]
- 48.Yamasaki, S., T. Okino, N. G. Chakraborty, W. O. Adkisson, A. Sampieri, S. J. Padula, F. Mauri, and B. Mukherji. 1995. Presentation of synthetic peptide antigen encoded by the MAGE-1 gene by granulocyte/macrophage-colony-stimulating-factor-cultured macrophages from HLA-A1 melanoma patients. Cancer Immunol. Immunother. 40:268-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Youde, S. J., P. R. Dunbar, E. M. Evans, A. N. Fiander, L. K. Borysiewicz, V. Cerundolo, and S. Man. 2000. Use of fluorogenic histocompatibility leukocyte antigen-A*0201/HPV 16 E7 peptide complexes to isolate rare human cytotoxic T-lymphocyte-recognizing endogenous human papillomavirus antigens. Cancer Res. 60:365-371. [PubMed] [Google Scholar]
- 50.zur Hausen, H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2:342-350. [DOI] [PubMed] [Google Scholar]