Abstract
While Newcastle disease virus (NDV) causes serious infections in birds, it is apparently nonpathogenic in mammalian species, including humans. Previous observations and small-scale clinical trials indicated that NDV exerts oncolytic effects. Isolates of NDV were found to have selective affinity to transformed cells. We previously showed that the attenuated NDV strain MTH-68/H causes apoptotic cell death in cultures of PC12 rat pheochromocytoma cells. The aim of the present study was to extend MTH-68/H cytotoxicity testing with human tumor cell lines and to analyze certain biochemical aspects of its oncolytic effect. MTH-68/H was found to be able to kill a wide range of transformed cells by apoptosis. While caspase-8 and caspase-9 are not involved in MTH-68/H-induced apoptosis, activation of caspase-3 and caspase-12 was detected in virus-infected PC12 cells. A human glioblastoma cell line with repressible expression of the p53 protein did not show any difference in MTH-68/H sensitivity in its p53-expressing and p53-depleted states, indicating that the apoptotic process induced by MTH-68/H does not depend on p53. Apoptosis was accompanied by virus replication in two tumor cell lines tested (PC12 cells and HeLa human cervical cells), and signs of endoplasmic reticulum stress (phosphorylation of protein kinase R-like endoplasmic reticulum kinase and eIF2α) were also detected in transformed cells. In contrast, proliferation of nontransformed mouse and rat fibroblast cell lines and human primary fibroblasts was not affected by MTH-68/H treatment. MTH-68/H thus selectively kills tumor cell cultures by inducing endoplasmic reticulum stress leading to p53-independent apoptotic cell death.
Almost a century ago, Dock (18) and DePace (17) reported that their patients simultaneously suffering from gynecological cancers and vaccinated with Pasteur's rabies vaccine showed tumor regression, suggesting that vaccination can alter the progression of human cancers. Since then, a group of almost 40 DNA and RNA viruses with oncolytic potential have been described. Among them are found viruses of human diseases (such as smallpox, rabies, and mumps) and viruses infecting birds (58). Since the idea of their therapeutic use in humans arose in the early 1960s, the oncolytic potential of some of these viruses has been confirmed in several human trials involving patients with cancers resistant to traditional therapeutic modalities (4, 9, 22, 43). Two major mechanisms may account for the possible molecular basis of the oncolytic effect. Oncolytic viruses can cause cell death by their replication in tumor cells, although it is not clear why they show selectivity to neoplastic versus nontransformed cells. Defects in the interferon pathway may account for an increased susceptibility to virus infection (42). Alternatively, immunological processes induced by virus infection are responsible for the cytotoxicity observed (24, 33). Although oncolytic viruses represent a promising possibility for effective therapeutic strategies against resistant cancers, only limited investigations to explain the cellular mechanisms of these oncolytic effects have been conducted so far.
Although the group of oncolytic viruses contains potentially dangerous human viruses (e.g., mumps virus) that may not be appropriate for human therapy, some of the others, including the avian paramyxovirus Newcastle disease virus (NDV), are not human pathogens (9, 11). NDV was first described in the early 1900s as the contagious agent of the fatal avian disease known as chicken pest. It is a member of the Paramyxoviridae family and is closely related to the infectious agent of human mumps. The structure of NDV is well characterized (1-3). Particles contain a completely sequenced 15-kb long, single-stranded, nonsegmented negative-sense RNA genome coding for six viral proteins. To date, many NDV forms have been described. However, these “field isolates” cannot be distinguished as distinct serotypes; thus, their classification is based on their virulence (velogenic, mesogenic, or lentogenic forms) rather than on their serological differences. NDV causes serious infections in almost all birds, which in the case of the velogenic forms can lead to death of the animals. Upon NDV infection, apoptotic events appear in avian macrophages and lymphocytes of the peripheral blood (29, 30), although infections of the gastrointestinal and nervous systems are usually responsible for death. While NDV has a strong cytotoxic potential against different tumor cells, it is one of the few oncolytic viruses that do not naturally infect humans. No serious human infection, except mild conjunctivitis or tracheitis, was ever described for humans working with live NDV vaccines. Therapeutic trials using NDV vaccines have been performed with promising results (6, 10, 12-15, 32). In these early trials, different NDV isolates were effective in human tumors as diverse as hematological tumors, gastrointestinal cancers, and glioblastomas. NDV was also found to be cytotoxic for cultures of transformed avian and mammalian cells (19, 45, 53).
The NDV variant MTH-68/H used in the present study was generated by several passages in chicken embryos of the original Hertfordshire strain of NDV designated Herts'33, described in the early 1930s in England (16), and was selected for its oncolytic capacity. The attenuated, highly purified MTH-68/H strain had beneficial effects in patients with advanced cancers (12-15), and its apoptotic effect was demonstrated in PC12 rat pheochromocytoma cells (19, 53).
In the present work, we tested MTH-68/H in human cell lines, determined their relative sensitivities to MTH-68/H, and tried to identify molecular events responsible for the cellular responses to MTH-68/H infection. Our previous studies indicated that MTH-68/H did not require the function of the p53 tumor suppressor protein to induce apoptosis of PC12 cells (19, 53). Since the primary target for current chemo- and radiotherapies is the genomic DNA, and since DNA damage induces p53-dependent apoptosis, the p53 status of the cancer cell has a fundamental effect on the outcome of anticancer treatments (8, 28). Therefore, we also decided to analyze the role of p53 in MTH-68/H-induced apoptosis in several human tumor cell lines of different p53 status. In addition, the MTH-68/H sensitivity of a human glioblastoma cell line with tetracycline-regulated p53 expression was also tested.
Here, we present data indicating that MTH-68/H is capable of killing transformed cell lines via apoptosis, irrespective of their p53 status. The susceptible cancer cell types represent a wide range of tissue origins, indicating that NDV particles utilize a common molecular mechanism to kill tumor cells. On the other hand, substantial differences in the sensitivities of tumor cell lines toward MTH-68/H were found, emphasizing the importance of the genetic background of cancer cells. MTH-68/H-induced cell death in human cell lines was independent of the activation of initiator caspase-8 and caspase-9, but activation of caspase-3 and caspase-12 was detected in MTH-68/H-infected cells. Caspase activation was preceded by increased phosphorylation of the endoplasmic reticulum (ER)-resident alpha subunit of the eukaryotic initiation factor 2 of translation (eIF2α) kinase protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) and followed by phosphorylation and inactivation of the translation initiation factor eIF2α. Our results thus indicate that virus-induced ER stress plays an important role in the oncolytic effect of MTH-68/H.
MATERIALS AND METHODS
Materials.
All biochemicals and culture media were purchased from Sigma-Aldrich Hungary (Budapest, Hungary), unless otherwise stated. MTH-68/H is a highly purified, attenuated NDV strain developed for human use by United Cancer Research Institute (Fort Lauderdale, FL). Lyophilized MTH-68/H vaccine (minimal titer for effective infective dose, 108.8) was produced by Phylaxia-Sanofi (Budapest, Hungary). MTH-68/H was rehydrated by suspending it in the culture medium just prior to the infection experiments.
Cell lines and cultures.
The main characteristics of the cell lines used in this study are summarized in Table 1. PC12 cells were cultured in Dulbecco's modified Eagle medium (DMEM) with 4.5 g/liter glucose supplemented with 10% horse and 5% fetal bovine sera (FBS). HeLa, U373, and MCF-7 cells were cultured in DMEM containing 10% FBS; NIH 3T3 and Rat-1 fibroblasts were cultured in DMEM supplemented with 10% calf serum. Primary human fibroblasts were maintained in DMEM containing 20% FBS. PANC-1 cells were grown in RPMI 1640 with phenol red supplemented with MEM-nonessential amino acid solution and 10% FBS, 2 mM l-glutamine, and 10% MEM-sodium pyruvate. HCT-116, HT-25, HT-168-M1/9, HT-199, and WM983B cells were grown in RPMI 1640 containing 5% FBS and 2 mM l-glutamine. DU-145, PC-3, HT-29, and NCI-H460 cells were cultured in DMEM-Ham's F12 (1:1) with 10% FBS. A431 cells were grown in DMEM-Ham's F12 (1:1) with 5% FBS. HT-29, HT-168-M1/9, HT-199, and WM983B cells were kindly provided by J. Tímár (National Oncology Institute, Budapest, Hungary); PANC-1, HCT-116, DU-145, PC-3, HT-29, and NCI-H460 cells were provided by Schering AG (Berlin, Germany). The U373 cells and the primary human fibroblast culture were the kind gifts of G. Sáfrány (National Institute of Radiobiology, Budapest, Hungary). The human glioblastoma cell line LNZTA3WT4 was purchased from ATCC and cultured in DMEM containing 10% FBS, 2 mM l-glutamine, and 1 μg/ml tetracycline. LNZTA3WT4 cells have tetracycline-regulated expression of wild-type p53 (wtp53): it is expressed in cultures treated with tetracycline but not in the absence of the antibiotic (54).
TABLE 1.
Main characteristics of the cell lines used in this study
| Cell line | Tissue origin | p53 status (reference)a | Relative MTH-68/H sensitivityc |
|---|---|---|---|
| Primary fibroblast | Human | NA | None |
| NIH 3T3 | Mouse embryonic fibroblast | wtp53+ (34) | None |
| Rat-1 | Rat embryonic fibroblast | wtp53+ (38) | None |
| PC12 | Rat pheochromocytoma | wtp53+ (20) | ++ |
| HT-25 | Human colon carcinoma | NA | +++ |
| HT-29 | Human colorectal adenocarcinoma | CGT/CAT mutation in codon 273 (47) | ++++ |
| HCT-116 | Human colon carcinoma | wtp53+ (7) | +++ |
| DU-145 | Brain metastasis of human prostate adenocarcinoma | Both alleles are mutated: Pro223Leu and Val274Phe (23) | +++ |
| PC-3 | Bone metastasis of human prostate adenocarcinoma | One allele is deleted; a point mutation in codon 138 results in a frameshift leading to early termination (23) | ++++ |
| PANC-1 | Ductal epithelioid carcinoma of human pancreas | CGT/CAT mutation in codon 273 (40) | +++++ |
| MCF-7 | Human breast adenocarcinoma | wtp53+ (5) | ++ |
| HeLa | Human cervix adenocarcinoma | HPV16 E6+; low p53 expression (26) | +++++ |
| NCI-H460 | Pleural metastasis of human large-cell-type lung carcinoma | Elevated p53 mRNA expressionb | +++ |
| U373 | Human astrocytoma | CGT/CAT mutation in codon 273 (52) | +++ |
| LNZTA3WT4 | Human glioblastoma | Endogenous p53 is inactivated; the cell line is stably transfected by wtp53 cDNA driven by the CMV promoter and repressed by a tetracycline repressorb | ++++ |
| A431 | Human epidermoid carcinoma | CGT/CAT mutation in codon 273 (46) | ++++ |
| HT-168-M1/9 | Human melanoma | NA | ++++ |
| HT199 | Human melanoma | NA | ++++ |
| WM983B | Human melanoma | NA | ++++ |
NA, no data available; CMV, cytomegalovirus.
Based on the description of the American Tissue Culture Collection.
Assessment of the relative MTH-68/H sensitivities was based on MTH-68/H titers sufficient to induce 50% cytotoxicity (MOI50) as follows: +++++, 0.01 to 0.1; ++++, 0.1 to 0.2; +++, 0.2 to 1.0; ++, 1.0 to 10.0; 1, 10.0 to 200.0; None, >200.0.
Detection of cytotoxicity using the WST-1 cell-proliferation assay.
The cell proliferation reagent WST-1 was purchased from Roche (Roche Hungary Ltd., Budapest, Hungary). Cells were grown in tissue culture grade, 24-well plates, in 1 ml culture medium as described above and infected with MTH-68/H for 72 h. For positive apoptosis control, cells were treated for 24 h with 1 μg/ml anisomycin, whereas for the negative controls, cells were treated with vehicle. The WST-1 assays were performed according to the manufacturer's instructions in triplicate.
Apoptosis assays. (i) Electrophoretic detection of internucleosomal fragmentation of chromosomal DNA.
Analysis of DNA fragmentation was performed as described previously (19). Cells were cultured in DMEM containing sera according to their requirements (see above) for 24 h. Treatments were carried out as indicated in the figure legends. DNA fragments were separated by electrophoresis in 1.8% agarose gels and visualized on a UV transilluminator after being stained with SYBR Gold (Molecular Probes, Eugene, OR).
(ii) TUNEL assay.
For all treatments, 105 cells were seeded in 8-well chamber slides, cultured for 24 h, and treated with MTH-68/H. The cells were fixed in 0.14 M phosphate-buffered saline (PBS; pH 7.4) containing 4% paraformaldehyde and 2.5% dimethyl sulfoxide at 4°C for 60 min, washed in PBS three times for 5 min, and permeabilized in PBS containing 0.1% Triton X-100-0.1% sodium citrate at 4°C for 2 min. The cells were washed and stained, using fluorescein isothiocyanate (FITC)-labeled dUTP and terminal deoxynucleotide transferase at 37°C for 60 min. The terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) reaction was terminated by the addition of 2× SSC (0.3 M NaCl plus 0.03 M Na citrate) for 10 min, and the cells were counterstained with propidium iodide-RNase A solution for 10 min at room temperature. FITC-labeled dUTP, terminal deoxynucleotide transferase, and propidium iodide-RNase A solution were purchased from Roche Hungary Ltd. (Budapest, Hungary). Samples were washed with distilled water and covered, using Vectashield H-1000 mounting solution (Vector, Burlingame, CA).
Western blotting.
Immunoblot analysis using antibodies against the proteins indicated in the figures was performed according to established protocols. Protein (100 μg) for each cell extract was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in 10% or 18% gels. The proteins were transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech AB., Uppsala, Sweden) treated with appropriate antibodies, and immune complexes were visualized using an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech AB., Uppsala, Sweden) according to the manufacturer's instructions. The following antibodies from Cell Signaling (Beverly, MA) were used: phospho-Mdm2 (polyclonal antibody [pAb]), p53 (pAb), phospho-PTEN (Ser388; pAb), PTEN (pAb), caspase-8 (pAb), cleaved caspase-3 (pAb), cleaved caspase-9 (pAb), eIF2α (pAb), and phospho-eIF2α (Ser51), while PERK- and phospho-PERK-specific (Thr981) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Caspase-12-specific (pAb) antibody was obtained from MBL Laboratories (Nagoya, Japan). Antiactin (monoclonal antibody, AB-1) antibody was purchased from Oncogene (Merck Ltd., Budapest, Hungary).
Immunocytochemistry.
Cells were cultured on poly-l-lysine-coated cover glasses. Following the treatment, the cells were fixed with 4% freshly prepared paraformaldehyde at 4°C. Immunocytochemistry was performed according to the antibody manufacturers' instructions. Slides were mounted with ProLong Antifade mounting medium (Molecular Probes, Eugene, OR), dried for 24 h at 4°C, and analyzed using the Olympus FV-1000 laser confocal microscope system.
Electrophoretic mobility shift assay.
Nuclear extracts were prepared and gel retardation assays were performed as described by Xu and Cooper (61). 32P-labeled, double-stranded p53 oligonucleotide containing the consensus binding site for p53 (5′-TACAGAACATGTCTAAGCATGCTGGGGACT-3′) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). DNA-protein complexes were electrophoresed in 5% nondenaturing polyacrylamide gels, and the dried gels were analyzed by a Cyclone PhosphorImager (Packard Instrument Co. Inc., Meriden, CT).
Quantification of infective MTH-68/H particles.
Quantitative analysis was performed as described by Lomniczi (35). Primary chicken embryonic cells (6 × 108) were infected with 0.5 ml of the supernatants of cancer cells treated at different multiplicities of infection (MOI; expressed as particle-to-cell ratios) of MTH-68/H for 72 h. After 3 days of incubation, intracellular viruses were released by sonic treatment and titrated by plaque assay.
RESULTS
Selective sensitivity of cancer cell lines to MTH-68/H.
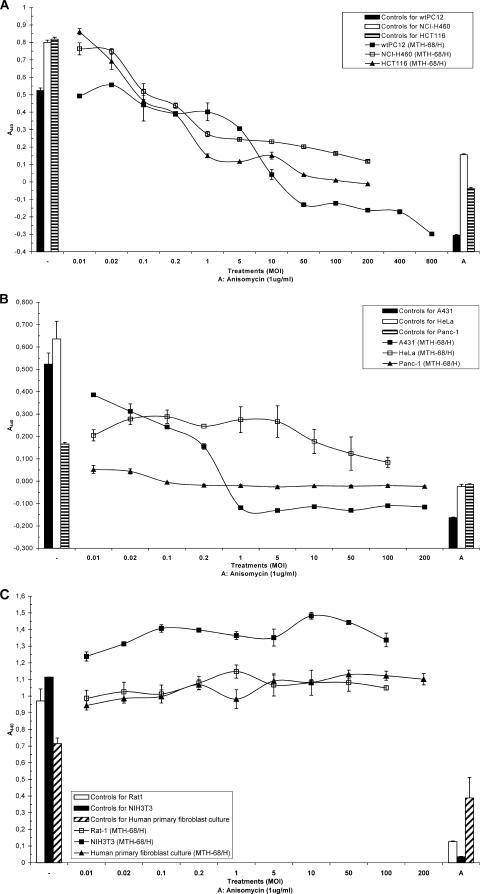
As previously reported, MTH-68/H induces apoptosis in nonhost PC12 rat pheochromocytoma cells (19, 53). To extend these studies, several other, mostly human, tumor cell lines with wild-type (Fig. 1A) or mutated (Fig. 1B) p53 genes as well as nontransformed fibroblast cell lines (Fig. 1C) were tested for MTH-68/H cytotoxicity (these cell lines are described in Table 1). Using the WST-1 proliferation assay, we tested MTH-68/H at a wide range of MOIs (from 0.01 to at least 200.0). WST-1 assay experiments with wtPC12 cells confirmed that the cytotoxic effect of MTH-68/H is dose dependent in this cell line and parallels the apoptosis-inducing effect observed previously. MTH-68/H exerted strong cytotoxicity at an MOI of 10.0. It is worth mentioning that DNA fragmentation assays (19, 53) and microscopic observations (data not shown) revealed massive cell death even at much lower virus titers (MOI, 0.2 to 1.0). These observations suggest that some of the dying cells might have relatively intact mitochondrial functions and, therefore, that the sensitivity of apoptosis assays may exceed that of the WST-1 test. p53-positive (p53+) human tumor cell lines (like the lung cancer cell line NCI-H460 and the colon carcinoma cell line HCT-116; Fig. 1A) and tumor cell lines with impaired p53 function (Fig. 1B and Table 1) were also sensitive to MTH-68/H-induced cytotoxicity. In two of the p53-deficient (p53−) cell lines (HeLa cervical cancer cells and PANC-1 pancreas carcinoma cells), even at the lowest titer tested (MOI, 0.01), MTH-68/H was strongly cytotoxic. Other cell lines with reduced p53 function (e.g., HT-29 colorectal adenocarcinoma and DU-145 and PC-3 metastatic cancer cells with prostate origin) were also sensitive to MTH-68/H infection, although quantitative variations among tumor cell lines were apparent (Table 1).
FIG. 1.
Cytotoxic effect of MTH-68/H on different cell lines. Cells (104, or in the case of the undifferentiated wild-type PC12 cells, 4 × 104) were cultured in 24-well tissue culture plates. Twenty-four hours after being plated, the cells were infected with different titers of MTH-68/H, as indicated. For the positive control, cells were treated with anisomycin (1 μg/ml); for the negative control, they were grown in culture medium without treatment. After 72 h of incubation, WST-1 assays were performed as described in Materials and Methods. Results for the untreated cells and the anisomycin controls are shown on the left (-) and right (A) sides of the charts, respectively. (A) p53-positive tumor cell lines; (B) p53-negative tumor cell lines; (C) nontransformed fibroblast cell lines.
These WST-1 assays indicated that all the human tumor cell lines tested in these experiments were sensitive to MTH-68/H (Table 1). In contrast, nontransformed fibroblasts (NIH 3T3 and Rat-1 rodent fibroblast cell lines and human primary fibroblast cultures) were highly resistant to MTH-68/H cytotoxicity: they continued their proliferation even at slightly higher rates than that of untreated control cells, while retaining their ability to undergo apoptosis in response to anisomycin treatment (Fig. 1C).
Replication of MTH-68/H in PC12, HeLa, and MCF-7 cells.
Two theories have been formulated in the past decades to explain viral oncolysis: active viral replication-mediated oncolysis and virus-induced antitumor immunity (48, 49). The tissue culture model used in our study provided an opportunity to directly address this problem. As described above, MTH-68/H triggered cell death in the absence of immune cells. To further analyze the mechanism of tumor cell killing, HeLa and MCF-7 cell cultures were treated with MTH-68/H (MOI, 0.01) and the production of infectious viruses in the media was studied. After 24 h of infection, the culture media were collected and transferred to fresh, noninfected cell cultures, incubated for an additional 24 h, and analyzed by phase-contrast microscopy. The results of this experiment (data not shown) indicated that the “preconditioned” media induced cell death in both HeLa and MCF-7 cells, suggesting the presence of a cytotoxic factor in the media of MTH-68/H-infected tumor cell cultures. The cytotoxic factor is either a proapoptotic agent secreted by tumor cells (e.g., interferon) or newly replicated MTH-68/H particles.
To distinguish between these two possibilities, supernatants of PC12, HeLa, NIH 3T3, and Rat-1 cell cultures infected for 72 h with the virus were retitrated to determine the numbers of infectious particles (Table 2). At very low MOIs (0.01 to 0.02), virus titers increased in both PC12 and HeLa cell cultures during the infection period, clearly indicating that virus replication took place in these cultures. In cultures infected with higher MTH-68/H titers, the numbers of particles did not change significantly or declined, most likely as a consequence of massive cell death at higher virus titers limiting the capacity of cultures to produce new virions. In contrast, no increase in the number of infective particles was detected in NIH 3T3 and Rat-1 fibroblasts (Table 2). The low numbers of particles infected with high initial virus titers (MOI, 5.0 or higher) found in fibroblast cultures at the end of the incubation period probably represent surviving virions rather than newly replicated particles. These observations suggest that MTH-68/H actively replicates in PC12 and HeLa cells but not in nontransformed fibroblasts.
TABLE 2.
Detection of infectious MTH-68/H particles in the culture media of tumor and control cells after 72 h of infection
| Treatment (MOI) | Virus titer (MTH-68/H particles/ml) for indicated cell type at indicated time postinfection:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| wtPC12
|
HeLa
|
NIH 3T3
|
Rat-1
|
|||||
| At the beginning of incubation | After 72 h | At the beginning of incubation | After 72 h | At the beginning of incubation | After 72 h | At the beginning of incubation | After 72 h | |
| Untreated | <101 | <101 | <101 | <101 | ||||
| Anisomycin (1 μg/ml) | <101 | <101 | <101 | <101 | ||||
| 0.01 | 4 × 102 | 4 × 103 | 102 | 6.3 × 104 | 102 | <101 | 102 | <101 |
| 0.02 | 8 × 102 | 4 × 103 | 2 × 102 | 1.5 × 104 | 2 × 102 | <101 | 2 × 102 | <101 |
| 0.1 | 4 × 103 | 4 × 103 | 103 | 1.5 × 104 | 103 | <101 | 103 | <101 |
| 0.2 | 8 × 103 | 4 × 103 | 2 × 103 | 2.5 × 104 | 2 × 103 | <101 | 2 × 103 | <101 |
| 1 | 4 × 104 | 2.5 × 104 | 104 | 1.5 × 104 | 104 | <101 | 104 | <101 |
| 5 | 2 × 105 | 2.5 × 104 | 5 × 104 | 1.5 × 104 | 5 × 104 | 1.3 × 102 | 5 × 104 | 6 × 101 |
| 10 | 4 × 105 | 4 × 104 | 105 | 105 | 105 | 2.1 × 102 | 105 | 8 × 101 |
| 50 | 2 × 106 | 1.5 × 104 | 5 × 105 | 105 | 5 × 105 | 1.4 × 103 | 5 × 105 | 7.5 × 102 |
| 100 | 4 × 106 | 1.5 × 105 | 106 | 1.5 × 105 | 106 | 1.9 × 103 | 106 | 7.2 × 102 |
wtPC12, HeLa (tumor cell lines), NIH 3T3, and Rat-1 cells (nontransformed fibroblasts) were infected with different numbers of infective MTH-68/H particles for 72 h. Following incubation, the supernatants were removed and retitrated to determine the numbers of infectious particles, as described in Materials and Methods.
MTH-68/H induces cell death in p53-expressing and p53-depleted human glioblastoma cells.
MTH-68/H induces cell death in cell lines expressing wild-type p53 (e.g., wtPC12, HCT116, and MCF-7 cells), mutated forms of p53 (e.g., DU-145, HT-29, and A431), or reduced levels of p53 (HeLa or PC-3) (26, 27, 46, 47). These cell lines, however, have very different genetic backgrounds. To further analyze the role of p53 protein in MTH-68/H-induced apoptosis, we made use of a cell line with inducible/repressible p53 expression.
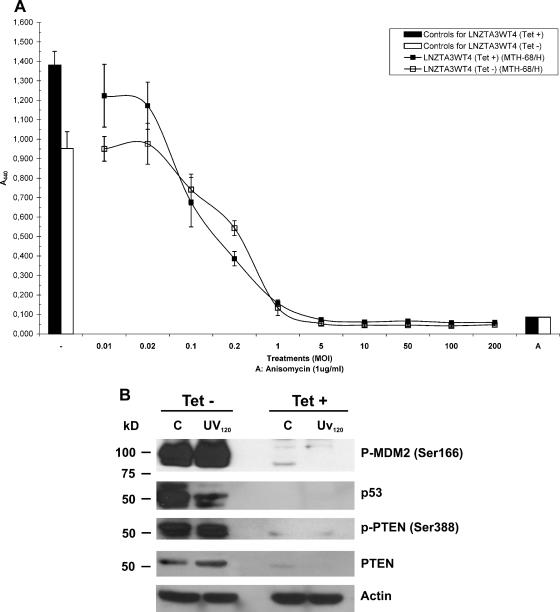
The human glioblastoma cell line LNZTA3WT4 has two useful features important for studies with MTH-68/H. First, due to genomic rearrangements, it does not have endogenous wtp53 expression but produces p53 protein encoded by a transfected cDNA under the control of a tetracycline-repressible promoter: p53 is expressed in the absence of tetracycline in the medium but not in its presence (54, 55). Second, glioblastoma belongs to the group of human malignancies that responded promisingly to MTH-68/H treatment in clinical trials (13, 15, 16). LNZTA3WT4 cells were infected with MTH-68/H in the presence or absence of tetracycline and analyzed by the WST-1 assay (Fig. 2A): the cells displayed very similar curves of MTH-68/H cytotoxicity in both the absence and the presence of tetracycline.
FIG. 2.
p53-independent cell death of human glioblastoma cells induced by MTH-68/H. (A) Analysis of the cytotoxic effects of MTH-68/H on p53-expressing and p53-depleted human glioblastoma cells. After LNZTA3WT4 cells were grown in the absence or presence of tetracycline (1 μg/ml) and infected with MTH-68/H at the MOIs indicated in the figure, WST-1 assays were performed. For further details, see Materials and Methods and the legend to Fig. 1. (B) Western blot analysis of proteins of the p53 network in LNZTA3WT4 cells. The cells were cultured either in the absence (Tet−) or presence (Tet+) of tetracycline to induce or repress exogenous p53 transcription. Cells with induced or repressed p53 expression were kept untreated (C) or were UV irradiated for 120 min (UV120) and subjected to Western blot analysis. To compare the signals obtained with the individual antibodies, membranes were stripped between the primary antibody incubations and reprobed. The primary antibodies used in these experiments are indicated on the right sides of the blots. Sample loading was controlled by using antiactin antibody as indicated. (C and D) DNA binding activity of p53 in MTH-68/H-infected cells. wtPC12 (p53+, panel C) and LNZTA3WT4 (with repressed p53, panel D) cells were infected with MTH-68/H, using an MOI of 10.0 for different times, as indicated. For untreated controls (samples 1), UV-irradiated (samples 2) or anisomycin-treated (sample 9 in panel D) cells were used. DNA binding reactions were performed using a 32P-labeled oligonucleotide carrying a p53 binding site, as described in Materials and Methods. Unlabeled competitor oligonucleotides containing specific (p53 oligonucleotide; sample 9 for panel C and 10 for panel D) or nonspecific (AP1 oligonucleotide; sample 10 for panel C; c-Myc oligonucleotide; sample 11 for panel D) consensus sequences were used in excess amounts with nuclear extracts of UV-irradiated PC12 cells or UV-irradiated LNZTA3WT4 cells kept in tetracycline-free media. Samples 11 (C) and 12 (D) served as no-protein controls.
To confirm that the LNZTA3WT4 cell line is p53− and p53+ in the presence and absence of tetracycline, respectively, under the experimental conditions used for MTH-68/H infection, cells were subjected to Western blot analysis (Fig. 2B), using antibodies against components of the p53-signaling network. As Fig. 2B shows, LNZTA3WT4 cells did not express the p53 protein in the presence of tetracycline, while in its absence, p53 expression was detected. Moreover, no significant p53 expression was found upon MTH-68/H infection in tetracycline-treated cells (data not shown).
Similar results were obtained for some of the components of the p53 regulatory circuit (Fig. 2B). A phosphatase and tensin homolog deleted on chromosome ten (PTEN), a lipid phosphatase responsible for the negative regulation of the Akt survival signaling pathway via the dephosphorylation of phosphatidylinositol-tris-phosphate, is involved in the regulation of p53 (21). Its activated form inhibits Akt-mediated Mdm2 phosphorylation, thereby increasing the stability of p53. On the other hand, PTEN is a p53-regulated tumor suppressor itself. Once p53 is stabilized, it increases transcription of the pten gene, elevating the PTEN protein level in the cell (21). Indeed, p53 and PTEN levels are tightly coregulated in LNZTA3WT4 glioblastoma cells: both are highly expressed in the absence of tetracycline but disappear from cells grown in tetracycline-containing medium (Fig. 2B). In summary, these results indicate that expression of the exogenous p53 cDNA is regulated by tetracycline under the experimental conditions used and that the p53 protein synthesized in the absence of tetracycline is functionally active. In the presence of tetracycline, expression of both p53 and PTEN is repressed.
To further analyze the significance of the p53 pathway in MTH-68/H-induced cytotoxicity, electrophoretic mobility shift assays were performed with nuclear extracts of p53-positive and p53-negative cells, using an oligonucleotide probe with a p53-binding site. Figures 2C and D show the results of such experiments with wtPC12 (p53+) and tetracycline-treated LNZTA3WT4 cells (p53−). In wtPC12 cells (Fig. 2C), UV irradiation, as expected, strongly stimulated the enhancer-binding activity of p53 protein (Fig. 2C, sample 2). In contrast, MTH-68/H infection only slightly enhanced the DNA binding of p53 (Fig. 2C, samples 3 to 8). A moderate increase in p53 activity upon MTH-68/H treatment was observed with HCT-116 colon carcinoma cells (p53+, data not shown). In contrast, neither basal level (Fig. 2C, sample 1), UV-induced (Fig. 2C, sample 2), nor MTH-68/H-induced (Fig. 2D, samples 3 to 8) p53 activity was observed in tetracycline-treated LNZTA3WT4 glioblastoma cells. Our data clearly indicate that MTH-68/H-induced cell death is independent of the presence of functional p53.
MTH-68/H induces apoptotic cell death in HeLa human cervical cancer cells.
MTH-68/H induces cell death in wtPC12 cells by apoptosis (19, 53). To demonstrate possible apoptotic processes upon viral infection in human tumor cells, HeLa cells infected with MTH-68/H were studied.
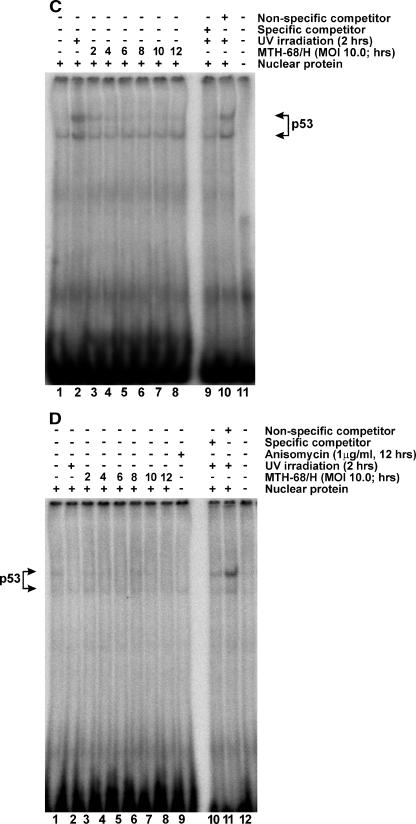
Electrophoretic analysis of DNA fragmentation revealed that MTH-68/H induced strong internucleosomal chromatin cleavage, a hallmark of apoptosis, in HeLa cells, even at low virus titers (Fig. 3A). A 30-min heat inactivation of MTH-68/H completely abolished virus-induced apoptosis, indicating that live virions are required to kill HeLa cells (Fig. 3A, lanes 10 to 12). Furthermore, HeLa cells were much more sensitive to MTH-68/H infection than wild-type PC12 cells in the DNA fragmentation assays: an MOI of 0.1 was sufficient to cause apoptotic DNA ladders (Fig. 3A), while the particle-to-cell ratio with the same effect in PC12 cells was 0.5 to 1.0 (19, 53). (The MOI of 0.01 used in the WST-1 assay [Fig. 1B] was also sufficient to cause full-blown DNA fragmentation in HeLa cells [data not shown].)
FIG. 3.
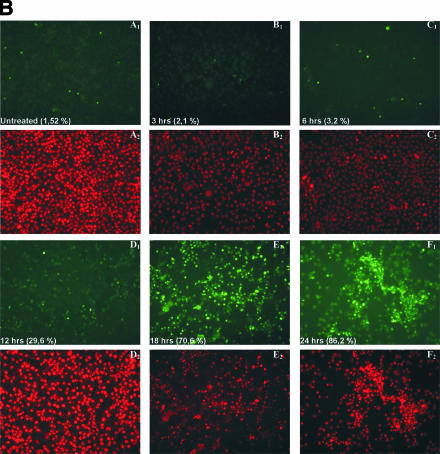
Apoptotic DNA fragmentation of HeLa cervical carcinoma cells treated with MTH-68/H. (A) Electrophoretic analysis of internucleosomal DNA fragmentation. HeLa cells were infected with MTH-68/H at the MOIs indicated in the figure (samples 1 to 9). In the same experiment, MTH-68/H particles were inactivated by boiling in culture medium for 30 min (samples 10 to 12). After 24 h of infection, DNA was extracted and examined by agarose gel electrophoresis as described in Materials and Methods. (B) Time kinetics of apoptosis in HeLa cells analyzed by TUNEL assay. TUNEL assays (panels A1 to F1), used to detect dying cells in HeLa cell cultures infected with MTH-68/H for various durations, were carried out as described in Materials and Methods. To make all the cell nuclei present in the culture visible, nuclei were counterstained with propidium iodide (panels A2 to F2). The fraction of TUNEL-positive cells is indicated in panels A1 to F1. Panels A1 and A2 show untreated, nearly confluent HeLa cell cultures. Panels C to F represent HeLa cultures treated with MTH-68/H vaccine at a 1:1 cell-to-particle ratio for the times indicated.
Quantitative analysis of MTH-68/H-induced apoptosis of HeLa cells by TUNEL assay confirmed the results of the electrophoretic analysis of DNA fragmentation (Fig. 3B). HeLa cells were infected with MTH-68/H (MOI, 1.0). Fragmented DNA was end-labeled with FITC-dUTP to identify the apoptotic cells. To determine the total cell number, cells were counterstained with propidium iodide. The fraction of apoptotic cells was determined at different time points after infection. TUNEL positivity of HeLa cultures started to increase from the basal level of 1 to 3% (Fig. 3B, panels A to C) after 12 h of treatment with MTH-68/H (almost 30%; Fig. 3B, panel D) and increased rapidly afterwards (70% and 86% at 18 and 24 h of treatment, respectively; Fig. 3B, panels E and F). A similar time course was observed with other human carcinoma cell lines (e.g., HT-29, LNZTA3WT4, and MCF-7; data not shown), suggesting that apoptotic processes are responsible for the cellular cytotoxic effect of MTH-68/H observed in the WST-1 assays.
MTH-68/H-induced apoptosis involves inactivation of eIF2α and activation of caspase-12 and caspase-3 but is independent of caspase-8 and caspase-9.
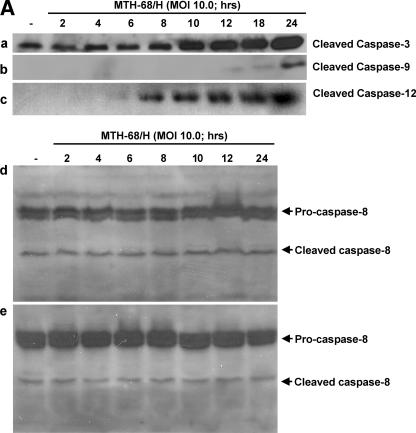
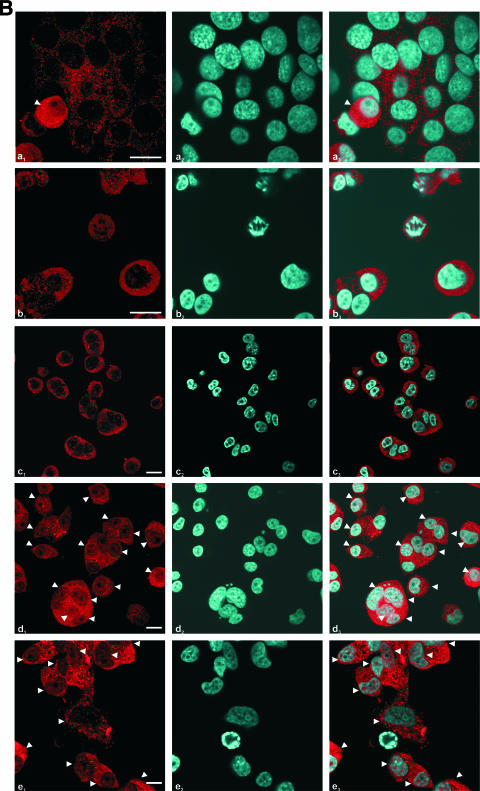
In order to determine the apoptotic pathways involved in MTH-68/H-induced apoptosis, we studied the activation of various caspases by immunoblot analysis and immunocytochemistry. Strong accumulation of cleaved caspase-3 was observed after 10 h of incubation in MTH-68/H-infected PC12 cells, but proteolytic activation of the initiator caspases caspase-8 in MCF-7 and DU-145 cells and caspase-9 in PC12 cells was not detected before the activation of effector caspase-3 (Fig. 4A). In contrast, activation (Fig. 4A) and nuclear translocation (Fig. 4B) of the endoplasmic reticulum enzyme caspase-12 correlated well with the accumulation of cleaved caspase-3 in PC12 cells.
FIG. 4.
Analysis of the involvement of caspases in MTH-68/H-induced apoptosis. Cultures were infected with MTH-68/H as indicated in the figure. (A) Analysis of the cleavage of caspase-3, caspase-9, and caspase-12 upon MTH-68/H infection in PC12 cells (blots a, b, and c, respectively) and analysis of caspase-8 activation upon MTH-68/H infection in MCF-7 (blot d) and DU-145 (blot e) human cancer cell lines. (B) Analysis of nuclear translocation of ER stress-activated caspase-12 by immunocytochemistry in PC12 cells. The primary anti-caspase-12 antibody was detected by Cy3-conjugated goat anti-rabbit secondary antibody (micrographs a1 to e1), while nuclei were counterstained using Hoechst 33258 dye (micrographs a2 to e2). Micrographs a3 to e3 show the Cy3 and Hoechst 33258 channels merged. Micrograph a shows untreated cells, while b, c, d, and e show PC12 cells infected with MTH-68/H for 4, 6, 10, and 24 h, respectively. White arrowheads indicate cells with significant nuclear translocation of caspase-12. Scale bars, 15 μm.
MTH-68/H infection stimulates eIF2α kinase PERK and subsequent phosphorylation of eIF2α.
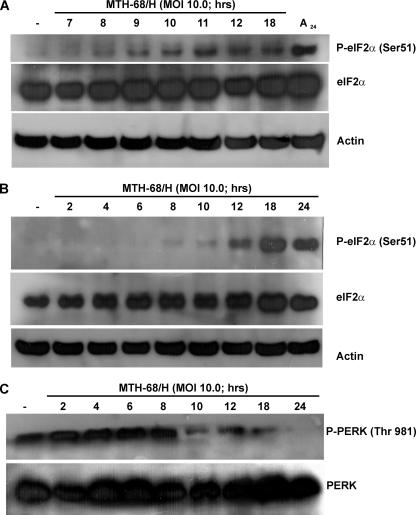
Activation of caspase-12 suggests that MTH-68/H disturbs endoplasmic reticulum functions in virus-infected cells. To determine if ER stress is involved in MTH-68/H-induced cellular responses, we analyzed ER stress-related proteins of MTH-68/H-infected cells, including eIF2α, the key regulator of eukaryotic protein synthesis, and its ER-localized kinase, PERK. eIF2α is regulated by several protein kinases via phosphorylation of Ser51, leading to a decrease in the initiation of translation (for a review, see reference 44). Phosphorylation of eIF2α was detected in all MTH-68/H-infected cell lines tested, including PC12 (Fig. 5A) and MCF-7 cells (Fig. 5B). Phosphorylation of PERK was detected in the very early phase of MTH-68/H infection in PC12 cells (Fig. 5C), preceding the phosphorylation of eIF2α. Our data suggest that MTH-68/H infection rapidly activates ER stress-related mechanisms, including fast activation of PERK with subsequent shutdown of translation by inactivation of eIF2α and activation of caspase-12 and caspase-3.
FIG. 5.
Western blot analysis of eIF2α and PERK in MTH-68/H-infected tumor cell lines. Cultures of PC12 (A and C) and MCF-7 (B) cells were infected with MTH-68/H as indicated in the figure. The primary antibodies used in these experiments are indicated on the right sides of the blots. Sample loading was controlled by using antiactin antibody as indicated. (Lane A24 in panel A shows a sample of PC12 cells treated with anisomycin (1 μg/ml) for 24 h and used as the positive control).
DISCUSSION
Though cancer is one of the most intensively studied human diseases, current therapeutic approaches are still not fully effective. Modern radio- and chemotherapeutic protocols are able to extend the life spans of patients, but they often fail to eliminate all the cancer cells, allowing them to escape and form new colonies or cause serious damage to healthy tissues. One of the alternatives to curing human tumors resistant to conventional therapeutic approaches is virotherapy using either genetically modified infectious agents or viral particles showing natural oncolytic abilities (41, 51, 58). Newcastle disease virus is a likely candidate in the quest for effective virotherapeutic agents. As an avian paramyxovirus, NDV causes serious infections in birds but is nonpathogenic in mammals. During the past decades, clinical trials with promising results, involving patients suffering from cancers resistant to conventional anticancer treatments, were conducted (6, 9, 10, 12-15, 32, 45). Tissue culture and animal experiments also provided support to the notion that NDV has oncolytic potential (36, 37), but the cellular basis of NDV cytotoxicity remained unknown. In this paper, we present evidence that MTH-68/H, an attenuated, nonpathogenic NDV strain, is directly oncolytic in several tumor cell lines of diverse tissue origin: it is able to replicate in transformed cell lines but not in control cells, and its apoptosis-inducing effect does not depend on the presence of functional p53 protein in the infected tumor cell lines but is presumably exerted through ER-mediated cellular stress mechanisms.
MTH-68/H was previously found to have an apoptosis-inducing effect in the PC12 rat pheochromocytoma cell line (19, 53). Here, we demonstrated that MTH-68/H killed a wide range of human cancer cells in culture (Fig. 1 and Table 1) but was not cytotoxic for rat, mouse, or primary human fibroblast cultures. This selective oncolytic effect was not affected by the tissue origin of the infected cell line: pancreas, glioblastoma, melanoma, and cervical cancer cells were among the cell lines most susceptible to MTH-68/H-induced cell death. On the other hand, the individual MTH-68/H sensitivity of tumor cell lines varied widely (Fig. 1 and Table 1): for example, the MOIs required for 50% cell death of MCF-7 breast cancer and PANC-1 pancreas cancer cells were found to have 1:1 and >100:1 cell-to-particle ratios, respectively. Cancer cell lines thus have a common lesion that makes them susceptible to MTH-68/H-induced oncolysis, but the process is affected by differences in the genetic constitutions of the different tumor cells. These variations in MTH-68/H susceptibility may be further increased by in vivo tumor characteristics. All these conditions should be considered when virotherapy with MTH-68/H is planned.
Our results also suggest that MTH-68/H actively replicates in tumor cell lines (PC12, MCF-7, and HeLa) but not in nontransformed fibroblasts (NIH 3T3 and Rat-1) (Table 2). Thus, while in vivo immunomodulation induced by MTH-68/H may contribute to its antitumor effect (48, 49), direct cytotoxicity should be considered the key factor of oncolysis by this virus strain.
A possible candidate for a gene/protein whose functional state may have a strong impact on the response of cells to MTH-68/H infection is p53. The p53 protein is a stress-activated transcription factor that regulates genes involved in apoptosis, cell cycle control, and DNA repair (for reviews, see references 25 and 31), and its functional state is a main determinant of the response of tumors to chemo- and radiotherapy. Therefore, we wanted to test the role of p53 in MTH-68/H cytotoxicity. Previous observations using PC12 cell lines expressing a dominant inhibitory p53 mutant (20) or overexpressing the wild-type p53 protein indicated that the apoptosis-inducing effect of MTH-68/H was not influencedby the p53 status of this cell line (53). In line with this observation, human tumor cell lines of diverse p53 statuses tested in the present study were found to have comparable sensitivities to MTH-68/H (Fig. 1 and Table 1). Most importantly, a human glioblastoma cell line with controllable p53 expression displayed no difference in MTH-68/H-induced cytotoxicity in its p53− and p53+ states (Fig. 2). These observations may have very important clinical implications: MTH-68/H virotherapy may be a promising alternative treatment in patients with p53-negative, highly chemoresistant malignancies.
MTH-68/H infection induces apoptotic cell death in PC12 cells (19, 53). Apoptosis can be triggered by both intrinsic and extrinsic signals and leads to the elimination of cells without inflammation or even damage to neighboring cells (56, 57). This type of self destruction is essential in vital processes, including the antiviral and anticancer defense mechanisms. Upon viral infection, induction of apoptosis can take place both by the cells of the antiviral defense line of the immune system and by intracellular regulatory mechanisms such as the interferon pathway (59). Besides the extrinsic and intrinsic pathways, ER-mediated events are important in antiviral mechanisms (for reviews, see references 50 and 60). Results presented in this paper point in this direction. MTH-68/H-induced apoptosis was independent of caspase-8 and caspase-9 activation, processes involved in the mediation of receptor-mediated extrinsic and mitochondrial intrinsic apoptotic pathways, respectively (Fig. 4). In contrast, several ER stress-related proteins were affected by MTH-68/H infection, including caspase-12, eIF2α, and PERK (Fig. 4 and 5). Shortly after MTH-68/H infection, PERK became phosphorylated, followed by inhibitory phosphorylation of eIF2α and activation and release of caspase-12. Although our observations indicate that MTH-68/H induces apoptosis via ER stress-related pathways, since it is not expressed in humans (39), caspase-12 cannot be responsible for the induction of apoptosis in the tested human cell lines. Other possible candidates may be caspase-4 and caspase-5, but in preliminary experiments, we did not succeed in detecting their activation in the human cell lines tested so far (data not shown). Phosphorylation of eIF2α, however, was observed in different human cell lines, indicating that this event is probably universal in MTH-68/H-infected cells. eIF2α is the key element in the regulation of translation, and its phosphorylation leads to the shutdown of synthesis of most of the proteins (44). Several protein kinases phosphorylate eIF2α, including the double-stranded RNA-induced PKR, PERK, heme-regulated inhibitor, and GCN4 (44). Early stimulation of PERK phosphorylation and blocking of eIF2α may be an essential event in MTH-68/H-induced cell death.
Acknowledgments
The authors are grateful to G. Sáfrány and J. Tímár for providing cell lines, B. Lomniczi for the quantification of infective MTH-68/H particles, and G. Tigyi for critical reading of the manuscript.
The confocal images were generated by an Olympus Fluoview FV1000S-IX81 system. The purchase of this microscope was supported by grant GVOP-3.2.1-2004-04-0172/3.0 to the University of Pécs.
Footnotes
Published ahead of print on 10 January 2007.
REFERENCES
- 1.Alexander, D. J. 2000. Newcastle disease and other avian paramyxoviruses. Rev. Sci. Tech. 19:443-462. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, D. J., and P. Reeve. 1972. The proteins of Newcastle disease virus. 1. Structural proteins. Microbios 5:199-212. [PubMed] [Google Scholar]
- 3.Alexander, D. J., and P. Reeve. 1972. The proteins of Newcastle disease virus. 2. Virus-induced proteins. Microbios 5:247-257. [PubMed] [Google Scholar]
- 4.Asada, T. 1974. Treatment of human cancer with mumps virus. Cancer 34:1907-1928. [DOI] [PubMed] [Google Scholar]
- 5.Balcer-Kubiczek, E. K., J. Yin, K. Lin, G. H. Harrison, J. M. Abraham, and S. J. Meltzer. 1995. p53 mutational status and survival of human breast cancer MCF-7 cell variants after exposure to X rays or fission neutrons. Radiat. Res. 142:256-262. [PubMed] [Google Scholar]
- 6.Batliwalla, F. M., B. A. Bateman, D. Serrano, D. Murray, S. Macphail, V. C. Maino, J. C. Ansel, P. K. Gregersen, and C. A. Armstrong. 1998. A 15-year follow-up of AJCC stage III malignant melanoma patients treated postsurgically with Newcastle disease virus (NDV) oncolysate and determination of alterations in the CD8 T cell repertoire. Mol. Med. 4:783-794. [PMC free article] [PubMed] [Google Scholar]
- 7.Bunz, F., A. Dutriaux, C. Lengauer, T. Waldman, S. Zhou, J. P. Brown, J. M. Sedivy, K. W. Kinzler, and B. Vogelstein. 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282:1497-1501. [DOI] [PubMed] [Google Scholar]
- 8.Bunz, F., P. M. Hwang, C. Torrance, T. Waldman, Y. Zhang, L. Dillehay, J. Williams, C. Lengauer, K. W. Kinzler, and B. Vogelstein. 1999. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J. Clin. Investig. 104:263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassel, W. A., and R. E. Garrett. 1965. Newcastle disease virus as an antineoplastic agent. Cancer 18:863-868. [DOI] [PubMed] [Google Scholar]
- 10.Cassel, W. A., D. R. Murray, and H. S. Phillips. 1983. A phase II study on the postsurgical management of stage II malignant melanoma with a Newcastle disease virus oncolysate. Cancer 52:856-860. [DOI] [PubMed] [Google Scholar]
- 11.Csatary, L. K. 1971. Viruses in the treatment of cancer. Lancet ii:825. [DOI] [PubMed] [Google Scholar]
- 12.Csatary, L. K., and T. Bakacs. 1999. Use of Newcastle disease virus vaccine (MTH-68/H) in a patient with high-grade glioblastoma. JAMA 281:1588-1589. [DOI] [PubMed] [Google Scholar]
- 13.Csatary, L. K., S. Eckhardt, I. Bukosza, F. Czegledi, C. Fenyvesi, P. Gergely, B. Bodey, and C. M. Csatary. 1993. Attenuated veterinary virus vaccine for the treatment of cancer. Cancer Detect. Prev. 17:619-627. [PubMed] [Google Scholar]
- 14.Csatary, L. K., G. Gosztonyi, J. Szeberenyi, Z. Fabian, V. Liszka, B. Bodey, and C. M. Csatary. 2004. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J. Neurooncol. 67:83-93. [DOI] [PubMed] [Google Scholar]
- 15.Csatary, L. K., R. W. Moss, J. Beuth, B. Torocsik, J. Szeberenyi, and T. Bakacs. 1999. Beneficial treatment of patients with advanced cancer using a Newcastle disease virus vaccine (MTH-68/H). Anticancer Res. 19:635-638. [PubMed] [Google Scholar]
- 16.Czeglédi, A., E. Wehmann, and B. Lomniczi. 2003. On the origins and relationships of Newcastle disease virus vaccine strains Hertfordshire and Mukteswar, and virulent strain Herts'33. Avian Pathol. 32:271-276. [DOI] [PubMed] [Google Scholar]
- 17.DePace, N. G. 1912. Sulla scomparsa di un enorme cancro vegetante del callo dell'utero senza cura chirurgica. Ginecologica 9:82. [Google Scholar]
- 18.Dock, G. 1904. Rabies virus vaccination in a patient with cervical carcinoma. Am. J. Med. Sci. 127:563. [Google Scholar]
- 19.Fábián, Z., B. Torocsik, K. Kiss, L. K. Csatary, B. Bodey, J. Tigyi, C. Csatary, and J. Szeberenyi. 2001. Induction of apoptosis by a Newcastle disease virus vaccine (MTH-68/H) in PC12 rat phaeochromocytoma cells. Anticancer Res. 21:125-135. [PubMed] [Google Scholar]
- 20.Fábián, Z., M. Vecsernyes, M. Pap, and J. Szeberenyi. 2006. The effects of a mutant p53 protein on the proliferation and differentiation of PC12 rat phaeochromocytoma cells. J. Cell. Biochem. 99:1431-1441. [DOI] [PubMed] [Google Scholar]
- 21.Freeman, D. J., A. G. Li, G. Wei, H. H. Li, N. Kertesz, R. Lesche, A. D. Whale, H. Martinez-Diaz, N. Rozengurt, R. D. Cardiff, X. Liu, and H. Wu. 2003. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell 3:117-130. [DOI] [PubMed] [Google Scholar]
- 22.Gross, S. 1971. Measles and leukaemia. Lancet i:397-398. [DOI] [PubMed] [Google Scholar]
- 23.Gurova, K. V., O. W. Rokhlin, A. V. Budanov, L. G. Burdelya, P. M. Chumakov, M. B. Cohen, and A. V. Gudkov. 2003. Cooperation of two mutant p53 alleles contributes to Fas resistance of prostate carcinoma cells. Cancer Res. 63:2905-2912. [PubMed] [Google Scholar]
- 24.Halpern, M. S., E. Wade, E. Rucker, K. L. Baxter-Gabbard, A. S. Levine, and R. R. Friis. 1973. A study of the relationship of reticuloendotheliosis virus to the avian leukosis-sarcoma complex of viruses. Virology 53:287-299. [DOI] [PubMed] [Google Scholar]
- 25.Haupt, S., M. Berger, Z. Goldberg, and Y. Haupt. 2003. Apoptosis—the p53 network. J. Cell Sci. 116:4077-4085. [DOI] [PubMed] [Google Scholar]
- 26.Hoppe-Seyler, F., and K. Butz. 1993. Repression of endogenous p53 transactivation function in HeLa cervical carcinoma cells by human papillomavirus type 16 E6, human mdm-2, and mutant p53. J. Virol. 67:3111-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isaacs, W. B., B. S. Carter, and C. M. Ewing. 1991. Wild-type p53 suppresses growth of human prostate cancer cells containing mutant p53 alleles. Cancer Res. 51:4716-4720. [PubMed] [Google Scholar]
- 28.Johnstone, R. W., A. A. Ruefli, and S. W. Lowe. 2002. Apoptosis: a link between cancer genetics and chemotherapy. Cell 108:153-164. [DOI] [PubMed] [Google Scholar]
- 29.Lam, K. M. 1995. Apoptosis in chicken embryo fibroblasts caused by Newcastle disease virus. Vet. Microbiol. 47:357-363. [DOI] [PubMed] [Google Scholar]
- 30.Lam, K. M., and A. C. Vasconcelos. 1994. Newcastle disease virus-induced apoptosis in chicken peripheral blood lymphocytes. Vet. Immunol. Immunopathol. 44:45-56. [DOI] [PubMed] [Google Scholar]
- 31.Levine, A. J. 1997. p53, the cellular gatekeeper for growth and division. Cell 88:323-331. [DOI] [PubMed] [Google Scholar]
- 32.Liebrich, W., P. Schlag, M. Manasterski, B. Lehner, M. Stohr, P. Moller, and V. Schirrmacher. 1991. In vitro and clinical characterisation of a Newcastle disease virus-modified autologous tumour cell vaccine for treatment of colorectal cancer patients. Eur. J. Cancer. 27:703-710. [DOI] [PubMed] [Google Scholar]
- 33.Lindenmann, J. 1974. Viruses as immunological adjuvants in cancer. Biochim. Biophys. Acta 355:49-75. [DOI] [PubMed] [Google Scholar]
- 34.Ling, C. C., M. Guo, C. H. Chen, and T. Deloherey. 1995. Radiation-induced apoptosis: effects of cell age and dose fractionation. Cancer Res. 55:5207-5212. [PubMed] [Google Scholar]
- 35.Lomniczi, B. 1973. Studies on interferon production and interferon sensitivity of different strains of Newcastle disease virus. J. Gen. Virol. 21:305-313. [DOI] [PubMed] [Google Scholar]
- 36.Lorence, R. M., B. B. Katubig, K. W. Reichard, H. M. Reyes, A. Phuangsab, M. D. Sassetti, R. J. Walter, and M. E. Peeples. 1994. Complete regression of human fibrosarcoma xenografts after local Newcastle disease virus therapy. Cancer Res. 54:6017-6021. [PubMed] [Google Scholar]
- 37.Lorence, R. M., K. W. Reichard, B. B. Katubig, H. M. Reyes, A. Phuangsab, B. R. Mitchell, C. J. Cascino, R. J. Walter, and M. E. Peeples. 1994. Complete regression of human neuroblastoma xenografts in athymic mice after local Newcastle disease virus therapy. J. Natl. Cancer Inst. 86:1228-1233. [DOI] [PubMed] [Google Scholar]
- 38.Meek, D. W., and W. Eckhart. 1988. Phosphorylation of p53 in normal and simian virus 40-transformed NIH 3T3 cells. Mol. Cell. Biol. 8:461-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Momoi, T. 2004. Caspases involved in ER stress-mediated cell death. J. Chem. Neuroanat. 28:101-105. [DOI] [PubMed] [Google Scholar]
- 40.Moore, P. S., B. Sipos, S. Orlandini, C. Sorio, F. X. Real, N. R. Lemoine, T. Gress, C. Bassi, G. Kloppel, H. Kalthoff, H. Ungefroren, M. Lohr, and A. Scarpa. 2001. Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch. 439:798-802. [DOI] [PubMed] [Google Scholar]
- 41.Mullen, J. T., and K. K. Tanabe. 2002. Viral oncolysis. Oncologist 7:106-119. [DOI] [PubMed] [Google Scholar]
- 42.Muster, T., J. Rajtarova, M. Sachet, H. Unger, R. Fleischhacker, I. Romirer, A. Grassauer, A. Url, A. Garcia-Sastre, K. Wolff, H. Pehamberger, and M. Bergmann. 2004. Interferon resistance promotes oncolysis by influenza virus NS1-deletion mutants. Int. J. Cancer. 110:15-21. [DOI] [PubMed] [Google Scholar]
- 43.Parato, K. A., D. Senger, P. A. Forsyth, and J. C. Bell. 2005. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer 5:965-976. [DOI] [PubMed] [Google Scholar]
- 44.Proud, C. G. 2005. eIF2 and the control of cell physiology. Semin. Cell Dev. Biol. 16:3-12. [DOI] [PubMed] [Google Scholar]
- 45.Reichard, K. W., R. M. Lorence, C. J. Cascino, M. E. Peeples, R. J. Walter, M. B. Fernando, H. M. Reyes, and J. A. Greager. 1992. Newcastle disease virus selectively kills human tumor cells. J. Surg. Res. 52:448-453. [DOI] [PubMed] [Google Scholar]
- 46.Reiss, M., D. E. Brash, T. Munoz-Antonia, J. A. Simon, A. Ziegler, V. F. Vellucci, and Z. L. Zhou. 1992. Status of the p53 tumor suppressor gene in human squamous carcinoma cell lines. Oncol. Res. 4:349-357. [PubMed] [Google Scholar]
- 47.Rodrigues, N. R., A. Rowan, M. E. Smith, I. B. Kerr, W. F. Bodmer, J. V. Gannon, and D. P. Lane. 1990. p53 mutations in colorectal cancer. Proc. Natl. Acad. Sci. USA 87:7555-7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schirrmacher, V., T. Ahlert, T. Probstle, H. H. Steiner, C. Herold-Mende, R. Gerhards, E. Hagmuller, and H. H. Steiner. 1998. Immunization with virus-modified tumor cells. Semin. Oncol. 25:677-696. [PubMed] [Google Scholar]
- 49.Schirrmacher, V., C. Haas, R. Bonifer, T. Ahlert, R. Gerhards, and C. Ertel. 1999. Human tumor cell modification by virus infection: an efficient and safe way to produce cancer vaccine with pleiotropic immune stimulatory properties when using Newcastle disease virus. Gene Ther. 6:63-73. [DOI] [PubMed] [Google Scholar]
- 50.Schröder, M., and R. J. Kaufman. 2005. ER stress and the unfolded protein response. Mutat. Res. 569:29-63. [DOI] [PubMed] [Google Scholar]
- 51.Shah, A. C., D. Benos, G. Y. Gillespie, and J. M. Markert. 2003. Oncolytic viruses: clinical applications as vectors for the treatment of malignant gliomas. J. Neuro-Oncol. 65:203-226. [DOI] [PubMed] [Google Scholar]
- 52.Shono, T., P. J. Tofilon, T. S. Schaefer, D. Parikh, T. J. Liu, and F. F. Lang. 2002. Apoptosis induced by adenovirus-mediated p53 gene transfer in human glioma correlates with site-specific phosphorylation. Cancer Res. 62:1069-1076. [PubMed] [Google Scholar]
- 53.Szeberényi, J., Z. Fábián, B. Torocsik, K. Kiss, and L. K. Csatary. 2003. Newcastle disease virus-induced apoptosis in PC12 pheochromocytoma cells. Am. J. Ther. 10:282-288. [DOI] [PubMed] [Google Scholar]
- 54.Van Meir, E. G., T. Kikuchi, M. Tada, H. Li, A. C. Diserens, B. E. Wojcik, H. J. Huang, T. Friedmann, N. de Tribolet, and W. K. Cavenee. 1994. Analysis of the p53 gene and its expression in human glioblastoma cells. Cancer Res. 54:649-652. [PubMed] [Google Scholar]
- 55.Van Meir, E. G., P. J. Polverini, V. R. Chazin, H. J. Su Huang, N. de Tribolet, and W. K. Cavenee. 1994. Release of an inhibitor of angiogenesis upon induction of wild type p53 expression in glioblastoma cells. Nat. Genet. 8:171-176. [DOI] [PubMed] [Google Scholar]
- 56.Wang, S., Z. F. Boonman, H. C. Li, Y. He, M. J. Jager, R. E. Toes, and J. Y. Niederkorn. 2003. Role of TRAIL and IFN-gamma in CD4+ T cell-dependent tumor rejection in the anterior chamber of the eye. J. Immunol. 171:2789-2796. [DOI] [PubMed] [Google Scholar]
- 57.Wang, X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 15:2922-2933. [PubMed] [Google Scholar]
- 58.Webb, H. E., and C. E. Smith. 1970. Viruses in the treatment of cancer. Lancet i:1206-1208. [DOI] [PubMed] [Google Scholar]
- 59.Williams, B. R. 1999. PKR; a sentinel kinase for cellular stress. Oncogene 18:6112-6120. [DOI] [PubMed] [Google Scholar]
- 60.Xu, C., B. Bailly-Maitre, and J. C. Reed. 2005. Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Investig. 115:2656-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu, W., and G. M. Cooper. 1995. Identification of a candidate c-mos repressor that restricts transcription of germ cell-specific genes. Mol. Cell. Biol. 15:5369-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]