Abstract
We have investigated the morphogenesis of human and murine cytomegalovirus by transmission electron microscopy after high-pressure freezing, freeze substitution, and plastic embedding. We observed large tubular infoldings of the inner nuclear membrane that were free of lamina and active in primary envelopment and subsequent transport of capsids to the nuclear periphery. Semiquantitative determinations of the enlarged inner nuclear membrane area and the location of the primary envelopment of nucleocapsids demonstrated that this structure represents a virus-induced specialized membrane domain at which the particles are preferentially enveloped. This is a previously undescribed structural element relevant in cytomegalovirus morphogenesis.
Murine cytomegalovirus (MCMV) and human CMV (HCMV) are members of the Betaherpesvirinae. Both viruses encode more than 200 open reading frames (15). The morphogenesis of herpesviruses is a complex process involving multiple interactions between viral and cellular components, especially membranes, and thus is of high interest for both virology and cell biology. The stepwise assembly of the virion has been studied extensively in alphaherpesviruses (10, 11, 18, 20). However, much less is known about the morphogenesis of CMVs (3, 12). When the findings from alphaherpesviruses are compared with those from cytomegaloviruses, it has to be kept in mind that the sequence homology is only partial and that the latter viruses have a larger coding capacity. Since the size of all herpesvirus capsids prevents their transport into the cytoplasm through the nuclear pore complex, nuclear egress requires the penetration of the nuclear membranes and the nuclear lamina, probably through an envelopment/de-envelopment process, which is still under debate (11, 24). This may also be different for CMVs, since the involved alphaherpesviral kinase US3 is not conserved in betaherpesviruses (7, 16). For MCMV, it has been shown that the viral protein M50 inserts into the inner nuclear membrane and is aggregated by a second viral protein, M53, to form the putative capsid docking site (13). M50 then recruits cellular protein kinases for phosphorylation and dissolution of the nuclear lamina. Additionally, it has been shown for HCMV that pUL97 in concert with the cellular p32 acts by the redistribution of lamina components (9). While the list of viral proteins involved in this process is growing, little ultrastructural information on nuclear egress is available. After release to the cytoplasm, CMV capsids are tegumented and enveloped at Golgi apparatus-derived cisternae (6) by a wrapping process and released by fusion with the plasma membrane.
We have investigated cytomegalovirus nuclear egress by electron microscopy using high-pressure freezing, freeze substitution, and plastic embedding. Fibroblast cell monolayers (3T3 murine fibroblasts for MCMV or human foreskin fibroblasts for HCMV) were grown on carbon-coated sapphire discs (3-mm diameter; Engineering Office M. Wohlwend GmbH, Switzerland) and infected at a multiplicity of infection of 0.5 PFU/cell (MCMV Smith strain or HCMV AD169). High-pressure freezing was performed 2 days postinfection (p.i.) for MCMV and 3 to 4 days p.i. for HCMV with the new HPF 01 compact high-pressure freezing apparatus (Engineering Office M. Wohlwend GmbH, Switzerland). For this, the sapphire disc was immersed in 1-hexadecene and protected with two aluminum planchettes (21). Hexadecene is not miscible with water and thus does not serve as a cryoprotectant but is needed for the optimal transfer of pressure and cooling to the sample. Freeze substitution was done in acetone containing 1.6% (wt/vol) osmium tetroxide, 0.1% (wt/vol) uranyl acetate, and 5% (vol/vol) water (23) by slowly warming the samples from −90°C to 0°C over a period of 18 h with a specially designed computer-controlled substitution apparatus (A. Ziegler and W. Fritz, unpublished data). The samples were then kept at 0°C and at room temperature for 30 min, washed with acetone, and embedded in a two-step Epon series (Fluka, Germany) of 50% Epon in acetone for 1 h and 100% Epon for 6 h. The Epon was polymerized for 3 days at 60°C. Thin sections (approximately 60 to 80 nm) were cut with a Leica Ultracut UCT ultramicrotome using a diamond knife (Diatome, Switzerland) and collected on bare copper grids. The samples were imaged with a Zeiss EM 10 or a Philips 400 transmission electron microscope at an acceleration voltage of 80 kV. The stereological determination of the surface ratio of infoldings to the peripheral inner nuclear membrane was performed on MCMV-infected 3T3 fibroblasts by intersection counting using a square lattice grid of more than four infected nuclei on more than five thin sections in three experiments each (4, 8). The budding events were counted in the same samples.
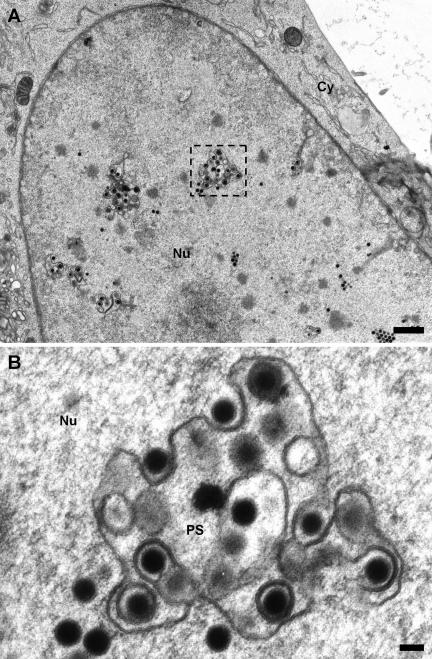
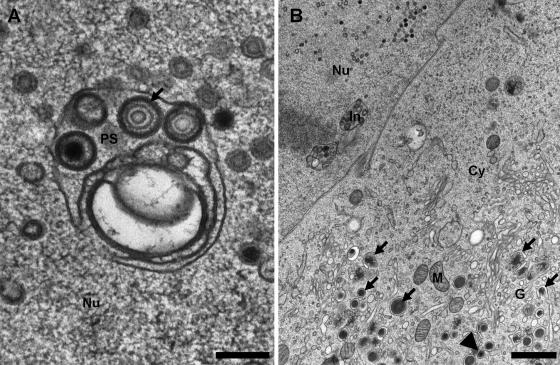
In both MCMV- and HCMV-infected cells, we could observe membranes inside the nuclei that were associated with nucleocapsids and active in their primary envelopment (Fig. 1 and 4A, respectively). In MCMV-infected cells, various budding intermediates of capsids were visible, including fully enveloped lumenal particles (Fig. 1A and B). In thin sections, these membranes were frequently connected with the inner nuclear membrane (Fig. 2A and B). These membranes represent tubular infoldings of the inner nuclear membrane, and their lumen is continuous with the perinuclear space. The infoldings possessed a complex tubular and sometimes branched structure with total lengths of up to 3 μm and variable diameters of up to 400 nm (Fig. 2A). In contrast to the unmodified inner nuclear membrane, they appear to be free of lamina (Fig. 2B, dotted lines), which is essential for their accessibility by the nucleocapsids in order to allow budding. In contrast to previous publications on pseudorabies virus morphogenesis (11), we very rarely found budding intermediates at the nuclear periphery but found them almost exclusively at the infoldings (Fig. 1A). To exclude the possibility that this observation was based on an unspecific effect due to the enlargement of the inner nuclear membrane area, a stereological estimation of the surface ratio of infolded to peripheral inner nuclear membrane was performed and combined with a statistic of the location of the primary envelopment (Table 1). The intersection counting on infected nuclei revealed that the infolded inner nuclear membrane represented 4.8% of the total inner nuclear membrane area, while 86% of the nucleocapsid budding profiles were located to it. Additionally, 93% of the primary enveloped capsids were observed in the lumen of the infoldings compared to the peripheral perinuclear space, which suggests an immediate fusion with the outer nuclear membrane at the stem of the infoldings. Interestingly, this massive structural alteration of the inner nuclear membrane had no visible effect on the integrity or shape of the outer nuclear membrane or the nuclear shape in general. Also, the nuclear pores appeared normal and not disrupted as previously reported for bovine herpesvirus 1 (24). In this context, it was surprising that in our samples, we never found primary enveloped virions in the process of fusion with the outer nuclear membrane; we found only cytoplasmic capsids in close proximity to the nucleus (Fig. 3A). Comparison of HCMV (AD169) and MCMV nuclear egresses in the two fibroblast systems showed some differences in appearance in spite of the high genomic sequence similarity of the two viruses. We observed that electron-dense material between the capsids and the primary envelope, the putative primary tegument, is more visible in HCMV than in MCMV virions (Fig. 4A). Additionally, in MCMV-infected 3T3 cells, the budding at the infolded inner nuclear membrane was exclusively initiated by C capsids (Fig. 1 and 2A), while in HCMV-infected HFF cells, enveloped B capsids could be also observed in the lumen of the infoldings (Fig. 4A). Accordingly, in the cytoplasm of the HCMV-infected fibroblasts, a significant amount of tegumented and fully enveloped B capsids was observed, while in MCMV-infected cells, capsids outside the nucleus were almost exclusively loaded with DNA (Fig. 3B). Additionally, MCMV did not form any dense bodies (Fig. 3B), in contrast to the high number seen in HCMV AD169-infected cells (Fig. 4B).
FIG. 1.
Infoldings of the inner nuclear membrane in MCMV-infected 3T3 fibroblasts 50 h postinfection. (A) The overview image of an infected nucleus shows several cross-sectioned infoldings with associated capsids. Note that only C capsids are observed to undergo primary envelopment, in contrast to HCMV (Fig. 4A) (bar, 1 μm). (B) Magnified cross section through an infolding with all intermediate stages of primary envelopment visible (bar, 100 nm). Cy, cytoplasm; Nu, nucleus; PS, perinuclear space.
FIG. 4.
HCMV morphogenesis in human fibroblast cells at 4 days p.i. (A) Cross section through an infolded inner nuclear membrane of a fibroblast cell infected with HCMV AD169. The inner nuclear membrane is complexly folded and harbors primary enveloped C and B capsids in which the putative primary tegument is well visible (arrow) (bar, 200 nm). (B) Overview of the cytoplasm of an infected cell showing two cross-sectioned infoldings of the inner nuclear membrane (In), cytoplasmic events of dense body formation (arrows), and an enveloped cisternal virion (arrowhead). Note the large amount of dense bodies (bar, 1 μm). Nu, nucleus; Cy, cytoplasm; G, Golgi complex and trans-Golgi network; M, mitochondria.
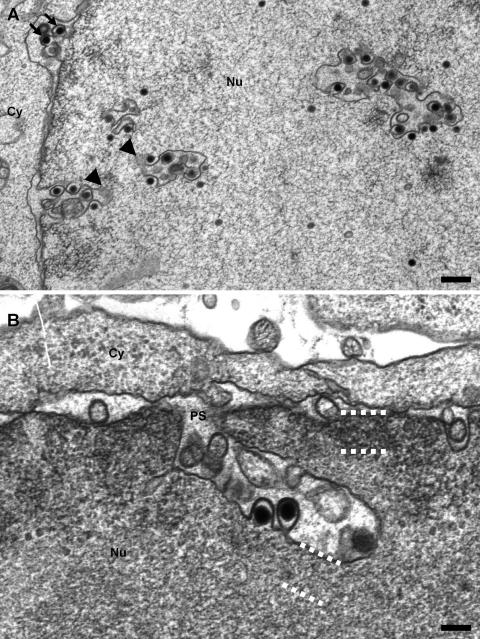
FIG. 2.
Continuity of the infoldings with the inner nuclear membrane in MCMV-infected 3T3 fibroblasts 50 h postinfection. (A) Longitudinal sections through the infoldings show the connectivity with the inner nuclear membrane and that the apparently detached vesicular profiles represent complex tubules connected out of section (arrowheads). As quantified in Table 1, most nucleocapsids undergo primary envelopment at the infolded inner nuclear membrane with a few exceptions that appear to bud at the nuclear periphery (arrows) (bar, 500 nm). (B) At higher magnifications, longitudinally sectioned infoldings clearly show the connection with the perinuclear space (PS) and the lack of lamina on the infolding (compare the areas at the nuclear periphery and the infolding framed by the dotted lines). Again, the stem of the infolding is narrow and apparently constricted by the nuclear lamina. Additionally, two budding capsids can be seen (bar, 200 nm). Nu, nucleus; Cy, cytoplasm.
TABLE 1.
MCMV primary envelopment specifically occurs at inner nuclear membrane infoldings
| Particle or intersection | No. | % |
|---|---|---|
| Particlesa | ||
| Budding at infoldings of INM | 105 | 86 |
| Budding at peripheral INM | 17 | 14 |
| Primary enveloped particles in infoldings of INM | 124 | 93 |
| Primary enveloped particles in peripheral perinuclear space | 9 | 7 |
| Intersections with:b | ||
| Infoldings of INM | 64 | 4.8 |
| Peripheral INM | 1,283 | 95.2 |
Localization of nuclear budding events and primary enveloped particles. The budding events at the infolded versus peripheral inner nuclear membranes (INM) and the location of primary enveloped virions in MCMV-infected 3T3 fibroblasts were counted.
Determination of infolded versus peripheral inner nuclear membrane areas. The ratio of the infolded versus peripheral inner nuclear membrane surface was estimated for the same samples by counting the intersections of the inner nuclear membranes with a square lattice grid.
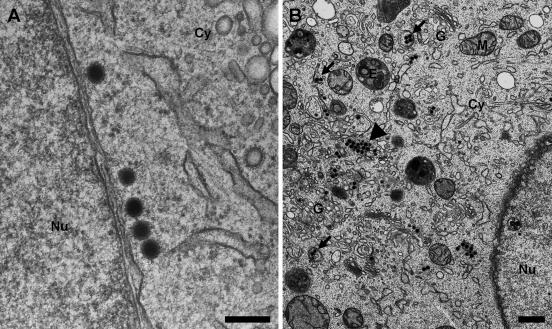
FIG. 3.
Cytoplasm of 3T3 fibroblasts infected with MCMV at 50 h p.i. (A) Cytoplasmic capsids in close proximity to the outer nuclear membrane probably newly released from the perinuclear space (bar, 200 nm). (B) Overview of the cytoplasm of an infected cell showing cytoplasmic capsid clusters typical of MCMV (arrowhead) and secondary envelopment events in the area of the Golgi complex (arrows). Note that no dense bodies are present (bar, 500 nm). Nu, nucleus; Cy, cytoplasm; E, late endosomes; G, Golgi complex and trans-Golgi network; M, mitochondria.
Our knowledge of cytomegalovirus morphogenesis and release from the infected cell is still incomplete. Most of the research on general herpesviral morphogenesis has been done on alphaherpesviruses, e.g., herpes simplex virus and pseudorabies virus. While these studies have greatly enhanced our understanding of the general features of this process, their results should be applied to cytomegaloviruses with caution, since the sequence homology is only partial and the coding capacity of cytomegaloviruses is significantly larger. Interestingly, the homology of the proteins involved in the initial steps of nuclear egress is relatively high but decreases for later events. In secondary tegumentation and envelopment, very few homologies are known, e.g., in the innermost tegument and the glycoproteins (10). Most models of herpesvirus morphogenesis rely on transmission electron microscopy (TEM) data acquired by chemical fixation of cell cultures, which has been shown to induce several artifacts (2, 14, 22). In recent years, cryofixation methods have been established for routine use and now represent a state of the art of biological specimen preparation for electron microscopy (5). With cryofixation by high-pressure freezing, a sample, such as infected cultivated cells, can be fixed from a defined physiological state within milliseconds (M. Müller and H. Moor, presented at the 42nd Annual Meeting of the Electron Microscopy Society of America, San Francisco, CA, 1984). In this study, we used high-pressure freezing followed by freeze substitution and plastic embedding, which allowed us to obtain ultrathin sections of cryofixed samples that are almost not beam sensitive and reveal a high contrast of membranous structures (23). An even more native state could be achieved by directly imaging the frozen samples by cryo-TEM (reviewed in reference 1). With this method, however, native cryosectioning of adherent cells is very difficult, and in addition, cryosections are very sensitive to beam damage so that the samples have to be imaged at low-dose conditions, which decreases the signal-to-noise ratio.
We were able to preserve large invaginations of the inner nuclear membrane reaching lengths of a few micrometers and diameters of up to several hundred nanometers (Fig. 2A and B). This additional intranuclear membrane surface was highly and specifically involved in the primary envelopment of CMV nucleocapsids, as estimated by statistical analysis of MCMV-infected cells (Table 1). Smith and De Harven (19) also showed modifications of the nuclear membranes connected to nuclear egress on chemically fixed samples, but these were not regarded as a crucial step in morphogenesis. Also, in human herpesvirus 6-infected cells, a further modification of both nuclear membranes was observed and termed the tegusome (17). The main differences between our infoldings and the tegusomes are that the latter are limited by a double membrane, and their lumen is equivalent to the cytoplasm, while the infoldings described here are single membraned, and the lumen is continuous with the perinuclear space.
In contrast to previous reports and the widely accepted pathway of herpesvirus nuclear egress, we rarely found capsids budding at the nuclear periphery but instead found them very frequently at the infoldings. It has been reported that in MCMV, cellular kinases are punctually recruited to the inner nuclear membrane by a complex of M50/M53, thus triggering the depolymerization of the nuclear lamina (13). We propose that this process is not responsible for the formation of individual budding sites for nucleocapsids but instead allows the formation of the observed membrane infoldings that allow CMV primary envelopment (Fig. 5). This hypothesis is supported by the observation that the reported recruitment of kinases occurs at approximately 10 to 15 points inside the nucleus (13), which is comparable to the frequency of invaginations that we have observed by TEM (Fig. 1A). The proposed mechanism has advantages over a budding at the nuclear periphery. First, the formation of the infoldings leads to an increase of inner nuclear membrane surface area that is free of lamina and thus is fully accessible for nucleocapsids to undergo primary envelopment (Fig. 1). Second, inside the tubular infolding, the transport is directed along the tubule, since it is continuous with the perinuclear space and not hindered by obstacles (e.g., chromatin). It can be expected that this mechanism enhances the efficiency of the transport of the primary enveloped capsids to the nuclear periphery, where they probably fuse with the outer nuclear membrane. Third, the observation that the density of the nuclear lamina appears to be reduced only on the infoldings while it is still visible at the nuclear periphery (Fig. 2B, dotted lines) has the consequence that the outer nuclear membrane is structurally unaffected, and the overall stability of the nucleus is retained. Interestingly, we never found primary enveloped virions in the process of fusion with the outer nuclear membrane. There are two possible explanations for this phenomenon. First, fusion intermediates are very-short-lived stages and more than one order of magnitude faster than the budding process. In this case, at a given time point, the total number of fusion profiles is very low, and thus, it is very improbable that such a profile would be observed in thin sections (60- to 80-nm thickness). Additionally, if fusion occurs within a few milliseconds, it might even be impossible to retain it, since it would be in the range of the immobilization time of the freezing process. Second, primary enveloped nucleocapsids do not fuse with the outer nuclear membrane but are transported along the secretory pathway. This would imply that naked cytoplasmic capsids arise by passage through disrupted nuclear pores as proposed previously by Wild et al. (24). We favor the first explanation because primary enveloped CMVs (Fig. 1B and 4A) possess a less dense tegument than mature virions, and the vast majority of the enveloped virions in the cytoplasm are heavily tegumented, as expected for secondary tegumentation and envelopment.
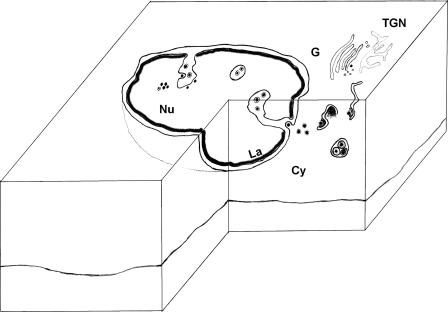
FIG. 5.
Model of cytomegalovirus nuclear egress. MCMV and HCMV morphogenesis proceeds by the loading of B capsids with viral DNA to yield the mature C capsid. In parallel, the nuclear lamina is locally dissolved to generate the delaminated infoldings of the inner nuclear membrane, which are forming long intranuclear tubules. Nucleocapsids then make contact to these membranes over a putative primary tegument to undergo primary envelopment. The primary enveloped virions are transported along the tubule to the nuclear periphery and probably fuse with the outer nuclear membrane in a fast process, releasing the naked capsids to the cytoplasm. There, the capsids acquire the two layers of secondary tegument and undergo wrapping by cisternae in the area of the Golgi complex, followed by secretion of the mature virion from the cell. Nu, nucleus; Cy, cytoplasm; PS, perinuclear space; G, Golgi complex; La, nuclear lamina; TGN, trans-Golgi network.
In summary, the advantages of high-pressure freezing followed by freeze substitution and plastic embedding are optimal for the visualization of short-lived and labile membrane structures in virus morphogenesis (5). As shown previously, the addition of 5% of water to the substitution medium enhances the visibility and retention of the cellular morphology, especially of membranes (23).
Acknowledgments
T.M. and D.M. are supported by the Landesstiftung Baden-Württemberg, Kompetenznetzwerk Resistenzentwicklung humanpathogener Erreger TP12. P.W. and T.M. are supported by the Deutsche Forschungsgemeinschaft within the Schwerpunktprogramm SPP 1175.
Footnotes
Published ahead of print on 27 December 2006.
REFERENCES
- 1.Al-Amoudi, A., J. J. Chang, A. Leforestier, A. McDowall, L. M. Salamin, L. P. Norlen, K. Richter, N. S. Blanc, D. Studer, and J. Dubochet. 2004. Cryo-electron microscopy of vitreous sections. EMBO J. 23:3583-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebersold, H. R., J. L. Cordier, and P. Luthy. 1981. Bacterial mesosomes: method dependent artifacts. Arch. Microbiol. 130:19-22. [DOI] [PubMed] [Google Scholar]
- 3.Gibson, W. 1996. Structure and assembly of the virion. Intervirology 39:389-400. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths, G. 1993. Fine structure immuno-cytochemistry. Springer-Verlag, Berlin, Germany.
- 5.Hohenberg, H. H., T. Müller-Reichert, H. Schwarz, and K. Zierold. 2003. Foreword to the Special Issue on High-Pressure Freezing. J. Microsc. 212:1-2.14516355 [Google Scholar]
- 6.Homman-Loudiyi, M., K. Hultenby, W. Britt, and C. Söderberg-Nauclér. 2003. Envelopment of human cytomegalovirus occurs by budding into Golgi-derived vacuole compartments positive for gB, Rab 3, trans-Golgi network 46, and mannosidase II. J. Virol. 77:3191-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2001. Effect of the pseudorabies virus US3 protein on nuclear membrane localization of the UL34 protein and virus egress from the nucleus. J. Gen. Virol. 82:2363-2371. [DOI] [PubMed] [Google Scholar]
- 8.Lucocq, J. 1993. Unbiased 3-D quantitation of ultrastructure in cell biology. Trends Cell Biol. 3:354-358. [DOI] [PubMed] [Google Scholar]
- 9.Marschall, M., A. Marzi, P. aus dem Siepen, R. Jochmann, M. Kalmer, S. Auerochs, P. Lischka, M. Leis, and T. Stamminger. 2005. Cellular p32 recruits cytomegalovirus kinase pUL97 to redistribute the nuclear lamina. J. Biol. Chem. 280:33357-33367. [DOI] [PubMed] [Google Scholar]
- 10.Mettenleiter, T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Res. 106:167-180. [DOI] [PubMed] [Google Scholar]
- 11.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mocarski, E., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 13.Muranyi, W., J. Haas, M. Wagner, G. Krohne, and U. H. Koszinowski. 2002. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science 297:854-857. [DOI] [PubMed] [Google Scholar]
- 14.Murk, J. L., G. Posthuma, A. J. Koster, H. J. Geuze, A. J. Verkleij, M. J. Kleijmeer, and B. M. Humbel. 2003. Influence of aldehyde fixation on the morphology of endosomes and lysosomes: quantitative analysis and electron tomography. J. Microsc. 212:81-90. [DOI] [PubMed] [Google Scholar]
- 15.Murphy, E., D. Yu, J. Grimwood, J. Schmutz, M. Dickson, M. A. Jarvis, G. Hahn, J. A. Nelson, R. M. Myers, and T. E. Shenk. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 100:14976-14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds, A. E., E. G. Wills, R. J. Roller, B. J. Ryckman, and J. D. Baines. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76:8939-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roffman, E., J. P. Albert, J. P. Goff, and N. Frenkel. 1990. Putative site for the acquisition of human herpesvirus 6 virion tegument. J. Virol. 64:6308-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roizman, B., and D. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2460. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 19.Smith, J. D., and E. De Harven. 1973. Herpes simplex virus and human cytomegalovirus replication in WI-38 cells. I. Sequence of viral replication. J. Virol. 12:919-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steven, A. C., and P. G. Spear. 1997. Herpesvirus capsid assembly and envelopment, p. 312-351. In W. Chiu, R. M. Burnett, and R. Garcea (ed.), Structural biology of viruses. Oxford University Press, New York, NY.
- 21.Studer, D., M. Michel, and M. Muller. 1989. High pressure freezing comes of age. Scanning Microsc. Suppl. 3:253-269. [PubMed] [Google Scholar]
- 22.Szczesny, P. J., P. Walther, and M. Muller. 1996. Light damage in rod outer segments: the effects of fixation on ultrastructural alterations. Curr. Eye Res. 15:807-814. [DOI] [PubMed] [Google Scholar]
- 23.Walther, P., and A. Ziegler. 2002. Freeze substitution of high-pressure frozen samples: the visibility of biological membranes is improved when the substitution medium contains water. J. Microsc. 208:3-10. [DOI] [PubMed] [Google Scholar]
- 24.Wild, P., M. Engels, C. Senn, K. Tobler, U. Ziegler, E. M. Schraner, E. Loepfe, M. Ackermann, M. Mueller, and P. Walther. 2005. Impairment of nuclear pores in bovine herpesvirus 1-infected MDBK cells. J. Virol. 79:1071-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]