Abstract
Morphological changes of liposomes caused by interactions between liposomal membranes and talin, a cytoskeletal submembranous protein, were studied by direct, real-time observation by using high-intensity dark-field microscopy. Surprisingly, when talin was added to a liposome solution, liposomes opened stable holes and were transformed into cup-shaped liposomes. The holes became larger with increasing talin concentration, and finally the cup-shaped liposomes were transformed into lipid bilayer sheets. These morphological changes were reversed by protein dilution, i.e., the sheets could be transformed back into closed spherical liposomes. We demonstrated that talin was localized mainly along the membrane verges, presumably avoiding exposure of its hydrophobic portion at the edge of the lipid bilayer. This is the first demonstration that a lipid bilayer can stably maintain a free verge in aqueous solution. This finding refutes the established dogma that all lipid bilayer membranes inevitably form closed vesicles and suggests that talin is a useful tool for manipulating liposomes.
Phospholipids spontaneously assemble into bilayer membranes in aqueous solution and necessarily form liposomes, which are closed-membrane vesicles (1). Liposomes often have been studied as simplified models of biological membranes (2–5) and are now used as such in a number of applications from pharmacology to bioengineering (6), for example, as carriers of DNA vectors or anticancer drugs for internal deliveries. However, studies of interaction mechanisms between liposome membranes and biological components, such as DNA or protein, are now still in progress (5, 7, 8), and the dynamic behavior of such complexes in solution has remained unclear. Therefore, real-time approaches by using optical microscopy to study the dynamic behavior of liposomes resulting from interactions between liposomal membranes and biological elements are very important.
Liposomes can be visualized with several types of optical microscopes. In this study, we used high-intensity dark-field microscopy (9–11), because dark-field microscopy is the best way to visualize the intact three-dimensional morphology and the dynamic behavior of individual lamellar liposomes in solution, and only this type of microscopy provides real-time, high-contrast images. In practice, other types of high-contrast microscopes, such as phase contrast or differential interference, still yield poor contrast for individual lamellar liposomes.
In this study, we investigated morphological changes of liposomes caused by talin. Talin is an actin-binding, peripheral-membrane protein that localizes at focal contacts in cells and that links actin filaments with plasma membranes through integrin (12–15). It has also been reported that talin can bind to membranes directly and can promote actin polymerization (16–18).
MATERIALS AND METHODS
Preparation and Observation of Liposomes.
Liposomes were prepared as described previously (9–11). Lipid films were generated by dissolving phospholipids in a chloroform/methanol solution, 98:2 (vol/vol). Ten microliters each of 10 mM phosphatidylethanolamine (PE) or phosphatidylcholine (PC) and phosphatidylglycerol (PG) or phosphatidylserine (PS) were mixed. The organic solvent was evaporated under a flow of nitrogen gas, and the lipids were further dried in vacuo for at least 90 min. Forty microliters of buffer A (5 mM Tris⋅HCl, pH 8.0/1 mM ATP/5 mM DTT) was then added to the dried lipid films at 4°C. Upon liquid addition, the lipid films immediately started swelling to form liposomes. Swelling was facilitated by occasionally agitating the test tubes by hand. After 30 min, the liposome suspensions were diluted 10-fold with buffer A containing talin at various concentrations. We added ATP in solution to examine the effect of actin on the talin activity, because ATP is required to maintain the native activity of actin.
Liposomes were observed at 25°C with a dark-field microscope (BHF, Olympus, Tokyo). Images were recorded by using an SIT video camera (C-2400-08, Hamamatsu Photonics, Hamamatsu City, Japan) and were further processed with a digital image analyzer (IBAS, Zeiss) to enhance contrast.
Protein.
Talin was isolated from chicken gizzard according to the method of Muguruma et al. (19). Samples were dialyzed against 20 mM Tris⋅HCl, pH 8.0/0.5 mM DTT/0.5 mM phenylmethylsulfonyl fluoride (PMSF) and were then used.
To make a concentration gradient of talin for microscope specimens, we used a flow cell made of a glass slide and a coverslip that were firmly fixed together with spacers. To apply talin to liposomes, a drop of talin in buffer A was placed on an open side of the flow cell, which had been filled with the liposome solution. A gentle flow was then induced in the cell, thereby moving liposomes at various speeds. Slowly moving liposomes were followed in the microscope, and transformations of liposomes at the buffer front containing talin were monitored. Conversely, to dilute talin, a drop of buffer A was placed on an open side of the flow cell that had been filled with transformed liposomes, and the reverse processes were monitored as described above.
Actin was purified from rabbit skeletal muscle as described previously (20). Anti-talin monoclonal antibody (T 3287) was purchased from Sigma (21) and was used after 100- to 1,000-fold dilution with buffer A.
Assay of Talin Activities.
The binding activity of talin to liposomes was determined by the cosedimentation method. Talin was added to a liposome solution and incubated at 25°C for 1 hr. Unbound and bound proteins were separated by centrifugation at 300,000 × g for 15 min. To evaluate the effects of talin, the frequency of three kinds of membrane structures—spherical liposomes, cup-shaped, and sheet-shaped membranes—was determined by observation under dark-field microscopy.
Visualization of Talin Localization.
To determine the localization of talin in transformed membranes, talin was labeled with rhodamine iodoacetamide (R-492, Molecular Probes). Three micromolar talin was incubated with 15 μM rhodamine iodoacetamide at 37°C for 15 min. The reaction was stopped by addition of DTT to a final concentration of 5 mM, dialyzed against 20 mM Tris⋅HCl, pH 8.0/0.5 mM DTT/0.5 mM PMSF to remove the remaining fluorescent dye, and then concentrated by using a small volume concentrator (Centricon, Amicon). The dye-to-protein molar ratio was 0.3. The labeled talin had the same activity as unlabeled talin as judged by the capability to transform liposomes. The localization of the labeled talin on liposomes was determined with fluorescence optics assembled into a dark-field microscope (BHF, Olympus) (20).
RESULTS
Morphological Change of Liposomes by Talin.
Liposomes made from neutral and acidic phospholipids usually take a spherical shape at neutral pH and low-salt conditions, and they show no morphological change (Fig. 1A). The line-like images of liposomes under dark-field illumination are sufficiently bright and thick to look as if they are multilamellar. However, analysis of dark-field images indicates that liposomes prepared by our method are unilamellar. The light intensity scattered from liposomes depends very strongly on the number of membrane layers. It has already been shown that when unilamellar liposomes are transformed into double-lamellar liposomes, the line-like images became more than 10-fold brighter (9). The line-like images of liposomes used in this study have a uniform brightness (Fig. 1A), and this brightness is almost identical to that of single-layered membranes from erythrocyte ghosts whose associated proteins have been removed by protease treatment (9).
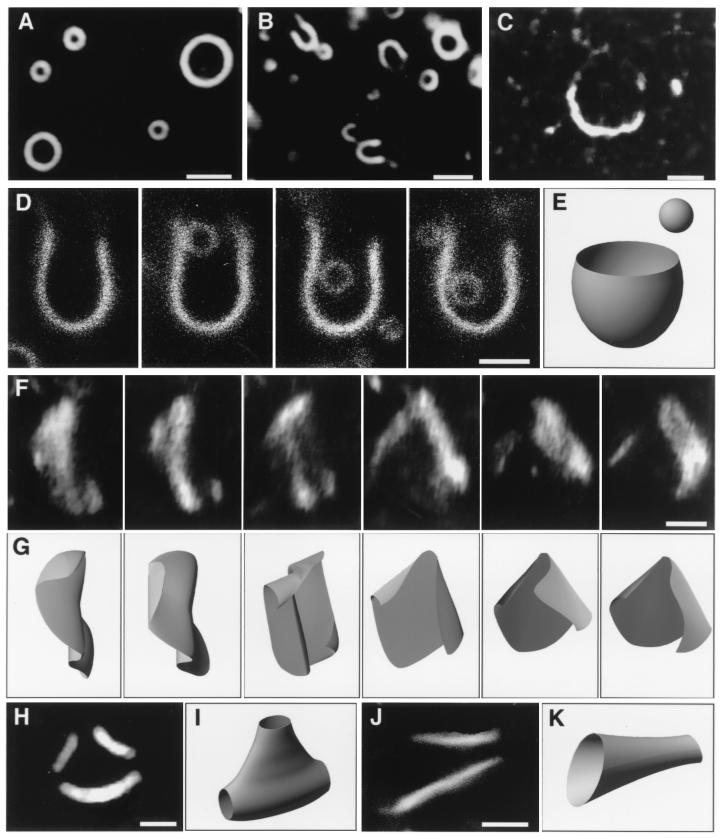
Figure 1.
Transformed liposomes observed by dark-field microscopy in the presence of talin. Liposomes were prepared from PE and PG (1:1, mol:mol). (A) Spherical liposomes obtained in the absence of talin. (B) Cup-shaped liposomes observed in the presence of 0.4 μM talin; at this talin concentration, the molar ratio of protein to total phospholipids was about 1/1,000. (C) A sheet-shaped membrane has stuck to the coverslip in 1.5 μM talin. (D) A sequence of photographs showing a small spherical liposome entering a cup-shaped membrane through its opening; the spherical liposome was trapped for several minutes and then escaped through the same opening (data not shown). (E) Three-dimensional model of a cup-shaped membrane with a small spherical liposome nearby. (F) Sequential images of a freely fluctuating lipid bilayer membrane, taken at 67-ms intervals. (G) Models of the membrane corresponding to each photograph of F. (H and J) Liposomes that have three or two opening holes, respectively, observed in the presence of 1.5 μM talin. (I and K) Models corresponding to liposomes shown in photographs H and J, respectively. (Bars = 2 μm.)
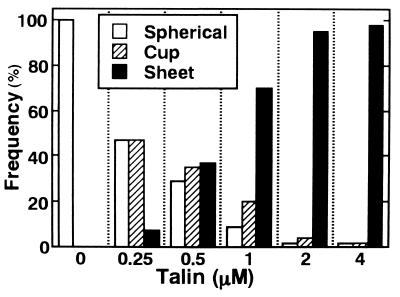
When talin was added to the liposome solution, cup-shaped liposomes, which resembled a “U” in the dark-field image (Fig. 1B), and sheet-shaped membranes (Fig. 1C) were produced in a talin concentration-dependent manner (Fig. 2). The cup-shaped forms were observed more frequently than sheet-shaped membranes at talin concentrations lower than 0.5 μM; conversely, sheet-shaped membranes predominated at higher talin concentrations. Fragmentation of sheet forms started at talin concentrations around 2 μM, so that the sizes of membrane sheets became smaller at talin concentrations of 4 μM, when the molar ratio of protein to total phospholipids was 1/100. Each free-floating, U-shaped membrane had a rotational symmetry around its axis, giving a circular image when its axis was oriented parallel to the illumination axis of the microscope, i.e., when it was perpendicular to the glass slide. In some cases, smaller spherical liposomes entered and exited the U-shaped membranes through their openings (Fig. 1D). These results indicated that the U-shaped membranes actually correspond in three dimensions to cup-like shapes (Fig. 1E). The outlines of sheet-shaped membranes, and especially their verges, were not clearly observed by dark-field microscopy and fluctuated actively (Fig. 1 F and G), indicating that the sheet-shaped membranes are very thin and flexible structures and, therefore, are thought to be opened lipid bilayer sheets. At high concentrations of talin, liposomes possessing multiple opening sites were occasionally observed (Fig. 1 H–K).
Figure 2.
Frequency of membrane structure types at various talin concentrations. Spherical liposomes, cup-shaped forms, and membrane sheets are shown by open, hatched, and solid bars, respectively. At each talin concentration, 200 structures were observed. Lipid composition was PE and PG (1:1, mol:mol).
Because a lipid bilayer membrane has a thickness of only several nanometers, the entire morphological image of a unilamellar liposome cannot be obtained at once by dark-field microscopy. The membrane part that is oriented perpendicular to the illumination axis does not scatter light and so is completely transparent. Only the part oriented parallel to the illumination axis gives a clear, line-like image. In this connection, it should be noted that a U-shaped image always appears as an open cup and never appears as a lidded cup. If U-shaped liposomes are composed of two collapsed bilayers (similar to stomatocyte red blood cells that are formed from biconcave shapes by asymmetrical deep hollowing of their central parts on one side), the brim of the cup should appear as a lid-like line image because membrane folding at the brim would generate a membrane part oriented parallel to the illumination axis. Therefore, the U-shaped images found in our study clearly indicate that cup-shaped liposomes are made of one bilayer with an open end.
For the same reason, although the circumferential outlines of disk- or other flat-shaped liposomes can be observed by dark-field microscopy regardless of their orientations (9, 11), those of lipid bilayer sheets that are oriented perpendicular to the illumination axis are completely invisible by dark-field microscopy. Note that the visible parts of membrane sheets [Figs. 1 C, F, and G, 3H (small arrows), and 4 (a dark-field image of the far-right column)] were parallel to the illumination axis.
Figure 3.
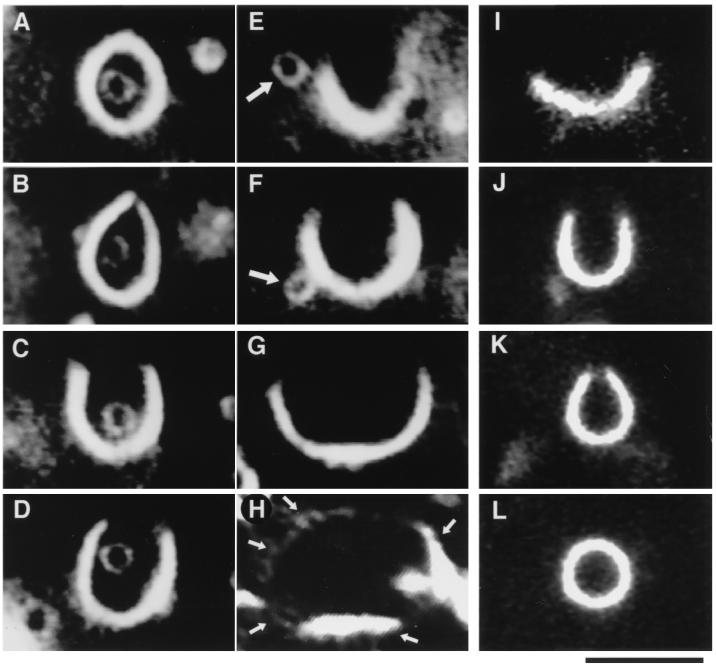
(A–H) A sequence of photographs showing morphological changes of liposomes at talin concentrations increased from 0 to 2 μM over a 7-min interval. This particular liposome contained another smaller liposome inside it. (A) Before talin addition, both liposomes were spherical, and the encapsulated liposome was unable to escape to the outside. (B) Immediately after talin addition, the lipid bilayer membrane of the outer liposome started to open a hole. (C and D) As the talin concentration increased, the hole became larger and the outer liposome transformed into a cup shape. (E and F) The encapsulated liposome (large arrow) escaped from the outer cup-shaped one through its widely opened hole. (G) The hole grew larger, and the outer cup-shaped liposome began to transform into an open membrane sheet. (H) Finally, at 7 min, the outer liposome was fully transformed into a lipid bilayer sheet. Because the lipid bilayer was very thin and fluctuated vigorously (Fig. 1 F and G), its verge was only partly observed by dark-field microscopy (small arrows). Note that we confirmed that these two liposomes were unilamellar from each of their scattering intensities. In this sequence, because the focus planes of these two liposomes were slightly different from each other and because we focused primarily on the outer liposome, the light intensity of the inner liposome appeared to be lower than that of the outer one and was not constant. (I–L) A sequence of photographs showing the reverse transformation caused by dilution of talin. (I) A fluctuating membrane sheet before talin dilution began. (J and K) After talin dilution, the sheet transformed back into a cup shape, and its verge became smaller with decreasing talin concentration. (L) Finally, after 4 min, the verge of the membrane disappeared, the hole closed completely, and thereby its shape returned to spherical. Lipid composition was PE and PG (1:1, mol:mol). (Bar = 5 μm.)
These morphological changes depended both on the salt concentration of the solution and on the lipid composition of the liposomes. Talin elicited these changes at concentrations lower than 30 mM KCl. Liposomes made from PC and PG (1:1, mol:mol) were transformed at similar talin concentrations as those made from PE and PG (1:1, mol:mol). Liposomes made from PC and PS (1:1, mol:mol) required about three times higher concentration of talin for transformation, even though talin has a higher affinity for liposomes containing PS than for those containing PG. This result indicates that the binding of talin to membranes is not in itself sufficient to make holes in liposomes.
Process of Liposome Transformation.
To characterize the transformation process, we monitored morphological changes of liposomes in the presence of increasing concentrations of talin. As shown in Fig. 3A, some liposomes prepared in the absence of talin contained smaller liposomes within them; these encapsulated liposomes never escaped in the absence of talin. When talin was added, however, the outer liposomes opened (Fig. 3B), and with increasing talin concentrations, the holes became larger and the outer liposomes were transformed into cup shapes (Fig. 3 C and D). When the holes became large enough, the encapsulated liposomes escaped (Fig. 3 E and F). Because liposomes prepared by our method are unilamellar as described above, and because these two liposomes were also unilamellar as determined by their scattering intensities, this is conclusive evidence that talin generates holes in closed lipid bilayers and thereby exposes a free verge. As the talin concentration increased to about 2 μM, the size of holes and, in rare cases, the number of holes increased, until the cup-shaped liposomes (Fig. 3G) were finally transformed into sheet shapes (Fig. 3H). In rare cases, liposomes with multiple holes, such as those shown in Fig. 1 H and J, were obtained.
These transformation processes were reversible. By dilution of talin with buffer, the cup- and sheet-shaped membranes were transformed back into original spherical-shaped liposomes (Fig. 3 I–L), indicating that binding between talin and liposome membranes is in equilibrium.
Talin Localization in Transformed Membranes.
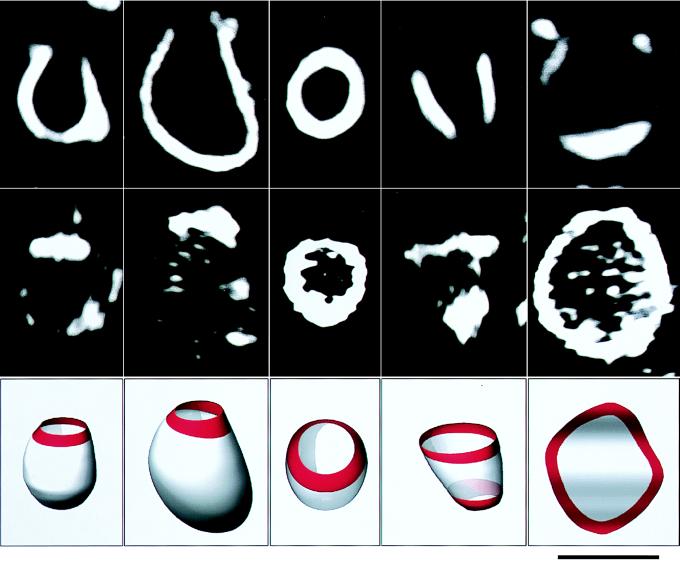
We next determined the localization of talin in transformed membranes by using a fluorescent label. The fluorescence of talin was observed mainly along verges of big holes on cup-shaped membranes, verges of big holes on liposomes with multiple opening sites, and verges of lipid bilayer sheets (Fig. 4). Fluorescence was observed only weakly throughout the surface of liposomes. The concentrated fluorescence around the hole verges was clearly seen especially in images of free-floating, cup-shaped membranes in microscope specimens (for example, Fig. 4, the center column).
Figure 4.
Talin localization on transformed liposomes. Localization was determined by using fluorescently labeled talin. The talin concentration was 1 μM, and the lipid composition was PE and PG (1:1, mol:mol). The photographs show, from left to right, three cup shapes, a cylindrical shape with two holes, and a membrane sheet attached to the glass surface by its central portion. The top and middle rows show the dark-field and fluorescent micrographs, respectively; the side views are shown, except for the cup-shaped one in the center column, which is a top view. The bottom row illustrates the three-dimensional shape of each sample. The talin localization on each sample is shown in red. (Bar = 5 μm.)
In addition, binding between an anti-talin antibody (21) and talin elicited an increase in light scattering, making membrane verges visible in dark-field microscopy. Using this antibody, we confirmed the results shown in Fig. 4 that talin is localized along the membrane verges (data not shown).
DISCUSSION
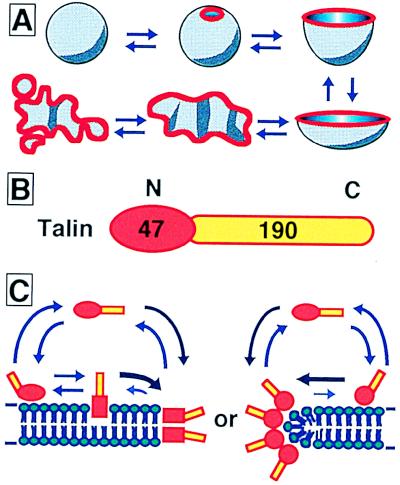
Talin has a 47-kDa N-terminal domain and a 190-kDa C-terminal domain (Fig. 5B). The N-terminal domain shows homology with the membrane-attachment domains of band 4.1 and ezrin, which are also peripheral membrane proteins that connect actin filaments to the plasma membrane and are thought to be responsible for membrane binding (22, 23). Although the C-terminal domain of talin binds to cytoskeletal proteins, including actin (19, 22, 24), as well as to anti-talin antibody (21), neither actin nor the anti-talin antibody had any effect on membranes already transformed by talin. Therefore, the C-terminal domain of talin may not participate in interactions with membranes. It seems likely that the N-terminal domain of talin penetrates into, or associates with, the lipid bilayer to make a hole in the liposomal membrane and expose a free verge (Fig. 5C). Note that, although the anti-talin antibody had no effect on liposomes already transformed by talin, talin solutions preabsorbed with a molar excess of this antibody were also unable to open holes in liposomes, confirming that talin is truly responsible for the transformation.
Figure 5.
Models showing the liposome transformation process, the domain structure of talin, and working models for protein association. (A) A closed spherical liposome transforms reversibly into an opened lipid bilayer membrane fragment, depending on the talin concentration. Red lines show talin localization, which is primarily along the free verge of the membrane. (B) Talin has a 47-kDa N-terminal membrane attachment domain and a 190-kDa C-terminal domain rich in α-helix. Actin and anti-talin monoclonal antibody interact with the C-terminal domain. The N- and C-terminal domains are colored red and yellow, respectively. (C) Talin penetrates into (Left) or associates with (Right) the membrane and is avoiding exposure of the hydrophobic portion at the edge of the lipid bilayer.
In the absence of lipid membranes, talin exists as a soluble protein, and hence some conformational change must occur in its structure, especially in its N-terminal region, when it binds to a lipid bilayer. Because the membrane transformation can be reversed by dilution of talin, such a conformational change of talin must be reversible. It has been shown previously that talin can take either of two different conformations, depending on the KCl concentration (25). This conformational change of talin may be involved in its membrane-transformation activity, because that activity also depends on salt conditions. When the talin concentration was increased, most liposomes maintained a single hole, although that hole became larger (Fig. 3). Only in rare cases, particularly when high concentrations of talin were added quickly, were multiple opening sites made (Fig. 1 H and J). These results indicate that talin accumulates cooperatively along the free verge of membrane (Fig. 5C).
Liposomes can be opened transiently by osmotic shock, and high-voltage electric pulses can make instantaneous holes in liposomal membranes (26). However, holes opened by osmotic shock or electroporation are unstable and it is difficult to control their size and duration. Talin, on the other hand, maintains stable large holes in liposomes, and these holes can be closed reversibly. Therefore, talin will prove to be a powerful tool for manipulating liposomes, and, as examples, encapsulation of macro molecules (such as DNA), cell organelles, and microbeads into liposomes will become more facile.
The remarkable membrane-breaking ability of talin observed in this study potentially is very dangerous for living cells. On the other hand, membranous structures such as cell organelles are always transforming, dividing, or fusing, and their topology must change. Therefore, it is possible that the property of talin to transform membranes may function favorably for topological membrane changes within living cells. Of course, it is not likely that talin functions as such a membrane regulator in vivo, because we were unable to observe such membrane breaking at physiological salt concentrations, but it seems likely that in vivo there are other proteins with activities toward lipid bilayers that are similar to talin, and cells may have evolved mechanisms for regulating the activity of such proteins.
Acknowledgments
We thank Dr. Robert Macnab (Yale University) and Dr. C. R. Calladine (University of Cambridge, U.K.) for comments on the manuscript.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: PC, phosphatidylcholine; PG, phosphatidylglycerol; PE, phosphatidylethanolamine; PS, phosphatidylserine; PMSF, phenylmethylsulfonyl fluoride.
References
- 1.Bangham A D. BioEssays. 1995;17:1081–1088. doi: 10.1002/bies.950171213. [DOI] [PubMed] [Google Scholar]
- 2.Lipowsky R. Nature (London) 1991;349:475–481. doi: 10.1038/349475a0. [DOI] [PubMed] [Google Scholar]
- 3.Canham P B. J Theor Biol. 1970;26:61–81. doi: 10.1016/s0022-5193(70)80032-7. [DOI] [PubMed] [Google Scholar]
- 4.Evans E A. Biophys J. 1974;14:923–931. doi: 10.1016/S0006-3495(74)85959-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sackmann E. Science. 1996;271:43–48. doi: 10.1126/science.271.5245.43. [DOI] [PubMed] [Google Scholar]
- 6.Lasic D D. In: Handbook of Biological Physics, 1a, Structure and Dynamics of Membranes. Lipowsky R, Sackmann E, editors. Amsterdam: Elsevier Science; 1995. pp. 491–519. [Google Scholar]
- 7.Rädler J O, Koltover I, Salditt T, Safinya R C. Science. 1997;275:810–814. doi: 10.1126/science.275.5301.810. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs H, Gesner R, Tauber R, Ghosh R. Biochemistry. 1995;34:6196–6207. doi: 10.1021/bi00018a024. [DOI] [PubMed] [Google Scholar]
- 9.Hotani H. J Mol Biol. 1984;178:113–120. doi: 10.1016/0022-2836(84)90234-1. [DOI] [PubMed] [Google Scholar]
- 10.Hotani H, Miyamoto H. Adv Biophys. 1990;26:135–156. doi: 10.1016/0065-227x(90)90010-q. [DOI] [PubMed] [Google Scholar]
- 11.Miyata H, Hotani H. Proc Natl Acad Sci USA. 1992;89:11547–11551. doi: 10.1073/pnas.89.23.11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burridge K, Connell L. J Cell Biol. 1983;97:359–367. doi: 10.1083/jcb.97.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horwitz A, Duggan K, Buck C, Beckerle M C, Burridge K. Nature (London) 1986;320:531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- 14.Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- 15.Burn P, Kupher A, Singer S J. Proc Natl Acad Sci USA. 1988;85:497–501. doi: 10.1073/pnas.85.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufmann S, Piekenbrock T, Goldmann H, Bärmann M, Isenberg G. FEBS Lett. 1991;284:187–191. doi: 10.1016/0014-5793(91)80681-r. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann S, Käs J, Goldmann H, Sackmann E, Isenberg G. FEBS Lett. 1992;314:203–205. doi: 10.1016/0014-5793(92)80975-m. [DOI] [PubMed] [Google Scholar]
- 18.Goldmann W H, Niggli V, Kaufmann S, Isenberg G. Biochemistry. 1992;31:7665–7671. doi: 10.1021/bi00148a030. [DOI] [PubMed] [Google Scholar]
- 19.Muguruma M, Matsumura S, Fukazawa T. Biochem Biophys Res Commun. 1990;171:1217–1223. doi: 10.1016/0006-291x(90)90815-5. [DOI] [PubMed] [Google Scholar]
- 20.Takiguchi K. J Biochem. 1991;109:520–527. doi: 10.1093/oxfordjournals.jbchem.a123414. [DOI] [PubMed] [Google Scholar]
- 21.Otey C, Griffiths W, Burridge K. Hybridoma. 1990;9:57–62. doi: 10.1089/hyb.1990.9.57. [DOI] [PubMed] [Google Scholar]
- 22.Rees D, Ades S, Singer S J, Hynes R. Nature (London) 1990;347:685–689. doi: 10.1038/347685a0. [DOI] [PubMed] [Google Scholar]
- 23.Tempel M, Goldmann H, Isenberg G, Sackmann E. Biophys J. 1995;69:228–241. doi: 10.1016/S0006-3495(95)79894-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiger B. Cell. 1979;18:193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- 25.Molony L, McCaslin D, Abernethy J, Paschal B, Burridge K. J Biol Chem. 1987;262:7790–7795. [PubMed] [Google Scholar]
- 26.Dimitrov D S. In: Handbook of Biological Physics, 1b, Structure and Dynamics of Membranes. Lipowsky R, Sackmann E, editors. Amsterdam: Elsevier Science; 1995. pp. 851–901. [Google Scholar]