Abstract
CD8+ T lymphocytes appear to play a role in controlling human immunodeficiency virus (HIV) replication, yet routine immunological assays do not measure the antiviral efficacy of these cells. Furthermore, it has been suggested that CD8+ T cells that recognize epitopes derived from proteins expressed early in the viral replication cycle can be highly efficient. We used a functional in vitro assay to assess the abilities of different epitope-specific CD8+ T-cell lines to control simian immunodeficiency virus (SIV) replication. We compared the antiviral efficacies of 26 epitope-specific CD8+ T-cell lines directed against seven SIV epitopes in Tat, Nef, Gag, Env, and Vif that were restricted by either Mamu-A*01 or Mamu-A*02. Suppression of SIV replication varied depending on the epitope specificities of the CD8+ T cells and was unrelated to whether the targeted epitope was derived from an early or late viral protein. Tat28-35SL8- and Gag181-189CM9-specific CD8+ T-cell lines were consistently superior at suppressing viral replication compared to the other five SIV-specific CD8+ T-cell lines. We also investigated the impact of viral escape on antiviral efficacy by determining if Tat28-35SL8- and Gag181-189CM9-specific CD8+ T-cell lines could suppress the replication of an escaped virus. Viral escape abrogated the abilities of Tat28-35SL8- and Gag181-189CM9-specific CD8+ T cells to control viral replication. However, gamma interferon (IFN-γ) enzyme-linked immunospot and IFN-γ/tumor necrosis factor alpha intracellular-cytokine-staining assays detected cross-reactive immune responses against the Gag escape variant. Understanding antiviral efficacy and epitope variability, therefore, will be important in selecting candidate epitopes for an HIV vaccine.
CD8+ T lymphocytes are an important component of the cellular immune response and play a role in controlling human immunodeficiency virus/simian immunodeficiency virus (HIV/SIV) replication. The depletion of circulating CD8+ lymphocytes in SIV-infected macaques results in an increase in plasma viral concentrations (25, 42, 59). CD8+ T lymphocytes have been shown to exert selective pressure on viral sequences in vivo; immune escape variants are observed in both the acute (3, 7, 50) and chronic (6, 15, 23, 54) phases of HIV/SIV infection. Furthermore, recent studies suggest that escape from CD8+ T-cell responses exacts a cost in viral fitness, since transmitted escape variants are not maintained in the absence of the selecting major histocompatibility complex (MHC) class I allele (2, 17, 33).
Several studies have shown associations between certain MHC class I alleles and slow or rapid HIV/SIV disease progression, implying that these alleles restrict CD8+ T-cell responses of varying effectiveness (8, 23, 26, 47, 48, 52, 61, 73, 74). However, it is still unknown which of the many HIV-specific CD8+ T lymphocytes actually contribute to the control of viral replication. Despite technological advances and new methodologies to detect and enumerate CD8+ T-lymphocyte responses against HIV/SIV, most of the current cellular assays do not actually measure antiviral efficacy, the ability to suppress viral replication (70).
Initial reports using such functional assays demonstrated that CD8+ cells (63) and virus-specific cytotoxic T lymphocytes (71) inhibited immunodeficiency virus replication in vitro. Dendritic cells pulsed with inactivated autologous virus initiated the expansion of virus-specific CD8+ T cells that controlled HIV replication (39). Based on these viral-replication inhibition assays, it has been suggested that CD8+ T lymphocytes directed against epitopes derived from early-expressed proteins, particularly Nef and Rev, are more efficacious than CD8+ T lymphocytes directed against epitopes in late-expressed viral proteins (1, 65, 66, 72). However, a recent investigation demonstrated that Pol-specific CD8+ T cells were also effective at suppressing HIV replication (62). While most data suggest that differences exist in the antiviral efficacies of CD8+ T-cell populations, current HIV studies are limited to a small number of well-defined clones.
Initially, we studied two immunodominant epitopes, Tat28-35SL8 and Gag181-189CM9, which are bound by the commonly studied Indian rhesus macaque MHC class I molecule Mamu-A*01 (3-6, 9, 17, 18, 32, 36, 45, 50-53, 60, 69). CD8+ T-cell lines directed against the Tat28-35SL8 epitope were consistently more effective at suppressing SIVmac239 replication than CD8+ T-cell lines directed against the Gag181-189CM9 epitope in our functional in vitro assay (36). This finding supported the notion that CD8+ T cells directed against early proteins are more efficacious than their counterparts directed against late proteins (1, 65, 66, 72).
In this study, we have conducted a broadened investigation to identify additional SIV-specific CD8+ T-cell responses with strong antiviral activity. Furthermore, we have directly compared the virus-suppressive abilities of CD8+ T cells that recognize early proteins to those of cells that recognize late-expressed proteins. Using the previously developed viral-suppression assay (VSA), we compared the abilities of 26 SIV-specific CD8+ T-cell populations directed against seven epitopes in five SIV proteins, Tat, Nef, Gag, Env, and Vif, to suppress viral replication. We found that suppression of SIV replication varies depending on the epitope specificities of the CD8+ T-cell lines and cannot be generalized by protein location or time of protein expression. The Tat28-35SL8- and Gag181-189CM9-specific CD8+ T-cell lines were the most effective at suppressing viral replication. Interestingly, low plasma viremia in macaques used to generate the SIV-specific CD8+ T-cell lines was not predictive of effective viral suppression.
We also investigated the effect viral escape had on the abilities of Tat28-35SL8- and Gag181-189CM9-specific CD8+ T cells to suppress SIV replication. Recent studies of HIV have observed the ability of antigen-specific CD8+ T lymphocytes to recognize variant peptides by using gamma interferon (IFN-γ) production as the readout, providing encouragement that the immune system may be able to cope with viral escape (22, 24, 46, 64). Similarly, we detected cytokine responses to a variant Gag peptide in both IFN-γ enzyme-linked immunospot (ELISPOT) and IFN-γ/tumor necrosis factor alpha (TNF-α) intracellular-cytokine-staining (ICS) assays. However, the cross-reactive cytokine response to the variant Gag peptide did not predict the abilities of Gag181-189CM9-specific CD8+ T-cell lines to suppress viral replication of an SIV escape variant. This illustrates the profound effect of viral escape on abrogating the ability of CD8+ T lymphocytes to suppress viral replication. Researchers have also claimed the detection of cross-clade HIV-specific CD8+ T-cell responses on the basis of cytokine production assays alone (13, 21, 68). Our results suggest that such findings may be misleading unless confirmed by functional assays.
MATERIALS AND METHODS
Animals and viruses.
Indian rhesus macaques (Macaca mulatta) were identified as Mamu-A*01+ and/or Mamu-A*02+ by sequence-specific primer DNA amplification (PCR-SSP), as previously described (32, 37). SIV-specific CD8+ T-cell lines were derived from Mamu-A*01+ and/or Mamu-A*02+ macaques infected with the molecularly cloned virus SIVmac239, GenBank accession no. M33262 (28). Naïve macaques were used as a source of peripheral blood mononuclear cells (PBMC) for in vitro SIV infections.
For in vitro SIV infections, CD8-depleted PBMC were infected with SIVmac239 (28) or an engineered escape variant virus based on SIVmac239 (17). This variant virus contained point mutations in two known Mamu-A*01 epitopes, Tat28-35SL8 and Gag181-189CM9, and contained the previously identified compensatory mutations required for replication of the Gag mutant (18, 53).
SIV-infected animals were maintained at the National Primate Research Center (University of Wisconsin—Madison) and cared for according to the regulations and guidelines of the University of Wisconsin Institutional Animal Care and Use Committee.
Quantification of vRNA in plasma.
Viral RNA (vRNA) was isolated from EDTA-anticoagulated plasma and detected in quantitative reverse transcription-PCR (QRT-PCR) using a modification of a published protocol with a one-step QRT-PCR kit (Invitrogen, Carlsbad, CA) (12). The final reaction mixtures (20-μl total volume) contained 0.2 mM of each deoxynucleoside triphosphate, 5 mM MgSO4, 0.015% bovine serum albumin (Sigma, St. Louis, MO), 150 nanograms random hexamer primers (Promega, Madison, WI), 0.8 μl SuperScript III reverse transcriptase and Platinum Taq DNA polymerase in a single enzyme mix, 600 nM of each amplification primer—forward (SIV1552), 5′-GTCTGCGTCATCTGGTGCATTC-3′; reverse (SIV1635), 5′-CACTAGCTGTCTCTGCACTATGTGTTTTG-3′—and 100 nM probe 5′-6-carboxyfluorescein-CTTCCTCAGTGTGTTTCACTTTCTCTTCTGCG-6-carboxytetramethylrhodamine-3′. Temperature cycling was performed on the LightCycler 1.2 (Roche, Indianapolis, IN) with slightly altered parameters. The reverse transcriptase reaction was performed at 37°C for 15 min and then at 50°C for 30 min. An activation temperature of 95°C for 2 min was followed by 50 amplification cycles of 95°C for 15 seconds and 62°C for 1 min with ramp times set to 3° per second. Data were acquired and analyzed using LightCycler 4.0 software.
Each QRT-PCR assay was run with a set of internal standards made up of a dilution series of synthetic transcript containing a fragment of the SIV gag gene. The theoretical concentrations of these standards range from 15 million copy eq to 3 copy eq per sample. Copy numbers for samples were determined by interpolation onto the standard curve using the LightCycler software version 4.0.
The interassay variation in observed crossing points for each point on the standard curve is less than 2%. Each assay is also run with an internal plasma standard to control for both vRNA isolation and detection in QRT-PCR. The interassay variability in the crossing point for this standard is 2.8%, and its observed concentration (average, 179,000 copy eq/ml) varies by 48%. Under normal assay conditions, the quantification threshold for this assay is 30 vRNA copy eq/ml.
Generation and maintenance of CD8+ T-cell lines.
Peptide-specific CD8+ T-cell lines were generated using previously described methods (67). Briefly, PBMC were separated from whole heparin- or EDTA-treated blood by Ficoll-Paque PLUS (GE Health Sciences, Piscataway, NJ) density centrifugation. CD8+ cells were separated from freshly isolated PBMC using the CD8 nonhuman primate microbead kit (Miltenyi Biotec, Auburn, CA) and the Miltenyi Biotec AutoMACS magnetic cell separation unit according to the manufacturer's protocol. Fresh, unseparated PBMC were also used to start CD8+ T-cell lines. Autologous B-lymphoblastoid cell lines (BLCLs) were used as antigen-presenting cells. BLCLs were pulsed with 1 μM of the relevant SIV-specific peptide for 1 to 2 h at 37°C, washed twice, and irradiated (9,000 rads). BLCLs were then mixed with either whole or CD8-enriched PBMC at a ratio of 1:1 in RPMI 1640 (Cambrex, Walkersville, MD) supplemented with l-glutamine (Mediatech, Herndon, VA), antibiotic-antimycotic solution (Mediatech), and 15% fetal bovine serum (R15; HyClone, Logan, UT) with 10 ng/ml of recombinant human interleukin-7 (Sigma-Aldrich, St. Louis, MO) and incubated for 48 h. The cells were cultured with R15 containing 100 units of interleukin-2/ml (NIH AIDS Research and Reference Reagent Program, Germantown, MD) every 3 to 5 days thereafter. The CD8+ T-cell lines were restimulated using peptide-pulsed, irradiated BLCL every 7 to 14 days. CD8+ T-cell lines were routinely tested for epitope specificity after >14 days in culture by either ICS or MHC class I tetramer assays. MHC class I tetramer staining was performed as previously described (38).
VSA.
We performed in vitro VSAs as previously described (36). Briefly, the target population consisted of CD8-depleted, phytohemagglutinin-stimulated lymphocytes that were infected with SIVmac239 (28), or an engineered CD8+ T-cell escape variant virus based on SIVmac239 (17), at a multiplicity of infection of 5 × 10−5. We used in vitro-stimulated epitope-specific CD8+ T-cell lines that were sorted or grown to a high specificity (>91%) as effector cells. All CD8+ T-cell lines used were less than 6 months old, and more than half of the data were derived from primary cell lines that underwent in vitro stimulation for approximately 2 months. Under these conditions, the age of the CD8+ T-cell line did not appear to impact its ability to suppress SIV replication (data not shown).
We added 5.0 × 105 CD8-depleted, phytohemagglutinin-stimulated lymphocytes (targets) to each well of a 24-well plate. The effector cells (CD8+ T-cell lines) were added to wells at effector-to-target (E:T) ratios of 1:10 and 1:20. These E:T ratios reflect the maximum possible number of potentially infectible target cells. The final volume of cell culture medium was 2 ml and contained 50 U/ml of interleukin-2 (NIH AIDS Research and Reference Reagent Program, Germantown, MD). The cocultures were maintained for 8 days. Every 2 days, 0.5 ml supernatant was collected and replaced with fresh medium to determine vRNA concentrations. At the end of the assay (day 8), intracellular Gag p27 staining was performed on the coculture to measure SIVmac239 infection, as previously described (36).
We cocultured MHC-mismatched effector and target cells as controls in each assay (36). Using MHC-mismatched effector and target cells, we observed negligible reduction in SIV replication compared to wells that contained only infected target cells (data not shown).
ICS assay.
SIV-specific CD8+ T-cell lines were used in TNF-α and IFN-γ ICS assays as previously described (67). Briefly, each test contained 2 × 105 CD8+ T cells and 0.5 × 105 to 1 × 105 autologous BLCLs. As a positive control, phorbol myristate acetate (1 μg/ml) with ionomycin (2 μg/ml; Sigma-Aldrich) was used. Individual Mamu-A*01- or Mamu-A*02-restricted minimal optimal peptides were used at a concentration of 5 μM or in serial 10-fold dilutions from 5 μM to 5 pM. Approximately 2 × 105 lymphocyte-gated events were acquired on a FACSCalibur (BD Biosciences) and analyzed using FlowJo software 6.4.1 (TreeStar). All values were normalized by subtracting the background level staining (negative control of CD8+ T-cell lines in media without stimulation).
IFN-γ ELISPOT assay.
PBMC were separated from whole heparin- or EDTA-treated blood by Ficoll-Paque PLUS (GE Health Sciences) density centrifugation. The PBMC were used directly in precoated ELISpotPLUS kits (MABTECH Inc., Mariemont, OH) for the detection of monkey IFN-γ according to the manufacturer's protocols. Briefly, 1.0 × 105 PBMC were used per well and incubated for 14 to 18 h at 37°C in 5% CO2. As a positive control, 5 μg/ml of concanavalin A (Sigma Chemical, St. Louis, MO) was added to the cells, and a negative control of no peptide was also included on each plate. Peptides were used at 10 μM or in serial 10-fold dilutions from 10 μM to 10 pM. All tests were performed in triplicate.
Wells were imaged with an AID ELISPOT reader (AID, Strassberg, Germany), counted with AID EliSpot Reader version 3.2.3, and analyzed as previously described (38). Spots were counted by an automated system with set parameters for size, intensity, and gradient. Background (the mean of wells without peptide stimulation) levels were subtracted from each well on the plate. A response was considered positive if the mean number of spot-forming cells (SFC) from triplicate sample wells exceeded background plus 2 standard deviations.
Assay results are shown as SFC per 1 × 106 cells. Responses of <50 SFC per 1 × 106 cells were not considered positive. Wells containing concanavalin A (positive control) were always greater than 1,000 SFC per 1 × 106 PBMC.
RESULTS
Characteristics of CD8+ T-cell populations against seven CD8+ T-cell epitopes in the viral-suppression assay.
Previously, we developed an in vitro VSA to examine the antiviral efficacies of CD8+ T-cell populations that recognized two immunodominant Mamu-A*01-restricted epitopes, Tat28-35SL8 and Gag181-189CM9 (36). We found that the Tat28-35SL8-specific CD8+ T-cell response was highly effective in suppressing SIV replication compared to CD8+ T cells directed against the Gag181-189CM9 epitope. This supported the previously suggested idea that CD8+ T lymphocytes directed against early proteins are better at controlling viral replication than CD8+ T lymphocytes directed against proteins expressed later in the viral replication cycle (1, 65, 66, 72). We sought to identify additional CD8+ T-cell populations capable of effective virus suppression. Mamu-A*01+ and Mamu-A*02+ macaques are routinely studied in vaccine and pathogenesis experiments due to the high frequency of these alleles, the knowledge of several well-defined CD8+ T-cell epitopes, and the availability of MHC class I tetrameric reagents (3-6, 9, 17, 18, 32, 36, 38, 45, 49, 51-53, 58, 60, 69).
We selected seven common SIV-specific CD8+ T-cell epitopes restricted by Mamu-A*01 and Mamu-A*02 for our investigation (Table 1). These epitopes are located within two early-expressed SIV proteins (Tat and Nef) and three late-expressed viral proteins (Gag, Env, and Vif) (30, 31, 55, 57). Previous investigations detected viral sequence variation in all of these CD8+ T-cell epitopes (3, 5, 6, 38, 45, 49-51, 67). The timing of viral escape ranged from 4 weeks postinfection, in the cases of the Tat and Nef epitopes, to approximately 1 year postinfection for selected Gag and Vif epitopes (Table 1).
TABLE 1.
Characteristics of epitope-specific CD8+ T cells studied in the VSA
| Protein | Amino acid positions | Short name | Sequence | MHC class I restriction | Earliest detected viral variation (wk) |
|---|---|---|---|---|---|
| Tat | 28-35 | SL8 | STPESANL | Mamu-A*01 | 4 |
| Vif | 97-104 | WY8 | WTDVTPNY | Mamu-A*02 | 57 |
| Nef | 159-167 | YY9 | YTSGPGIRY | Mamu-A*02 | 4 |
| Gag | 71-79 | GY9 | GSENLKSLY | Mamu-A*02 | 59 |
| Gag | 181-189 | CM9 | CTPYDINQM | Mamu-A*01 | 18 |
| Env | 788-795 | RY8 | RTLLSRVY | Mamu-A*02 | 32 |
| Env | 620-628 | TL9 | TVPWPNASL | Mamu-A*01 | 14 |
As a marker for disease progression status, SIV viral loads (vRNA copies/ml of plasma) at the time of CD8+ T-cell line generation are indicated in Table 2 for each macaque. We generated CD8+ T-cell lines from at least two animals for each of the seven SIV-specific CD8+ T-cell epitopes (Table 2). With the exception of the Env620-628TL9 epitope, each epitope-specific CD8+ T-cell line was derived from at least one elite-controller (EC) macaque, an animal maintaining chronic-phase SIV viremia below 1,000 vRNA copies/ml (73). Some previously published data on Tat28-35SL8- and Gag181-189CM9-specific CD8+ T-cell viral suppression was incorporated for the disease progression comparison (36).
TABLE 2.
Disease statuses of SIV-infected macaques used to generate CD8+ T-cell lines and representative antiviral efficacies of the CD8+ T-cell lines on day 8 of the VSA at an E:T ratio of 1:10
| Animal | SIV-specific CD8+ T-cell line | Parameters near time of CD8+ T-cell line generation
|
Percent reduction in SIV:
|
||
|---|---|---|---|---|---|
| No. of wk infected | Viral load (no. of vRNA copies/ml) | vRNA copies/ml | Gag p27+ cells | ||
| 95061a | Gag 181-189 CM9 | 253 | <50 | 83 | 76 |
| 95061a | Tat 28-35 SL8 | 290 | <50 | 77 | 55 |
| 95061a | Env 788-795 RY8 | 288 | <50 | 61 | 0 |
| 95061a | Nef 159-167 YY9 | 288 | <50 | 1 | 0 |
| 95061a | Gag 71-79 GY9 | 266 | 109 | 72 | 24 |
| 98014a | Nef 159-167 YY9 | 96 | <50 | 0 | 0 |
| 98016a | Vif 97-104 WY8 | 111 | <50 | 2 | 2 |
| 98016a | Gag 71-79 GY9 | 96 | 323 | 7 | 0 |
| 98016a | Nef 159-167 YY9 | 96 | 323 | 0 | 0 |
| 98016a | Env 788-795 RY8 | 136 | 353 | 0 | 0 |
| 2125 | Tat 28-35 SL8 | 42 | 158,000 | 99 | 99 |
| 2125 | Gag 181-189 CM9 | 42 | 158,000 | 90 | 84 |
| 2125 | Env 622-630 TL9 | 42 | 158,000 | 47 | 0 |
| 2128 | Tat 28-35 SL8 | 18 | 190,000 | 98 | 98 |
| 2128 | Env 788-795 RY8 | 47 | 457,000 | 28 | 14 |
| 2128 | Nef 159-167 YY9 | 47 | 457,000 | 10 | 0 |
| 2128 | Gag 71-79 GY9 | 71 | 461,000 | 52 | 44 |
| 01008 | Env 788-795 RY8 | 71 | 40,400 | 0 | 0 |
| 01008 | Vif 97-104 WY8 | 71 | 40,400 | 0 | 0 |
| 01008 | Env 622-630 TL9 | 53 | 89,300 | 0 | 0 |
| 97110 | Gag 181-189 CM9 | 39 | 65,100 | 45 | 32 |
| 97110 | Env 788-795 RY8 | 32 | 126,000 | 0 | 0 |
| 97110 | Tat 28-35 SL8 | 16 | 569,000 | 38 | 0 |
| 01034 | Env 622-630 TL9 | 54 | 837,000 | 44 | 14 |
| 97044 | Env 788-795 RY8 | 66 | 1,610,000 | 64 | 28 |
| 2095 | Tat 28-35 SL8 | 172 | 2,580,000 | 98 | 90 |
Elite-controller macaque (maintaining chronic-phase viral load of <1,000 viral RNA copies/ml).
Effective suppression of viral replication by two SIV-specific CD8+ T-cell populations.
Previous studies showed differences in antiviral efficacy between HIV-specific CD8+ T-cell clones directed against early proteins versus late proteins (1, 65, 66, 72). Therefore, we hypothesized that CD8+ T cells directed against SIV epitopes in early proteins might be more effective at suppressing viral replication than those directed against epitopes in late proteins. We generated CD8+ T-cell lines that recognized two early-protein epitopes (Tat28-35SL8 and Nef159-167YY9) and five late-protein epitopes (Gag181-189CM9, Gag71-79GY9, Env620-628TL9, Env788-795RY8, and Vif97-104WY8). Epitopes within Gag, Nef, and Env were of particular interest due to the inclusion of the genes in recent CD8+ T-cell-based vaccines (4-6, 9, 19, 34, 41, 43, 45, 60, 69).
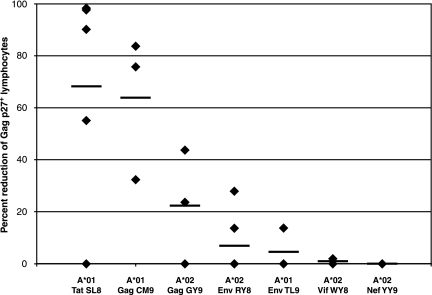
Our analysis included seven SIV epitopes restricted by Mamu-A*01 or Mamu-A*02 (Table 1). Tat28-35SL8-specific CD8+ T-cell populations were effective at viral suppression (Fig. 1 and Table 2), as we have described before (36). Tat28-35SL8-specific CD8+ T-cell lines from four of the five animals tested reduced viral production and Gag p27+ target cells by >55% (Fig. 1). CD8+ T cells directed against Gag181-189CM9 demonstrated the second-highest levels of viral suppression, in the range of 32 to 90% reduction. Env620-628TL9-specific CD8+ T cells did not suppress viral replication. Moreover, none of the four Mamu-A*02-restricted CD8+ T-cell responses consistently suppressed viral replication >50% in the VSA. These included four CD8+ T-cell lines directed against Nef159-167YY9, although Nef is an early SIV protein (Fig. 1). Effective suppression was not simply due to a higher frequency of epitope-specific CD8+ T cells in the coculture, as the frequencies were typically comparable when tested at the end of each assay (data not shown).
FIG. 1.
Percent reduction of Gag p27+ target cells following an 8-day coculture with SIV-specific CD8+ T-cell lines at an E:T ratio of 1:10. Twenty-six epitope-specific CD8+ T-cell lines directed against seven SIV epitopes (Tat28-35SL8, Gag181-189CM9, Gag71-79GY9, Env788-795RY8, Env620-628TL9, Vif97-104WY8, and Nef159-167YY9) were used in the VSA. These CD8+ T-cell lines were derived from 10 SIV-infected Indian rhesus macaques. The black bars indicate the average reduction for each SIV-specific CD8+ T-cell response. Exact values are listed in Table 2.
Of the five CD8+ T-cell populations directed against late-expressed proteins, effector cells recognizing Gag181-189CM9 displayed the highest levels of viral suppression (Fig. 1 and Table 2). Interestingly, the CD8+ T-cell response directed against Gag71-79GY9 was not as effective as the Gag181-189CM9-specific CD8+ T-cell population. These results suggest that antiviral efficacy may be independent of the viral protein from which the epitope is derived. Rather, antiviral efficacy should be evaluated for each epitope of interest. The Env620-628TL9-, Env788-795RY8-, and Vif97-104WY8-specific CD8+ T-cell lines were ineffective at suppressing SIV replication (typically <20% reduction).
Disease status does not appear to predict the ability of CD8+ T cells to suppress SIV replication.
We also investigated the potential impact of viral replication (vRNA copies/ml) at the time the CD8+ T-cell lines were generated on antiviral efficacy. We had previously observed some variability among animals in the ability of Tat28-35SL8-specific CD8+ T cells to suppress SIV replication (36). To address this question, we generated CD8+ T-cell lines from macaques in various stages of disease progression, including three EC macaques, 95061, 98014, and 98016, that controlled SIV replication at <1,000 vRNA copies/ml (Table 2). We hypothesized that CD8+ T-cell lines from EC macaques would be particularly effective at suppressing SIVmac239 replication.
Remarkably, CD8+ T-cell lines generated from elite-controller macaques were not always effective suppressors (Table 2). For instance, Tat28-35SL8-specific CD8+ T cells derived from animal 95061, with a viral load of <50 vRNA copies/ml, did not suppress SIV replication as effectively as Tat28-35SL8-specific CD8+ T-cell lines derived from progressor macaques 2125 and 2128. Despite the high plasma virus RNA concentrations at the time the CD8+ T-cell lines were generated, Tat28-35SL8-specific cell lines from macaques 2125 and 2128 reduced vRNA concentrations and Gag p27+ target cells >90% (Table 2). None of the four Mamu-A*02-restricted responses had appreciable levels of viral suppression, despite the derivation of several SIV-specific CD8+ T-cell lines from elite controllers. These included CD8+ T-cell lines directed against the early protein Nef159-167YY9. Similarly, no correlation was observed between CD4 counts at the time of cell line generation and the ability of CD8+ T cells to suppress SIV replication (data not shown).
Impact of viral escape on CD8+ T-cell antiviral efficacy.
It is well established that CD8+ T lymphocytes select for viral escape variants (3, 5-7, 15, 23, 50, 54). Recent reports demonstrated that CD8+ T lymphocytes can mount responses to variant peptides in cytokine production assays, implying that HIV/SIV-specific CD8+ lymphocytes can recognize mutant epitopes (22, 24, 46, 64). Recent studies also quantified the cross-clade reactivities of cellular immune responses to HIV using the same assays (13, 21, 68). However, since they use PBMC or epitope-specific CD8+ T-cell lines pulsed with excessive amounts of synthetic peptide, these routine immunological assays do not reflect natural antigen processing and presentation. Therefore, we investigated the abilities of our two most effective CD8+ T-cell lines, Tat28-35SL8 and Gag181-189CM9, to recognize escape variants commonly generated in vivo (3, 5, 6, 17, 18, 50, 51, 53).
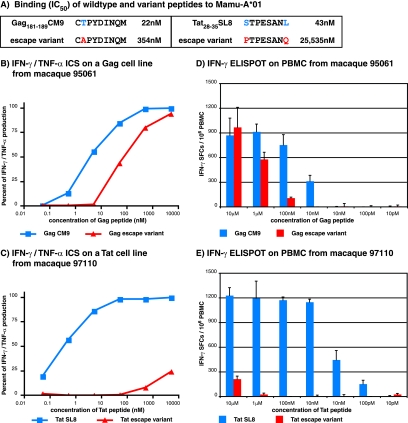
A previous study found that the two mutations at positions 1 and 8 of the Tat epitope (STPESANL to PTPESANQ) essentially abrogated peptide binding to Mamu-A*01 (Fig. 2A) (17). However, while the position 2 amino acid substitution in the Gag epitope (CTPYDINQM to CAPYDINQM) diminished MHC class I binding, this variant peptide still bound at a biologically relevant affinity of 354 nM (50% inhibitory concentration).
FIG. 2.
Recognition of wild-type and mutant epitopes in Gag and Tat as measured by IFN-γ/TNF-α ICS and IFN-γ ELISPOT assays. (A) Binding (50% inhibitory concentrations [IC50]) of the wild-type and variant peptides to Mamu-A*01 from a previous study (17). (B and C) IFN-γ/TNF-α ICS assay results for recognition of wild-type and escape variant peptides using Gag181-189CM9- (B) and Tat28-35SL8-specific (C) CD8+ T-cell lines derived from macaques 95061 and 97110, respectively. The data were normalized against the cytokine production when the highest wild-type peptide concentration was used. This value was considered the maximal cytokine response and was labeled as 100%. (D and E) Ex vivo IFN-γ ELISPOT assay results for recognition of wild-type and escape variant peptides using freshly isolated PBMC from macaque 95061 to test Gag reactivity (D) and from macaque 97110 to test Tat reactivity (E). Mean values from triplicate wells were calculated for each assay. The background, the mean of wells without peptide, was subtracted from each well. Mean responses of <50 SFC per 1 × 106 cells were not considered positive. The error bars in panels D and E indicate standard deviations for the triplicate wells.
Based upon the previous binding analysis of the escape variant epitopes, we hypothesized that we would observe some recognition of the Gag escape variant and none with the Tat escape variant. We first tested for peptide cross-reactivity in IFN-γ/TNF-α ICS assays using Gag181-189CM9- and Tat28-35SL8-specific CD8+ T-cell lines (Fig. 2B and C). The Gag variant peptide displayed cross-reactivity at a peptide concentration range of 10,000 nM to 100 nM, while the Tat escape variant was only weakly recognized at the highest peptide concentration (Fig. 2B and C). To confirm these results, we then tested the recognition of the variant peptides in ex vivo IFN-γ ELISPOT assays using freshly isolated PBMC. At a peptide concentration of 100 nM, the Gag escape variant induced cytokine secretion (Fig. 2D). Responses to the wild-type and variant Gag peptides were equal in magnitude at the highest peptide concentration. In contrast, the Tat escape variant showed minimal cytokine reactivity and only at the highest peptide concentration (Fig. 2E). The responses to variant peptides in IFN-γ ELISPOT were likely due to recognition of the variant peptide (cross-reactivity) and not the stimulation of a de novo response against the mutant peptides. When both peptides were tested in the same well, the number of SFC/106 PBMC did not exceed the number of SFC seen when either peptide was added alone (data not shown). Our results, in conjunction with previously published work (22, 24, 46, 64), suggested the possibility that the Gag181-189CM9-specific CD8+ T-cell lines might be effective at suppressing an SIV escape variant.
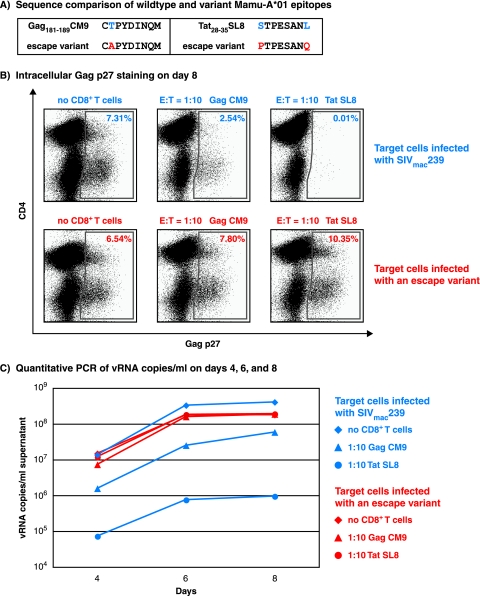
To directly assess the abilities of epitope-specific CD8+ T cells to control the replication of the mutant virus, we used a variant of SIVmac239 (17) that contained escape mutations in both Tat28-35SL8 and Gag181-189CM9 to infect target cells (Fig. 3). As a control to determine whether the CD8+ T cells used were still capable of suppressing viral replication, CD8− target cells were also infected with wild-type SIVmac239. As expected, both the Gag181-189CM9 and Tat28-35SL8-specific CD8+ T cells effectively suppressed wild-type SIVmac239 replication (Fig. 3). However, when the escape mutant SIV was used, neither of these CD8+ T-cell lines suppressed viral replication in four independent assays. We observed similar trends at an E:T ratio of 1:20 (data not shown). This poor suppression was particularly unexpected for the Gag181-189CM9-specific CD8+ T cells. The variant epitope still bound with a biologically relevant affinity, and the variant peptide stimulated both SIV-specific CD8+ T-cell lines and PBMC in IFN-γ/TNF-α ICS and IFN-γ ELISPOT assays, respectively (Fig. 2). This inability of Gag181-189CM9-specific CD8+ T cells to suppress the replication of the mutant virus stresses the importance of functional assays that incorporate natural antigen processing and presentation to evaluate the antiviral efficacies of epitope-specific CD8+ T-cell responses. These data also reemphasize the importance of viral escape in evading host immune responses.
FIG. 3.
Gag181-189CM9- and Tat28-35SL8-specific CD8+ T lymphocytes were unable to suppress the replication of an SIVmac239 escape variant. (A) Amino acid sequences of the wild-type and variant epitopes located in Gag and Tat. (B) Intracellular Gag p27 staining of representative day 8 VSA results using effector and target cells at 1:10 infected with either wild-type SIVmac239 or an escape variant, SIVmac239. (C) Quantitative PCR of viral RNA copies/ml on days 4, 6, and 8 of the same assay.
DISCUSSION
Unlike studies involving antibody responses in which neutralization assays distinguish effective antibodies from ineffective ones, most current assays involving CD8+ T cells do not measure antiviral efficacy (70). We recently developed a functional in vitro VSA to assess the ability of CD8+ T cells to control SIV replication and to study the impact of viral variation (36). This new assay has enabled us to expand upon previous HIV studies, which hinted at differences in antiviral efficacy among various CD8+ T-cell populations (1, 62, 65, 66, 71, 72). We used our viral-suppression assay to examine the antiviral efficacies of seven common CD8+ T-cell responses against epitopes restricted by two high-frequency MHC class I alleles, Mamu-A*01 and Mamu-A*02 (Table 1). These CD8+ T cells recognized epitopes from both early- and late-expressed viral proteins. Twenty-six epitope-specific CD8+ T-cell lines were derived from 10 SIVmac239-infected macaques with differing disease statuses, including three elite-controller macaques. We also examined the abilities of CD8+ T cells to suppress the replication of viruses containing common escape mutations in two immunodominant Mamu-A*01-restricted CD8+ T-cell epitopes.
We found that suppression of SIV replication varied, depending on the epitope specificity of the CD8+ T cell (Fig. 1 and Table 2). Furthermore, this suppressive ability was not related to whether the epitope was derived from an early- or late-expressed viral protein. Even in our broadened investigation, Tat28-35SL8-specific CD8+ T cells remained the most effective at suppressing viral replication, as we had shown in our initial studies (36). Tat28-35SL8-specific CD8+ T cells at an E:T ratio of 1:10 markedly reduced SIV replication (>55%) in four of the five CD8+ T-cell lines tested. Epitope-specific CD8+ T cells against Gag181-189CM9 were also effective and exhibited levels of suppression in the range of 32 to 90%. While Gag is generally viewed as a late protein, recent findings have shown that Gag-specific epitopes can be recognized as early as 2 hours after SIV infection (58a). This early presentation advantage may contribute to the ability of Gag181-189CM9 CD8+ T cells to effectively suppress SIV replication. The remaining five SIV-specific CD8+ T-cell responses, including CD8+ T-cell lines against an early SIV epitope, Nef159-167YY9, were largely ineffective at suppressing viral replication (Fig. 1). In vivo, Nef159-167YY9 elicits an immunodominant CD8+ T-cell response, with viral escape occurring as early as 4 weeks postinfection (50, 58, 67). However, Nef159-167YY9-specific CD8+ T-cell lines from four different macaques, including two elite controllers, failed to suppress viral replication. In addition, we found that CD8+ T lymphocytes, which recognize two different epitopes within the same viral protein, did not exhibit similar suppressive properties. Both an immunodominant Mamu-A*01-restricted epitope, Gag181-89CM9, and an immunodominant Mamu-A*02-restricted epitope, Gag71-79GY9, are located in Gag. However, only Gag181-189CM9-specific CD8+ T-cell lines consistently suppressed SIV replication >50% (Fig. 1 and Table 2). Similar to previous studies (11, 62, 72), the functional avidity of cytokine reactivity did not appear to correlate with antiviral efficacy in vitro (data not shown).
We also examined the impact that disease progression may have on the ability of CD8+ T cells to effectively suppress viral replication. While Tat28-35SL8-specific CD8+ T cells were more effective at suppressing SIV replication, we previously observed animal-to-animal variability (36). However, this variability did not appear to directly reflect differences in the disease statuses or viral loads of macaques at the time of cell line generation (Table 2).
The effect of viral escape from CD8+ T-cell responses is an important consideration for vaccine development. Certain high-frequency CD8+ T-cell responses rapidly select for viral escape, driving CD8+ T-cell-susceptible viral sequences to extinction within 4 weeks of infection (3, 50, 58, 67). Recent studies have also addressed the influence of escape on viral fitness (17, 27, 33, 40, 41). To explore the role of viral escape in antiviral efficacy, we generated a clone of SIVmac239 that contained escape mutations in two Mamu-A*01-restricted CD8+ T-cell epitopes, Tat28-35SL8 and Gag181-189CM9 (17). Tat28-35SL8- and Gag181-189CM9-specific CD8+ T cells did not suppress the replication of this escape variant virus (Fig. 3). This loss of antiviral efficacy was somewhat surprising for Gag181-189CM9-specific CD8+ T cells, because the variant of Gag181-189CM9 was well recognized in both IFN-γ/TNF-α ICS and IFN-γ ELISPOT assays (Fig. 2). Our data suggest that the currently employed routine immunological assays neither accurately predict the antiviral efficacy of CD8+ T cells nor measure the impact of viral escape on the ability of these cells to suppress viral replication. Furthermore, similar cytokine production readouts are currently being employed to detect cross-clade CD8+ T-cell responses induced by HIV infection and vaccine immunogens (13, 21, 68). Our results suggest that the correlation between cytokine production and antiviral function is neither direct nor consistent. Therefore, while CD8+ T cells may release IFN-γ in response to peptides representing several HIV clades, this does not necessarily indicate that they can effectively suppress the replication of viruses in these clades.
In this study, we used epitope-specific CD8+ T-cell lines rather than CD8+ T-cell clones to reduce potential bias that could result from studying cell populations with limited or no T-cell receptor (TCR) diversity. Epitope-specific CD8+ T-cell populations with diverse clonotypic repertoires are observed in HIV and SIV infection; indeed, TCR diversity may be beneficial (10, 14, 29, 56). Complementary studies in our laboratory using CD8+ T-cell clones have shown that different clones specific for a single peptide can vary in their abilities to suppress virus replication, underscoring the importance of TCR diversity (11).
Previous studies of HIV and SIV demonstrated that epitope-specific CD8+ T lymphocytes suppress viral replication primarily through direct cytolytic activity (20, 71, 72). In our investigation, we did not directly measure the cytolytic activities of our highly specific CD8+ T-cell lines. While we cannot account for potential variability in the cytolytic activity and its effect on our VSA readouts, results from VSA and ICS assays displayed similar trends over time (data not shown).
It is plausible that findings using our in vitro viral-suppression assay might be extrapolated to the ability to suppress HIV/SIV replication in vivo. However, future investigations of CD8+ T-cell antiviral efficacy might benefit from modifications of this assay. These include using higher multiplicities of infection to mimic the massive infection and rapid loss of CD4+ T cells at mucosal surfaces (35, 44). Emerging evidence also suggests that CD8+ T cells reactive to subdominant epitopes may contribute significantly to the control of HIV and SIV replication in vivo (16, 18a). More extensive evaluation of these T-cell populations will be necessary.
Overall, our results outline clear differences in CD8+ T-cell efficacy and suggest that the antiviral efficacy of CD8+ T cells should be measured for individual epitopes and not generalized by protein. We found no supporting evidence for the idea that CD8+ T cells that recognize epitopes in early-expressed viral proteins were more efficacious than those that recognize epitopes in late-expressed proteins. However, recent findings regarding Gag epitope expression show that these timing designations might not be as appropriate as was once thought (58a). Furthermore, our findings indicate that routine immunological assays are both indirect and inadequate for assessing T-cell function and cross-reactivity. While ELISPOT and ICS assays are widely accepted means to track CD8+ T-cell frequencies, these assays fail to differentiate among T-cell populations in terms of antiviral efficacy. The use of functional assays in addition to standard immunogenicity assays should enable us to more thoroughly evaluate candidate epitopes for inclusion in future HIV vaccines.
Acknowledgments
We thank William Rehrauer, Jess Maxwell, Chrystal Glidden, and Gretta Borchardt for MHC class I PCR-SSP typing and gratefully acknowledge Laura Valentine, Alex Ko, and Andrea Weiler for immunological assay assistance. David O'Connor and Adrian McDermott provided helpful discussions. We also thank the Immunology and Virology Core Laboratories at the National Primate Research Center, University of Wisconsin—Madison, for technical assistance.
This research was supported by NIH grants R01 AI049120, R01 AI052056, and R24 RR015371 to D.I.W., in addition to the NIH-National Center for Research Resources grant P51 RR000167 awarded to the Wisconsin National Primate Research Center (WNPRC). This work was conducted at a facility constructed with support from Research Facilities Improvement Grant numbers RR15459-01 and RR020141-01 (WNPRC).
Footnotes
Published ahead of print on 27 December 2006.
REFERENCES
- 1.Ali, A., R. Lubong, H. Ng, D. G. Brooks, J. A. Zack, and O. O. Yang. 2004. Impacts of epitope expression kinetics and class I downregulation on the antiviral activity of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes. J. Virol. 78:561-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. M., M. Altfeld, X. G. Yu, K. M. O'Sullivan, M. Lichterfeld, S. Le Gall, M. John, B. R. Mothe, P. K. Lee, E. T. Kalife, D. E. Cohen, K. A. Freedberg, D. A. Strick, M. N. Johnston, A. Sette, E. S. Rosenberg, S. A. Mallal, P. J. Goulder, C. Brander, and B. D. Walker. 2004. Selection, transmission, and reversion of an antigen-processing cytotoxic-T-lymphocyte escape mutation in human immunodeficiency virus type 1 infection. J. Virol. 78:7069-7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 4.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 5.Barouch, D. H., J. Kunstman, J. Glowczwskie, K. J. Kunstman, M. A. Egan, F. W. Peyerl, S. Santra, M. J. Kuroda, J. E. Schmitz, K. Beaudry, G. R. Krivulka, M. A. Lifton, D. A. Gorgone, S. M. Wolinsky, and N. L. Letvin. 2003. Viral escape from dominant simian immunodeficiency virus epitope-specific cytotoxic T lymphocytes in DNA-vaccinated rhesus monkeys. J. Virol. 77:7367-7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. [DOI] [PubMed] [Google Scholar]
- 7.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 8.Carrington, M., G. W. Nelson, M. P. Martin, T. Kissner, D. Vlahov, J. J. Goedert, R. Kaslow, S. Buchbinder, K. Hoots, and S. J. O'Brien. 1999. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science 283:1748-1752. [DOI] [PubMed] [Google Scholar]
- 9.Casimiro, D. R., F. Wang, W. A. Schleif, X. Liang, Z. Q. Zhang, T. W. Tobery, M. E. Davies, A. B. McDermott, D. H. O'Connor, A. Fridman, A. Bagchi, L. G. Tussey, A. J. Bett, A. C. Finnefrock, T. M. Fu, A. Tang, K. A. Wilson, M. Chen, H. C. Perry, G. J. Heidecker, D. C. Freed, A. Carella, K. S. Punt, K. J. Sykes, L. Huang, V. I. Ausensi, M. Bachinsky, U. Sadasivan-Nair, D. I. Watkins, E. A. Emini, and J. W. Shiver. 2005. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J. Virol. 79:15547-15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Z. W., Y. Li, X. Zeng, M. J. Kuroda, J. E. Schmitz, Y. Shen, X. Lai, L. Shen, and N. L. Letvin. 2001. The TCR repertoire of an immunodominant CD8+ T lymphocyte population. J. Immunol. 166:4525-4533. [DOI] [PubMed] [Google Scholar]
- 11.Chung, C., W. Lee, J. T. Loffredo, B. Burwitz, T. C. Friedrich, J. P. Giraldo Vela, G. Napoe, E. G. Rakasz, N. A. Wilson, D. B. Allison, and D. I. Watkins. 29. Nov. 2006. Not all cytokine-producing CD8+ T-cells suppress simian immunodeficiency virus replication. J. Virol. doi: 10.1128/JVI.01780-06. (Subsequently published, J. Virol. 81:1517-1523, 2007.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cline, A. N., J. W. Bess, M. Piatak, Jr., and J. D. Lifson. 2005. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J. Med. Primatol. 34:303-312. [DOI] [PubMed] [Google Scholar]
- 13.Coplan, P. M., S. B. Gupta, S. A. Dubey, P. Pitisuttithum, A. Nikas, B. Mbewe, E. Vardas, M. Schechter, E. G. Kallas, D. C. Freed, T. M. Fu, C. T. Mast, P. Puthavathana, J. Kublin, K. Brown Collins, J. Chisi, R. Pendame, S. J. Thaler, G. Gray, J. McIntyre, W. L. Straus, J. H. Condra, D. V. Mehrotra, H. A. Guess, E. A. Emini, and J. W. Shiver. 2005. Cross-reactivity of anti-HIV-1 T cell immune responses among the major HIV-1 clades in HIV-1-positive individuals from four continents. J. Infect. Dis. 191:1427-1434. [DOI] [PubMed] [Google Scholar]
- 14.Dong, T., G. Stewart-Jones, N. Chen, P. Easterbrook, X. Xu, L. Papagno, V. Appay, M. Weekes, C. Conlon, C. Spina, S. Little, G. Screaton, A. van der Merwe, D. D. Richman, A. J. McMichael, E. Y. Jones, and S. L. Rowland-Jones. 2004. HIV-specific cytotoxic T cells from long-term survivors select a unique T cell receptor. J. Exp. Med. 200:1547-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans, D. T., D. H. O'Connor, P. Jing, J. L. Dzuris, J. Sidney, J. da Silva, T. M. Allen, H. Horton, J. E. Venham, R. A. Rudersdorf, T. Vogel, C. D. Pauza, R. E. Bontrop, R. DeMars, A. Sette, A. L. Hughes, and D. I. Watkins. 1999. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat. Med. 5:1270-1276. [DOI] [PubMed] [Google Scholar]
- 16.Frahm, N., P. Kiepiela, S. Adams, C. H. Linde, H. S. Hewitt, K. Sango, M. E. Feeney, M. M. Addo, M. Lichterfeld, M. P. Lahaie, E. Pae, A. G. Wurcel, T. Roach, M. A. St John, M. Altfeld, F. M. Marincola, C. Moore, S. Mallal, M. Carrington, D. Heckerman, T. M. Allen, J. I. Mullins, B. T. Korber, P. J. Goulder, B. D. Walker, and C. Brander. 2006. Control of human immunodeficiency virus replication by cytotoxic T lymphocytes targeting subdominant epitopes. Nat. Immunol. 7:173-178. [DOI] [PubMed] [Google Scholar]
- 17.Friedrich, T. C., E. J. Dodds, L. J. Yant, L. Vojnov, R. Rudersdorf, C. Cullen, D. T. Evans, R. C. Desrosiers, B. R. Mothe, J. Sidney, A. Sette, K. Kunstman, S. Wolinsky, M. Piatak, J. Lifson, A. L. Hughes, N. Wilson, D. H. O'Connor, and D. I. Watkins. 2004. Reversion of CTL escape-variant immunodeficiency viruses in vivo. Nat. Med. 10:275-281. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich, T. C., C. A. Frye, L. J. Yant, D. H. O'Connor, N. A. Kriewaldt, M. Benson, L. Vojnov, E. J. Dodds, C. Cullen, R. Rudersdorf, A. L. Hughes, N. Wilson, and D. I. Watkins. 2004. Extraepitopic compensatory substitutions partially restore fitness to simian immunodeficiency virus variants that escape from an immunodominant cytotoxic-T-lymphocyte response. J. Virol. 78:2581-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Friedrich, T. C., L. E. Valentine, L. J. Yant, E. G. Rakasz, S. M. Piaskowski, J. R. Furlott, K. L. Weisgrau, B. Burwitz, G. E. May, E. J. León, T. Soma, G. Napoé, S. V. Capuano, N. A. Wilson, and D. I. Watkins. Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 19.Gallimore, A., M. Cranage, N. Cook, N. Almond, J. Bootman, E. Rud, P. Silvera, M. Dennis, T. Corcoran, J. Stott, et al. 1995. Early suppression of SIV replication by CD8+ Nef-specific cytotoxic T cells in vaccinated macaques. Nat. Med. 1:1167-1173. [DOI] [PubMed] [Google Scholar]
- 20.Gauduin, M. C., R. L. Glickman, R. Means, and R. P. Johnson. 1998. Inhibition of simian immunodeficiency virus (SIV) replication by CD8+ T lymphocytes from macaques immunized with live attenuated SIV. J. Virol. 72:6315-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geels, M. J., S. A. Dubey, K. Anderson, E. Baan, M. Bakker, G. Pollakis, W. A. Paxton, J. W. Shiver, and J. Goudsmit. 2005. Broad cross-clade T-cell responses to Gag in individuals infected with human immunodeficiency virus type 1 non-B clades (A to G): importance of HLA anchor residue conservation. J. Virol. 79:11247-11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillespie, G. M., R. Kaul, T. Dong, H. B. Yang, T. Rostron, J. J. Bwayo, P. Kiama, T. Peto, F. A. Plummer, A. J. McMichael, and S. L. Rowland-Jones. 2002. Cross-reactive cytotoxic T lymphocytes against a HIV-1 p24 epitope in slow progressors with B*57. AIDS 16:961-972. [DOI] [PubMed] [Google Scholar]
- 23.Goulder, P. J., R. E. Phillips, R. A. Colbert, S. McAdam, G. Ogg, M. A. Nowak, P. Giangrande, G. Luzzi, B. Morgan, A. Edwards, A. J. McMichael, and S. Rowland-Jones. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3:212-217. [DOI] [PubMed] [Google Scholar]
- 24.Iversen, A. K., G. Stewart-Jones, G. H. Learn, N. Christie, C. Sylvester-Hviid, A. E. Armitage, R. Kaul, T. Beattie, J. K. Lee, Y. Li, P. Chotiyarnwong, T. Dong, X. Xu, M. A. Luscher, K. MacDonald, H. Ullum, B. Klarlund-Pedersen, P. Skinhoj, L. Fugger, S. Buus, J. I. Mullins, E. Y. Jones, P. A. van der Merwe, and A. J. McMichael. 2006. Conflicting selective forces affect T cell receptor contacts in an immunodominant human immunodeficiency virus epitope. Nat. Immunol. 7:179-189. [DOI] [PubMed] [Google Scholar]
- 25.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaslow, R. A., M. Carrington, R. Apple, L. Park, A. Munoz, A. J. Saah, J. J. Goedert, C. Winkler, S. J. O'Brien, C. Rinaldo, R. Detels, W. Blattner, J. Phair, H. Erlich, and D. L. Mann. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2:405-411. [DOI] [PubMed] [Google Scholar]
- 27.Kawada, M., H. Igarashi, A. Takeda, T. Tsukamoto, H. Yamamoto, S. Dohki, M. Takiguchi, and T. Matano. 2006. Involvement of multiple epitope-specific cytotoxic-T-lymphocyte responses in vaccine-based control of simian immunodeficiency virus replication in rhesus macaques. J. Virol. 80:1949-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kestler, H., T. Kodama, D. Ringler, M. Marthas, N. Pedersen, A. Lackner, D. Regier, P. Sehgal, M. Daniel, N. King, et al. 1990. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science 248:1109-1112. [DOI] [PubMed] [Google Scholar]
- 29.Killian, M. S., R. L. Sabado, S. Kilpatrick, M. A. Hausner, B. D. Jamieson, and O. O. Yang. 2005. Clonal breadth of the HIV-1-specific T-cell receptor repertoire in vivo as determined by subtractive analysis. AIDS 19:887-896. [DOI] [PubMed] [Google Scholar]
- 30.Kim, S. Y., R. Byrn, J. Groopman, and D. Baltimore. 1989. Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. J. Virol. 63:3708-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klotman, M. E., S. Kim, A. Buchbinder, A. DeRossi, D. Baltimore, and F. Wong-Staal. 1991. Kinetics of expression of multiply spliced RNA in early human immunodeficiency virus type 1 infection of lymphocytes and monocytes. Proc. Natl. Acad. Sci. USA 88:5011-5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knapp, L. A., E. Lehmann, M. S. Piekarczyk, J. A. Urvater, and D. I. Watkins. 1997. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 50:657-661. [DOI] [PubMed] [Google Scholar]
- 33.Leslie, A. J., K. J. Pfafferott, P. Chetty, R. Draenert, M. M. Addo, M. Feeney, Y. Tang, E. C. Holmes, T. Allen, J. G. Prado, M. Altfeld, C. Brander, C. Dixon, D. Ramduth, P. Jeena, S. A. Thomas, A. S. John, T. A. Roach, B. Kupfer, G. Luzzi, A. Edwards, G. Taylor, H. Lyall, G. Tudor-Williams, V. Novelli, J. Martinez-Picado, P. Kiepiela, B. D. Walker, and P. J. Goulder. 2004. HIV evolution: CTL escape mutation and reversion after transmission. Nat. Med. 10:282-289. [DOI] [PubMed] [Google Scholar]
- 34.Letvin, N. L., J. R. Mascola, Y. Sun, D. A. Gorgone, A. P. Buzby, L. Xu, Z. Y. Yang, B. Chakrabarti, S. S. Rao, J. E. Schmitz, D. C. Montefiori, B. R. Barker, F. L. Bookstein, and G. J. Nabel. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 36.Loffredo, J. T., E. G. Rakasz, J. P. Giraldo, S. P. Spencer, K. K. Grafton, S. R. Martin, G. Napoe, L. J. Yant, N. A. Wilson, and D. I. Watkins. 2005. Tat28-35SL8-specific CD8+ T lymphocytes are more effective than Gag181-189CM9-specific CD8+ T lymphocytes at suppressing simian immunodeficiency virus replication in a functional in vitro assay. J. Virol. 79:14986-14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loffredo, J. T., J. Sidney, S. Piaskowski, A. Szymanski, J. Furlott, R. Rudersdorf, J. Reed, B. Peters, H. D. Hickman-Miller, W. Bardet, W. M. Rehrauer, D. H. O'Connor, N. A. Wilson, W. H. Hildebrand, A. Sette, and D. I. Watkins. 2005. The high frequency Indian rhesus macaque MHC class I molecule, Mamu-B*01, does not appear to be involved in CD8+ T lymphocyte responses to SIVmac239. J. Immunol. 175:5986-5997. [DOI] [PubMed] [Google Scholar]
- 38.Loffredo, J. T., J. Sidney, C. Wojewoda, E. Dodds, M. R. Reynolds, G. Napoe, B. R. Mothe, D. H. O'Connor, N. A. Wilson, D. I. Watkins, and A. Sette. 2004. Identification of seventeen new simian immunodeficiency virus-derived CD8+ T cell epitopes restricted by the high frequency molecule, Mamu-A*02, and potential escape from CTL recognition. J. Immunol. 173:5064-5076. [DOI] [PubMed] [Google Scholar]
- 39.Lu, W., and J. M. Andrieu. 2001. In vitro human immunodeficiency virus eradication by autologous CD8+ T cells expanded with inactivated-virus-pulsed dendritic cells. J. Virol. 75:8949-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Picado, J., J. G. Prado, E. E. Fry, K. Pfafferott, A. Leslie, S. Chetty, C. Thobakgale, I. Honeyborne, H. Crawford, P. Matthews, T. Pillay, C. Rousseau, J. I. Mullins, C. Brander, B. D. Walker, D. I. Stuart, P. Kiepiela, and P. Goulder. 2006. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J. Virol. 80:3617-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matano, T., M. Kobayashi, H. Igarashi, A. Takeda, H. Nakamura, M. Kano, C. Sugimoto, K. Mori, A. Iida, T. Hirata, M. Hasegawa, T. Yuasa, M. Miyazawa, Y. Takahashi, M. Yasunami, A. Kimura, D. H. O'Connor, D. I. Watkins, and Y. Nagai. 2004. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J. Exp. Med. 199:1709-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72:164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattapallil, J. J., D. C. Douek, A. Buckler-White, D. Montefiori, N. L. Letvin, G. J. Nabel, and M. Roederer. 2006. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J. Exp. Med. 203:1533-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 45.McDermott, A. B., D. H. O'Connor, S. Fuenger, S. Piaskowski, S. Martin, J. Loffredo, M. Reynolds, J. Reed, J. Furlott, T. Jacoby, C. Riek, E. Dodds, K. Krebs, M. E. Davies, W. A. Schleif, D. R. Casimiro, J. W. Shiver, and D. I. Watkins. 2005. Cytotoxic T-lymphocyte escape does not always explain the transient control of simian immunodeficiency virus SIVmac239 viremia in adenovirus-boosted and DNA-primed Mamu-A*01-positive rhesus macaques. J. Virol. 79:15556-15566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKinney, D. M., R. Skvoretz, B. D. Livingston, C. C. Wilson, M. Anders, R. W. Chesnut, A. Sette, M. Essex, V. Novitsky, and M. J. Newman. 2004. Recognition of variant HIV-1 epitopes from diverse viral subtypes by vaccine-induced CTL. J. Immunol. 173:1941-1950. [DOI] [PubMed] [Google Scholar]
- 47.Migueles, S. A., M. S. Sabbaghian, W. L. Shupert, M. P. Bettinotti, F. M. Marincola, L. Martino, C. W. Hallahan, S. M. Selig, D. Schwartz, J. Sullivan, and M. Connors. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 97:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muhl, T., M. Krawczak, P. Ten Haaft, G. Hunsmann, and U. Sauermann. 2002. MHC class I alleles influence set-point viral load and survival time in simian immunodeficiency virus-infected rhesus monkeys. J. Immunol. 169:3438-3446. [DOI] [PubMed] [Google Scholar]
- 49.Newberg, M. H., K. J. McEvers, D. A. Gorgone, M. A. Lifton, S. H. Baumeister, R. S. Veazey, J. E. Schmitz, and N. L. Letvin. 2006. Immunodomination in the evolution of dominant epitope-specific CD8+ T lymphocyte responses in simian immunodeficiency virus-infected rhesus monkeys. J. Immunol. 176:319-328. [DOI] [PubMed] [Google Scholar]
- 50.O'Connor, D. H., T. M. Allen, T. U. Vogel, P. Jing, I. P. DeSouza, E. Dodds, E. J. Dunphy, C. Melsaether, B. Mothe, H. Yamamoto, H. Horton, N. Wilson, A. L. Hughes, and D. I. Watkins. 2002. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat. Med. 8:493-499. [DOI] [PubMed] [Google Scholar]
- 51.O'Connor, D. H., A. B. McDermott, K. C. Krebs, E. J. Dodds, J. E. Miller, E. J. Gonzalez, T. J. Jacoby, L. Yant, H. Piontkivska, R. Pantophlet, D. R. Burton, W. M. Rehrauer, N. Wilson, A. L. Hughes, and D. I. Watkins. 2004. A dominant role for CD8+-T-lymphocyte selection in simian immunodeficiency virus sequence variation. J. Virol. 78:14012-14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Connor, D. H., B. R. Mothe, J. T. Weinfurter, S. Fuenger, W. M. Rehrauer, P. Jing, R. R. Rudersdorf, M. E. Liebl, K. Krebs, J. Vasquez, E. Dodds, J. Loffredo, S. Martin, A. B. McDermott, T. M. Allen, C. Wang, G. G. Doxiadis, D. C. Montefiori, A. Hughes, D. R. Burton, D. B. Allison, S. M. Wolinsky, R. Bontrop, L. J. Picker, and D. I. Watkins. 2003. Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J. Virol. 77:9029-9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peyerl, F. W., H. S. Bazick, M. H. Newberg, D. H. Barouch, J. Sodroski, and N. L. Letvin. 2004. Fitness costs limit viral escape from cytotoxic T lymphocytes at a structurally constrained epitope. J. Virol. 78:13901-13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phillips, R. E., S. Rowland-Jones, D. F. Nixon, F. M. Gotch, J. P. Edwards, A. O. Ogunlesi, J. G. Elvin, J. A. Rothbard, C. R. Bangham, C. R. Rizza, and A. J. McMichael. 1991. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 354:453-459. [DOI] [PubMed] [Google Scholar]
- 55.Pomerantz, R. J., D. Trono, M. B. Feinberg, and D. Baltimore. 1990. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell 61:1271-1276. [DOI] [PubMed] [Google Scholar]
- 56.Price, D. A., S. M. West, M. R. Betts, L. E. Ruff, J. M. Brenchley, D. R. Ambrozak, Y. Edghill-Smith, M. J. Kuroda, D. Bogdan, K. Kunstman, N. L. Letvin, G. Franchini, S. M. Wolinsky, R. A. Koup, and D. C. Douek. 2004. T cell receptor recognition motifs govern immune escape patterns in acute SIV infection. Immunity 21:793-803. [DOI] [PubMed] [Google Scholar]
- 57.Ranki, A., A. Lagerstedt, V. Ovod, E. Aavik, and K. J. Krohn. 1994. Expression kinetics and subcellular localization of HIV-1 regulatory proteins Nef, Tat and Rev in acutely and chronically infected lymphoid cell lines. Arch. Virol. 139:365-378. [DOI] [PubMed] [Google Scholar]
- 58.Robinson, S., W. A. Charini, M. H. Newberg, M. J. Kuroda, C. I. Lord, and N. L. Letvin. 2001. A commonly recognized simian immunodeficiency virus Nef epitope presented to cytotoxic T lymphocytes of Indian-origin rhesus monkeys by the prevalent major histocompatibility complex class I allele Mamu-A*02. J. Virol. 75:10179-10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58a.Sacha, J. B., C. Chung, E. G. Rakasz, S. P. Spencer, A. K. Jonas, A. T. Bean, W. Lee, B. J. Burwitz, J. J. Stephany, J. T. Loffredo, D. B. Allison, S. Adnan, A. Hoji, N. A. Wilson, T. C. Friedrich, J. D. Lifson, O. O. Yang, and D. I. Watkins. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J. Immunol., in press. [DOI] [PMC free article] [PubMed]
- 59.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 60.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 61.Tang, J., S. Tang, E. Lobashevsky, A. D. Myracle, U. Fideli, G. Aldrovandi, S. Allen, R. Musonda, and R. A. Kaslow. 2002. Favorable and unfavorable HLA class I alleles and haplotypes in Zambians predominantly infected with clade C human immunodeficiency virus type 1. J. Virol. 76:8276-8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tomiyama, H., M. Fujiwara, S. Oka, and M. Takiguchi. 2005. Cutting edge: epitope-dependent effect of Nef-mediated HLA class I down-regulation on ability of HIV-1-specific CTLs to suppress HIV-1 replication. J. Immunol. 174:36-40. [DOI] [PubMed] [Google Scholar]
- 63.Tsubota, H., C. I. Lord, D. I. Watkins, C. Morimoto, and N. L. Letvin. 1989. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J. Exp. Med. 169:1421-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turnbull, E. L., A. R. Lopes, N. A. Jones, D. Cornforth, P. Newton, D. Aldam, P. Pellegrino, J. Turner, I. Williams, C. M. Wilson, P. A. Goepfert, M. K. Maini, and P. Borrow. 2006. HIV-1 epitope-specific CD8+ T cell responses strongly associated with delayed disease progression cross-recognize epitope variants efficiently. J. Immunol. 176:6130-6146. [DOI] [PubMed] [Google Scholar]
- 65.van Baalen, C. A., C. Guillon, M. van Baalen, E. J. Verschuren, P. H. Boers, A. D. Osterhaus, and R. A. Gruters. 2002. Impact of antigen expression kinetics on the effectiveness of HIV-specific cytotoxic T lymphocytes. Eur. J. Immunol. 32:2644-2652. [DOI] [PubMed] [Google Scholar]
- 66.Van Baalen, C. A., M. Schutten, R. C. Huisman, P. H. Boers, R. A. Gruters, and A. D. Osterhaus. 1998. Kinetics of antiviral activity by human immunodeficiency virus type 1-specific cytotoxic T lymphocytes (CTL) and rapid selection of CTL escape virus in vitro. J. Virol. 72:6851-6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vogel, T. U., T. C. Friedrich, D. H. O'Connor, W. Rehrauer, E. J. Dodds, H. Hickman, W. Hildebrand, J. Sidney, A. Sette, A. Hughes, H. Horton, K. Vielhuber, R. Rudersdorf, I. P. De Souza, M. R. Reynolds, T. M. Allen, N. Wilson, and D. I. Watkins. 2002. Escape in one of two cytotoxic T-lymphocyte epitopes bound by a high-frequency major histocompatibility complex class I molecule, Mamu-A*02: a paradigm for virus evolution and persistence? J. Virol. 76:11623-11636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weaver, E. A., Z. Lu, Z. T. Camacho, F. Moukdar, H. X. Liao, B. J. Ma, M. Muldoon, J. Theiler, G. J. Nabel, N. L. Letvin, B. T. Korber, B. H. Hahn, B. F. Haynes, and F. Gao. 2006. Cross-subtype T-cell immune responses induced by a human immunodeficiency virus type 1 group M consensus Env immunogen. J. Virol. 80:6745-6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson, N. A., J. Reed, G. S. Napoe, S. Piaskowski, A. Szymanski, J. Furlott, E. J. Gonzalez, L. J. Yant, N. J. Maness, G. E. May, T. Soma, M. R. Reynolds, E. Rakasz, R. Rudersdorf, A. B. McDermott, H. O'Connor, D. T. C. Friedrich, D. B. Allison, A. Patki, L. J. Picker, D. R. Burton, J. Lin, L. Huang, D. Patel, G. Heindecker, J. Fan, M. Citron, M. Horton, F. Wang, X. Liang, J. W. Shiver, D. R. Casimiro, and D. I. Watkins. 2006. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 80:5875-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang, O. O. 2003. Will we be able to ‘spot’ an effective HIV-1 vaccine? Trends Immunol. 24:67-72. [DOI] [PubMed] [Google Scholar]
- 71.Yang, O. O., S. A. Kalams, A. Trocha, H. Cao, A. Luster, R. P. Johnson, and B. D. Walker. 1997. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 71:3120-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang, O. O., P. T. Sarkis, A. Trocha, S. A. Kalams, R. P. Johnson, and B. D. Walker. 2003. Impacts of avidity and specificity on the antiviral efficiency of HIV-1-specific CTL. J. Immunol. 171:3718-3724. [DOI] [PubMed] [Google Scholar]
- 73.Yant, L. J., T. C. Friedrich, R. C. Johnson, G. E. May, N. J. Maness, A. M. Enz, J. D. Lifson, D. H. O'Connor, M. Carrington, and D. I. Watkins. 2006. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 80:5074-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang, Z. Q., T. M. Fu, D. R. Casimiro, M. E. Davies, X. Liang, W. A. Schleif, L. Handt, L. Tussey, M. Chen, A. Tang, K. A. Wilson, W. L. Trigona, D. C. Freed, C. Y. Tan, M. Horton, E. A. Emini, and J. W. Shiver. 2002. Mamu-A*01 allele-mediated attenuation of disease progression in simian-human immunodeficiency virus infection. J. Virol. 76:12845-12854. [DOI] [PMC free article] [PubMed] [Google Scholar]