Abstract
The saimiri transforming protein oncogene, called STP-A, of herpesvirus saimiri (HVS) subgroup A is not required for viral replication but is required for lymphoid cell immortalization in culture and lymphoma induction in primates. Here we report that STP-A interacts with cellular tumor necrosis factor receptor-associated factors (TRAF2 and TRAF6) and Src family protein tyrosine kinases (SF-PTKs) in a genetically and functionally separable manner and that each interaction constitutively elicits independent cellular signal transduction. The amino-terminal and central proline-rich motifs of STP-A were responsible for TRAF6 and TRAF2 interactions, respectively, and STP-A and TRAF6 interaction contributed to the majority of NF-κB activation, whereas STP-A and TRAF2 interaction played a minor role in NF-κB activation. On the other hand, interaction of STP-A with SF-PTKs through its SH2 binding motif effectively elicited AP-1 and NF-AT transcription factor activity. One cellular gene targeted by STP-A is intercellular adhesion molecule 1 (ICAM-1), which participates in a wide range of inflammatory and immune responses. Both TRAF and SF-PTK signal transductions induced by STP-A were required for the marked increase of ICAM-1 expression. These results demonstrate that the viral oncogene STP-A independently targets two vital cellular signaling molecules and that these activities likely contribute to HVS-mediated lymphoid cell immortalization in culture and lymphoma induction in primates.
Upon lymphocyte activation, many cellular proteins, including T-cell receptor and B-cell receptor subunits, adaptor proteins, and other effector molecules, are phosphorylated and subsequently involved in the formation of molecular complexes at the site of activation (42). Particularly, the earliest signaling event is the sequential activation of the nonreceptor protein tyrosine kinases (PTKs) of the Src and Syk families. Activated Src family PTKs (SF-PTKs) Lck and Fyn in T lymphocytes subsequently phosphorylate tyrosine residues within a consensus sequence termed the immunoreceptor tyrosine-based activation motif in the cytosolic tails of the T-cell receptor (TCR) subunits. The phosphorylated immunoreceptor tyrosine-based activation motifs of TCR in turn recruit the Syk family PTKs ZAP-70 and Syk through their SH2 domains (31). Together with Lck and Fyn, the ZAP-70 and Syk kinases then promote the phosphorylation of many intracellular signaling molecules, including phospholipase C-γ1, Cbl, Vav, linker for activation of T cells, and SLP-76 (SH2-containing leukocyte protein 76) (10, 43, 46, 48). Phosphorylation of these cellular signaling molecules ultimately induces various cellular events, such as cytoskeletal alteration, intracellular Ca2+ influx, and NF-AT, AP-1, and NF-κB transcription factor activation (31, 42, 46).
Another key signal transduction pathway of T and B lymphocytes is mediated by members of the tumor necrosis factor receptor (TNF-R) and Toll-like receptor family. The key components of these receptor signaling pathways are the cytoplasmic adapter proteins known as TNF-R-associated factors (TRAFs) (8, 55). For example, CD40, a member of the TNF-R family, is expressed on antigen-presenting cells and provides key activation signals in T-cell-dependent B-cell activation by using TRAF signal transduction pathways (44). Several proteins carried by microbes also induce TRAF-mediated signaling pathways. A notable example is the latent membrane protein 1 (LMP-1) produced by the Epstein-Barr virus (EBV) (33). The TRAF proteins are characterized by the presence of a unique TRAF domain at the C terminus, which consists of a coiled-coil domain followed by a conserved TRAF-C domain. The TRAF domain plays an important role in TRAF function by mediating self-association and interaction with upstream receptors and other signaling proteins (8). The N-terminal portions of most TRAF proteins contain a RING finger and several zinc finger motifs, which are important for downstream signaling events. Many of the biological effects of TRAF signaling appear to be mediated through the activation of the NF-κB transcription factor (4, 55).
Herpesvirus saimiri (HVS) is a member of the gammaherpesvirus family, which includes human EBV and human herpesvirus 8, also called Kaposi's sarcoma-associated herpesvirus. HVS infection is endemic and nonpathogenic in its natural host, squirrel monkeys (Saimiri sciureus) (15). However, HVS infections of other species of New World primates result in rapidly progressing fatal T-cell lymphomas and leukemias (13, 23). Besides its potent oncogenicity in vivo, HVS can immortalize peripheral blood mononuclear cells of humans, rhesus monkeys, common marmosets, and rabbits to interleukin-2 (IL-2)-independent growth in vitro (1, 2, 5). Cell lines derived from peripheral blood mononuclear cells of common marmosets by in vitro immortalization with HVS represent a restricted lymphocyte subpopulation that is CD2+ CD8+ CD4− CD56+, indicating that these cells are likely derived from suppressor/cytotoxic T lymphocytes (24).
Mutational analyses have demonstrated that the leftmost open reading frames (ORFs) of HVS are not required for viral replication but are essential for lymphoma induction in vivo and immortalization of lymphoid cells in culture (13, 14). At this location, HVS subgroup A viruses contain an ORF encoding saimiri transforming protein A (STP-A). A previous study has shown that deletion of STP-A yields a virus that is no longer capable of inducing fatal lymphomas in common marmosets (16). STP-A has a highly acidic amino terminus, nine nonconsecutive collagen-like repeats (Gly-X-Y, where X and/or Y is proline) in the central region, and a hydrophobic stretch at the carboxyl terminus sufficient for membrane targeting (2). Our previous studies have shown that STP-A contains binding motifs for TRAFs (TRAF1, -2, and -3), Src tyrosine kinase, and STAT3 (9, 28, 29). Interaction of STP-A with Src and STAT3 has been shown to induce Src-dependent activation of STAT3 activity and elevation of certain proteins involved in proliferation and antiapoptosis, such as cyclin D1, c-Fos, and Bcl-xL (9).
Despite our previous study of STP-A and TRAF interaction, however, this interaction has not been well characterized for the activation of the NF-κB signaling pathway (28). Here we investigate the downstream cellular signaling pathways and consequences of STP-A-mediated activation of cellular TRAF and Src activities. We found that interaction of STP-A with TRAF6 induced robust NF-κB transcription factor activation, whereas interaction of STP-A with SF-PTKs primarily elicited strong AP-1 and NF-AT transcription factor activation. Ultimately, both STP-A-mediated signal transductions resulted in strong induction of ICAM-1 expression and IL-2 promoter activity. These results indicate that the STP-A oncoprotein constitutively activates TRAF- and SF-PTK-mediated signal transductions in genetically and functionally separable manners, which likely plays an important role in HVS-induced pathogenesis.
MATERIALS AND METHODS
Cells and transfection.
Human Jurkat T E6.1 cells were maintained in RPMI 1640 medium containing 10% fetal bovine serum, 2 mM glutamine, and antibiotics (200 units/ml penicillin and 200 μg/ml streptomycin) (Invitrogen, San Diego, CA). The Jurkat.κB.GFP cell line was kindly provided by Xin Lin (M. D. Anderson, TX) (9, 54). Cells were transfected with STP-A expression vectors by electroporation as described previously (30). The human embryonic kidney (HEK) 293T cell line was maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and antibiotics (200 units/ml penicillin and 200 μg/ml streptomycin). 293T cells were transfected by calcium phosphate transfection as described previously (30). Escherichia coli strains Top10 and TKX1 were obtained from Invitrogen and Stratagene (La Jolla, CA), respectively.
DNA constructs.
Wild-type (WT) and mutant STP-A mammalian expression plasmids were constructed by cloning N-terminally hemagglutinin (HA)-tagged STP-A PCR products into the pEF.IRES.puro vector. In addition, the STP-A coding sequence was cloned into the EcoRI/NotI sites of pGEX4T-1 (Clontech, Palo Alto, CA) for the expression of bacterial glutathione S-transferase (GST) fusion proteins in E. coli. In vitro site-directed mutagenesis was carried out to construct various STP-A mutants. Each STP-A mutant was completely sequenced to verify the presence of the mutation and the absence of any other changes. The Myc-tagged Lck and Fyn proteins were previously described (30). The NF-κB luciferase reporter plasmid was purchased from Stratagene. The NF-AT luciferase reporter plasmid was purchased from Biomyx Technology (San Diego, CA). The IL-2 promoter was previously described (11). The AP-1 luciferase reporter plasmid (pGL3-2×AP-1) and intercellular adhesion molecule 1 (ICAM-1) luciferase reporter construct containing the 1.3-kb full-length ICAM-1 gene promoter (pGL1.3) were kindly provided by Tomas Parks (Cellegy Phamaceuticals, Inc., San Francisco, CA). The WT and kinase-dead (KD) Src constructs were purchased from Upstate Biotechnology (Lake Placid, NY). The WT and KD Fyn constructs were kindly provided by Hamid Band (Northwestern University, Chicago, IL). The dominant-negative (DN) forms used here were TRAF2 ΔRing and TRAF6 ΔRingΔZn finger mutants (20, 33, 51), Ubc13 C87A (33), a TAK1 kinase-dead (K63W) mutant (40), and an IκBα S32A/36A mutant (47). These constructs were kindly provided by E. Kieff (Harvard University, Boston, MA), J. Chen (University of Texas Southwestern, Dallas, TX), and Y. W. Choi (University of Pennsylvania, Philadelphia, PA).
Antibodies and reagents.
A horseradish peroxidase-conjugated phospho-specific antibody against tyrosine phosphorylation was obtained from Upstate Biotechnology. A phospho-specific antibody against tyrosine phosphorylation was purchased from Sigma (St. Louis, MO). Monoclonal antibodies against Myc, Fyn, GST, HA, IκBα, TAK1, and tubulin and a polyclonal antibody against Src were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A monoclonal antibody against Ubc13 was obtained from Zymed (San Francisco, CA). Glutathione-conjugated beads and beads conjugated with anti-HA were obtained from Clontech and Santa Cruz Biotechnology. Horseradish peroxidase-conjugated anti-HA antibody was obtained from Covance (San Diego, CA).
Luciferase reporter assays.
HEK 293T cells grown in six-well plates were transfected with STP-A expression plasmids (0.5 μg) together with a luciferase reporter construct (0.5 μg) and a constitutive β-galactosidase (β-Gal) expression construct (pGK-β-gal; 0.1 μg). At 36 h posttransfection, a luciferase assay was performed as described previously (30).
Fluorescence-activated cell sorter analysis.
The induction of ICAM-1 surface expression in transfected cells was assayed by immunofluorescence staining of viable cells, followed by flow cytometry using a Becton Dickinson FACSCalibur analyzer. Briefly, Jurkat T cells were transfected with pEF.IRES.puro vectors carrying STP-A by electroporation. At 24 h postelectroporation, Ficoll-Histopaque was used to remove dead cells. The live cells were incubated for an additional 16 h at 37°C and then stained with a phycoerythrin (PE)-conjugated antibody against human ICAM-1 (Pharmingen, San Diego, CA). Fluorescence-activated cell sorter analysis was performed with Cell Quest software (Becton Dickinson).
End-point RT-PCR analysis of ICAM-1 mRNA.
Total RNA was isolated from Jurkat T cells transiently transfected with STP-A expression vectors using an RNeasy kit (QIAGEN, Chatsworth, CA). RNA was DNase treated, reverse transcribed, and PCR amplified using a one-step reverse transcription-PCR (RT-PCR) kit (Roche, Indianapolis, IN). The primer sequences were 5′-AGA GGT CTC AGA AGG GAC CG (forward) and 5′-GGG CCA TAC AGG ACA CGA AC (backward) for human ICAM-1 cDNA (228 bp) and 5′-TGT TGC CAT CAA TGA CCC CTT (forward) and 5′-CTC CAC GAC GTA CTC AGC G (backward) for human glyceraldehyde-3-phosphate dehydrogenase cDNA (202 bp).
IP and IB.
Cell lysates were prepared in 1% NP-40 lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1% NP-40) containing protease inhibitors. Immunoprecipitation (IP) and immunoblotting (IB) were carried out as described previously (9, 30). Immunoprecipitates were separated by 12% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). Membranes were incubated with appropriate antibodies in 5% skim milk-phosphate-buffered saline-Tween 20 (or 3% bovine serum albumin-Tris-buffered saline-Tween 20 for phosphotyrosine blots). The proteins were visualized with chemiluminescent detection reagents (Pierce, Rockford, IL) and detected with a Fuji phosphorimager (LAS-3000; Fuji Film Co., Tokyo, Japan).
GST pull-down assays.
GST fusion proteins were purified from either the E. coli Top10 or TKX1 strain carrying a mammalian elk tyrosine kinase gene expression vector. Whole-cell lysates (WCLs) of HEK 293T cells transfected with either the Lck, Fyn, or Src expression vector were precleared with glutathione-Sepharose-conjugated beads for 2 h, followed by incubation with glutathione bead-immobilized GST-STP-A fusion proteins in binding buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1% NP-40, and protease inhibitors) at 4°C for 2 h. The glutathione beads were then washed four times with binding buffer, and the proteins associated with the beads were analyzed by 12% SDS-PAGE and subjected to immunoblot assay with a phosphorimager.
RESULTS
HVS STP-A oncoprotein is efficiently phosphorylated and interacts with SF-PTKs.
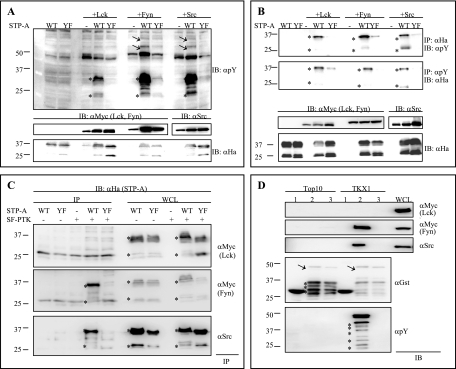
We previously reported that the tyrosine residue in the Y115AEV SH2 binding motif of STP-A, a major oncoprotein of HVS subgroup A strain 11, is efficiently phosphorylated by and interacts with Src kinase (29). In contrast, the STP-A Y115F (YF) mutant is neither tyrosine phosphorylated by nor interacts with Src kinase (9, 29). Here we further examined whether STP-A is phosphorylated by Lck and Fyn, which are major SF-PTKs of T cells. To do this, we expressed STP-A in 293T cells either alone or together with SF-PTKs and then immunoblotted WCLs with an antiphosphotyrosine antibody. WT STP-A, but not the Y115F (YF) mutant, was strongly tyrosine phosphorylated by either Fyn or Src and moderately tyrosine phosphorylated by Lck (Fig. 1A and B). Furthermore, STP-A expression appeared to increase the levels of cellular tyrosine phosphorylation induced by Fyn and Src but not by Lck (Fig. 1A, arrows).
FIG. 1.
STP-A phosphorylation by and interaction with SF-PTKs. (A) SF-PTKs phosphorylate STP-A. 293T cells were transfected with HA-tagged WT STP-A or Y115F mutant (YF) STP-A, Myc-tagged Lck, Myc-tagged Fyn, and Src expression vectors, as indicated at the top of figure. At 48 h posttransfection, whole-cell lysates (WCLs) were used for immunoblotting (IB) with antiphosphotyrosine (αpY) antibody to detect tyrosine-phosphorylated proteins (top panel) and with anti-HA antibody to detect the level of STP-A expression (bottom panel). IBs with anti-Myc, anti-Src, and anti-HA antibodies show the relative expression of SF-PTKs and STP-A (middle and bottom panels). Arrows indicate phosphorylated cellular proteins, and asterisks indicate the STP-A protein. (B) Tyrosine phosphorylation of STP-A. 293T cells were transfected as described above. At 48 h posttransfection, half of the 293T WCLs were subjected to immunoprecipitation (IP) with anti-HA antibody, followed by IB with antiphosphotyrosine (αpY) antibody. The other half of the WCLs were used for IP with antiphosphotyrosine (αpY) antibody, followed by IB with anti-HA antibody. IBs with anti-Myc, anti-Src, and anti-HA antibodies show the relative expression levels of Lck, Fyn, Src, and STP-A. (C) Interaction of STP-A with Fyn and Src but not with Lck. The same WCLs used for panel A were subjected to IP with anti-Myc antibody (top and middle panels) or anti-Src antibody (bottom panel), followed by IB with anti-HA antibody for STP-A. Asterisks indicate STP-A. (D) In vitro interaction of tyrosine-phosphorylated STP-A with Fyn and Src but not with Lck. Glutathione-Sepharose beads containing GST (lanes 1), GST-STP-A WT (lanes 2), or GST-STP-A Y115F (YF) mutant (lanes 3) were purified from either E. coli strain Top10 or TKX1 and then used for GST pull-down with WCLs of 293T cells transfected with either Myc-Lck, Myc-Fyn, or Src expression vector. Proteins associated with GST and GST-STP-A fusion proteins were separated by 12% SDS-PAGE and subjected to IB with either anti-Myc antibody, for Lck and Fyn, or anti-Src antibody. WCLs were included as controls. GST and GST-STP-A fusion proteins purified from either E. coli strain Top10 or TKX1 were resolved by SDS-PAGE and subjected to immunoblotting with an anti-GST antibody and an antiphosphotyrosine (αpY) antibody. Arrows and asterisks indicate full-length GST-STP-A and degraded GST-STP-A, respectively.
We also found that WT STP-A effectively interacted with Fyn and Src but not with Lck (Fig. 1C). This interaction was specific because the Y115F (YF) mutant was not able to interact with Fyn and Src under the same conditions (Fig. 1C). To further confirm this binding, a GST pull-down assay was performed using bacterial GST fusion proteins. Unphosphorylated GST-STP-A was produced from the E. coli Top10 strain, and tyrosine-phosphorylated GST-STP-A was produced from the E. coli TKX1 strain, which contains the elk tyrosine kinase gene. The Elk tyrosine kinase has broad specificity and efficiently phosphorylates many mammalian proteins in E. coli. We found that over 60% of GST-STP-A protein purified from E. coli TKX1 was tyrosine phosphorylated by Elk kinase (data not shown). The GST pull-down assay showed that both Fyn and Src kinases effectively interacted with phosphorylated GST-STP-A but not with unphosphorylated GST-STP-A protein (Fig. 1D). In striking contrast, Lck was not capable of interacting with either phosphorylated or unphosphorylated GST-STP-A at a detectable level (Fig. 1D). These results suggest that Lck phosphorylates but does not detectably interact with STP-A, whereas both Fyn and Src phosphorylate and interact with STP-A.
STP-A interaction with SF-PTKs induces NF-AT and AP-1 activation.
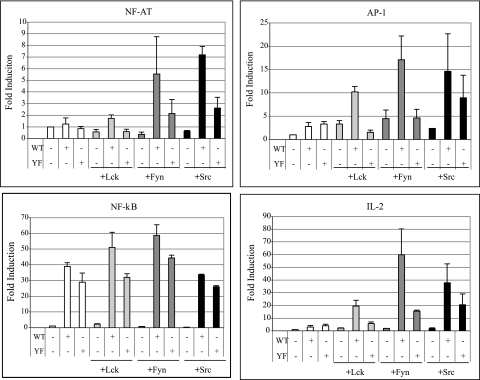
To delineate STP-A interactions with SF-PTKs, we investigated whether these interactions affect cellular NF-AT, AP-1, and NF-κB transcription factor activities. While STP-A alone did not significantly increase NF-AT and AP-1 activities, coexpression of Fyn and Src resulted in a marked increase of NF-AT and AP-1 activities (Fig. 2). Lck coexpression also induced STP-A-mediated activation of NF-AT and AP-1 activities, but the activation levels were significantly lower than those induced by Fyn and Src coexpression, indicating that STP-A interactions with Fyn and Src strongly potentiate NF-AT and AP-1 activation (Fig. 2). In addition, this activation was dependent on STP-A and SF-PTK interaction, since the STP-A Y115F (YF) mutant resulted in reduced levels of NF-AT and AP-1 transcription factor activity regardless of SF-PTK expression (Fig. 2). In contrast, STP-A-mediated NF-κB activation was independent of SF-PTK expression (Fig. 2). This was further supported by the result that the STP-A Y115F (YF) mutant induced NF-κB activation as efficiently as WT STP-A (Fig. 2). Finally, luciferase activity driven from the IL-2 promoter, which contains NF-AT-, AP-1-, and NF-κB-responsive elements, was markedly enhanced by the coexpression of SF-PTKs with WT STP-A compared with that for the STP-A Y115F (YF) mutant coexpressed with SF-PTKs (Fig. 2).
FIG. 2.
Activation of transcription factor activities by STP-A. 293T cells were transfected with 0.5 μg of pEF.IRES.puro, pEF.IRES.puro-STP-A WT, pEF.IRES.puro-STP-A Y115F (YF) mutant, Lck, Fyn, and Src expression vectors in various combinations along with 0.5 μg of a luciferase reporter construct and 0.1 μg of a transfection control β-Gal expression construct (pGK-β-gal). After 36 h, cells were harvested, and luciferase and β-Gal values were determined and reported as x-fold increased luciferase activity divided by β-Gal activity relative to that of vector-transfected cells. Bars represent means ± standard errors of the means (SEM) for three independent experiments. WCLs used for luciferase assays were subjected to IB with anti-Myc, anti-Src, and anti-HA antibodies to detect Lck and Fyn, Src, and STP-A expression, respectively (data not shown).
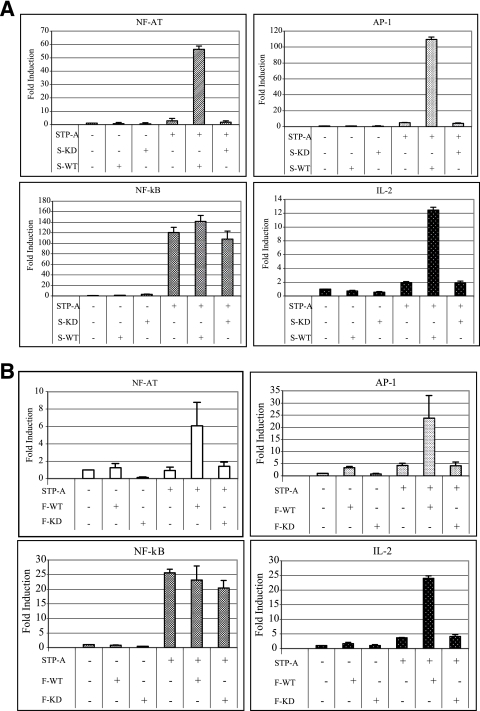
To further test whether Src and Fyn kinase activities are necessary to induce STP-A-mediated activation of NF-AT and AP-1 activities, a Src KD mutant and Fyn KD mutant were cotransfected with STP-A. This showed that coexpression of Src and Fyn kinase-dead mutants was not able to induce STP-A-mediated NF-AT, AP-1, and IL-2 promoter activation (Fig. 3A and B). In contrast, STP-A robustly induced NF-κB activity even in the presence of Src and Fyn KD mutant expression (Fig. 3A and B). STP-A, SF-PTKs, and their mutants were equivalently expressed (data not shown). These results demonstrate that STP-A interactions with SF-PTKs contribute to the activation of NF-AT and AP-1 transcription factor activities but not to NF-κB transcription factor activity and that the kinase enzymatic activities of Src and Fyn are necessary for the ability of STP-A to induce NF-AT and AP-1 transcription factor activities.
FIG. 3.
Activation of NF-AT and AP-1 transcription factor activities by STP-A requires SF-PTK activity. (A) Requirement of Src kinase activity. 293T cells were transfected with 0.5 μg of pEF.IRES.puro or pEF.IRES.puro-STP-A WT and 1 μg of Src WT (S-WT) or Src kinase-dead (S-KD) expression vector, along with 0.5 μg of a luciferase reporter construct and 0.1 μg of a transfection control β-Gal expression construct (pGK-β-gal). After 36 h, cells were harvested, and luciferase and β-Gal values were determined and reported as x-fold increased luciferase activity divided by β-Gal activity relative to that of vector-transfected cells. (B) Requirement of Fyn kinase activity. Samples and procedures were the same as those described for panel A, except for the use of Fyn WT (F-WT) and Fyn kinase-dead (F-KD) expression vectors. Bars represent means ± SEM for three independent experiments. WCLs used for luciferase assays were subjected to IB with anti-Src, anti-Fyn, and anti-HA antibodies to detect Src, Fyn, and STP-A expression, respectively (data not shown).
Interaction of STP-A oncoprotein with TRAFs.
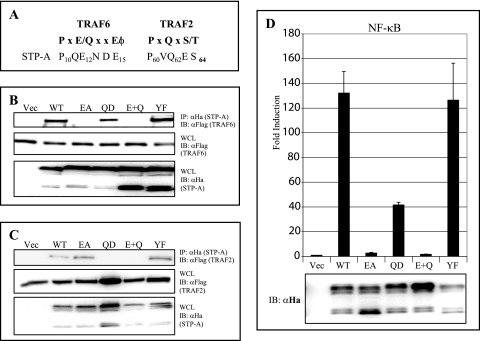
Our previous study showed that HVS STP-A has a P60VQ62ES64 TRAF2 binding sequence similar to that in EBV LMP-1, CD30, CD40, and CD27 and that ΔP60 and Q62D mutations in this TRAF2 binding motif abolished complex formation with TRAF2 (28). The structural determination of the peptide-TRAF6 interaction identified a PXEXXE/φ (X may be any amino acid and φ is an acidic/aromatic residue) TRAF6 binding motif, which is present in CD40 and the TRANCE receptor (56). Close inspection reveals that STP-A also contains a consensus TRAF6 binding motif, P10QE12NDE15, in its amino-terminal region (Fig. 4A) (28). The potential interaction of STP-A with TRAF6 was therefore investigated by transfecting 293T cells with expression vectors for HA-tagged STP-A and Flag-tagged TRAF6. The presence of TRAF6 was readily evident in the STP-A immunoprecipitates (Fig. 4B). To further investigate whether the putative TRAF6 binding motif of STP-A is required for TRAF6 association, the STP-A E12A mutant was evaluated for TRAF6 binding activity. Additionally, STP-A Q62D and E12A/Q62D mutants were included to differentiate TRAF2 and TRAF6 binding. The E12A mutation in the STP-A TRAF6 binding motif abolished TRAF6 binding without affecting TRAF2 binding, whereas the Q62D mutation in the STP-A TRAF2 binding motif abolished TRAF2 binding without affecting TRAF6 binding (Fig. 4B and C). Finally, the STP-A E12A/Q62D double mutation abrogated both TRAF2 and TRAF6 binding (Fig. 4B and C). These results indicate that STP-A interacts differentially with TRAF6 and TRAF2, through its N-terminal P10QE12NDE15 motif and central P60VQ62ET64 motif, respectively.
FIG. 4.
STP-A interaction with TRAF2 and TRAF6. (A) Sequence alignment of STP-A TRAF binding motifs. STP-A interactions with TRAF6 (B) and TRAF2 (C) are shown. 293T cells were transfected with vector (49) HA-STP-A WT or its mutants (E12A, Q62D, E12A/Q62D [E+Q], and Y115F), together with Flag-tagged TRAF2 or TRAF6. At 36 h posttransfection, WCLs were used for IP with beads conjugated with anti-HA antibody, followed by IB with anti-Flag antibody. WCLs were used for IB with anti-HA and anti-Flag antibodies to detect STP-A and TRAFs. (D) NF-κB activation by STP-A and its mutants. 293T cells were transfected with 0.5 μg of pEF.IRES.puro, pEF.IRES.puro-STP-A WT, or pEF.IRES.puro-STP-A mutant along with 0.5 μg of NF-κB luciferase reporter construct and 0.1 μg of a transfection control β-Gal expression construct (pGK-β-gal). After 36 h, cells were harvested, and luciferase and β-Gal values were determined and reported as x-fold increased luciferase activity divided by β-Gal activity relative to that of vector-transfected cells. Bars represent means ± SEM for three independent experiments. WCLs used for luciferase assays were subjected to IB with anti-HA antibody to detect STP-A expression.
To further investigate the effects of STP-A and TRAF2/6 interactions on NF-κB activation, 293T cells were transfected with WT STP-A or its mutants along with an NF-κB-driven luciferase reporter plasmid and a control β-galactosidase expression plasmid, pGK-β-gal. At 36 h posttransfection, relative luciferase values were measured and normalized with β-galactosidase activity for transfection efficiency. WT STP-A induced a robust activation of NF-κB activity, the STP-A Q62D mutant showed a reduced activation of NF-κB activity, the STP-A E12A mutant showed a drastically reduced activation of NF-κB activity, and the E12A/Q62D mutant showed little or no activation of NF-κB activity (Fig. 4D). Finally, the STP-A Y115F mutant induced NF-κB activity as strongly as did WT STP-A (Fig. 4D). These results demonstrate that STP-A and TRAF6 interaction primarily contributes to NF-κB activation, whereas STP-A and TRAF2 interaction plays a minor role in NF-κB activation, and that STP-A and SF-PTK interaction does not contribute to NF-κB activation.
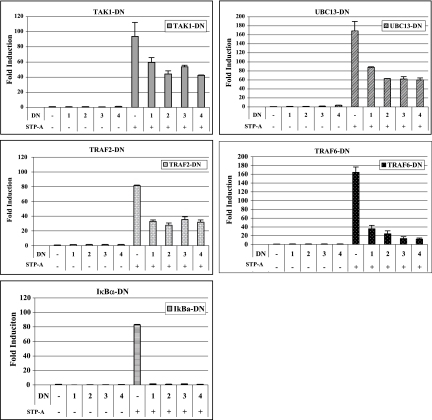
Effects of dominant-negative mutants of TRAF, TAK1, Ubc13, and IκBα on STP-A-mediated NF-κB activation.
Both TRAF2/6 ubiquitin (Ub) ligase and Ub-conjugating enzyme Ubc13-Uev1A complexes mediate the activation of protein kinases, such as transforming growth factor beta-activated kinase (TAK1) and IκB kinase (IKK), by catalyzing the formation of a unique polyubiquitin chain linked through Lys-63 of Ub (7). To test the roles of signaling components of the TRAF-TAK1-IKK pathway, we expressed their DN forms with STP-A, followed by measuring NF-κB activation. Luciferase assays showed that the expression of DN forms of TRAF2, TRAF6, TAK1, Ubc13, and IκBα markedly suppressed STP-A-mediated activation of NF-κB activity (Fig. 5). In particular, TRAF6 and IκBα dominant-negative mutants almost completely abolished STP-A-mediated activation of NF-κB activity (Fig. 5). Immunoblotting showed the equivalent expression of STP-A and dominant-negative mutants (data not shown). These results suggest that functional activities of TAK1, Ubc13, TRAF2, TRAF6, and IκBα are required for STP-A-mediated activation of NF-κB activity.
FIG. 5.
Inhibition of STP-A-induced NF-κB activation by DN mutants. 293T cells were transfected with 0.5 μg of pEF.IRES.puro or pEF.IRES.puro-STP-A WT, together with increasing amounts (μg) of the DN form of TAK1, UBC13, TRAF2, TRAF6, or IκBα. An NF-κB-luciferase reporter construct (0.5 μg) and 0.1 μg of a transfection control β-Gal expression construct (pGK-β-gal) were also included. After 36 h, cells were harvested, and luciferase and β-Gal values were determined and reported as x-fold increased luciferase activity divided by β-Gal activity relative to that of vector-transfected cells. Bars represent means ± SEM for three independent experiments. WCLs used for luciferase assays were subjected to IB with anit-TAK1, anti-Ubc13, anti-IκBα, and anti-Flag antibodies to detect TAK1, Ubc13, IκBα, TRAF2, and TRAF6 DN mutant expression, respectively (data not shown).
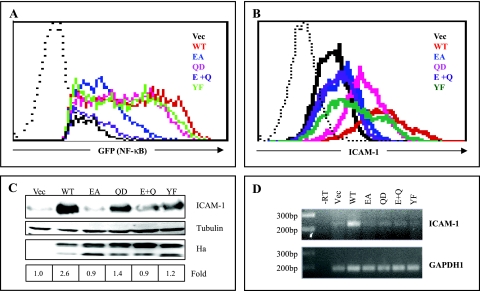
Induction of NF-κB activation and ICAM-1 surface expression in T cells by STP-A.
To further examine STP-A-induced NF-κB activation in T cells, we measured NF-κB activation using the Jurkat.κB.GFP cell line, a variant of the Jurkat T-cell line carrying the green fluorescent protein (GFP) gene under the control of an NF-κB-dependent promoter (54). These cells were electroporated with WT STP-A and its mutants, followed by measuring the GFP-positive cell population by flow cytometry analysis. Consistent with the NF-κB luciferase assays shown in Fig. 4D, WT STP-A strongly increased the GFP-positive cell population (Fig. 6A). The STP-A Q62D and STP-A Y115F mutants also increased the GFP-positive cell population, almost as strongly as did WT STP-A, whereas STP-A E12A increased the population only weakly (Fig. 6A). Finally, the STP-A E12A/Q62D mutant showed little or no increase in the GFP-positive cell population (Fig. 6A).
FIG. 6.
STP-A-mediated NF-κB activation and ICAM-1 surface expression in T cells. (A) STP-A-mediated NF-κB activation in T cells. Jurkat.κB.GFP cells were electroporated with STP-A WT and its mutants. At 24 h postelectroporation, Ficoll-Histopaque was used to remove dead cells. The live cells were incubated for an additional 16 h at 37°C, followed by measuring the GFP-positive cell population by flow cytometry analysis. The dotted line indicates the isotype control. (B) STP-A-mediated ICAM-1 surface expression on T cells. Jurkat.κB.GFP cells were electroporated with STP-A WT and its mutants. At 24 h postelectroporation, Ficoll-Histopaque was used to remove dead cells. The live cells were incubated for an additional 16 h at 37°C and then stained with a PE-conjugated antibody against human ICAM-1, followed by measuring the PE-positive cell population by flow cytometry analysis. The dotted line indicates the isotype control. (C) Enhanced expression of ICAM-1 protein by STP-A. WCLs of Jurkat.κB.GFP cells electroporated with STP-A WT and its mutants were subjected to IB with anti-HA antibody (αHa) and anti-human ICAM-1 antibody (αICAM-1) to detect STP-A and ICAM-1 expression, respectively, and with anti-tubulin antibody (αTubulin) as a loading control. The bottom panel indicates the induction of the ICAM-1 protein level in STP-A-transfected cells over that in vector-transfected cells. (D) Induction of ICAM-1 mRNA by STP-A. Total RNA isolated from Jurkat T cells transiently expressing STP-A was subjected to end-point RT-PCR analysis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control for quality and equivalent loading of total RNA. −RT, RT-PCR assay of total RNA isolated from vector-transfected cells without the addition of reverse transcriptase.
A number of surface molecules on lymphocytes are induced upon stimulation (27, 41). To test whether STP-A affects the expression of cell surface molecules on T cells, we transfected Jurkat.κB.GFP cells with a STP-A expression vector and surveyed GFP-positive cells for the expression of various cell surface molecules stained with PE-conjugated antibodies by flow cytometry. This revealed that WT STP-A robustly enhanced the surface expression of ICAM-1 on these cells (Fig. 6B). In contrast, the expression patterns of STP-A E12A and E12A/Q62D mutants that were defective in NF-κB activation showed little or no increase of ICAM-1 surface expression (Fig. 6B). Interestingly, unlike their ability to induce NF-κB-mediated GFP expression, STP-A Q62D and Y115F (YF) mutants only weakly increased ICAM-1 surface expression (Fig. 6A and B). In correlation with the increase of ICAM-1 surface expression, the ICAM-1 protein level was also upregulated effectively upon WT STP-A expression, weakly upon STP-A Q62D and Y115F mutant expression, and minimally upon E12A and E12A/Q62D mutant expression (Fig. 6C). WT STP-A and its mutants were expressed at equivalent levels in Jurkat.κB.GFP cells (Fig. 6C). Finally, end-point RT-PCR also showed a significant increase of ICAM-1 mRNA upon WT STP-A expression (Fig. 6D). These results indicate that STP-A effectively induces NF-κB activation in T cells in a TRAF signaling-dependent manner, whereas both Src- and TRAF-mediated signaling activities induced by STP-A are necessary to efficiently upregulate ICAM-1 expression in T cells.
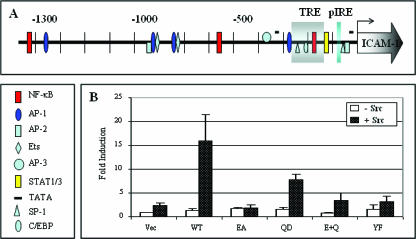
Upregulation of ICAM-1 promoter activity by STP-A.
It is well documented that the regulation of ICAM-1 gene expression occurs primarily at the transcriptional level, which involves different signaling pathways and several enhancer elements (27, 41). As shown in a schematic diagram of the 5′-flanking region of the ICAM-1 gene, the 1.3-kb full-length ICAM-1 gene promoter contains binding sites for numerous transcription factors, such as NF-κB, AP-1, C/EBP, Ets, STAT, and SP-1 (Fig. 7A) (41). To study the effect of STP-A on ICAM-1 promoter activity, we transfected 293T cells with WT STP-A or its mutants along with a luciferase reporter construct containing the 1.3-kb full-length ICAM-1 promoter (i.e., pGL1.3) (38, 41). As shown in Fig. 7B, a weak increase in the ICAM-1 promoter activity was obtained with STP-A expression alone, whereas a much greater increase in the ICAM-1 promoter activity was obtained by STP-A and Src coexpression. Furthermore, it appeared that STP-A binding with both TRAFs, particularly TRAF6, and Src was necessary to fully activate ICAM-1 promoter activity (Fig. 7B). These results suggest that TRAF- and Src-mediated signal transductions induced by STP-A cooperate to upregulate ICAM-1 promoter activity.
FIG. 7.
Activation of ICAM-1 promoter activity and enhanced expression of ICAM-1 protein by STP-A. (A) Schematic diagram of the 1.3-kb full-length ICAM-1 gene promoter region. The individual transcription factor binding sites are as follows: the TRE box indicates the TNF-α response element region containing Sp1, C/EBP, Ets, and NF-κB sites, and pIRE indicates the interferon response element. (B) Activation of ICAM-1 promoter by STP-A, with or without Src coexpression. 293T cells were transfected with 0.5 μg of pEF.IRES.puro, pEF.IRES.puro-STP-A WT, or its mutant and 1 μg of Src expression vector, along with 0.5 μg of a 1.3-kb full-length ICAM-1-luciferase reporter construct (pGL1.3) and 0.1 μg of a constitutive β-Gal expression construct (pGK-β-gal). After 36 h, cells were harvested, and luciferase and β-Gal values were determined and reported as x-fold increased luciferase activity divided by β-Gal activity. Bars represent means ± SEM for three independent experiments.
DISCUSSION
STP is considered to be a major viral oncoprotein of HVS since it is not required for viral replication but is required for viral pathogenesis in tissue culture and in primates (13, 14). We previously demonstrated that HVS STP-C, a major oncoprotein of HVS subgroup C virus strain 488, induces the activation of NF-κB via interaction with TRAF molecules (28). In this study, we demonstrated that STP-A, a major oncoprotein of HVS subgroup A viruses, also induces robust NF-κB activation through association with TRAF molecules. We further identified that besides the NF-κB transcription factor, the AP-1 and NF-AT transcription factors are downstream signaling targets of STP-A. STP-A-mediated activation of AP-1 and NF-AT activities mainly depends on its ability to interact with SF-PTKs, whereas STP-A-mediated activation of NF-κB activity relies primarily on its TRAF interaction, not SF-PTK interaction. This indicates that STP-A interacts with cellular TRAFs and SF-PTKs in a genetically and functionally separable manner and that each interaction constitutively elicits independent cellular signal transductions. Ultimately, the activation of NF-κB, AP-1, and NF-AT activities by STP-A results in the upregulation of ICAM-1 and IL-2 promoter activities, which likely are associated with HVS pathogenesis.
Since its discovery, TRAF2 has become the prototypical member of the TRAF family. TRAF2 plays a protective role, which was demonstrated by the premature death of TRAF2-deficient mice owing to severe runting (20). The linear sequence that mediates interaction between TRAF2 and other receptors is PXQXS/T (X may be any amino acid). This sequence has been identified in CD27, CD30, CD40, EBV LMP-1, and HVS STP-A and STP-C (28). TRAF6 was initially identified as a signal transducer for IL-1 receptor (6). TRAF6 binds to the linear sequence PXEXXE/φ (φ is an aromatic amino acid) that has been mapped to the membrane-proximal region of CD40 (56). We showed that STP-A interacted with TRAF2 and TRAF6 through two independent binding motifs, indicating that STP-A mimics the cellular IL-1 receptor and CD40, which interact with both TRAF2 and TRAF6 via different sites. We also found that STP-A interaction with TRAF6 had a more pronounced effect on NF-κB activation than its interaction with TRAF2, which is similar to EBV LMP-1. EBV LMP-1 enables constitutive NF-κB activation through two cytoplasmic C-terminal activation regions (CTARs; also called transformation effector sites [TESs]) (17, 19, 26). CTAR1/TES1 engages TRAF1, -2, -3, and -5 through a consensus PXQXS/T motif, whereas CTAR2/TES2 engages TRAF6 through TRADD and RIP binding (17, 19, 26). Like STP-A, LMP-1 CTAR2-mediated activation of TRAF6 gives a more significant contribution to NF-κB activation than its CTAR1-mediated activation of TRAF2 (17, 19, 26). However, unlike LMP-1, which indirectly interacts with TRAF6 through complex formation with TRADD and RIP, STP-A directly interacts with TRAF6 (17, 19, 26). This indicates that while both HVS STP-A and EBV LMP-1 oncoproteins effectively activate cellular NF-κB signal transduction by mimicking ligand-bound, activated forms of CD40 and TNF-R, they have also acquired distinctive strategies to target the cellular TRAF6 molecule. This indicates that the TRAF6 molecule plays an important role in cellular NF-κB signal transduction. In fact, studies with numerous knockout mice support this notion (25, 32, 39). Thus, TRAF molecules are common targets for gammaherpesvirus oncoproteins, and particularly, TRAF6 is targeted by LMP-1 and STP-A, with different binding strategies.
TRAF6 can activate IKK through two intermediary complexes, namely, TRAF6-regulated IKK activators 1 and 2 (TRIKA1 and -2) (3, 7, 12, 50, 53). TRIKA1 is a dimeric ubiquitin-conjugating enzyme complex composed of Ubc13 and the Ubc-like protein Uev1A (7, 53). The TRIKA1 complex, together with TRAF6, has been shown to catalyze the formation of a Lys-63-linked polyubiquitin chain which mediates IKK activation. TRIKA2, which is composed of TAK1, TAB1, and TAB2, phosphorylates and activates IKK and c-Jun N-terminal kinase in a manner that depends on TRIKA1 (22). Using DN mutant forms of these signaling molecules, we showed that inhibition of TRIKA1 or TRIKA2 complexes markedly abolished STP-A-mediated NF-κB activation. Furthermore, we showed that DN TRAF6 displayed a more pronounced effect on STP-A than did DN TRAF2, further suggesting that TRAF6 plays a major role in STP-A-induced NF-κB activation. In fact, despite their structural and functional similarities, TRAF2 and TRAF6 exhibit distinctive roles in cellular signal transduction: TRAF2 is involved in NF-κB activation induced by TNF-α but not by IL-1, whereas TRAF6 is involved in NF-κB activation induced by IL-1 but not by TNF-α (52). This implies that HVS STP-A more closely mimics the cellular IL-1 signaling pathway than the TNF-α signaling pathway.
We have previously shown that STP-A interacts with and activates the Stat3 transcription factor and Src kinase through its central proline-rich motif and C-terminal SH2 binding motif (9). Furthermore, we have shown that Src kinase associated with STP-A phosphorylates the Y705 residue of Stat3, which induces Stat3 nuclear localization and transcriptional activation. Both the STP-A Y115F (YF) mutant, which is defective in Src interaction, and the D1 mutant, which is defective in Stat3 interaction, showed diminished levels of Stat3 transcriptional activation, whereas the STP-A YF/D1 mutant, defective in both Stat3 and Src interactions, showed little or no activation of Stat3 transcription activity (9). This indicates that STP-A functions as an adaptor to link Src kinase and the Stat3 transcription factor: STP-A recruits Stat3 in the vicinity of Src kinase to allow Stat3 tyrosine phosphorylation and, thereby, its subsequent transcriptional activation. Here we also found that while Fyn and Lck phosphorylated STP-A as strongly as did Src, only Fyn, not Lck, interacted with STP-A as effectively as Src kinase. Furthermore, STP-A interaction likely increased their kinase activities, since STP-A expression enhanced overall cellular tyrosine phosphorylation when coexpressed with Src or Fyn kinase but not with Lck kinase. Consistently, Src and Fyn coexpression markedly increased STP-A-mediated AP-1 and NF-AT activities, whereas Lck coexpression did so only marginally, indicating the direct correlation between the enhancement of overall tyrosine phosphorylation and the increase of AP-1 and NF-AT activities. This suggests that STP-A functions not only as an adaptor to link Src kinase and the Stat3 transcription factor, leading to Stat3 transcription activation, but also as an activator to increase Src and Fyn kinase activities, resulting in AP-1 and NF-AT transcription activation. It is intriguing that the HVS subgroup A oncoprotein STP-A interacts only weakly with Lck, which is a major T-cell non-receptor tyrosine kinase. Besides STP-C, HVS subgroup C carries an additional ORF encoding Tip at the same locus as that encoding STP-A of HVS subgroup A. STP-C interacts with TRAFs but not with tyrosine kinase. Instead, the Tip protein interacts strongly with Lck but only marginally with Fyn, showing a reverse correlation between STP-A and Tip in the repertoire of cellular tyrosine kinase binding efficiencies. In fact, while both STP-A and Tip target SF-PTKs, their modes of interaction are different: STP-A interacts with Fyn and Src through its SH2 binding motif, whereas Tip interacts with Lck through its SH3 binding motif (21). Despite our interesting findings, however, a major drawback of this study is the lack of demonstration of STP-A interactions with TRAF and SF-PTK molecules in virus-transformed T cells. This is because, unlike the STP-C and Tip genes, which are significantly expressed in HVS subgroup C-transformed T cells, STP-A gene expression is completely shut off in HVS subgroup A-transformed T cells (18, 36, 37). This suggests that during viral evolution, HVS subgroup A and C transformation-essential proteins selected Fyn/Src and Lck, respectively, as their major binding partners through different modes of interaction and chose their different manners of gene expression, which may ultimately lead to similar but different biological activities. In fact, HVS subgroups have been shown to display distinctive transforming activities and host spectra for pathogenesis (15). Further analysis lies ahead to understand how this biochemical difference potentially delineates biological differences.
ICAM-1, an inducible cell adhesion glycoprotein, can be upregulated by a variety of stimuli, including bacterial lipopolysaccharide, phorbol esters, oxidative stress, and proinflammatory cytokines, such as TNF-α, IL-1β, and gamma interferon (27, 41, 45). ICAM-1 serves as a counterreceptor for a number of cellular molecules, such as LFA-1 (CD11a) and MAC-1 (CD11b), and plays a central role in a wide range of inflammatory and immune responses (25). The regulation of ICAM-1 gene expression occurs at multiple levels, but primarily at the transcription level. This phenomenon involves different signaling pathways and several enhancer elements, such as the palindromic gamma interferon-responsive element (pIRE), NF-κB, Ets, C/EBP, AP-1, SP-1, and the retinoic acid response element (Fig. 7) (27, 45). NF-κB has been reported to play a pivotal role in ICAM-1 gene regulation; for example, human T-cell leukemia virus type 1 Tax and EBV LMP-1 activate NF-κΒ activity to upregulate ICAM-1 expression, which is thought to facilitate viral transmission by promoting adhesion to neighboring cells and to induce cell proliferation by activating signal transduction (27, 34, 35). However, we found that unlike the case for these viral oncoproteins, HVS STP-A-mediated activation of NF-κB activity was not sufficient to induce ICAM-1 expression in T cells. Cooperation between TRAF-mediated NF-κB activation and SF-PTK-mediated AP-1 activation was necessary to fully induce ICAM-1 expression. This indicates that while viral oncoproteins target similar cellular signaling pathways, their downstream outcomes are different to meet their own needs. In summary, our previous and current studies have revealed that HVS STP-A targets three different important cellular signaling molecules, namely, SF-PTK, Stat3, and TRAFs, in genetically separable manners and that these interactions constitutively activate diverse cellular signaling pathways, which may ultimately contribute to HVS-induced pathogenesis.
Acknowledgments
We thank E. Kieff, J. Chen, H. Band, T. Parks, and Y. W. Choi for providing reagents.
This work was supported by U.S. Public Health Service grants CA109697, CA31363, CA106156, and RR00168.
Footnotes
Published ahead of print on 20 December 2006.
REFERENCES
- 1.Albrecht, J. C., I. Muller-Fleckenstein, M. Schmidt, B. Fleckenstein, and B. Biesinger. 2005. Tyrosine phosphorylation of the Tio oncoprotein is essential for transformation of primary human T cells. J. Virol. 79:10507-10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biesinger, B., J. J. Trimble, R. C. Desrosiers, and B. Fleckenstein. 1990. The divergence between two oncogenic herpesvirus saimiri strains in a genomic region related to the transforming phenotype. Virology 176:505-514. [DOI] [PubMed] [Google Scholar]
- 3.Blonska, M., P. B. Shambharkar, M. Kobayashi, D. Zhang, H. Sakurai, B. Su, and X. Lin. 2005. TAK1 is recruited to the tumor necrosis factor-alpha (TNF-alpha) receptor 1 complex in a receptor-interacting protein (RIP)-dependent manner and cooperates with MEKK3 leading to NF-kappaB activation. J. Biol. Chem. 280:43056-43063. [DOI] [PubMed] [Google Scholar]
- 4.Bradley, J. R., and J. S. Pober. 2001. Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20:6482-6491. [DOI] [PubMed] [Google Scholar]
- 5.Cabanillas, J. A., R. Cambronero, A. Pacheco-Castro, M. C. Garcia-Rodirguez, J. M. Martin-Fernandez, G. Fontan, and J. R. Regueiro. 2002. Characterization of herpesvirus saimiri-transformed T lymphocytes from common variable immunodeficiency patients. Clin. Exp. Immunol. 127:366-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao, Z., M. Tanaka, C. Regnier, M. Rothe, A. Yamit-hezi, J. D. Woronicz, M. E. Fuentes, M. H. Durnin, S. A. Dalrymple, and D. V. Goeddel. 1999. NF-kappa B activation by tumor necrosis factor and interleukin-1. Cold Spring Harbor Symp. Quant. Biol. 64:473-483. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Z. J. 2005. Ubiquitin signalling in the NF-kappaB pathway. Nat. Cell Biol. 7:758-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung, J. Y., Y. C. Park, H. Ye, and H. Wu. 2002. All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J. Cell Sci. 115:679-688. [DOI] [PubMed] [Google Scholar]
- 9.Chung, Y. H., N. H. Cho, M. I. Garcia, S. H. Lee, P. Feng, and J. U. Jung. 2004. Activation of Stat3 transcription factor by herpesvirus saimiri STP-A oncoprotein. J. Virol. 78:6489-6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crespo, P., K. E. Schuebel, A. A. Ostrom, J. S. Gutkind, and X. R. Bustelo. 1997. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature 385:169-172. [DOI] [PubMed] [Google Scholar]
- 11.Cron, R. Q., S. J. Bort, Y. Wang, M. W. Brunvand, and D. B. Lewis. 1999. T cell priming enhances IL-4 gene expression by increasing nuclear factor of activated T cells. J. Immunol. 162:860-870. [PubMed] [Google Scholar]
- 12.Deng, L., C. Wang, E. Spencer, L. Yang, A. Braun, J. You, C. Slaughter, C. Pickart, and Z. J. Chen. 2000. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103:351-361. [DOI] [PubMed] [Google Scholar]
- 13.Desrosiers, R. C., A. Bakker, J. Kamine, L. A. Falk, R. D. Hunt, and N. W. King. 1985. A region of the herpesvirus saimiri genome required for oncogenicity. Science 228:184-187. [DOI] [PubMed] [Google Scholar]
- 14.Desrosiers, R. C., R. L. Burghoff, A. Bakker, and J. Kamine. 1984. Construction of replication-competent herpesvirus saimiri deletion mutants. J. Virol. 49:343-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desrosiers, R. C., and L. A. Falk. 1982. Herpesvirus saimiri strain variability. J. Virol. 43:352-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desrosiers, R. C., D. P. Silva, L. M. Waldron, and N. L. Letvin. 1986. Nononcogenic deletion mutants of herpesvirus saimiri are defective for in vitro immortalization. J. Virol. 57:701-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devergne, O., E. Hatzivassiliou, K. M. Izumi, K. M. Kaye, M. F. Kleijnen, E. Kieff, and G. Mosialos. 1996. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-kappaB activation. Mol. Cell. Biol. 16:7098-7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fickenscher, H., B. Biesinger, A. Knappe, S. Wittmann, and B. Fleckenstein. 1996. Regulation of the herpesvirus saimiri oncogene stpC, similar to that of T-cell activation genes, in growth-transformed human T lymphocytes. J. Virol. 70:6012-6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He, Z., B. Xin, X. Yang, C. Chan, and L. Cao. 2000. Nuclear factor-kappaB activation is involved in LMP1-mediated transformation and tumorigenesis of rat-1 fibroblasts. Cancer Res. 60:1845-1848. [PubMed] [Google Scholar]
- 20.Ivanov, V. N., O. Fodstad, and Z. Ronai. 2001. Expression of ring finger-deleted TRAF2 sensitizes metastatic melanoma cells to apoptosis via up-regulation of p38, TNFalpha and suppression of NF-kappaB activities. Oncogene 20:2243-2253. [DOI] [PubMed] [Google Scholar]
- 21.Jung, J. U., S. M. Lang, U. Friedrich, T. Jun, T. M. Roberts, R. C. Desrosiers, and B. Biesinger. 1995. Identification of Lck-binding elements in tip of herpesvirus saimiri. J. Biol. Chem. 270:20660-20667. [DOI] [PubMed] [Google Scholar]
- 22.Kanayama, A., R. B. Seth, L. Sun, C. K. Ea, M. Hong, A. Shaito, Y. H. Chiu, L. Deng, and Z. J. Chen. 2004. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol. Cell 15:535-548. [DOI] [PubMed] [Google Scholar]
- 23.Kaschka-Dierich, C., I. Bauer, B. Fleckenstein, and R. C. Desrosiers. 1981. Episomal and nonepisomal herpesvirus DNA in lymphoid tumor cell lines. Haematol. Blood Transfus. 26:197-203. [DOI] [PubMed] [Google Scholar]
- 24.Kiyotaki, M., R. C. Desrosiers, and N. L. Letvin. 1986. Herpesvirus saimiri strain 11 immortalizes a restricted marmoset T8 lymphocyte subpopulation in vitro. J. Exp. Med. 164:926-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi, N., Y. Kadono, A. Naito, K. Matsumoto, T. Yamamoto, S. Tanaka, and J. Inoue. 2001. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 20:1271-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam, N., and B. Sugden. 2003. CD40 and its viral mimic, LMP1: similar means to different ends. Cell Signal. 15:9-16. [DOI] [PubMed] [Google Scholar]
- 27.Ledebur, H. C., and T. P. Parks. 1995. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-kappa B site and p65 homodimers. J. Biol. Chem. 270:933-943. [DOI] [PubMed] [Google Scholar]
- 28.Lee, H., J. K. Choi, M. Li, K. Kaye, E. Kieff, and J. U. Jung. 1999. Role of cellular tumor necrosis factor receptor-associated factors in NF-kappaB activation and lymphocyte transformation by herpesvirus saimiri STP. J. Virol. 73:3913-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, H., J. J. Trimble, D. W. Yoon, D. Regier, R. C. Desrosiers, and J. U. Jung. 1997. Genetic variation of herpesvirus saimiri subgroup A transforming protein and its association with cellular src. J. Virol. 71:3817-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, S. H., Y. H. Chung, N. H. Cho, Y. Gwack, P. Feng, and J. U. Jung. 2004. Modulation of T-cell receptor signal transduction by herpesvirus signaling adaptor protein. Mol. Cell. Biol. 24:5369-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin, J., and A. Weiss. 2001. T cell receptor signalling. J. Cell Sci. 114:243-244. [DOI] [PubMed] [Google Scholar]
- 32.Lomaga, M. A., W. C. Yeh, I. Sarosi, G. S. Duncan, C. Furlonger, A. Ho, S. Morony, C. Capparelli, G. Van, S. Kaufman, A. van der Heiden, A. Itie, A. Wakeham, W. Khoo, T. Sasaki, Z. Cao, J. M. Penninger, C. J. Paige, D. L. Lacey, C. R. Dunstan, W. J. Boyle, D. V. Goeddel, and T. W. Mak. 1999. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13:1015-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luftig, M., E. Prinarakis, T. Yasui, T. Tsichritzis, E. Cahir-McFarland, J. Inoue, H. Nakano, T. W. Mak, W. C. Yeh, X. Li, S. Akira, N. Suzuki, S. Suzuki, G. Mosialos, and E. Kieff. 2003. Epstein-Barr virus latent membrane protein 1 activation of NF-kappaB through IRAK1 and TRAF6. Proc. Natl. Acad. Sci. USA 100:15595-15600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehl, A. M., J. E. Floettmann, M. Jones, P. Brennan, and M. Rowe. 2001. Characterization of intercellular adhesion molecule-1 regulation by Epstein-Barr virus-encoded latent membrane protein-1 identifies pathways that cooperate with nuclear factor kappa B to activate transcription. J. Biol. Chem. 276:984-992. [DOI] [PubMed] [Google Scholar]
- 35.Mori, N., S. Murakami, S. Oda, and S. Eto. 1994. Human T-cell leukemia virus type I tax induces intracellular adhesion molecule-1 expression in T cells. Blood 84:350-351. [PubMed] [Google Scholar]
- 36.Murthy, S., J. Kamine, and R. C. Desrosiers. 1986. Viral-encoded small RNAs in herpes virus saimiri induced tumors. EMBO J. 5:1625-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murthy, S. C., J. J. Trimble, and R. C. Desrosiers. 1989. Deletion mutants of herpesvirus saimiri define an open reading frame necessary for transformation. J. Virol. 63:3307-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myers, C. L., S. J. Wertheimer, J. Schembri-King, T. Parks, and R. W. Wallace. 1992. Induction of ICAM-1 by TNF-alpha, IL-1 beta, and LPS in human endothelial cells after downregulation of PKC. Am. J. Physiol. 263:C767-C772. [DOI] [PubMed] [Google Scholar]
- 39.Naito, A., H. Yoshida, E. Nishioka, M. Satoh, S. Azuma, T. Yamamoto, S. Nishikawa, and J. Inoue. 2002. TRAF6-deficient mice display hypohidrotic ectodermal dysplasia. Proc. Natl. Acad. Sci. USA 99:8766-8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ninomiya-Tsuji, J., K. Kishimoto, A. Hiyama, J. Inoue, Z. Cao, and K. Matsumoto. 1999. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398:252-256. [DOI] [PubMed] [Google Scholar]
- 41.Parks, T. P., and M. E. Gerritsen. 2001. Regulation of intercellular adhesion molecule (ICAM) gene expression, p. 109-173. In Tucker Collins (ed.), Leukocyte recruitment, endothelial cell adhesion molecules, and transcriptional control: insights for drug discovery. Kluwer Academic Publishers, Boston, MA.
- 42.Peterson, E. J., J. L. Clements, N. Fang, and G. A. Koretzky. 1998. Adaptor proteins in lymphocyte antigen-receptor signaling. Curr. Opin. Immunol. 10:337-344. [DOI] [PubMed] [Google Scholar]
- 43.Pivniouk, V. I., T. R. Martin, J. M. Lu-Kuo, H. R. Katz, H. C. Oettgen, and R. S. Geha. 1999. SLP-76 deficiency impairs signaling via the high-affinity IgE receptor in mast cells. J. Clin. Investig. 103:1737-1743. [PMC free article] [PubMed] [Google Scholar]
- 44.Pullen, S. S., T. T. Dang, J. J. Crute, and M. R. Kehry. 1999. CD40 signaling through tumor necrosis factor receptor-associated factors (TRAFs). Binding site specificity and activation of downstream pathways by distinct TRAFs. J. Biol. Chem. 274:14246-14254. [DOI] [PubMed] [Google Scholar]
- 45.Roy, J., M. Audette, and M. J. Tremblay. 2001. Intercellular adhesion molecule-1 (ICAM-1) gene expression in human T cells is regulated by phosphotyrosyl phosphatase activity. Involvement of NF-kappaB, Ets, and palindromic interferon-gamma-responsive element-binding sites. J. Biol. Chem. 276:14553-14561. [DOI] [PubMed] [Google Scholar]
- 46.Samelson, L. E. 1999. Adaptor proteins and T-cell antigen receptor signaling. Prog. Biophys. Mol. Biol. 71:393-403. [DOI] [PubMed] [Google Scholar]
- 47.Schouten, G. J., A. C. Vertegaal, S. T. Whiteside, A. Israel, M. Toebes, J. C. Dorsman, A. J. van der Eb, and A. Zantema. 1997. IkappaB alpha is a target for the mitogen-activated 90 kDa ribosomal S6 kinase. EMBO J. 16:3133-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Secrist, J. P., L. Karnitz, and R. T. Abraham. 1991. T-cell antigen receptor ligation induces tyrosine phosphorylation of phospholipase C-gamma 1. J. Biol. Chem. 266:12135-12139. [PubMed] [Google Scholar]
- 49.Szomolanyi, E., P. Medveczky, and C. Mulder. 1987. In vitro immortalization of marmoset cells with three subgroups of herpesvirus saimiri. J. Virol. 61:3485-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takaesu, G., R. M. Surabhi, K. J. Park, J. Ninomiya-Tsuji, K. Matsumoto, and R. B. Gaynor. 2003. TAK1 is critical for IkappaB kinase-mediated activation of the NF-kappaB pathway. J. Mol. Biol. 326:105-115. [DOI] [PubMed] [Google Scholar]
- 51.Takeuchi, M., M. Rothe, and D. V. Goeddel. 1996. Anatomy of TRAF2. Distinct domains for nuclear factor-kappaB activation and association with tumor necrosis factor signaling proteins. J. Biol. Chem. 271:19935-19942. [DOI] [PubMed] [Google Scholar]
- 52.Wajant, H., F. Henkler, and P. Scheurich. 2001. The TNF-receptor-associated factor family: scaffold molecules for cytokine receptors, kinases and their regulators. Cell Signal. 13:389-400. [DOI] [PubMed] [Google Scholar]
- 53.Wang, C., L. Deng, M. Hong, G. R. Akkaraju, J. Inoue, and Z. J. Chen. 2001. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412:346-351. [DOI] [PubMed] [Google Scholar]
- 54.Wang, D., Y. You, S. M. Case, L. M. McAllister-Lucas, L. Wang, P. S. DiStefano, G. Nunez, J. Bertin, and X. Lin. 2002. A requirement for CARMA1 in TCR-induced NF-kappa B activation. Nat. Immunol. 3:830-835. [DOI] [PubMed] [Google Scholar]
- 55.Wu, H., and J. R. Arron. 2003. TRAF6, a molecular bridge spanning adaptive immunity, innate immunity and osteoimmunology. Bioessays 25:1096-1105. [DOI] [PubMed] [Google Scholar]
- 56.Ye, H., J. R. Arron, B. Lamothe, M. Cirilli, T. Kobayashi, N. K. Shevde, D. Segal, O. K. Dzivenu, M. Vologodskaia, M. Yim, K. Du, S. Singh, J. W. Pike, B. G. Darnay, Y. Choi, and H. Wu. 2002. Distinct molecular mechanism for initiating TRAF6 signalling. Nature 418:443-447. [DOI] [PubMed] [Google Scholar]