Abstract
CD8 T cells exert their antiviral function through cytokines and lysis of infected cells. Because hepatocytes are susceptible to noncytolytic mechanisms of viral clearance, CD8 T-cell antiviral efficiency against hepatotropic viruses has been linked to their capacity to produce gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α). On the other hand, intrahepatic cytokine production triggers the recruitment of mononuclear cells, which sustain acute and chronic liver damage. Using virus-specific CD8 T cells and human hepatocytes, we analyzed the modulation of virus-specific CD8 T-cell function after recognition peptide-pulsed or virally infected hepatocytes. We observed that hepatocyte antigen presentation was generally inefficient, and the quantity of viral antigen strongly influenced CD8 T-cell antiviral function. High levels of hepatitis B virus production induced robust IFN-γ and TNF-α production in virus-specific CD8 T cells, while limiting amounts of viral antigen, both in hepatocyte-like cells and naturally infected human hepatocytes, preferentially stimulated CD8 T-cell degranulation. Our data document a mechanism where virus-specific CD8 T-cell function is influenced by the quantity of virus produced within hepatocytes.
Virus-specific CD8 T cells play a major role in viral clearance and disease pathogenesis during infection with hepatitis B virus (HBV) and hepatitis C virus (HCV) (6). The noncytopathic nature of both viruses and their ability to infect hepatocytes (1, 23, 27, 31, 39) necessitate a coordinated mechanism to reach viral clearance and avoid liver destruction (21, 64). This is likely facilitated by the fact that virally infected hepatocytes are largely resistant to perforin/granzyme-mediated killing (30) but sensitive to cytokine (gamma interferon [IFN-γ] and tumor necrosis factor alpha [ΤΝF-α])-mediated control of viral replication (9, 21).
In mouse and chimpanzee models of HBV infection, IFN-γ produced by virus-specific CD8 T cells correlates with a profound reduction in serum and liver HBV DNA (22, 23, 25, 63) and helps to reduce hepatocyte damage by inducing cyto-protective proteins that confer resistance to granzyme B-mediated killing (4). Similar correlations have been observed in HCV-infected humans and experimentally infected chimpanzees, where the appearance of IFN-γ-producing CD8 T cells coincides with decreases in HCV RNA (51, 52). However, intrahepatic IFN-γ production is also responsible for triggering the recruitment of inflammatory cells to the liver, which contribute to liver pathology (2, 32, 45).
It has often been assumed that the extent of liver damage in chronic viral hepatitis was directly proportional to the intensity of the virus-specific CD8 response. However, the frequency of circulating HBV- and HCV-specific CD8 T cells correlates more with protection than liver damage (18, 29, 34, 35, 58). Furthermore, virus-specific CD8 T cells represent a small minority of the total intrahepatic population in chronic active hepatitis B and C, and their frequencies are inversely related to hepatic damage (20, 24, 38, 46).
The relative frequency of intrahepatic virus-specific CD8 T cells varies in chronically infected patients due to inflammatory infiltrate, but the absolute number of intrahepatic HBcAg-specific CD8 T cells was found to be similar, irrespective of liver inflammation or the level of HBV replication (38). Further to this point, it is important to note that the level of viral replication can influence liver inflammation. The inhibition of HBV replication via antiviral drugs results in a clear improvement of alanine aminotransferase values and liver histology in chronic hepatitis B patients (16, 17, 33), despite the absence of any durable changes in virus-specific T-cell immunity (11), while viral rebound occurring after termination of therapy often results in sudden episodes of hepatitis reactivation (33). We thus hypothesized that the intrahepatic virus-specific CD8 T-cell population can exercise different functions when encountering hepatocytes producing different quantities of virus.
Using HBV-specific CD8 T-cell clones and HLA-A2+ primary hepatocytes, either healthy or chronically infected with HBV, and normal or tetracycline-regulated HBV-transfected hepatocyte-like cell lines, we investigated the efficiency and outcome of hepatocyte-mediated CD8 T-cell activation. Our results demonstrated that hepatocytes were less efficient than professional antigen-presenting cells (APC) at presenting stimulatory epitopes to CD8 T cells, and only high levels of intrahepatic viral antigen production achieved robust CD8 T-cell IFN-γ production. The quantity of viral antigen and hepatocyte modulation of virus-specific CD8 T-cell function will be discussed in the context of the pathogenesis of viral hepatitis.
MATERIALS AND METHODS
Cell lines and medium.
Epstein-Barr virus-transformed B cells (EBV B cells; HLA-A0201+) and HepG2 cells (HLA-0201+) were grown and maintained in RPMI 1640 (Autogen Bioclear, Wiltshire, United Kingdom) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 20 mM HEPES, 0.5 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, MeM amino acids plus l-glutamine, MeM nonessential amino acids (Invitrogen Ltd., Paisley, United Kingdom), and 5 μg/ml Plasmocin (InvivoGen, San Diego, CA) to prevent mycoplasma contamination. Huh-7, RH-1 (muscle sarcoma), and MG-63 (osteosarcoma cell line) cells were maintained in Dulbecco's modified Eagle's medium (Autogen Bioclear, Wiltshire, United Kingdom) supplemented as above for RPMI 1640. Monocytes were prepared from peripheral blood mononuclear cells (PBMC) using the monocyte isolation kit II (Miltenyi Biotech, Surrey, United Kingdom). Where applicable, monocytes were cultured in Aim-V 2% AB serum (Invitrogen Ltd., Paisley, United Kingdom).
HBV-expressing HepG2.105 cells and the vector control parent line, HepG2TA2-7, were grown in Dulbecco's modified Eagle's medium supplemented with 10% tetracycline-approved FBS (BD Biosciences, San Diego, CA), 20 mM HEPES, 0.5 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, MeM nonessential amino acids (Invitrogen Ltd., Paisley, United Kingdom), 200 μg/ml G418 sulfate, 80 μg/ml Hygromycin B (AutogenBioclear, Wiltshire, United Kingdom). Doxycycline (0.1 to 100 ng/ml) was added to cultures to regulate HBV expression. HBeAg/HBcAg in the cell culture supernatants was measured by a commercial HBeAg enzyme-linked immunosorbent assay (ELISA; Enzygnost; Dade-Behring) as previously described (49). Quantitative determination of HBcAg was performed using home-made ELISAs as detailed below.
HBc18-27 (clone C18-3)-specific and HBp455-63 (clone P3-52)-specific CD8 T-cell clones were generated from resolved HLA-A2+ HBV patients. PBMC were isolated from fresh heparinized blood by Ficoll-Paque density gradient centrifugation. Short-term lines were made by stimulating PBMC in Aim-V 2% human AB serum with 1 μM of either HBc18-27 or HBp455-63 peptides plus 20 U/ml interleukin-2- (IL-2; R&D Systems, Abingdon, United Kingdom) for 10 days.
HBc18-27-specific CD8 T cells were labeled with phycoerythrin (PE)-conjugated HLA-A2 class I pentamers (Proimmune, Oxford, United Kingdom) bearing the HBc18-27 epitope for 30 min at 37°C and purified via magnetic cell sorting using anti-PE microbeads (Miltenyi Biotech, Surrey, United Kingdom). CD8 T cells were then cloned by limiting dilution assay, and clonal populations were expanded in Aim-V, 2% AB serum, 20 U/ml IL-2, 10 ng/ml IL-7, and 10 ng/ml IL-15 (R&D Systems, Abingdon, United Kingdom) with 1.5 μg/ml phytohemagglutinin (Sigma-Aldrich, Dorset, United Kingdom) using allogeneic irradiated PBMC as feeder cells.
HBp455-63-specific CD8 T cells were purified using the IFN-γ secretion assay per the manufacturer's instructions (Miltenyi Biotech, Surrey, United Kingdom). IFN-γ-positive cells were purified by magnetic sorting and cloned via limiting dilution assay. Clonal populations of HBp455-63-specific CD8 T cells were expanded as above.
Cytomegalovirus (CMV)-specific short-term lines (10 days) were grown from healthy, HLA-0201 donors by stimulating PBMC with 1 μM CMV pp65 peptide (NLVPMVATV) in Aim-V 2% AB serum supplemented with 20 U/ml IL-2. After 10 days of expansion, the ability of EBV B cells or HepG2 cells to activate CMV-specific cytotoxic T-lymphocyte (CTL) degranulation and IFN-γ production by these short-term lines was tested as described below.
Quantification of HBcAg in HepG2.105 cells.
HepG2.105 cells were maintained in 10-cm-diameter dishes in medium containing no doxycycline or with 100 ng/ml of doxycycline. After 5 days, supernatants were collected. The cells were detached from the plates using trypsin, resuspended in phosphate-buffered saline (PBS; 14 ml), and the cell number was determined. Cells were washed two more times with PBS (10 ml and 1.5 ml) to remove non-cell-associated HBV antigens and lysed in 700 μl lysis buffer (50 mM Tris-Cl−, 100 mM NaCl, pH 7.5). Aliquots of these cytoplasmic lysates were analyzed by ELISA. Ninety-six-well ELISA plates (Maxisorp; Nunc) were coated overnight with a mixture of 1 μl each of polyclonal rabbit antisera raised against recombinant particulate and denatured HBcAg (10) in 100 μl of 0.0175 M Na phosphate buffer, pH 7.8, per well. The wells were washed several times with PBS containing 0.05% NP-40 and blocked with PBS containing 2% bovine serum albumin (BSA) plus 0.05% NP-40. HBcAg was detected using the monoclonal antibody mc312, recognizing a linear epitope (amino acids 78 to 83 of the core protein [44]) conjugated with horseradish peroxidase and tetramethylbenzidine-hydrogen peroxide as substrates. A dilution series of recombinant HBcAg (amino acids 1 to 149) particles served to establish a calibration curve. Two to three differently sized aliquots of the cytoplasmic cell lysates were analyzed in duplicate by measuring the absorbance at 450 nm. Background absorbance was determined using PBS containing 1% BSA. The amounts of HBcAg were determined using the calibration curves from background-corrected absorbance values of each sample. Copy numbers of core protein/cell were derived, assuming a molecular mass of 21,000 Da/core protein subunit, by dividing these values by the number of cells.
Purification of primary human hepatocytes.
Normal human liver tissue was obtained from healthy tissue surrounding liver metastases under informed consent according to ethical and moral guidelines of the institution. Tissue sections were collected in William's E medium and maintained at 4°C for a maximum of 2 h before cell isolation. Hepatocytes were isolated by enzyme perfusion described for use with human liver by Strain et al. with some modifications (47). A section of liver of about 100 to 200 g was cut, and exposed vessels on a single surface were cannulated with 3-mm internal diameter tubing. The tissue was perfused sequentially at 50 ml/min with 500 ml PBS-HEPES wash solution to remove William's E medium, 500 ml PBS-HEPES-0.5 mM EGTA, and again with 500 ml PBS-HEPES wash solution. Finally, 300 ml enzyme solution (0.05% collagenase [Roche, Indianapolis, IN], 0.012% hyaluronidase [Sigma, Dorset, United Kingdom], 0.025% Dispase II [Roche], 0.005% DNase [Roche] containing 5 mM CaC12), maintained at 41°C, was perfused with recirculation, and enzymatic digestion continued for 10 to 20 min until the liver was judged to be substantially softened. Tissue was mechanically dissociated in 200 ml Hanks' balanced salt solution (Invitrogen) containing 10% FBS, 5 mM CaCl2. The cell suspension was filtered through a 60-μm cell strainer and pelleted at 37 × g for 10 min at 4°C. Cells were washed three times in Hanks' balanced salt solution containing 10% FBS, 5 mM CaCl2, after which viability and yield were assessed. Purified hepatocytes were used immediately after isolation or from frozen stocks thawed from 70% University of Washington solution, 20% FBS, and 10% dimethyl sulfoxide freezing medium. Isolated hepatocytes did not contain lymphocytes/monocytes as assessed by flow cytometry and appeared greater than 90% pure by microscopic analysis and forward scatter versus side scatter by flow cytometry. Contamination with some endothelial cells could not be ruled out; however, endothelial cell surface markers (VCAM-1 and HLA-DR) were undetectable by fluorescence-activated cell sorter staining.
Naturally infected hepatocytes were purified from chronically HBV-infected patients with low-level HBV replication (∼105 HBV DNA copies/ml) with a HBc18-27 viral sequence identical to the HBc18-27 epitope recognized by the CD8 T-cell clone (D. Brown and G. Dusheicko, personal communication).
Flow cytometry.
Adherent cell lines (HepG2, Huh-7, RH-1, and MG-63) were detached using 5 mM EDTA, 0.2% BSA solution. Cells were washed and resuspended in staining buffer (PBS, 1% BSA, 0.1% azide). HepG2 cells incubated for 24 h in 100 U/ml recombinant human IFN-γ (AL-Immunotools, Friesoythe, Germany) were stained in parallel with HepG2 cells cultured in medium alone. Each target cell line was incubated with HLA-A,B,C-fluorescein isothiocyanate (FITC), HLA-DR-PE, immunoglobulin G1 (IgG1)-FITC, IgG2b-FITC, IgG1-PE (BD Pharmingen, San Diego, CA), CD80-FITC, CD95-PE (FAS), IgG1 (Serotec, Kidlington, United Kingdom), CD86-PE (Santa Cruz Biotechnology, Santa Cruz, CA), and CD54 (ICAM-1; Beckman Coulter, High Wycombe, United Kingdom) for 30 min on ice. Cells were washed and analyzed immediately or after resuspension in 2% formaldehyde. For unconjugated antibodies, cells were washed to remove primary antibody and stained with anti-mouse Ig-FITC (Serotec, Kidlington, United Kingdom) for 30 min on ice prior to acquisition. To exclude the possibility that collagenase digestion during hepatocyte purification altered phenotypic staining, HepG2 cells were incubated with 5 mg/ml collagenase at 37°C for 30 min. A total of 10,000 events were collected for each stain using a FACScan flow cytometer (BD Biosciences, San Diego, CA) and analyzed using CellQuest software. Isotype values were subtracted to determine specific mean fluorescent intensity (MFI).
For intracellular cytokine staining, following 5 h of incubation with targets, CD8 T cells were washed, stained with Cy-chrome-conjugated anti-CD8 (BD Pharmingen, San Diego, CA), and then permeabilized and fixed using Cytofix/Cytoperm (BD Pharmingen, San Diego) according to the manufacturer's instructions. Cells were washed and incubated with PE-conjugated anti-human IFN-γ antibody (R&D Systems, Abingdon, United Kingdom) for 30 min on ice and then washed and analyzed by flow cytometry.
For degranulation assays, CD107a-PE antibody (BD Pharmingen, San Diego) was added to all wells at the beginning of the 5-h incubation with T cells (7). Following the incubation, cells were washed and labeled with Cy-chrome-anti-CD8. For triple staining, FITC-anti-IFN-γ (R&D Systems, Abingdon, United Kingdom) or TNF-α—Alexa 488 (BD Pharmingen) was used for intracellular cytokine staining.
Cytokine measurement.
Cell culture supernatants were collected at 5 and 24 h (parallel duplicate cultures) from cocultures of HBc18-27- and HBp455-63-specific T-cell clones and either EBV B cells or HepG2 cells. Cell culture supernatants were tested for the production of IFN-γ, TNF-α, IL-2, IL-4, IL-10, and IL-6 using the TH1/TH2 CBA assay (BD Biosciences) according to the manufacturer's instructions.
CD8 T-cell activation by targets.
Target cell lines were prepared 1 day prior to the experiment, except primary hepatocytes were used immediately after purification or thawing. Target cells were detached, if necessary, using trypsin-Versene (Invitrogen Ltd., Paisley, United Kingdom), washed, and resuspended in complete RPMI at 106 cells/ml. Trypsin had no effect on cell surface expression of HLA-A2 as determined by flow cytometry (data not shown). Targets were pulsed with various concentrations of HBc18-27 peptide (FLPSDFFPSV) or HBp455-63 peptide (GLSRYVARL) for 1 h on ice. Cells were washed three times with RPMI to remove peptide and resuspended with complete RPMI at 106 cells/ml. A total of 100,000 target cells/well were added to 96-well plates and incubated overnight at 37°C, 5% CO2 to permit adherence if necessary. For IFN-γ-treated targets, 48 h prior to the experiment, HepG2 cells were treated with 100 U/ml IFN-γ. After 24 h of incubation, HepG2 cells were detached and pulsed with peptide and plated as described above, and exposure to IFN-γ was maintained until the addition of T-cell clones. A total of 75,000 HBc18-27- or HBp455-63-specific CD8 T-cell clones were added to respective wells for 5 h with 10 μg/ml brefeldin A (Sigma-Aldrich, Dorset, United Kingdom) for intracellular cytokine staining or anti-CD107a-PE antibody for degranulation.
Cytotoxicity assay.
Target cells were labeled with 0.75 μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) for 10 min at 37°C. Cells were washed, and CFSE-labeled targets were pulsed with either 10−8 M HBc18-27 or 5 × 10−8 M HBp455-63 for 1 h on ice. Unlabeled (0 M peptide) and CFSE-labeled (plus peptide) targets were mixed together in a 1:1 ratio, 2 × 105 of each target, in 24-well plates and incubated overnight to permit HepG2 adherence. A total of 4 × 105 T-cell clones (effector/target ratio, 1:1) were added to the targets and incubated for 5 h at 37°C. Following incubation, HepG2 cells were collected via trypsinization and T cells were labeled with CD11a-allophycocyanin (BD Pharmingen) to exclude them from target cell gating. Cytotoxicity was determined by comparing the ratio of peptide-pulsed targets to unpulsed targets in wells with or without the addition of T-cell clones. There was no observed lysis of either unlabeled, unpulsed, or CFSE-labeled, unpulsed target cells (data not shown).
RESULTS
Phenotypes of primary human hepatocytes.
Previous reports examined the expression of major histocompatibility complex (MHC) class I and II and/or ICAM-1 on human hepatocytes but lacked quantitative techniques and focused on differences between malignant and nonmalignant hepatocytes without relative comparisons to other, more thoroughly characterized cell types (3, 14, 15, 19, 56). To gain a fundamental understanding of the expression profile for the primary receptors involved in antigen presentation we determined the immunological phenotype of (i) healthy primary human hepatocytes purified from sections of surgically removed livers, (ii) hepatocyte-like cell lines (HepG2 and Huh-7), (iii) professional antigen-presenting cells (monocytes and EBV B cells), and (iv) unrelated tumor cell lines (RH-1 and MG-63).
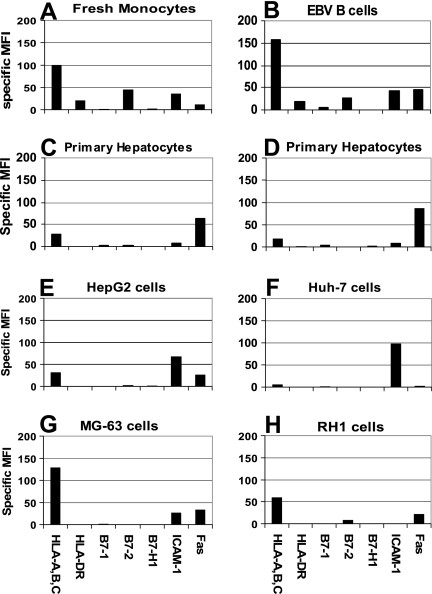
As expected, we found that professional APC, monocytes, and EBV-transformed B cells expressed relatively high levels of MHC-I, HLA-DR, and B7-1 and -2 with moderate expression of ICAM-1 (Fig. 1A and B). In contrast, we found that expression of nearly all primary receptors involved in antigen presentation was low on healthy primary human hepatocytes (Fig. 1C and D). We were unable to detect HLA-DR and found only extremely low levels of B7-1 and B7-2 on primary hepatocytes. B7-H1, a receptor known to inhibit CD8 T-cell effector function in the mouse liver (26), was not detected on healthy hepatocytes or other cell types; however, the expression of B7-H1 could be induced on monocytes following IFN-γ treatment (data not shown).
FIG. 1.
Phenotypic analysis of professional antigen-presenting cells and hepatocytes. (A) Monocytes; (B) EBV B cells; (C and D) primary human hepatocytes; (E) HepG2 cells; (F) Huh-7 cells; (G) MG-63 cells; (H) RH1 cells. Cells were stained for primary receptors involved in antigen presentation. All MFI values expressed have the isotype value subtracted. Each panel is representative of at least two separate experiments.
The expression of MHC-I, HLA-DR, and costimulatory molecules on hepatocyte-like cell lines was similar to primary hepatocytes with the exception of significantly higher ICAM-1 expression (Fig. 1E and F). The unrelated tumor cell lines RH-1, a muscle sarcoma, and MG-63, an osteosarcoma, were included as a comparison for receptor expression on other solid organs/tissues. Most notably, these cell lines expressed moderate to high levels of MHC-I (Fig. 1G and H), supporting the idea that MHC class I expression on normal hepatocytes is low.
To ensure that enzymatic processing of liver tissue did not affect receptor expression on hepatocytes, HepG2 cells were treated with collagenase for 30 min and receptor expression was evaluated. Collagenase did not alter the expression of the primary receptors involved in antigen presentation (data not shown).
Hepatocytes are less efficient at stimulating CD8 effector functions.
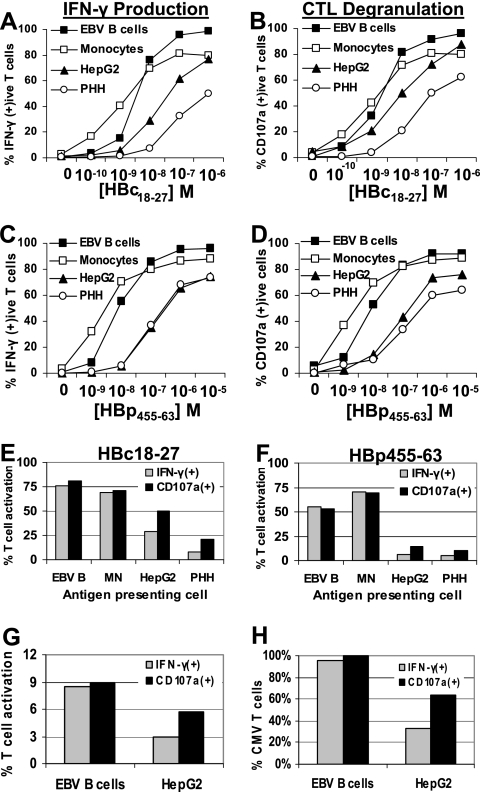
Cytolytic and noncytolytic CD8 T-cell effector functions are critical for the resolution of viral infections. To perform these functions, CD8 T cells are required to secrete stored lytic molecules as well as synthesize and release IFN-γ upon target cell recognition. We tested the relative ability of hepatocytes to activate CD8 T-cell cytolytic and noncytolytic functions using CD8 T-cell clones specific for HBV core (HBc18-27) and polymerase (HBp455-63) HLA-A2-restricted epitopes. IFN-γ production (assessed by intracellular cytokine staining) and CTL degranulation (assessed by CD107a staining [7]) were measured in HBV-specific CD8 T-cell clones incubated with HLA-A2+ EBV-transformed B cells, freshly purified monocytes, HepG2 cells, and primary human hepatocytes (PHH) pulsed with different concentrations of HBc18-27 or HBp455-63 peptides. After 5 h of coincubation, the frequencies of CD8 T cells producing IFN-γ or positive for CD107a were analyzed.
APC efficiencies were measured by their ability to activate 50% of CD8 T-cell clones. In doing so, we found that hepatocytes, compared to EBV B cells and monocytes, required 100-fold or greater peptide concentrations to stimulate 50% HBc18-27- or HBp455-63-specific T-cell clones to produce IFN-γ (Fig. 2A and C). The difference between professional APC and hepatocytes was reduced when degranulation was measured, although hepatocytes still required 10-fold-higher peptide concentrations to stimulate granule release in 50% of T-cell clones (Fig. 2B and D). The stimulatory efficiency of HepG2 cells was within these two extremes. RH-1 cells, an HLA-A2+ muscle sarcoma, pulsed with identical peptide concentrations activated HBc18-27- and HBp455-63-specific T-cell clones with nearly equal or greater efficiency as that observed with professional APC, suggesting that hepatocytes are a particularly inefficient nonprofessional APC (data not shown).
FIG. 2.
Primary hepatocytes can stimulate CD8 T-cell IFN-γ production and degranulation. (A to D) HBc18-27 T-cell IFN-γ production (A) and HBc18-27 T-cell degranulation (B) or HBp455-63 T-cell IFN-γ production (C) and HBp455-63 T-cell degranulation (D) after 5 h of stimulation by EBV B cells, monocytes, HepG2 cells, and PHH pulsed with increasing concentrations of HBc18-27 or HBp455-63 peptide. Data are displayed as the frequency of IFN-γ- or CD107a-positive T-cell clones at each peptide concentration. (E and F) T-cell clone response to target cells pulsed with 10−8 M peptide. IFN-γ production and degranulation were measured in parallel. The percentages of IFN-γ-positive T cells were plotted against the percentage of CD107a-positive T cells for each target cell line. Each panel is representative of at least three separate experiments. (G) Activation of CMV-specific short-term line. EBV B cells and HepG2 cells were pulsed with 1 μM CMV peptide, and CD107a and IFN-γ production levels were measured after 5 h. (H) Percentage of CMV-specific T-cell activation. CD107a+ T cells stimulated by EBV B cells were set at 100% CMV-specific activation. Results for remaining conditions were divided by this number to obtain the percentage of CMV-specific activation under different conditions.
The differences observed between the ability of hepatocytes to stimulate T-cell IFN-γ production (≈100-fold less efficient) and degranulation (≈10-fold less efficient) would suggest that CD8 T-cell effector functions were differentially activated by hepatocytes. This was particularly evident when target cells were pulsed with low concentrations (10−8 M) of HBc18-27 or HBp455-63 peptides. While EBV B cells and monocytes pulsed with 10−8 M peptide stimulated equivalent percentages of T-cell clones to make IFN-γ and degranulate (Fig. 2E and F), the frequency of IFN-γ-producing CD8 T cells was half that of CD107a-positive T cells following activation by HepG2 cells (Fig. 2E and F). The discrepancy was more pronounced with primary hepatocytes which, in the case of HBc18-27-specific T cells, stimulated three times more T cells to degranulate (21%) than they did to make IFN-γ (7%) (Fig. 2E). Again, RH-1 cells stimulated T-cell responses similar to professional APC, resulting in nearly equivalent percentages of T-cell clones positive for IFN-γ and CD107a (data not shown).
To confirm that the use of CTL clones did not bias these assays, we prepared CMV-specific short-term lines from an HLA-0201+ donor. EBV B cells pulsed with CMV peptide stimulated an equal percentage of CD8 T cells to secrete cytotoxic granules and produce IFN-γ (9%) (Fig. 2G and H). However, consistent with the previous data, HepG2 cells stimulated fewer CD8 T cells (6%) than EBV B cells and activated nearly twofold more CMV-specific T cells to degranulate than produced IFN-γ (CD107a+, 6%; IFN-γ+, 3%) (Fig. 2G and H).
Hepatocytes bias the CD8 T-cell response towards cytotoxicity.
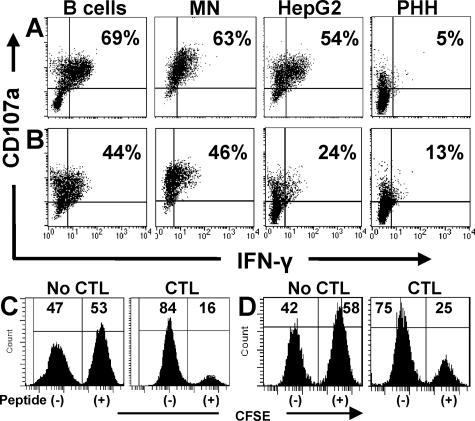
To further analyze possible CD8 T-cell divergence between degranulation and IFN-γ production, we determined if each T cell could degranulate and secrete IFN-γ in response to different target cells or if individual T cells preferentially elicited one type of effector response. We simultaneously stained cocultures for CD107a expression and IFN-γ and, as shown in Fig. 3A, when B cells or monocytes were used to activate HBc18-27-specific T cells, more than 60% of CD8 T cells positive for CD107a were also positive for IFN-γ (B cells, 69%; monocytes, 63%). Just over half (54%) of T cells positive for CD107a also produced IFN-γ when HepG2 cells stimulated HBc18-27 clones. Primary hepatocytes were the least efficient: only 5% of the CD107a-positive cells were also positive for IFN-γ (Fig. 3A). Polymerase-specific responses, while lower, followed a trend identical to core-specific T cells (Fig. 3B). These data demonstrated that HBV-specific CD8 T cells responded to targets by secreting cytotoxic granules and producing IFN-γ; however, the response to primary hepatocytes was clearly biased towards degranulation.
FIG. 3.
Hepatocytes bias the CD8 T-cell response towards degranulation. (A and B) Staining for IFN-γ and CD107a was performed on HBc18-27 (A) and HBp455-63 (B) T-cell clones after 5 h of incubation with peptide-pulsed target cells (10−8 M HBc18-27 and 5 × 10−8 M HBp455-63). Percentages displayed in the upper right quadrant indicate the percent CD107a-positive CD8 T cells that were positive for IFN-γ. Each panel is representative of at least two separate experiments. (C and D) Cytotoxic function of HBc18-27-specific (C) and HBp455-63-specific (D) CTL clones. CFSE-labeled HepG2 cells pulsed with either HBc18-27 (10−8 M) or HBp455-63 (5 × 10−8 M) peptide were mixed with unpulsed HepG2 cells and cultured in the absence or presence of CTL clones (effector/target ratio, 1:1) for 5 h. Cytotoxicity was determined by the disappearance of CFSE-labeled targets.
CD8 T-cell degranulation can kill infected target cells via the release of perforin/granzyme or the directional externalization of FasL stored in secretory lysosomes (12). To correlate cytotoxic function with degranulation, each T-cell clone was cocultured with unlabeled target cells or CFSE-labeled target cells pulsed with suboptimal peptide concentrations. Cytotoxicity was determined by the disappearance of CFSE-labeled (peptide-pulsed) cells. Figures 3C and D demonstrate that virus-specific CTL can efficiently kill HepG2 cells pulsed with low concentrations of peptide, a concentration that activated IFN-γ production in a minority of T cells (Fig. 3B and D).
Inefficiency of hepatocytes to stimulate CTL cytokine production extends beyond IFN-γ and does not compensate over time.
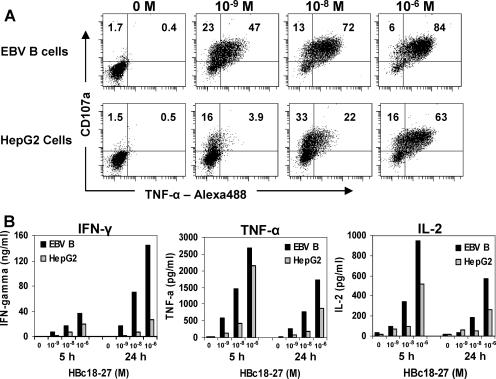
To determine if differences seen between professional APC and hepatocytes to stimulate cytokine production were limited to IFN-γ, we analyzed the production of TNF-α in CTL clones using triple staining for CD8, CD107a, and TNF-α. As seen in Fig. 4A, a majority of HBc18-27-specific T-cell clones were double positive for CD107a and TNF-α (47% and 72%, respectively) when professional APC were pulsed with low concentrations of peptide (10−9 and 10−8 M). In contrast, HepG2 cells stimulated fewer CTL clones in general and at low peptide concentrations, 10−9 and 10−8 M, and a minority of CTL clones were double positive for CD107a and TNF-α (Fig. 4A; 3.9% and 22%, respectively). Only at optimal peptide concentrations (10−6 M) were HepG2 cells able to stimulate TNF-α production by a majority of CTL clones, confirming results observed with IFN-γ (Fig. 4A).
FIG. 4.
Reduced T-cell cytokine production stimulated by hepatocytes extends to TNF-α and IL-2 and is not altered by the duration of T-cell activation. (A) HBc18-27 T-cell CD107a and TNF-α production measured by double staining after 5 h of incubation with EBV B cells or HepG2 cells pulsed with the indicated peptide concentrations. (B) IFN-γ, TNF-α, and IL-2 measured in the supernatant of HBc18-27 T-cell clones cocultured with EBV B cells or HepG2 cells for 5 or 24 h. Data presented are representative of at least two individual experiments.
It is possible that cytokine production occurs at a slower rate when hepatocytes present suboptimal concentrations of peptide and that longer incubation times may be required for efficient T-cell activation. Thus, we cocultured HBc18-27- and HBp455-63-specific T-cell clones with either EBV B cells or HepG2 cells pulsed with suboptimal (10−8 and 10−9 M) or optimal (10−6 M) peptide concentrations for 5 h (time of intracellular cytokine staining) and 24 h. We collected supernatant and measured the production of IFN-γ, TNF-α, IL-2, IL-10, IL-6, and IL-4 for each CTL clone. As seen in Fig. 4B, HBc18-27-specific T cells produced IFN-γ, TNF-α, and IL-2 in response to peptide. No IL-10, IL-6, or IL-4 was measured in the supernatants of either CTL clone (data not shown). HepG2 cells were significantly less efficient at stimulating HBc18-27-specific T-cell cytokine production, and the difference became even more dramatic at the longer incubation time of 24 h (Fig. 4B). In the case of IFN-γ, there was only a minimal increase in IFN-γ (1.4-fold) in the supernatant of HepG2-stimulated clones beyond 5 h of incubation (5 h, 19.1 ng/ml; 24 h, 26.2 ng/ml). In contrast, EBV B-cell-stimulated CTL clones continued to produce IFN-γ beyond 5 h, with nearly a fourfold increase at 24 h (5 h, 36.8 ng/ml; 24 h, 144.8 ng/ml). Similar results were observed using the HBp455-63-specific T-cell clones (data not shown). Therefore, in addition to requiring more peptide to activate CD8 T cells, hepatocyte-mediated T-cell cytokine production was not sustained beyond the brief window of activation.
Inflammation does not alter the balance between degranulation and cytokine production.
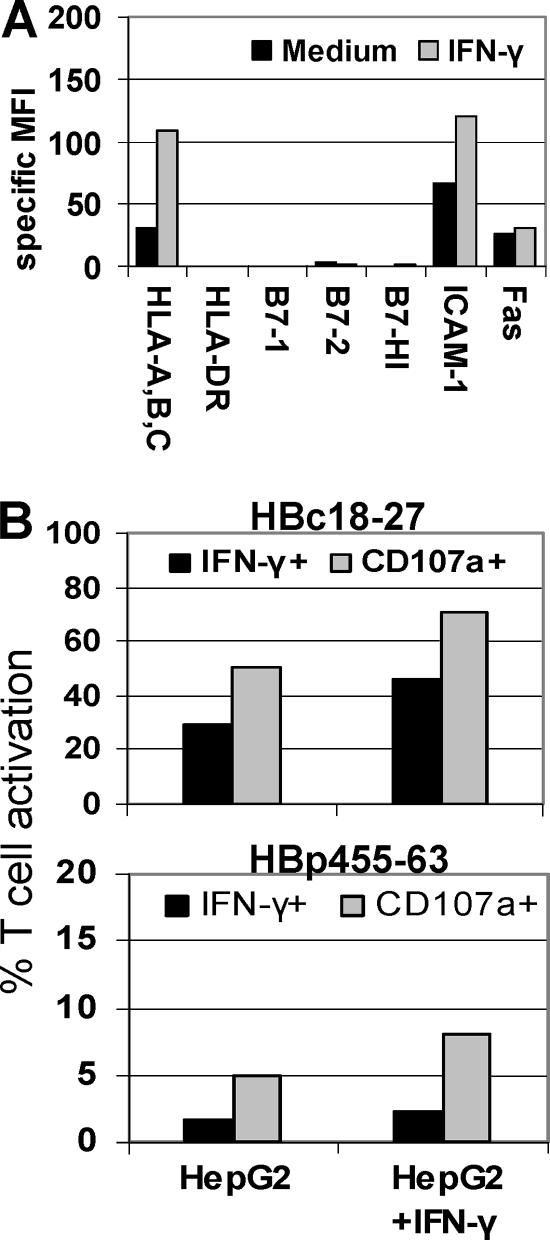
During natural infection, inflammation will likely affect T-cell responses to infected hepatocytes. Because IFN-γ appears to be important for the resolution of both HBV and HCV infections and is known to have a profound impact on APC phenotype, we tested CD8 T-cell responses to peptide-pulsed HepG2 cells exposed to IFN-γ for 24 h prior to peptide loading. As seen in Fig. 5A, IFN-γ treatment of HepG2 cells nearly tripled MHC-I expression and doubled ICAM-1 expression. Despite the substantial increase in MHC-I, at this limiting peptide concentration (10−8 M) there was minimal impact on the balance between CD8 T-cell degranulation and IFN-γ production. Both HBc18-27 and HBp455-63 responses increased in IFN-γ-treated HepG2 cells, but the balance between CD8 T cells positive for IFN-γ and CD107a remained almost unchanged (Fig. 5B). Therefore, the relatively low expression of HLA-A,B,C by hepatocytes was not the sole determinant governing the balance of CD8 T-cell responses and would suggest that the amount of viral antigen was a significant factor determining virus-specific CD8 T-cell responses in the human liver.
FIG. 5.
Increased MHC-I expression does not shift the balance in T-cell activation. (A) Phenotype of HepG2 cells with or without 100 U/ml IFN-γ for 24 h. (B) HBc18-27 and HBp455-63 T-cell IFN-γ production and degranulation in response to HepG2 cells pulsed with 10−8 M peptide after 24 h of incubation with IFN-γ.
Level of viral antigen produced dictates the quality of the CD8 T-cell response.
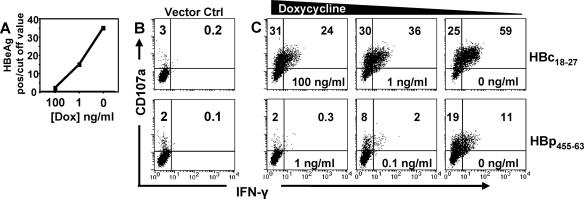
The results described above show that normal and “inflamed” hepatocytes pulsed with low concentrations of synthetic peptide induced IFN-γ production in a small fraction of virus-specific CD8 T cells (Fig. 3 and 5). However, an important question to address was if naturally processed viral epitopes derived from viral antigens synthesized within infected cells would identically skew CD8 T cells towards potential cytolytic function. We took advantage of a recently established HepG2 cell line, HepG2.105, which was stably transfected with a tetracycline-dependent transactivator (tTA) and a complete HBV genome such that production of the pregenomic RNA, which serves as mRNA for the core and polymerase proteins, is under the control of a tetracycline-responsive promoter (49). Using the tetracycline analog doxycycline, we could tune the level of HBV antigen production by varying the concentration of doxycycline. Consistent with previous data (49), in the absence of doxycycline, high levels of HBeAg reactivity (not caused by authentic precore-derived HBeAg but by cross-reactivity of HBcAg and/or HBcAg degradation products) were detectable in the cell culture supernatants. The presence of 1 ng/ml doxycycline markedly reduced, and that of 100 ng/ml nearly abolished, HBe reactivity (Fig. 6A) (49). Direct intracellular quantification showed that cells kept in the absence of doxycycline contained approximately 25 fg/cell of HBcAg (or 7 × 105 molecules/cell) but only between 0.047 and 0.187 fg/cell (1.3 × 103 to 5.0 × 103 molecules) in the presence of 100 ng/ml of doxycycline. Hence, intracellular HBcAg levels were adjustable at least 150-fold. These ratios are fully congruent with the levels of HBV DNA observed in the presence versus absence of doxycycline (49), likely mimicking the different levels of HBV replication in infected hepatocytes within the liver (61).
FIG. 6.
Viral replication within hepatocytes governs the quality of CD8 T-cell responses. (A) HBeAg-HBcAg production with different concentrations of doxycycline. (B) HBc18-27 (top) or HBp455-63 (bottom) T-cell clones incubated with the HepG2 cell line containing only tTA. (C) HBc18-27 (top row) or HBp455-63 (bottom row) T-cell clone activation after coculture with HepG2.105 cells expressing different levels of HBV. Panels are representative of three separate experiments.
Challenged against target cells expressing the tTA alone, neither HBc18-27- nor HBp455-63-specific CD8 T cells displayed any activation (Fig. 6B). In contrast, HBc18-27-specific CD8 T cells efficiently recognized HBV-expressing HepG2 cells cultured in decreasing concentrations of doxycycline (increasing HBV production), and the balance between CD8 T cells positive for CD107a and IFN-γ was directly related to the level of viral antigen (Fig. 6C, top row). When HBV antigen production was minimal at a high dose of doxycycline (100 ng/ml), only 24% of T-cell clones were double positive for CD107a and IFN-γ. As viral antigen within target cells increased, the percentage of CD107a and IFN-γ double-positive HBc18-27 CD8 T cells similarly increased, reaching a maximum of 59% under full induction of HBV expression.
Polymerase-specific T-cell responses to HBV-expressing HepG2 cells were low. Very few HBp455-63 CD8 T cells became double positive for CD107a and IFN-γ when HBV expression was fully induced (11% with 0 ng/ml doxycycline) and even fewer in the presence of 0.1 ng/ml doxycycline (2%), while HBV-transfected HepG2 cells cultured with 1 ng/ml doxycycline were not recognized at all (Fig. 6C, bottom row). This is likely a reflection of the lower level of polymerase expression than that of the core protein by transfected HepG2 cells. Consistent with the data for hepatocytes pulsed with low concentrations of HBp455-63 peptide, even full induction of HBV in transfected HepG2 cells activated a minority of CD107a-positive HBp455-63 CD8 T cells to synthesize IFN-γ (Fig. 6C, bottom row).
Activation of HBV-specific CD8 T cells by naturally infected hepatocytes.
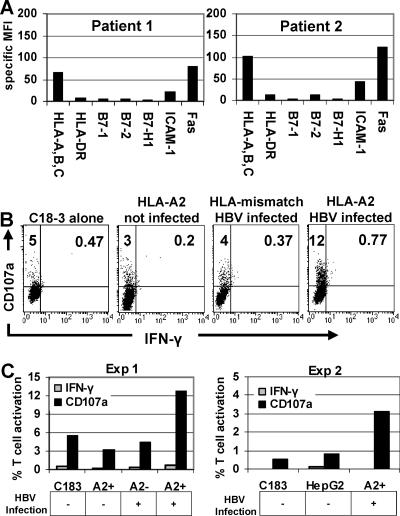
To further investigate the functional behavior of virus-specific CD8 cells after encountering naturally infected hepatocytes, we tested the HBc18-27-specific CD8 T-cell response to chronically HBV-infected primary hepatocytes. These hepatocytes were purified from sections of explanted liver and expressed a phenotypic profile consistent with intrahepatic inflammation. As seen in the two patients presented, similar to IFN-γ-treated HepG2 cells, hepatocyte MHC-I and ICAM-1 expression were increased two- to threefold compared to healthy hepatocytes, while the expression of HLA-DR, costimulatory molecules, and B7-H1 remained low despite the chronic status of the infection (Fig. 7A).
FIG. 7.
HBc18-27 T-cell recognition of naturally infected hepatocytes. (A) Phenotypes of chronically HBV infected hepatocytes from two individual patients. (B) HBc18-27-specific T-cell clone degranulation and IFN-γ production after incubation alone with HLA-A2+ uninfected, with HLA-mismatched HBV infected, or with HLA-A2+ HBV-infected PHH. (C) Graphical representation of CD107a and IFN-γ data from panel B (Exp 1) and an additional experiment with HLA-A2+, chronically HBV-infected PHH (Exp 2). A2+ and A2− on the x axis indicate whether PHH were used for the experiment.
When CD8 T-cell responses to naturally infected hepatocytes were measured, the level of activation of HBc18-27 CD8 T cells incubated with either HLA-A2+ uninfected or HLA-mismatched, HBV-infected hepatocytes was similar to that detected when HBc18-27 CD8 T cells were incubated alone (5%) (Fig. 7B). In contrast, 12% of HBc18-27 CD8 T cells were activated by HLA-A2+ HBV-infected primary hepatocytes and only expressed CD107a without IFN-γ synthesis (Fig. 7B). A graphical summary of these results, including data from a second patient, is presented in Fig. 7C.
Data obtained with primary hepatocytes, taken from the site of natural infection, confirmed previous observations that, despite the increased expression of MHC-I on chronically infected hepatocytes, low viral replication stimulated an HBc18-27-specific response that was exclusively cytotoxic in nature.
DISCUSSION
The difficulty in obtaining suitable clinical samples has hindered our understanding of the pathogenesis of viral hepatitis, limiting analysis to correlations made between circulating and intrahepatic pools of virus-specific CD8 T cells and parameters measuring liver function (5). Studies have shown that prolonged exposure to increased quantities of viral antigens, such as HBeAg+ patients with persistently high levels of HBV replication, results in phenotypic alterations and a hierarchical loss of cytokine production, anergy, and ultimately deletion of virus-specific CD8 T cells (41, 58, 60, 62, 65). In this work, we investigated a different aspect of viral hepatitis, the immediate consequences of fluctuations in viral load on virus-specific CD8 T-cell function, situations associated with liver damage in both acute and chronic HBV infection.
Our data demonstrate that the quantity of viral antigen available for presentation on hepatocytes was a major factor determining the type of T-cell response, while the level of MHC-I expression appeared to have little impact on the balance between CD8 T-cell cytokine production and degranulation. High concentrations of peptide (10−6 to 10−5 M) or high viral replication were necessary for hepatocytes to stimulate robust cytokine production by HBV-specific CD8 T cells, whereas limiting antigen/peptide concentrations preferentially stimulated T-cell degranulation. This phenomenon was demonstrated in naturally infected hepatocytes, where MHC-I expression was increased but HBV replication was low (∼105 HBV DNA copies/ml) as a result of antiviral therapy.
The combination of experiments (peptide-pulsed primary hepatocytes/HepG2 cells, HepG2.105 cells, and naturally infected primary hepatocytes) illustrates how the quantity of virus within hepatocytes can impact the activation of intrahepatic virus-specific CD8 T cells and their cytolytic/noncytolytic functional balance during chronic and acute viral hepatitis. This is reminiscent of the recent report showing that individual CD8 T-cell functions can be triggered by varying antigen concentrations presented by PBMC of human immunodeficiency virus- and CMV-infected patients, with only degranulation triggered by low peptide doses (2 × 10−11 to 2 × 10−10 M) (8, 55). Therefore, this phenomenon is not specific to hepatocytes but is more pronounced due to the inefficiency of hepatocyte antigen presentation. Data in Fig. 2, using four different targets, illustrate this point. Fresh blood monocytes were able to stimulate equivalent percentages of T-cell clones to produce IFN-γ and degranulate to as low as 10−10 M peptide, whereas EBV B cells stimulated equivalent effector functions at peptide concentrations of 10−8 M. This was 100- to 1,000-fold more sensitive than primary hepatocytes, which in the case of HBc18-27-specific T-cell clones still did not equally stimulate T-cell effector functions at 10−6 M peptide, suggesting that efficient T-cell activation in the liver requires high viral replication and the production of large amounts of viral antigen.
The underlying mechanism contributing to hepatocyte inefficiency is not clear. The fact that the level of MHC-I expression did not shift the balance in T-cell activation suggests that additional regulatory interactions may be operating. It appears unlikely that B7-H1-PD-1 interactions are involved, as neither HBV-specific CD8 T-cell clone expressed PD-1 (data not shown) and B7-H1 was nearly undetectable on all target cell types (Fig. 1), including chronically infected primary hepatocytes (Fig. 6).
Although we lack direct evidence, our results suggest that CD8 T-cell-mediated cytolytic clearance of infected hepatocytes could dominate in chronic hepatitis B patients during antiviral treatment, perhaps contributing to the very slow rate of decline in infected cells observed during the later stages of therapy (36, 54). In contrast, efficient IFN-γ production by CD8 T cells stimulated in the presence of high quantities of antigen fits the observed prevalence of rapid noncytolytic, cytokine-mediated viral clearance in the early phases of acute HBV and HCV infections (23, 52, 59).
In the setting of high viral replication, when most hepatocytes are likely to be infected, virus-specific CD8 T cells will be activated to secrete IFN-γ and TNF-α, clearing virus from neighboring infected hepatocytes (9, 22) but also driving the inflammatory mononuclear infiltrate (32). The decreasing quantity of viral antigen could then result in an almost exclusive stimulation of CD8 T-cell degranulation (48) with the resulting scenario being direct hepatocyte damage by virus-specific CD8 T cells. This may not cause massive liver failure, due to the likely lower number of infected hepatocytes and decreased number of virus-specific CD8 T cells which characterizes the resolving phase of acute HBV and HCV infection (35, 40, 58).
Experiments in chimpanzees reflect this possible scenario and show that efficient IFN-γ production is present only at the peak in viral replication, while cytolytic events can be observed when viral replication is decreasing (23, 63). Furthermore, the oscillation between noncytolytic and cytolytic T-cell function recently observed after adoptive transfer of HBeAg-specific CD8 T cells to HBV transgenic mice could be explained by the quantity of viral antigens expressed on hepatocytes at different times (25).
Our results might also explain why fluctuations of viral load during the natural history of chronic hepatitis B infection are often associated with inflammatory reactivation of chronic hepatitis (13, 37, 53), while the inhibition of viral replication during antiviral treatment of chronic hepatitis B and C results in amelioration of liver inflammation (16, 17, 33, 57). These two opposite pathogenic events have both been associated with an increase in virus-specific T-cell immunity, but it remains difficult to understand why changes in T-cell immunity can be associated with either an increase or decrease in disease activity (11, 28, 42, 43, 50, 53, 57).
Instead, our data showing that the quantity of viral antigen presented by hepatocytes can regulate the amount of cytokines produced by virus-specific CD8 T cells might reconcile these observations. Reactivation of virus replication will likely boost cytokine production by virus-specific CD8 T cells present within the chronically infected liver (38), leading to recruitment of inflammatory cells and hepatocyte damage. On the other hand, drug-mediated inhibition of viral replication could switch the function of intrahepatic virus-specific CD8 T cells towards hepatocyte lysis without sustaining inflammatory cell recruitment.
In conclusion, we have analyzed the ability of hepatocytes to activate virus-specific CD8 T cells and the effect of viral load on the ensuing T-cell response. Our data demonstrate how simply the nature of the target and the quantity of antigen might modulate virus-specific CD8 T-cell functions without the necessary involvement of complex regulatory networks.
Acknowledgments
We acknowledge all those who helped and provided input during the course of this project. In particular, we acknowledge Myrddin Rees and Wei-Wen Teo for help obtaining and purifying hepatocytes, as well as Dave Brown and Geoff Dusheicko for sequencing the HBc18-27 epitope.
This work was supported by The Wellcome Trust.
We have no conflicting financial interests.
Footnotes
Published ahead of print on 3 January 2007.
REFERENCES
- 1.Agnello, V., G. Abel, G. B. Knight, and E. Muchmore. 1998. Detection of widespread hepatocyte infection in chronic hepatitis C. Hepatology 28:573-584. [DOI] [PubMed] [Google Scholar]
- 2.Ando, K., L. G. Guidotti, S. Wirth, T. Ishikawa, G. Missale, T. Moriyama, R. D. Schreiber, H. J. Schlicht, S. N. Huang, and F. V. Chisari. 1994. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J. Immunol. 152:3245-3253. [PubMed] [Google Scholar]
- 3.Barbatis, C., J. Woods, J. A. Morton, K. A. Fleming, A. McMichael, and J. O. McGee. 1981. Immunohistochemical analysis of HLA (A, B, C) antigens in liver disease using a monoclonal antibody. Gut 22:985-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrie, M. B., H. W. Stout, M. S. Abougergi, B. C. Miller, and D. L. Thiele. 2004. Antiviral cytokines induce hepatic expression of the granzyme B inhibitors, proteinase inhibitor 9 and serine proteinase inhibitor 6. J. Immunol. 172:6453-6459. [DOI] [PubMed] [Google Scholar]
- 5.Bertoletti, A., and C. Ferrari. 2003. Kinetics of the immune response during HBV and HCV infection. Hepatology 38:4-13. [DOI] [PubMed] [Google Scholar]
- 6.Bertoletti, A., and M. K. Maini. 2000. Protection or damage: a dual role for the virus-specific cytotoxic T lymphocyte response in hepatitis B and C infection? Curr. Opin. Immunol. 12:403-408. [DOI] [PubMed] [Google Scholar]
- 7.Betts, M. R., J. M. Brenchley, D. A. Price, S. C. De Rosa, D. C. Douek, M. Roederer, and R. A. Koup. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65-78. [DOI] [PubMed] [Google Scholar]
- 8.Betts, M. R., D. A. Price, J. M. Brenchley, K. Lore, F. J. Guenaga, A. Smed-Sorensen, D. R. Ambrozak, S. A. Migueles, M. Connors, M. Roederer, D. C. Douek, and R. A. Koup. 2004. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J. Immunol. 172:6407-6417. [DOI] [PubMed] [Google Scholar]
- 9.Biermer, M., R. Puro, and R. J. Schneider. 2003. Tumor necrosis factor alpha inhibition of hepatitis B virus replication involves disruption of capsid integrity through activation of NF-κB. J. Virol. 77:4033-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnbaum, F., and M. Nassal. 1990. Hepatitis B virus nucleocapsid assembly: primary structure requirements in the core protein. J. Virol. 64:3319-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boni, C., A. Penna, A. Bertoletti, V. Lamonaca, I. Rapti, G. Missale, M. Pilli, S. Urbani, A. Cavalli, S. Cerioni, R. Panebianco, J. Jenkins, and C. Ferrari. 2003. Transient restoration of anti-viral T cell responses induced by lamivudine therapy in chronic hepatitis B. J. Hepatol. 39:595-605. [DOI] [PubMed] [Google Scholar]
- 12.Bossi, G., and G. M. Griffiths. 1999. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat. Med. 5:90-96. [DOI] [PubMed] [Google Scholar]
- 13.Brunetto, M. R., M. M. Giarin, F. Oliveri, E. Chiaberge, M. Baldi, A. Alfarano, A. Serra, G. Saracco, G. Verme, H. Will, et al. 1991. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc. Natl. Acad. Sci. USA 88:4186-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu, J. H., W. Y. Lui, H. M. Chang, C. C. Loong, L. H. Wu, H. L. Kao, and C. W. Wu. 1997. Class I and class II major histocompatibility complex antigens expression on human hepatocytes and hepatoma cells: an approach with high sensitivity and specificity. Cytometry 30:317-323. [PubMed] [Google Scholar]
- 15.Chu, C. M., and Y. F. Liaw. 1993. Coexpression of intercellular adhesion molecule-1 and class I major histocompatibility complex antigens on hepatocyte membrane in chronic viral hepatitis. J. Clin. Pathol. 46:1004-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dienstag, J. L., R. D. Goldin, E. J. Heathcote, H. W. Hann, M. Woessner, S. L. Stephenson, S. Gardner, D. F. Gray, and E. R. Schiff. 2003. Histological outcome during long-term lamivudine therapy. Gastroenterology 124:105-117. [DOI] [PubMed] [Google Scholar]
- 17.Dienstag, J. L., R. P. Perrillo, E. R. Schiff, M. Bartholomew, C. Vicary, and M. Rubin. 1995. A preliminary trial of lamivudine for chronic hepatitis B infection. N. Engl. J. Med. 333:1657-1661. [DOI] [PubMed] [Google Scholar]
- 18.Freeman, A. J., Y. Pan, C. E. Harvey, J. J. Post, M. G. Law, P. A. White, W. D. Rawlinson, A. R. Lloyd, G. Marinos, and R. A. Ffrench. 2003. The presence of an intrahepatic cytotoxic T lymphocyte response is associated with low viral load in patients with chronic hepatitis C virus infection. J. Hepatol. 38:349-356. [DOI] [PubMed] [Google Scholar]
- 19.Fukusato, T., M. A. Gerber, S. N. Thung, S. Ferrone, and F. Schaffner. 1986. Expression of HLA class I antigens on hepatocytes in liver disease. Am. J. Pathol. 123:264-270. [PMC free article] [PubMed] [Google Scholar]
- 20.Grabowska, A. M., F. Lechner, P. Klenerman, P. J. Tighe, S. Ryder, J. K. Ball, B. J. Thomson, W. L. Irving, and R. A. Robins. 2001. Direct ex vivo comparison of the breadth and specificity of the T cells in the liver and peripheral blood of patients with chronic HCV infection. Eur. J. Immunol. 31:2388-2394. [DOI] [PubMed] [Google Scholar]
- 21.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 22.Guidotti, L. G., T. Ishikawa, M. V. Hobbs, B. Matzke, R. Schreiber, and F. V. Chisari. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4:25-36. [DOI] [PubMed] [Google Scholar]
- 23.Guidotti, L. G., R. Rochford, J. Chung, M. Shapiro, R. Purcell, and F. V. Chisari. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284:825-829. [DOI] [PubMed] [Google Scholar]
- 24.He, X. S., B. Rehermann, F. X. Lopez-Labrador, J. Boisvert, R. Cheung, J. Mumm, H. Wedemeyer, M. Berenguer, T. L. Wright, M. M. Davis, and H. B. Greenberg. 1999. Quantitative analysis of hepatitis C virus-specific CD8+ T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl. Acad. Sci. USA 96:5692-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isogawa, M., Y. Furuichi, and F. V. Chisari. 2005. Oscillating CD8+ T cell effector functions after antigen recognition in the liver. Immunity 23:53-63. [DOI] [PubMed] [Google Scholar]
- 26.Iwai, Y., S. Terawaki, M. Ikegawa, T. Okazaki, and T. Honjo. 2003. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp. Med. 198:39-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jilbert, A. R., T. T. Wu, J. M. England, P. M. Hall, N. Z. Carp, A. P. O'Connell, and W. S. Mason. 1992. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. J. Virol. 66:1377-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung, M. C., N. Gruner, R. Zachoval, W. Schraut, T. Gerlach, H. Diepolder, C. A. Schirren, M. Page, J. Bailey, E. Birtles, E. Whitehead, J. Trojan, S. Zeuzem, and G. R. Pape. 2002. Immunological monitoring during therapeutic vaccination as a prerequisite for the design of new effective therapies: induction of a vaccine-specific CD4+ T-cell proliferative response in chronic hepatitis B carriers. Vaccine 20:3598-3612. [DOI] [PubMed] [Google Scholar]
- 29.Jung, M. C., B. Hartmann, J. T. Gerlach, H. Diepolder, R. Gruber, W. Schraut, N. Gruner, R. Zachoval, R. Hoffmann, T. Santantonio, M. Wachtler, and G. R. Pape. 1999. Virus-specific lymphokine production differs quantitatively but not qualitatively in acute and chronic hepatitis B infection. Virology 261:165-172. [DOI] [PubMed] [Google Scholar]
- 30.Kafrouni, M. I., G. R. Brown, and D. L. Thiele. 2001. Virally infected hepatocytes are resistant to perforin-dependent CTL effector mechanisms. J. Immunol. 167:1566-1574. [DOI] [PubMed] [Google Scholar]
- 31.Kajino, K., A. R. Jilbert, J. Saputelli, C. E. Aldrich, J. Cullen, and W. S. Mason. 1994. Woodchuck hepatitis virus infections: very rapid recovery after a prolonged viremia and infection of virtually every hepatocyte. J. Virol. 68:5792-5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kakimi, K., T. E. Lane, S. Wieland, V. C. Asensio, I. L. Campbell, F. V. Chisari, and L. G. Guidotti. 2001. Blocking chemokine responsive to gamma-2/interferon (IFN)-gamma inducible protein and monokine induced by IFN-gamma activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. J. Exp. Med. 194:1755-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai, C. L., R. N. Chien, N. W. Leung, T. T. Chang, R. Guan, D. I. Tai, K. Y. Ng, P. C. Wu, J. C. Dent, J. Barber, S. L. Stephenson, D. F. Gray, et al. 1998. A one-year trial of lamivudine for chronic hepatitis B. N. Engl. J. Med. 339:61-68. [DOI] [PubMed] [Google Scholar]
- 34.Lauer, G. M., E. Barnes, M. Lucas, J. Timm, K. Ouchi, A. Y. Kim, C. L. Day, G. K. Robbins, D. R. Casson, M. Reiser, G. Dusheiko, T. M. Allen, R. T. Chung, B. D. Walker, and P. Klenerman. 2004. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology 127:924-936. [DOI] [PubMed] [Google Scholar]
- 35.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewin, S. R., R. M. Ribeiro, T. Walters, G. K. Lau, S. Bowden, S. Locarnini, and A. S. Perelson. 2001. Analysis of hepatitis B viral load decline under potent therapy: complex decay profiles observed. Hepatology 34:1012-1020. [DOI] [PubMed] [Google Scholar]
- 37.Lok, A. S., and B. J. McMahon. 2001. Chronic hepatitis B. Hepatology 34:1225-1241. [DOI] [PubMed] [Google Scholar]
- 38.Maini, M. K., C. Boni, C. K. Lee, J. R. Larrubia, S. Reignat, G. S. Ogg, A. S. King, J. Herberg, R. Gilson, A. Alisa, R. Williams, D. Vergani, N. V. Naoumov, C. Ferrari, and A. Bertoletti. 2000. The role of virus-specific CD8+ cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191:1269-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Major, M. E., K. Mihalik, J. Fernandez, J. Seidman, D. Kleiner, A. A. Kolykhalov, C. M. Rice, and S. M. Feinstone. 1999. Long-term follow-up of chimpanzees inoculated with the first infectious clone for hepatitis C virus. J. Virol. 73:3317-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rehermann, B., C. Ferrari, C. Pasquinelli, and F. V. Chisari. 1996. The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat. Med. 2:1104-1108. [DOI] [PubMed] [Google Scholar]
- 41.Reignat, S., G. J. Webster, D. Brown, G. S. Ogg, A. King, S. L. Seneviratne, G. Dusheiko, R. Williams, M. K. Maini, and A. Bertoletti. 2002. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J. Exp. Med. 195:1089-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossol, S., G. Marinos, P. Carucci, M. V. Singer, R. Williams, and N. V. Naoumov. 1997. Interleukin-12 induction of Th1 cytokines is important for viral clearance in chronic hepatitis B. J. Clin. Investig. 99:3025-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothman, A. L., C. Morishima, H. L. Bonkovsky, S. J. Polyak, R. Ray, A. M. Di Bisceglie, K. L. Lindsay, P. F. Malet, M. Chang, D. R. Gretch, D. G. Sullivan, A. K. Bhan, E. C. Wright, and M. J. Koziel. 2005. Associations among clinical, immunological, and viral quasispecies measurements in advanced chronic hepatitis C. Hepatology 41:617-625. [DOI] [PubMed] [Google Scholar]
- 44.Sallberg, M., U. Ruden, L. O. Magnius, H. P. Harthus, M. Noah, and B. Wahren. 1991. Characterisation of a linear binding site for a monoclonal antibody to hepatitis B core antigen. J. Med. Virol. 33:248-252. [DOI] [PubMed] [Google Scholar]
- 45.Sitia, G., M. Isogawa, K. Kakimi, S. F. Wieland, F. V. Chisari, and L. G. Guidotti. 2002. Depletion of neutrophils blocks the recruitment of antigen-nonspecific cells into the liver without affecting the antiviral activity of hepatitis B virus-specific cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 99:13717-13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spangenberg, H. C., S. Viazov, N. Kersting, C. Neumann-Haefelin, D. McKinney, M. Roggendorf, F. von Weizsacker, H. E. Blum, and R. Thimme. 2005. Intrahepatic CD8+ T-cell failure during chronic hepatitis C virus infection. Hepatology 42:828-837. [DOI] [PubMed] [Google Scholar]
- 47.Strain, A. J., T. Ismail, H. Tsubouchi, N. Arakaki, T. Hishida, N. Kitamura, Y. Daikuhara, and P. McMaster. 1991. Native and recombinant human hepatocyte growth factors are highly potent promoters of DNA synthesis in both human and rat hepatocytes. J. Clin. Investig. 87:1853-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Summers, J., A. R. Jilbert, W. Yang, C. E. Aldrich, J. Saputelli, S. Litwin, E. Toll, and W. S. Mason. 2003. Hepatocyte turnover during resolution of a transient hepadnaviral infection. Proc. Natl. Acad. Sci. USA 100:11652-11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun, D., and M. Nassal. 2006. Stable HepG2- and Huh7-based human hepatoma cell lines for efficient regulated expression of infectious hepatitis B virus. J. Hepatol. 45:636-645. [DOI] [PubMed] [Google Scholar]
- 50.Tang, K. H., E. Herrmann, H. Cooksley, N. Tatman, S. Chokshi, R. Williams, S. Zeuzem, and N. V. Naoumov. 2005. Relationship between early HCV kinetics and T-cell reactivity in chronic hepatitis C genotype 1 during peginterferon and ribavirin therapy. J. Hepatol. 43:776-782. [DOI] [PubMed] [Google Scholar]
- 51.Thimme, R., J. Bukh, H. C. Spangenberg, S. Wieland, J. Pemberton, C. Steiger, S. Govindarajan, R. H. Purcell, and F. V. Chisari. 2002. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc. Natl. Acad. Sci. USA 99:15661-15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai, S. L., P. J. Chen, M. Y. Lai, P. M. Yang, J. L. Sung, J. H. Huang, L. H. Hwang, T. H. Chang, and D. S. Chen. 1992. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens. Implications for hepatitis B e antigen seroconversion. J. Clin. Investig. 89:87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsiang, M., J. F. Rooney, J. J. Toole, and C. S. Gibbs. 1999. Biphasic clearance kinetics of hepatitis B virus from patients during adefovir dipivoxil therapy. Hepatology 29:1863-1869. [DOI] [PubMed] [Google Scholar]
- 55.Valitutti, S., S. Muller, M. Dessing, and A. Lanzavecchia. 1996. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J. Exp. Med. 183:1917-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van den Oord, J. J., R. de Vos, and V. J. Desmet. 1986. In situ distribution of major histocompatibility complex products and viral antigens in chronic hepatitis B virus infection: evidence that HBc-containing hepatocytes may express HLA-DR antigens. Hepatology 6:981-989. [DOI] [PubMed] [Google Scholar]
- 57.Vertuani, S., M. Bazzaro, G. Gualandi, F. Micheletti, M. Marastoni, C. Fortini, A. Canella, M. Marino, R. Tomatis, S. Traniello, and R. Gavioli. 2002. Effect of interferon-alpha therapy on epitope-specific cytotoxic T lymphocyte responses in hepatitis C virus-infected individuals. Eur. J. Immunol. 32:144-154. [DOI] [PubMed] [Google Scholar]
- 58.Webster, G. J., S. Reignat, D. Brown, G. S. Ogg, L. Jones, S. L. Seneviratne, R. Williams, G. Dusheiko, and A. Bertoletti. 2004. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J. Virol. 78:5707-5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Webster, G. J., S. Reignat, M. K. Maini, S. A. Whalley, G. S. Ogg, A. King, D. Brown, P. L. Amlot, R. Williams, D. Vergani, G. M. Dusheiko, and A. Bertoletti. 2000. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology 32:1117-1124. [DOI] [PubMed] [Google Scholar]
- 60.Welsh, R. M. 2001. Assessing CD8 T cell number and dysfunction in the presence of antigen. J. Exp. Med. 193:F19-F22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Werle-Lapostolle, B., S. Bowden, S. Locarnini, K. Wursthorn, J. Petersen, G. Lau, C. Trepo, P. Marcellin, Z. Goodman, W. E. t. Delaney, S. Xiong, C. L. Brosgart, S. S. Chen, C. S. Gibbs, and F. Zoulim. 2004. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 126:1750-1758. [DOI] [PubMed] [Google Scholar]
- 62.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wieland, S., R. Thimme, R. H. Purcell, and F. V. Chisari. 2004. Genomic analysis of the host response to hepatitis B virus infection. Proc. Natl. Acad. Sci. USA 101:6669-6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wieland, S. F., and F. V. Chisari. 2005. Stealth and cunning: hepatitis B and hepatitis C viruses. J. Virol. 79:9369-9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou, S., R. Ou, L. Huang, G. E. Price, and D. Moskophidis. 2004. Differential tissue-specific regulation of antiviral CD8+ T-cell immune responses during chronic viral infection. J. Virol. 78:3578-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]