Abstract
Early control of virus replication by the innate immune response is essential to allow time for the generation of a more effective adaptive immune response. As an important component of innate immunity, complement has been shown to be necessary for protection against numerous microbial infections. This study was undertaken to investigate the role of complement in neutralizing influenza virus. Results demonstrated that the classical pathway of complement mediated serum neutralization of influenza virus. Although nonimmune serum neutralized influenza virus, the mechanism of virus neutralization (VN) required antibody, as sera from RAG1-deficient mice lacked VN activity; moreover, purified natural immunoglobulin M (IgM) restored VN activity to antibody-deficient sera. The mechanism of VN by natural IgM and complement was associated with virion aggregation and coating of the viral hemagglutinin receptor; however, viral lysis did not significantly contribute to VN. Additionally, reconstitution of RAG1-deficient mice with natural IgM resulted in delayed morbidity during influenza virus infection. Collectively, these results provide evidence that natural IgM and the early components of the classical pathway of complement work in concert to neutralize influenza virus and that this interaction may have a significant impact on the course of influenza viral pneumonia.
As a first-line defense against pathogens, innate immunity is critically important in impeding microbial invasion and alerting other components of the body's defense system. The complement system, a key component of the innate immune response, protects mucosal surfaces and is present in human serum at very high concentrations (greater than 1 mg/ml). Complement was first identified as a heat-labile component of normal serum that killed bacteria (24). Subsequently, this factor was designated “complement” when it was found to complement the action of antibody directed against bacteria and erythrocytes. Since these initial discoveries were made, well over a century ago, numerous effector and regulatory components have been identified as constituents of the complement system. To date, three main pathways of complement activation have been identified: the classical, lectin, and alternative pathways. Activation of the classical pathway occurs when C1 binds to antibody or directly to activating surfaces; recognition by mannose binding lectin (MBL) of carbohydrate residues found primarily on microbes leads to activation of the lectin pathway, whereas the alternative pathway is initiated when C3 binds to a suitable activating surface.
With the generation of complement-deficient mice, the interactions of complement components with other molecules of innate and adaptive immunity are beginning to be more appreciated. Recently, an important role for complement component C3 in priming T cells was discovered (23). C3-deficient mice were shown to be more susceptible to influenza virus infection, which corresponded with attenuated T-cell priming and migration to the lungs. Similarly, C3 deficiency in humans correlates with recurrent infections primarily of the upper and lower respiratory tract (34). Complement can also play an important role in neutralizing viruses. All three complement pathways have been shown to be activated by viruses to various degrees (1, 10, 21). That complement can bind to influenza virus in the presence of specific antibody has long been known (5). Indeed, immune sera are usually heated to inactivate interfering complement components during assays for virus-neutralizing serum antibodies that inhibit the hemagglutination (HA) activity of influenza virus. Interestingly, these complement components are also partly responsible for background hemagglutination inhibition in nonimmune serum (16). Thus, while there is evidence that serum complement can neutralize an important human pathogen such as influenza virus, the mechanism of this process has not been investigated in the absence of virus-specific antibody. We undertook this study to further understand the role of complement in neutralizing influenza virus.
MATERIALS AND METHODS
Virus.
Influenza A virus A/PR/8/34 (PR8) was obtained from Charles River Laboratories. PR8 stock virus was prepared by growing the virus for 2 days in the allantoic cavity of 10-day-old embryonated hen eggs. Allantoic fluid containing virus was harvested, filtered, and centrifuged to remove cellular debris. For some assays, virus stock was further purified by centrifugation of the PR8 stock virus through a discontinuous sucrose gradient at 45,000 × g for 45 min at 4°C. The milky virus band located in the upper half of the gradient was harvested, washed in phosphate-buffered saline (PBS), and centrifuged at 85,000 × g for 45 min at 4°C. The virus pellet was gradually resuspended in PBS, and aliquots were stored at −70°C. Virus stocks were quantified by a hemagglutination assay to obtain hemagglutination units (HAU).
Mice and virus infection.
C3−/−, C4−/−, C1q−/−, and RAG1−/− mice (all on a C57BL/6 background) and C57BL/6 (B6) mice were bred at the Warren Alpert Animal Facility (Harvard Medical School). Secretory-immunoglobulin M (IgM)-deficient (sIgM−/−) mice (6) (on a C57BL/6 background) were obtained from Hidde Ploegh (MIT, Cambridge, MA). C5-deficient mice (B10.D2/oSnJ) and wild-type controls (B10.D2/nSnJ) were purchased from Jackson Laboratory. All mice were used at 8 to 10 weeks of age. Experiments were performed in accordance with federal and institutional guidelines. For in vivo experiments, RAG1−/− mice were injected intraperitoneally with 500 μg of natural IgM (nIgM) or 400 μl of PBS on days −1, 0, and 5 of infection. On day 0, all mice were anesthetized with 300 μg 2,2,2-tribromoethanol (Sigma) per gram of body weight and infected intranasally with 300 50% tissue culture infective doses (TCID50) of PR8 stock virus in a volume of 30 μl.
For the determination of morbidity, mice were weighed on the day of infection and at regular intervals thereafter. The percent changes in body weight from the original weights were calculated.
Serum and antibody purification.
To obtain serum for virus neutralization (VN) assays, mice were sacrificed by CO2 inhalation and blood was harvested from the renal artery. Freshly harvested blood was allowed to clot at 4°C, and serum was obtained after centrifugation at 2,700 × g for 5 min at 4°C. Aliquots of sera were stored at −70°C until needed. Freshly thawed serum aliquots were used in each assay.
To obtain small quantities of natural IgM, commercially available serum (Cedarlane) was precipitated overnight with saturated ammonium sulfate. After centrifugation at 3,000 × g for 30 min, the precipitate was solubilized in PBS and dialyzed overnight. The dialysate was concentrated by centrifugation using a Vivaspin concentrator (Vivascience) and then purified using an ImmunoPure IgM purification kit (Pierce) according to the manufacturer's instructions. This method utilizes immobilized MBL in an affinity-based protocol to purify mouse IgM from serum. Purified natural IgM was stored in aliquots at −70°C.
To obtain large quantities of nIgM for in vivo studies, a modified IgM purification procedure was used (2). Mouse serum was precipitated by dialysis in 2 mM NaPO4 buffer for 2 days with regular changes of buffer. The precipitate was pelleted by centrifugation at 11,000 × g for 15 min and washed 2 times with buffer. After resuspension of the precipitate in column buffer (10 mM Tris-HCl, 150 mM NaCl, 0.5 mM EDTA) and filtration through a 0.2-um filter, the solution was passed through a gel filtration column. Fractions containing IgM were determined by Western blotting and enzyme-linked immunosorbent assays (ELISA). IgM eluted in the first large peak, whereas IgG was primarily found in the second peak, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Fractions containing IgM were passed through a protein G column to remove residual IgG and concentrated by centrifugation. Protein concentration was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis.
The H220-17-4 hybridoma produces a mouse IgM antibody that binds to the HA of influenza virus of the H1 subtype. Antibody was purified from culture supernatants by overnight precipitation with 50% saturated ammonium sulfate. After centrifugation at 3,000 × g for 30 min, the precipitate was resuspended in a small volume of PBS and dialyzed overnight against PBS. The dialysate was concentrated by centrifugation and loaded onto an MBL column. The eluate was concentrated, and aliquots were stored at −70°C.
The H36-4-5.2 hybridoma produces a mouse IgG2a antibody that neutralizes PR8 virus by binding to the Sb/B site on HA (27). To obtain purified antibody, culture supernatants were precipitated by saturated ammonium sulfate precipitation. After dialysis against PBS, the solution was added to a protein G (Pharmacia) column and the IgG eluate was biotinylated with biotin N-hydroxysuccinimide ester (Sigma).
Both H220-17-4 and H36-4-5.2 hybridomas were kind gifts from Walter Gerhard (Wistar Institute, PA).
VN assays.
VN assays, measuring the reduction of virus titer, were performed according to a modification of a standard procedure (11). MDCK cells were grown in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin (all from Invitrogen). Trypsinized MDCK cells (1.5 × 104) were added to the wells of a flat-bottom, 96-well microtiter plate and incubated at 37°C in 5% CO2 overnight. PR8 stock virus was added to sera diluted in PBS-CM (PBS, 0.15 mM CaCl2, 0.5 mM MgSO4). When purified antibody was used, an incubation with virus at room temperature for 15 min was performed before virus was added to diluted serum. To heat inactivate serum, sera were incubated at 56°C for 30 min before being used in VN assays. Upon the addition of serum, sample tubes were incubated at 37°C for 60 min, after which they were diluted 10-fold in MM media (RPMI-1640, 0.01% bovine serum albumin [BSA], 2 mM l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin) and added (100 μl) to confluent MDCK cultures that had been washed with serum-free RPMI-1640. Each dilution was used to infect eight replicate wells. Cultures were incubated for 2 h at 37°C in 5% CO2, after which the supernatant was aspirated and 200 μl of MMT media (MM media, 0.000125% trypsin) was added. Incubation of cultures continued for 3 days, after which virus growth was measured by a standard hemagglutination assay. Supernatant (100 μl) was transferred to a round-bottom, 96-well microtiter plate to which 100 μl of red blood cell solution (0.5% chicken red blood cells in PBS) was added, and agglutination patterns were scored. Virus titer was calculated as the log10 dilution that resulted in infection of 50% of the replicate cultures (log10 TCID50), according to the Reed and Muench method (33). The assay had a 102.1 minimum threshold of virus detection.
ELISA.
Virus-binding antibodies in mouse sera were detected by ELISA. Polyvinyl 96-well microtiter plates were coated with purified PR8 stock virus (1,000 HAU/ml in PBS) at 50 μl/well. Sera diluted in PBST-BSA (PBS, 0.05% Tween 20, 0.01% BSA) were added to wells that had been blocked with 0.1% BSA, and plates were incubated for 2 h at room temperature. Alkaline phosphatase-conjugated goat anti-mouse IgM (Southern Biotech) was used to detect antibodies bound to virus-coated wells, and plates were developed using alkaline phosphatase substrate (Sigma) and read at 405 nm.
EM.
For the visualization of virus particles by electron microscopy (EM), purified, UV-inactivated PR8 virus was obtained from W. Gerhard (Wistar Institute, PA). Ten microliters of virus (10 μg/ml) was added to mouse serum (30%) previously diluted in CaMg saline (145 mM NaCl, 0.836 mM MgCl, 0.270 mM CaCl2), followed by incubation at 37°C for 60 min. In some instances, virus was treated with purified nIgM (40 μg/ml) before the addition of serum. Samples were stained with uranyl formate (0.7%) and then visualized by transmission EM.
Complement deposition and antibody-blocking assays.
To measure complement deposition on viral particles, polyvinyl 96-well microtiter plates were coated with 50 μl/well of purified PR8 stock virus (1,000 HAU/ml in PBS). Wells were blocked with 0.1% BSA in PBS, followed by a 30-min incubation with PBST-BSA or nIgM diluted in PBST-BSA. Sera diluted to 30% in PBS-MgCl2 (PBS, 0.5 mM MgSO4, 0.15 mM CaCl2) were added to wells for a 20-min incubation period at 37°C. Plates were washed, and antibodies specific for C3 and C4 were used to detect complement deposition. Goat anti-C3, directly conjugated to peroxidase (Cappel, ICN Biomedicals), was used (1:500 dilution in PBST-BSA) to detect C3 deposition. C4 deposition was determined by adding rabbit anti-human C4c (Dako) (1:500 dilution in PBST-BSA) to the plates for a 60-min incubation, followed by a peroxidase-labeled goat anti-rabbit IgG for 60 min at room temperature. Plates were developed with ABTS [2,2′-azinobis(3 ethylbenzthiazolinesulfonic acid)] solution (Moss, Inc.). Background-corrected (no virus) readings at 405 nm were obtained. These antibodies produced background level readings in wells that lacked serum.
To measure binding of anti-HA antibody to influenza viral particles, an assay similar to the complement deposition assay was performed with 10-fold dilutions of PR8 virus. After incubation with sera, biotin-labeled H36-4-5.2 was added for a 30-min incubation period, followed by incubation with streptavidin-alkaline phosphatase for 10 min. Plates were developed using alkaline phosphatase substrate (Sigma) as before. Percent H36-4-5.2 binding was calculated by dividing the absorbance readings of samples treated with serum by the absorbance readings of respective samples not treated with serum.
RESULTS
Influenza virus is sensitive to neutralization by serum complement.
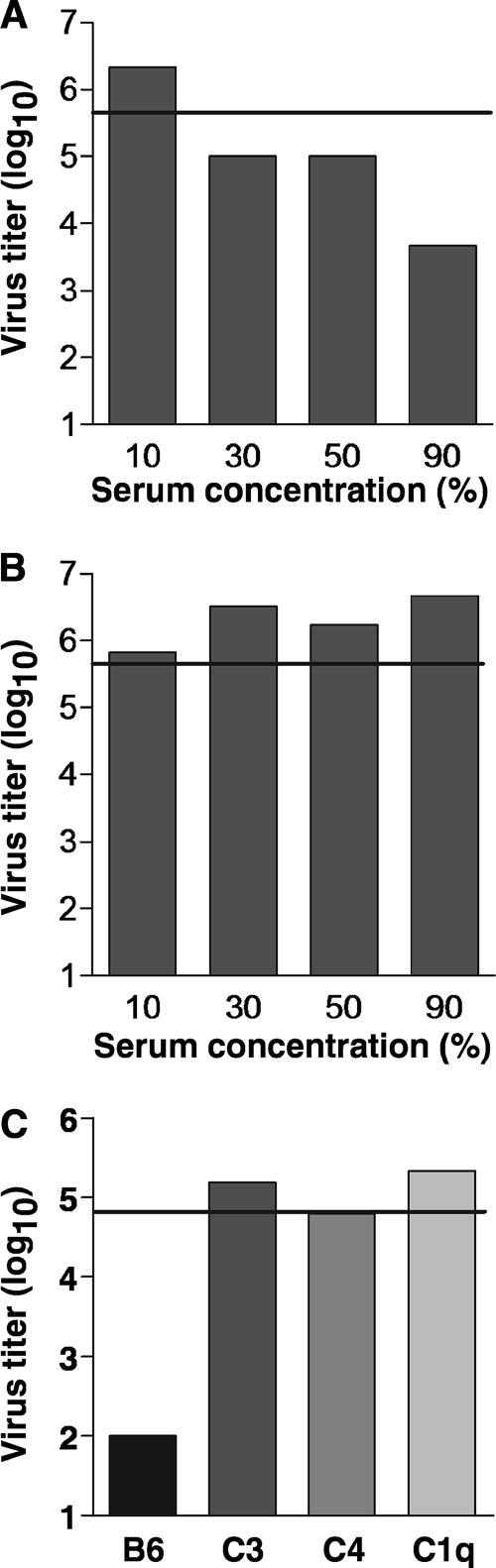
To determine the sensitivity of influenza virus to the neutralizing activity of serum, a mouse-adapted human influenza A virus, A/PR/8/34 (PR8), was incubated with increasing concentrations of pooled mouse serum prepared from normal B6 mice (Fig. 1). The extent of VN was ascertained by measuring the amount of infective virus remaining in the samples after culture on virus-permissive MDCK cells. With 90% serum, PR8 was neutralized by approximately 2 logs; 50% and 30% serum neutralized PR8 by approximately 1 log; however, PR8 was not neutralized by 10% serum (Fig. 1A). To examine the contribution of complement to VN, we took advantage of the heat-labile property of serum complement. PR8 was incubated with increasing concentrations of heat-inactivated serum, and VN was determined as before. As shown in Fig. 1B, heat-inactivated serum was deficient in VN activity at all of the concentrations tested. Preliminary results indicated that the sensitivity of the VN assay was dependent not only upon the concentration of serum but also on the amount of virus used in the assay. Optimal sensitivity was obtained when 30% serum and a 10-fold dilution of stock virus were used. These conditions were employed in subsequent VN assays.
FIG. 1.
Neutralization of influenza virus is dependent on the classical pathway of complement. (A) PR8 virus was incubated with various concentrations of serum (B6 mice), and the levels of viable virus remaining in the samples were determined by measuring growth, in 10-fold dilutions, on MDCK cells (log10 TCID50). (B) The contribution of complement to virus neutralization was determined by incubating PR8 virus with heat-inactivated (HI) normal serum. (C) The role of classical pathway complements in neutralizing influenza virus was measured by incubating PR8 virus with sera (30%) from B6, C3−/−, C4−/−, and C1q−/− mice. Representative results for two similar experiments are shown. The horizontal line indicates the level of stock PR8 virus used in the assay (0% serum).
The classical pathway of complement mediates neutralization of influenza virus.
To verify that VN was specifically dependent on complement, PR8 was incubated with sera from mice deficient in the central component of complement, C3. Similarly to heat-inactivated serum, C3-deficient serum was unable to neutralize virus (Fig. 1C). Complement activation can proceed by three known pathways, the classical pathway, the lectin pathway, and the alternative pathway. The classical and lectin pathways are C4 dependent, whereas the alternative pathway is C4 independent. To determine whether VN was mediated by either the classical/lectin pathways or the alternative pathway, PR8 was incubated with sera from C4-deficient mice. C4-deficient serum was unable to neutralize PR8 (Fig. 1C). Thus, neutralization of influenza virus is dependent on the classical or lectin pathways of complement.
The classical and lectin pathways are initiated by C1 and MBL, respectively. Previous studies have shown that MBL can recognize mannose groups present on influenza virus surface glycoproteins (18). To examine the contribution of MBL and to rule out the participation of C1 in VN, PR8 was incubated with C1q-deficient serum. Surprisingly, PR8 was not neutralized by C1q-deficient serum (Fig. 1C). Therefore, neutralization of influenza virus by complement is initiated by C1-mediated activation of the classical pathway.
Influenza virus neutralization is dependent on the presence of natural IgM in normal mouse serum.
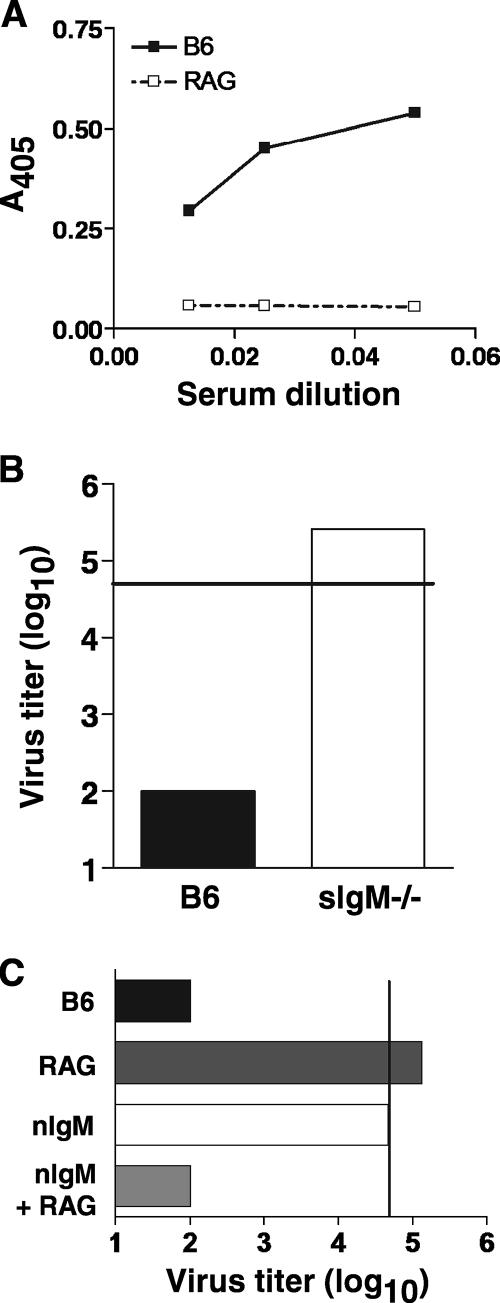
C1q activation results from direct binding to an activating surface or to complement-fixing immunoglobulins, such as IgM and IgG, that are bound to antigens. Therefore, the VN activity of complement may be mediated by immunoglobulins bound to influenza virus. Since sera that were used in the above-described experiments were from mice that had not been exposed to influenza virus, the involvement of influenza virus-specific antibodies in VN could not be accounted for. However, it has previously been shown that sera from naïve mice contains nIgM that binds to viruses (29). Indeed, nIgM has been implicated in enhancing the adaptive immune response during influenza virus infection and virus-reactive natural antibody has been demonstrated to bind to various strains of influenza virus (4). Similarly, we found detectable binding of IgM to PR8 in sera from naive B6 mice (Fig. 2A). Comparable levels of PR8-reactive IgM were also detected in the sera of C3- and C4-deficient mice (data not shown). To determine whether VN was mediated by nIgM, PR8 was incubated with sera from secretory-IgM-deficient (sIgM−/−) mice. These mice express IgM on the surfaces of B cells but do not possess detectable levels of serum IgM; however, other immunoglobulin isotypes are present in the sera of these mice (6). As shown in Fig. 2B, sera from sIgM−/− mice did not neutralize PR8. To further dissect the role of nIgM in neutralizing influenza virus, nIgM was purified from serum and used in VN assays. On its own, nIgM did not neutralize PR8 to an appreciable extent (Fig. 2C). However, when a source of complement (RAG1−/− serum) was also added, VN activity was fully restored. As expected, RAG1−/− serum alone did not exhibit VN activity. Together, these results demonstrate that both complement and nIgM are necessary for neutralization of influenza virus by serum.
FIG. 2.
Complement-dependent neutralization of influenza virus is mediated by natural IgM. (A) Detection of influenza virus-binding nIgM in mouse serum by ELISA. Diluted sera from B6 (▪) or RAG1−/− (□) mice were added to microtiter wells coated with PR8 virus. IgM binding was detected by goat anti-mouse IgM, and absorbance readings were graphed. Representative results for at least three similar experiments are shown. (B) Influenza virus neutralization by sera (30%) from mice lacking secreted IgM (sIgM−/−) was measured in a VN assay. Sera from B6 mice were included as a control. (C) Purified IgM (40 μg/ml) from normal mouse sera was used in a VN assay in combination with sera (30%) from RAG1−/− mice as a source of antibody-free complement. Representative data for at least two similar experiments are shown. The vertical line indicates the level of stock PR8 virus used in the assay.
C5-dependent lysis of influenza virus is not a mechanism of virus neutralization.
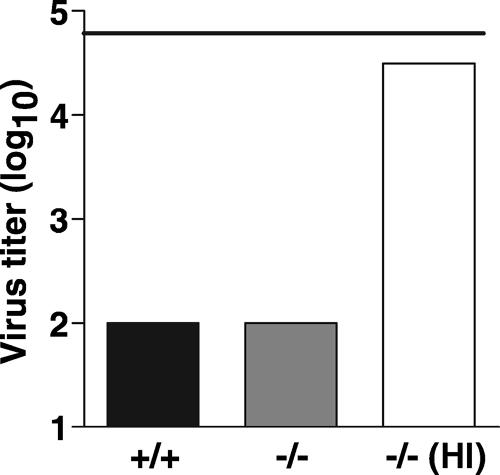
Viruses can be directly neutralized by complement and antibody in at least three ways: virus coating, aggregation, and lysis. Full activation of the complement pathway can lead to viral lysis by formation of the terminal membrane attack complex, which consists of C5b-C9. Deposition of the membrane attack complex on the surface of an enveloped virus, such as influenza virus, produces membrane-spanning pores that can alter the stability of the virion. To determine whether serum neutralization of influenza virus occurs by complement-mediated lysis, PR8 was incubated with sera from C5-deficient mice. As shown in Fig. 3, C5-deficient serum demonstrated full VN activity. The VN activity of C5-deficient serum was complement dependent, as heat-inactivated serum was devoid of VN activity. This suggests that viral lysis by serum complement is an unlikely process for influenza virus neutralization.
FIG. 3.
Neutralization of influenza virus occurs by a process that is independent of C5-mediated viral lysis. Sera (30%) from mice with a spontaneous mutation in C5 (−/−) were used in a VN assay. Sera from C5-sufficient mice (+/+) were used as positive controls. C5-deficient serum was also heat inactivated (HI) to demonstrate that the virus-neutralizing activity of this serum was dependent on complement. The horizontal line indicates the level of stock PR8 virus used in the assay.
Aggregation and coating of influenza virions by complement and natural IgM.
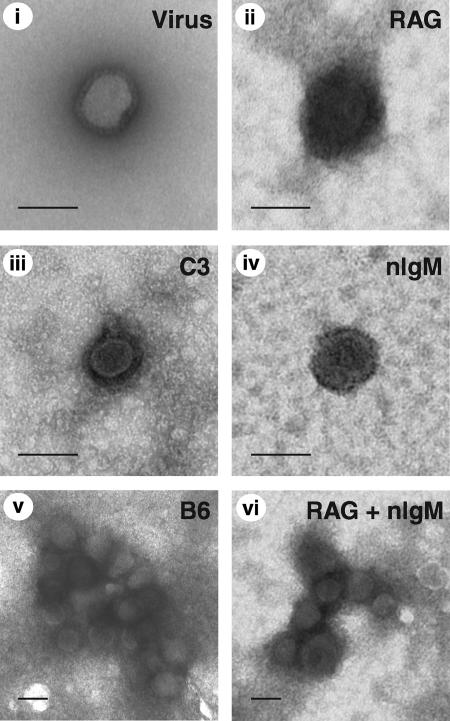
Complement deposition on the surfaces of virions has been shown by electron microscopy to produce a substantial coat of material surrounding viral particles. Such a process is thought to interfere with the ability of the virus receptor to bind to its corresponding cellular ligand, thus neutralizing the virus. Additionally, aggregates of viral particles have been demonstrated to form upon incubation with complement proteins and virus-specific antibody. Aggregation reduces the number of individual infectious units, thereby essentially neutralizing the virus. To examine the contribution of nIgM and complement to neutralization of influenza virus by either coating or aggregating, purified PR8 was incubated with nIgM and sera from RAG1−/− mice. Visualization of the viral particles by transmission electron microscopy demonstrated the presence of numerous aggregates (Fig. 4). Such aggregates did not form when virus was treated with sera from RAG1−/− or C3−/− mice alone, nor were they detected with nIgM treatment alone. However, PR8 virions were clearly coated with material in the presence of sera from either RAG1−/− or C3−/− mice. A similar coat of electron-dense material was apparent on viral particles that formed aggregates in samples treated with nIgM and RAG1−/− sera. Similarly coated aggregates were observed when viral particles were treated with sera from B6 mice. Interestingly, addition of nIgM alone produced viral particles that displayed a regular, punctate pattern of electron density on the virion surface. Collectively, these results demonstrate that nIgM and complement work synergistically to form coated viral aggregates; however, coating of the virions with serum components alone is not sufficient to neutralize influenza virus.
FIG. 4.
Influenza virus is coated with serum components and forms aggregates in the presence of natural IgM and serum. Electron micrographs of PR8 virus particles incubated with sera (RAG1−/−, C3−/−, B6) and nIgM are shown. Samples were stained with uranyl formate before analysis by transmission electron microscopy. Bars represent 100 nm.
Qualitative and quantitative differences in virus coating produced by natural IgM.
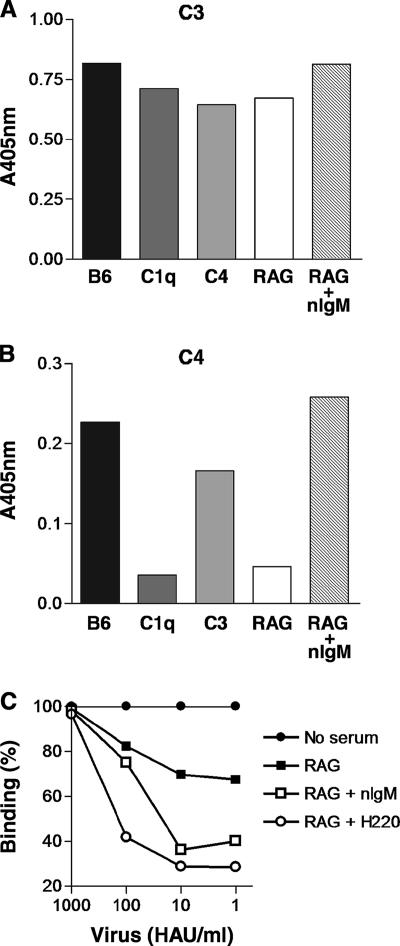
In electron micrographs of viral particles treated with sera from RAG1−/− and C3−/− mice, the difference in the pattern of coating with serum components was striking. To further understand the composition of the serum components deposited on the virions, sera were added to microtiter plates coated with purified virus and the presence of deposited complement components C3 and C4 was measured (Fig. 5). C3 protein was detected on viral particles treated with sera from B6 mice (Fig. 5A); however, while C3 protein was also deposited by sera from C1q−/−, C4−/−, and RAG1−/− mice, the levels were slightly reduced compared with B6 levels (1.2-, 1.3-, and 1.2-fold lower, respectively). Addition of sera from RAG1−/− mice to PR8 treated with purified natural IgM restored C3 deposition to levels observed with B6 serum. Similarly, C4 was deposited on viral particles by all sera tested (Fig. 5B), with the exception of C4-deficient serum (data not shown). As observed for C3, levels of C4 deposition were greatest in sera from B6 mice. However, sera deficient in C1q and antibody (RAG1−/−) had 6.3-fold and 4.9-fold lower levels, respectively, of C4 deposition than wild-type serum. Sera from C3-deficient mice also had lower levels of C4 deposition, although the difference was not as large (1.4-fold lower). As noticed with C3 deposition, when sera from RAG1−/− mice were added to nIgM-treated viral particles, C4 deposition was restored to levels observed with B6 serum. Thus, deposition of C4 on influenza virus particles is primarily dependent on natural antibody and C1q, whereas C3 deposition is principally independent of the classical pathway; nevertheless, C3 deposition can be enhanced by natural antibody.
FIG. 5.
Complement deposition on influenza virus particles is enhanced by natural IgM and restricts access to the virus receptor, HA. (A and B) PR8 virus-coated microtiter wells were treated with purified nIgM (10 μg/ml) and sera (30%) from B6, C1q−/−, C4−/−, C3−/−, and RAG1−/− mice. Deposition of C3 (A) and C4 (B) was detected by complement-specific antibodies, and absorbance readings were taken. Background absorbance from non-virus-coated microtiter wells was subtracted. (C) Microtiter wells coated with dilutions of PR8 virus were treated with nIgM (10 μg/ml) or H220-17-4 (H220; 1.0 μg/ml), an HA-specific IgM antibody. Subsequently, sera (30%) from RAG1−/− mice were added as a source of complement. To determine the level of HA accessibility, biotinylated H36-4-5.2, an HA-specific IgG2a antibody, was added to the wells (2.4 μg/ml), and the level of binding by this antibody was determined by comparison with similarly treated wells that did not receive serum. An irrelevant control IgM antibody allowed binding of H36-4-5.2 similar to that observed with sera (RAG1−/−) alone (data not shown). None of the antibodies alone blocked H36-4-5.2 in the absence of serum. Results are representative of two similar experiments.
Although these results demonstrated enhanced deposition of complement proteins on influenza virus by nIgM, how complement binding influences VN is not clear. We sought to determine whether enhanced complement deposition was associated with increased blockade of the viral receptor (HA). The availability of HA to be recognized by a neutralizing IgG2a antibody (H36-4-5.2) that binds to the Sb portion of HA (27) was examined after treatment with serum. Sera from RAG1−/− mice alone reduced the binding of H36-4-5.2 to PR8 virus particles by 33% (Fig. 5C). Addition of natural IgM further reduced H36-4-5.2 binding by 30% to a level of 36%. A HA-specific IgM antibody (H220-17-4) was similarly effective at reducing binding of H36-4-5.2 to PR8 (to 29%). H220-17-4 alone has low VN activity but neutralized PR8 to a greater extent (2 logs) in the presence of sera from RAG1−/− mice in VN assays (data not shown). In the presence of RAG1−/− sera, an irrelevant control IgM antibody was equivalent to RAG1−/− sera alone in inhibiting H36-4-5.2 binding (data not shown). These results suggest that enhanced nIgM-mediated complement deposition on HA may significantly block the accessibility of the viral receptor for its cellular ligand.
Collectively, these results demonstrate that the presence of natural antibody quantitatively and qualitatively alters serum components that coat influenza virus. Such differences are known to have important consequences for VN, as discussed below.
The contribution of natural IgM to protection during influenza virus infection.
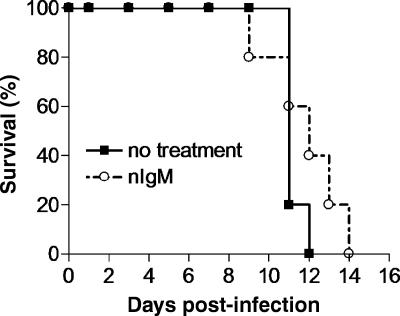
Previous studies that have demonstrated an important role for nIgM in protecting mice during viral infection utilized sIgM−/− mice. However, in such studies, the direct role of nIgM in protecting mice is complicated by the contribution of an otherwise intact adaptive immune response. To investigate the direct contribution of nIgM to protection during the course of an influenza virus infection, RAG1−/− mice were infected with influenza virus following reconstitution with nIgM. RAG1−/− mice received 0.5 mg of nIgM on days −1, 0, and 5. Reconstitution of mice with 0.5 mg of nIgM results in approximately 250 μg/ml of circulating Ig, which is within the normal range for mice, i.e., 200 to 800 μg/ml. By day 3 of infection, the mean body weight of infected mice was higher for the mice that received nIgM (Table 1) . This difference was also apparent on day 5 of infection and increased in significance. However, by day 7, morbidity levels were similar in both groups of mice. Compared with results for the untreated group, survival was prolonged by approximately 1 to 2 days among mice reconstituted with nIgM (Fig. 6). Together, these results suggest a significant role for nIgM in early protection that may have a notable impact on survival during influenza virus infection.
TABLE 1.
Effects of nIgM treatment on morbidity in RAG1−/− mice
| Day postinfection | % original body wta with indicated treatment
|
Pb | |
|---|---|---|---|
| PBS | nIgM | ||
| 3 | 86.9 ± 3.937 | 97.0 ± 2.052 | 0.0528 |
| 5 | 79.7 ± 1.583 | 86.8 ± 1.974 | 0.0224 |
| 7 | 74.04 ± 1.199 | 74.76 ± 1.718 | 0.7400 |
Mice were injected with 500 μg nIgM or 400 μl PBS intraperitoneally on days −1, 0, and 5. All mice received a dose of 300 TCID50 PR8 virus administered intranasally. The mice were weighed on day 0 and on the indicated days postinfection. The percentages of the original (day 0) body weights are given (± standard errors of the means). Five mice were used in each treatment group.
P value was based upon the difference between the control and treatment groups as determined by an unpaired t test.
FIG. 6.
Survival of influenza virus-infected mice reconstituted with natural IgM. Groups of RAG1−/− mice received PBS (▪) or 500 μg of purified nIgM (○) on days −1, 0, and 5. All mice were infected intranasally with 300 TCID50 PR8 virus on day 0.
DISCUSSION
This study was undertaken to identify the mechanisms by which complement mediates neutralization of influenza virus. Importantly, neutralization was mediated by the classical pathway of complement and required the presence of virus-reactive nIgM. VN by complement and nIgM was associated with aggregating of virions and coating with complement; however, virolysis was not found to contribute significantly to VN. Moreover, nIgM was partially protective in antibody-deficient mice (RAG1−/−) during the early phase of infection.
It is generally thought that coating of virions with complement proteins can neutralize virus by nonspecifically blocking virus receptors. Although virus-specific antibody plays an important role in complement-mediated VN, virions can activate complement in the absence of antibody by way of the classical, lectin, or alternative complement pathway (1, 10, 21). Such activation usually results in deposition of complement proteins on surface structures, yet this does not necessarily lead to virus neutralization. Indeed, complement deposition on influenza virus particles by activation of the alternative pathway has previously been demonstrated; however, this did not correspond with VN (5, 26). Similarly, in the present study, C3 deposition by the alternative pathway was detected on influenza virus particles in the apparent absence of neutralization, although levels of C4 deposition were reduced. Through the sequential addition of purified complement proteins, studies have shown that the activation of early classical-pathway proteins alone is sufficient to neutralize some viruses (16, 25, 31). Notably, early complement molecules can be activated by either the classical or the lectin pathway. The lectin pathway is initiated when MBL binds to specific carbohydrate residues present on the surfaces of many microbes. Indeed, MBL has been shown to bind strains of influenza virus to various degrees based upon the level of glycosylation of the HA molecule (32). In the present study, sera from C1q-deficient and antibody-deficient (RAG1−/−) mice, both of which have intact MBL pathways, were unable to neutralize influenza virus under the conditions used. This suggests that the lectin pathway of complement activation does not significantly contribute to VN in our in vitro model. Importantly, the presence of nIgM contributed to greater levels of C3 and C4 deposition on influenza virus particles, which correlated with virus neutralization.
While the in vivo contribution of natural antibody in providing protection against influenza virus infection has recently been studied (4, 17), little is known of its direct contribution to VN. The present study demonstrates an important role for natural antibody in contributing to complement-mediated influenza virus neutralization. Neutralization was dependent on nIgM since serum lacking only immunoglobulins of the IgM isotype (sIgM−/−) was devoid of VN activity, yet serum containing only IgM (from activation-induced cytidine deaminase (AID)-deficient mice) possessed full VN activity (data not shown). An elegant study by Ochsenbein et al. demonstrated an important antiviral role for natural antibodies in vivo (29). Surprisingly, while the control of early viral distribution was mediated by nIgM, the response was found to be independent of complement. Nevertheless, it is now well appreciated that numerous viruses have adapted to complement-mediated VN by incorporating complement-neutralizing molecules into their virions (15). Such molecules are used particularly by large DNA viruses that spread systemically. Influenza virus has not been shown to interfere with complement activation, possibly due in part to limitations imposed by its small RNA genome.
Characterization of many natural antibodies produced by B-cell hybridomas has established that they can recognize self molecules (13, 37). There is mounting evidence that these antibodies are involved in homeostasis and in maintaining tolerance to self (7). Enveloped viruses, such as influenza virus, inherently possess material derived from the infected host cell. Hence, it is conceivable that self-reactive natural antibodies may mediate VN by reacting with altered auto-antigens presented on the surfaces of budding viruses. Indeed, anti-phosphorylcholine natural antibodies are abundantly present in human serum and have been shown to recognize cells undergoing apoptosis due to changes in the lipid composition of the external layer of the cell membrane (35). Interestingly, these self-reactive antibodies play an important role in protection against streptococcal infections (8). Additionally, self-reactive antibodies have been shown to be polyreactive (13, 28). Thus, while a certain species of natural antibody may react with a self molecule, it may also cross-react with pathogen-encoded epitopes (3, 20). In this study, nIgM did not possess virus-neutralizing activity on its own, therefore leaving open the possibility that nIgM may recognize a non-virally encoded molecule on the influenza virion. Hence, while the specificities of nIgM antibodies that react with several influenza virus strains (4) have not been reported, recognition of both virus- and host-derived molecules is a possibility.
In our study, virus aggregating and coating were associated with the VN activity of nIgM and the classical pathway of complement. Specifically, VN was mediated by the first four components of the classical complement pathway. The importance of C1, C4, C2, and C3 in mediating virus neutralization has been demonstrated in a number of reports. Hook et al. have recently shown that human nIgM and complement together neutralize herpes simplex virus (22). In results similar to ours, VN did not require the terminal complement components but was dependent on the activation of the classical complement pathway. Linscott and Levinson reported that nIgM to Newcastle disease virus was present in human serum and that C1-C3 was sufficient for virus neutralization in the presence of early IgM (25). Similarly, Oldstone et al. demonstrated the involvement of C1-C3 in the enhancement of polyoma virus neutralization in the presence of virus-specific antibody (31). VN was shown to be associated with aggregation of polyoma virions (30).
Although virus aggregation does not actually neutralize virus but reduces the virus yield instead, this process may nevertheless be physiologically important. By aggregating virus, neutralization is attained in that nonphagocytic cells do not readily endocytose large aggregates and are thus protected from infection. In contrast, phagocytic cells readily endocytose aggregated material, particularly when such material is coated with complement, considering that many professional phagocytic cells possess complement receptors (30). Indeed, aggregation has been shown to be used by another important family of innate molecules, collectins, to enhance endocytosis of influenza virus by neutrophils (19).
Even though we found direct evidence for viral aggregation, we cannot exclude the possible contribution of virion coating by complement to VN in this study. In the presence of immunoglobulin-free serum, virions were clearly coated, presumably by components of complement proteins C1, C2, C4, and C3. In a similar report, using human sera, Beebe et al. demonstrated that the classical complement pathway mediated neutralization of influenza virus in the absence of viral lysis (5). VN was shown to be dependent on the presence of a nonneutralizing virus-specific IgG present in a majority of human sera. The authors concluded that VN was caused by complement deposition on the virus envelope. We found both quantitative and qualitative differences in complement deposition on influenza virus particles in the presence of nIgM that correlated with VN. In particular, C4 deposition was greatly elevated by nIgM, and more importantly, coating of the influenza virus HA was similarly enhanced. It is conceivable that binding of nIgM to specific molecules on the surface of the virion may focus complement deposition onto the viral receptor and other molecules important in the infection process. In a report by Feng et al., C1 significantly enhanced the VN activity of an influenza virus HA-specific antibody that possessed low direct VN activity (16). Binding of HA to sialic acid residues on red blood cells was more significantly affected, suggesting that C1q deposition on or near the HA molecule contributed to this enhancement. Taken together, in addition to promoting aggregation, complement and nIgM could conceivably also mediate VN by inhibiting binding of influenza virus to cellular receptors or by interfering with a postattachment process (14, 22).
The contribution of nIgM to the outcome of infection with influenza virus has only recently begun to be appreciated. A study by Baumgarth et al. (4) demonstrated that nIgM produced by B1 cells is important in enhancing the response of B2 cells during influenza virus infection. Additionally, Harada et al. showed that nonmutated IgM was able to protect AID-deficient mice infected with a lethal dose of influenza virus (17). However, these studies used mice with partially intact adaptive immune responses that could mask the individual effect of nIgM. Therefore, to evaluate the direct contribution of nIgM during influenza virus infection, RAG1−/− mice, which lack lymphocytes, were used in the present study. Passive transfer of nIgM significantly reduced the initial level of early morbidity and slightly extended the survival of infected mice. Watford et al. have demonstrated the existence of complement proteins in the respiratory tracts of healthy individuals (36). Importantly, C3 deposition on Streptococcus pneumoniae by constituents of bronchoalveolar lavage fluid was found to be mediated by the classical complement pathway, thus demonstrating the presence of a functional complement pathway in the respiratory tract. Similarly, pulmonary infection with Streptococcus pneumoniae is more severe in C1q-deficient and secretory-IgM-deficient mice, suggesting an important role for the early presence of these components in protection against acute pulmonary infections (9). In addition, the presence of abundant quantities of complement and nIgM in serum may increasingly contribute to immunity in the lower respiratory tract as infection spreads and inflammation ensues. Interestingly, a lack of factor B in relation to other complement components in the lungs of healthy individuals (36) suggests that while the classical and lectin complement pathways may function effectively early on, the alternative pathway may be inoperative.
Exposure to influenza virus usually results in lifelong immunity to the same strain of virus (12). Protection is thought to be mediated by directly neutralizing virus-specific antibody. However, the fact that a majority of human sera from individuals exposed to H1N1 influenza viruses possess nonneutralizing antibody specific for H1N1 influenza viruses (5) indicates that the complement-dependent nature of VN by these sera is physiologically important. The present study suggests a similar mechanism by which virus-binding, nonneutralizing natural antibody mediates neutralization of influenza virus in the presence of complement.
Acknowledgments
We thank Walter Gerhard (Wistar Institute) for providing reagents and for expert technical advice, Tom Schneider (Merrimack) and Priti Kumar (CBR Institute) for critical reading of the manuscript, Maria Ericsson and Elizabeth Benecchi (Harvard Medical School) for expert technical assistance with EM work, and Becca Yeamans for excellent assistance with animals.
This work was supported by grants from the National Institutes of Health.
Footnotes
Published ahead of print on 3 January 2007.
REFERENCES
- 1.Anders, E. M., C. A. Hartley, P. C. Reading, and R. A. Ezekowitz. 1994. Complement-dependent neutralization of influenza virus by a serum mannose-binding lectin. J. Gen. Virol. 75:615-622. [DOI] [PubMed] [Google Scholar]
- 2.Arnold, J. N., M. R. Wormald, D. M. Suter, C. M. Radcliffe, D. J. Harvey, R. A. Dwek, P. M. Rudd, and R. B. Sim. 2005. Human serum IgM glycosylation: identification of glycoforms that can bind to mannan-binding lectin. J. Biol. Chem. 280:29080-29087. [DOI] [PubMed] [Google Scholar]
- 3.Baccala, R., T. V. Quang, M. Gilbert, T. Ternynck, and S. Avrameas. 1989. Two murine natural polyreactive autoantibodies are encoded by nonmutated germ-line genes. Proc. Natl. Acad. Sci. USA 86:4624-4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumgarth, N., O. C. Herman, G. C. Jager, L. E. Brown, L. A. Herzenberg, and J. Chen. 2000. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 192:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beebe, D. P., R. D. Schreiber, and N. R. Cooper. 1983. Neutralization of influenza virus by normal human sera: mechanisms involving antibody and complement. J. Immunol. 130:1317-1322. [PubMed] [Google Scholar]
- 6.Boes, M., C. Esau, M. B. Fischer, T. Schmidt, M. Carroll, and J. Chen. 1998. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 160:4776-4787. [PubMed] [Google Scholar]
- 7.Boes, M., T. Schmidt, K. Linkemann, B. C. Beaudette, A. Marshak-Rothstein, and J. Chen. 2000. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc. Natl. Acad. Sci. USA 97:1184-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briles, D. E., M. Nahm, K. Schroer, J. Davie, P. Baker, J. Kearney, and R. Barletta. 1981. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J. Exp. Med. 153:694-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, J. S., T. Hussell, S. M. Gilliland, D. W. Holden, J. C. Paton, M. R. Ehrenstein, M. J. Walport, and M. Botto. 2002. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl. Acad. Sci. USA 99:16969-16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper, N. R., F. C. Jensen, R. M. Welsh, Jr., and M. B. Oldstone. 1976. Lysis of RNA tumor viruses by human serum: direct antibody-independent triggering of the classical complement pathway. J. Exp. Med. 144:970-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cottey, R., C. A. Rowe, and B. S. Bender. 2001. Current protocols in immunology: influenza virus. John Wiley and Sons, Inc., New York, NY. [DOI] [PubMed]
- 12.Couch, R. B. 2003. An overview of serum antibody responses to influenza virus antigens. Dev. Biol. (Basel) 115:25-30. [PubMed] [Google Scholar]
- 13.Dighiero, G., P. Lymberi, J. C. Mazie, S. Rouyre, G. S. Butler-Browne, R. G. Whalen, and S. Avrameas. 1983. Murine hybridomas secreting natural monoclonal antibodies reacting with self antigens. J. Immunol. 131:2267-2272. [PubMed] [Google Scholar]
- 14.Edwards, M. J., and N. J. Dimmock. 2001. Hemagglutinin 1-specific immunoglobulin G and Fab molecules mediate postattachment neutralization of influenza A virus by inhibition of an early fusion event. J. Virol. 75:10208-10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Favoreel, H. W., G. R. Van de Walle, H. J. Nauwynck, and M. B. Pensaert. 2003. Virus complement evasion strategies. J. Gen. Virol. 84:1-15. [DOI] [PubMed] [Google Scholar]
- 16.Feng, J. Q., K. Mozdzanowska, and W. Gerhard. 2002. Complement component C1q enhances the biological activity of influenza virus hemagglutinin-specific antibodies depending on their fine antigen specificity and heavy-chain isotype. J. Virol. 76:1369-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada, Y., M. Muramatsu, T. Shibata, T. Honjo, and K. Kuroda. 2003. Unmutated immunoglobulin M can protect mice from death by influenza virus infection. J. Exp. Med. 197:1779-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartshorn, K. L., K. Sastry, M. R. White, E. M. Anders, M. Super, R. A. Ezekowitz, and A. I. Tauber. 1993. Human mannose-binding protein functions as an opsonin for influenza A viruses. J. Clin. Investig. 91:1414-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartshorn, K. L., M. R. White, V. Shepherd, K. Reid, J. C. Jensenius, and E. C. Crouch. 1997. Mechanisms of anti-influenza activity of surfactant proteins A and D: comparison with serum collectins. Am. J. Physiol. 273:L1156-L1166. [DOI] [PubMed] [Google Scholar]
- 20.Haynes, B. F., J. Fleming, E. W. St. Clair, H. Katinger, G. Stiegler, R. Kunert, J. Robinson, R. M. Scearce, K. Plonk, H. F. Staats, T. L. Ortel, H. X. Liao, and S. M. Alam. 2005. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 308:1906-1908. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch, R. L., J. A. Winkelstein, and D. E. Griffin. 1980. The role of complement in viral infections. III. Activation of the classical and alternative complement pathways by Sindbis virus. J. Immunol. 124:2507-2510. [PubMed] [Google Scholar]
- 22.Hook, L. M., J. M. Lubinski, M. Jiang, M. K. Pangburn, and H. M. Friedman. 2006. Herpes simplex virus type 1 and 2 glycoprotein C prevents complement-mediated neutralization induced by natural immunoglobulin M antibody. J. Virol. 80:4038-4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopf, M., B. Abel, A. Gallimore, M. Carroll, and M. F. Bachmann. 2002. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat. Med. 8:373-378. [DOI] [PubMed] [Google Scholar]
- 24.Lachmann, P. 2006. Complement before molecular biology. Mol. Immunol. 43:496-508. [DOI] [PubMed] [Google Scholar]
- 25.Linscott, W. D., and W. E. Levinson. 1969. Complement components required for virus neutralization by early immunoglobulin antibody. Proc. Natl. Acad. Sci. USA 64:520-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McSharry, J. J., R. J. Pickering, and L. A. Caliguiri. 1981. Activation of the alternative complement pathway by enveloped viruses containing limited amounts of sialic acid. Virology 114:507-515. [DOI] [PubMed] [Google Scholar]
- 27.Mozdzanowska, K., J. Feng, and W. Gerhard. 2003. Virus-neutralizing activity mediated by the Fab fragment of a hemagglutinin-specific antibody is sufficient for the resolution of influenza virus infection in SCID mice. J. Virol. 77:8322-8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naparstek, Y., J. Andre-Schwartz, T. Manser, L. J. Wysocki, L. Breitman, B. D. Stollar, M. Gefter, and R. S. Schwartz. 1986. A single germline VH gene segment of normal A/J mice encodes autoantibodies characteristic of systemic lupus erythematosus. J. Exp. Med. 164:614-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochsenbein, A. F., T. Fehr, C. Lutz, M. Suter, F. Brombacher, H. Hengartner, and R. M. Zinkernagel. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286:2156-2159. [DOI] [PubMed] [Google Scholar]
- 30.Oldstone, M. B. 1975. Virus neutralization and virus-induced immune complex disease. Virus-antibody union resulting in immunoprotection or immunologic injury—two sides of the same coin. Prog. Med. Virol. 19:84-119. [PubMed] [Google Scholar]
- 31.Oldstone, M. B., N. R. Cooper, and D. L. Larson. 1974. Formation and biologic role of polyoma virus-antibody complexes. A critical role for complement. J. Exp. Med. 140:549-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reading, P. C., L. S. Morey, E. C. Crouch, and E. M. Anders. 1997. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J. Virol. 71:8204-8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoint. Am. J. Hyg. 27:493-497. [Google Scholar]
- 34.Reis, E. S., D. A. Falcao, and L. Isaac. 2006. Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand. J. Immunol. 63:155-168. [DOI] [PubMed] [Google Scholar]
- 35.Shaw, P. X., C. S. Goodyear, M. K. Chang, J. L. Witztum, and G. J. Silverman. 2003. The autoreactivity of anti-phosphorylcholine antibodies for atherosclerosis-associated neo-antigens and apoptotic cells. J. Immunol. 170:6151-6157. [DOI] [PubMed] [Google Scholar]
- 36.Watford, W. T., A. J. Ghio, and J. R. Wright. 2000. Complement-mediated host defense in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L790-L798. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, M., W. G. Austen, Jr., I. Chiu, E. M. Alicot, R. Hung, M. Ma, N. Verna, M. Xu, H. B. Hechtman, F. D. Moore, Jr., and M. C. Carroll. 2004. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc. Natl. Acad. Sci. USA 101:3886-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]