Abstract
Ectromelia virus (ECTV), a natural mouse pathogen and the causative agent of mousepox, is closely related to variola virus (VARV), which causes smallpox in humans. Mousepox is an excellent surrogate small-animal model for smallpox. Both ECTV and VARV encode a multitude of host response modifiers that target components of the immune system and that are thought to contribute to the high mortality rates associated with infection. Like VARV, ECTV encodes a protein homologous to the ectodomain of the host gamma interferon (IFN-γ) receptor 1. We generated an IFN-γ binding protein (IFN-γbp) deletion mutant of ECTV to study the role of viral IFN-γbp (vIFN-γbp) in host-virus interaction and also to elucidate the contribution of this molecule to the outcome of infection. Our data show that the absence of vIFN-γbp does not affect virus replication per se but does have a profound effect on virus replication and pathogenesis in mice. BALB/c mice, which are normally susceptible to infection with ECTV, were able to control replication of the mutant virus and survive infection. Absence of vIFN-γbp from ECTV allowed the generation of an effective host immune response that was otherwise diminished by this viral protein. Mice infected with a vIFN-γbp deletion mutant virus, designated ECTV-IFN-γbpΔ, produced increased levels of IFN-γ and generated robust cell-mediated and antibody responses. Using several strains of mice that exhibit differential degrees of resistance to mousepox, we show that recovery or death from ECTV infection is determined by a balance between the host's ability to produce IFN-γ and the virus' ability to dampen its effects.
The eradication of variola virus (VARV), the causative agent of smallpox, almost 30 years ago marked the most successful vaccination campaign against an infectious agent (19). However, VARV continues to be a threat as a potential agent of bioterrorism (21). As remarkable as its eradication was, little is known about the molecular factors associated with its high case-fatality rates or what contributes to an effective immune response to infection. VARV is strictly a pathogen of humans and has no natural animal reservoir (5). Therefore, much of our understanding of poxvirus infections comes from animal models using closely related poxviruses. VARV is a member of the genus Orthopoxvirus and is closely related to other members, such as ectromelia virus (ECTV), which causes a disease similar to smallpox, termed mousepox, in mice (13, 18).
ECTV is a natural pathogen of mice, and inbred strains of laboratory mice have been found to exhibit differential degrees of resistance to mousepox. Some inbred strains of mice, including C57BL/6, AKR, and certain 129 strains, have been classified as resistant to the lethal effects of ECTV infection, while A/J and BALB/c mice are susceptible (7, 47). Other strains, for example, CBA/H, have an intermediate phenotype (3). Mechanisms necessary for the recovery of resistant mice from primary ECTV infection include interferons (alpha/beta interferon [IFN-α/β] and IFN-γ), natural killer (NK) cells, activated mononuclear phagocytes, inducible nitric oxide production, and virus-specific cytotoxic-T-lymphocyte (CTL) and antibody responses (6, 7, 12, 17, 24-26, 34, 35).
In the face of a hostile host immune response, poxviruses have evolved genes whose products directly interfere with various components of the host immune system to allow their successful replication and propagation (37). Both VARV and ECTV also encode viral IFN-γ binding protein (vIFN-γbp) with amino acid sequence similarity to the extracellular binding domain of IFN-γ receptor 1 and target host IFN-γ (31, 38, 40). The ECTV IFN-γbp, which maps to open reading frame (ORF) 158 of the ECTV genome (9), is an orthologue of the B8R gene in VARV and vaccinia virus (VACV) (28).
ECTV IFN-γbp is a 266-amino-acid glycoprotein that exists both within the infected cell and as a secreted molecule (1, 8, 9, 33). It binds with very high affinity to murine and human IFN-γ and prevents ligation of the cytokine with specific cell surface receptors (31, 40). Studies to investigate the biological roles of other poxvirus IFN-γbps have yielded conflicting results (41, 43, 46). In two separate studies, deletion of the VACV B8R gene was found to result in loss of virus virulence in a mouse (46) and a rabbit (41) model. On the other hand, a third study found that loss of the VACV B8R gene did not affect virulence (43). Since the natural host of VACV is unknown and the vIFN-γbp orthologue binds with high affinity to rabbit, but not mouse, IFN-γ (31), the discrepancies between the above-mentioned studies may reflect the incompatibilities between the virus and host systems employed. Therefore, to better understand the function of this host immune response modifier, a combination of a host with its natural pathogen is necessary. A good model for this is ECTV, which, like VARV, has a very restricted host range and causes systemic infection and disease and where virus and host are thought to have coevolved (5, 13, 18). Most importantly, the vIFN-γbp encoded by ECTV is known to bind mouse IFN-γ with high affinity (31). Further, unlike other animal models, the effect of this viral protein on the host immune response can be extensively studied in mice.
In order to study the role of vIFN-γbp in host-virus interactions, we generated an IFN-γbp deletion mutant of ECTV. We showed previously that recovery of mice from mousepox is strictly dependent on host IFN-γ (7, 25, 34). Here, we show that although vIFN-γbp is not required for ECTV replication in vitro, it profoundly influences virulence in the host. Absence of vIFN-γbp from ECTV resulted in lower virus loads in infected animals and allowed otherwise normally susceptible mouse strains to survive infection. This was concomitant with augmented IFN-γ production and elevated NK cell, CTL, and antibody responses. Thus, a single virus-encoded protein strongly influenced both innate and adaptive immune responses and the outcome of infection.
MATERIALS AND METHODS
Mice.
Inbred specific-pathogen-free female mice were used at 6 to 8 weeks of age. A/J, BALB/c, CBA/H, C57BL/6, IFN-γ gene knockout (C57BL/6.IFN-γ−/−) (11), and Rag-1 knockout (C57BL/6.Rag-1−/−) (29) mice on a C57BL/6 background were obtained from the Animal Services Division of the John Curtin School of Medical Research and the Australian Phenomics Facility (Canberra, Australia). All experiments were carried out under protocols approved by the Institutional Animal Ethics and Experimentation Committee and in accordance with the guidelines of the Australian code of practice for the care and use of animals for scientific purposes.
Cell lines and cell cultures.
BSC-1, an epithelial kidney cell line from African green monkeys, and the murine cell lines L929 (H-2k), P-815 (H-2d), MC57G (H-2b), and YAC-1 were obtained from the American Type Culture Collection (Rockville, MD). DC2.4 (H-2b) cells (39) were a gift from David Tscharke. All cells were maintained in Eagle's minimum essential medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 120 μg/ml penicillin, and 200 μg/ml streptomycin and neomycin sulfate.
Viruses and infection.
Wild-type ECTV Moscow strain (10), designated ECTV-WT, and a vIFN-γbp deletion mutant virus, designated ECTV-IFN-γbpΔ, were used. ECTV-IFN-γbpΔ was generated using a general strategy for constructing mutant poxviruses in which the IFN-γbp gene was replaced with β-gal, and knockout virus was selected using Escherichia coli gpt with mycophenolic acid selection and blue-white screening (14, 15). A revertant virus, ECTV-IFN-γbpΔR, was generated using the same transient-dominant selection method used to generate the knockout virus. The vIFN-γbp gene was reinserted in place of the β-gal selection marker, and the revertant virus was selected using E. coli gpt and mycophenolic acid selection, together with a blue-white screen. The genotypes of both genetically modified viruses were confirmed by DNA sequencing. All ECTV strains were propagated in BSC-1 cells, and titers were determined by viral plaque assay (24). Mice were inoculated subcutaneously in the left hind footpad under anesthesia at the virus doses indicated. At various times postinfection (p.i.), organs were removed aseptically and processed for determination of virus titers by viral plaque assay.
Synthetic peptides.
Peptides synthesized, as described previously (45), at the John Curtin School of Medical Research Biomolecular Resources Facility (Australian National University, Canberra, Australia) were resuspended at 5 μM in dimethyl sulfoxide and diluted in culture medium to the required concentrations. The amino acid sequences for the peptides of interest are as follows: B8R20-27, TSYKFESV; A19L47-55, VSLDYINTM; A47L138-146, AAFEFINSL; A42R88-96, YAPVSPIVI; A3L270-277, KSYNYMLL; A8R189-196, ITYRFYLI; B2R54-62, YSQVNKRYI; A23R297-305, IGMFNLTFI; J3R289-296, SIFRFLNI; and L2R53-61, VIYIFTVRL (32, 45). A range of peptide concentrations (0.01, 0.1, and 1 μg/ml) were tested in CTL and intracellular cytokine staining assays to determine the optimal immunogenic concentrations. Based on this, the B8R20-27, A19L47-55, A47L138-146, and A42R88-96 peptides were used at 10 nM and the other six peptides were used at 100 nM. A herpes simplex virus type 1 (HSV-1) glycoprotein B-derived peptide, HSV-1gB498-505 (SSIEFARL), was used as an irrelevant control at a concentration of 10 nM.
CTL and NK cell activity assays.
CTL and NK cell cytolytic assays were performed as described elsewhere (7). To measure ex vivo CTL responses, spleen cells from infected animals were assessed for the ability to kill 51Cr-labeled virus-infected, peptide-pulsed, or uninfected syngeneic targets. YAC-1 target cells were used for NK cell lytic assays. MC57G, DC2.4, or P815 cells were used as targets to detect major histocompatibility complex class I (MHC-I)-restricted killing by splenic effector cells.
Intracellular cytokine staining for IFN-γ.
Antigen-specific IFN-γ-producing CD8 T cells were enumerated using intracellular cytokine staining as described elsewhere (45). Briefly, 106 splenocytes were incubated with 2 × 105 uninfected, virus-infected, or peptide-pulsed DC2.4 cells or with synthetic peptides. After 2 h, 10 μg/ml brefeldin A was added, and the cells were incubated for a further 3 h before being stained with anti-CD8α-allophycocyanin (clone 53-6.7; BD Biosciences) and anti-IFN-γ-phycoerythrin (clone XMG1.2; BD Biosciences) as previously described. Total events for cells were acquired using a FACSort flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star Inc.). Antigen-specific IFN-γ production was calculated by subtracting the background values obtained with the irrelevant HSV-1 gB peptide.
Anti-ECTV antibody determination.
Serum samples were assayed by enzyme-linked immunosorbent assay (ELISA) for ECTV-specific immunoglobulin G (IgG) using purified ECTV as described previously (7). Sera were assayed at 1:200 dilution, based on a test range of 1:50 to 1:1,000.
IFN-γ ELISA.
Spleen cells from infected animals were cocultured with either ECTV-infected P815 cells or ECTV-infected splenocytes of the appropriate MHC-I haplotype for 6, 24, or 48 h. The culture supernatants were harvested, and IFN-γ levels were determined by capture ELISA as described previously (7).
Statistical analysis.
Statistical analyses of experimental data, employing parametric and nonparametric tests as indicated, were performed using GraphPad Prism (GraphPad Software, San Diego, CA).
RESULTS
vIFN-γbp is not required for viral replication in vitro.
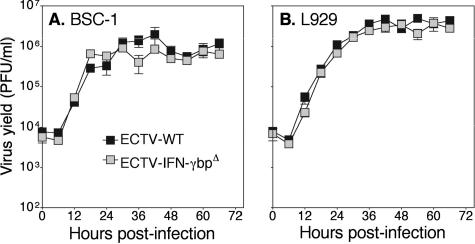
We investigated whether inactivation of the ECTV-ORF158 gene, encoding the viral IFN-γbp, affects virus replication in cell cultures. Monolayers of BSC-1 and L929 cells were infected at 0.1 PFU per cell with ECTV-WT or ECTV-IFN-γbpΔ. At selected times p.i., cells were harvested and virus yields were determined. Figure 1 shows that there was no difference between the capacities of wild-type and mutant viruses to replicate in either monkey BSC-1 cells or murine L929 cells. These results indicate that vIFN-γbp is not required for replication in vitro.
FIG. 1.
Kinetics of virus replication in vitro. BSC-1 cells (A) and L929 cells (B) were infected with ECTV-WT or ECTV-IFN-γbpΔ at 0.1 PFU per cell. At the times indicated, cells were harvested and the virus yield was determined. The data shown are means ± standard errors of the mean of triplicate samples and represent one of two separate experiments.
ECTV-IFN-γbpΔ replicates to lower titers in resistant C57BL/6 mice.
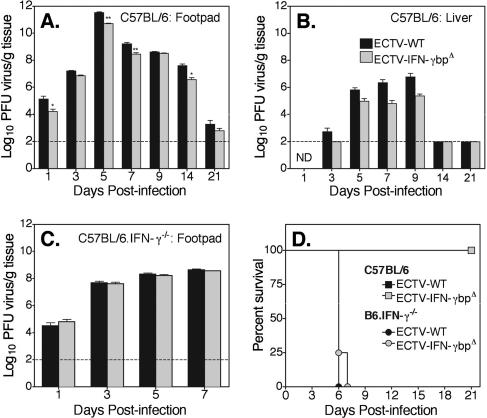
To determine whether vIFN-γbp affected the ability of ECTV to replicate within a host, C57BL/6 mice were infected with either ECTV-WT or ECTV-IFN-γbpΔ. The kinetics of virus replication in organs, including the footpad, popliteal lymph node, spleen, and liver, were determined at days 1, 3, 5, 7, 9, 14, and 21 p.i. We found that ECTV-IFN-γbpΔ replicated to significantly lower titers than ECTV-WT in the footpad on most days tested (Fig. 2A). Titers in the liver were significantly lower in the ECTV-IFN-γbpΔ group than in mice infected with ECTV-WT throughout the course of infection (Fig. 2B). Similar results were obtained in lymph nodes and spleen (data not shown). However, the animals were able to control both strains of virus effectively and to recover from infection. It is interesting that absence of vIFN-γbp resulted in lower viral titers at the site of infection even by day 1 p.i. (Fig. 2A), indicating that this viral protein acts early in infection.
FIG. 2.
Kinetics of virus replication in vivo. C57BL/6 (A, B, and D) or C57BL/6.IFN-γ−/− (C and D) mice were infected with 103 PFU of either ECTV-WT or ECTV-IFN-γbpΔ. (A to C) On the days indicated, groups of mice were sacrificed and tissue was collected for determination of virus titers. The data are representative of two separate animal experiments and are expressed as log10 PFU/gram tissue plus the standard error of the mean for five mice per group. The sensitivity of the viral plaque assay was 2 log10 PFU, shown by a dotted line. ECTV-IFN-γbpΔ replicated to lower titers than ECTV-WT in both footpads (A) and livers (B) of C57BL/6 mice (*, P < 0.02, and **, P < 0.003 by the Wilcoxon signed rank test). There was no difference in virus replication in the footpads of C57BL/6.IFN-γ−/− mice (C) up to day 7 p.i., after which the mice succumbed to infection. ND, not done. (D) Groups of five C57BL/6 and eight C57BL/6.IFN-γ−/− mice were monitored daily for 21 days. P values for survival proportions were obtained by the Kaplan-Meier log rank statistical test (P = 0.0055).
C57BL/6 mice deficient in IFN-γ, C57BL/6.IFN-γ−/− (11), are highly susceptible to ECTV infection (7). To ascertain that the difference in replication between the mutant and wild-type viruses was a function of the specific interaction between vIFN-γbp and host IFN-γ, we investigated virus replication in infected C57BL/6.IFN-γ−/− mice. Figure 2C shows that infection with either ECTV-WT or ECTV-IFN-γbpΔ resulted in similar viral titers in the primary site of infection (footpad). In addition, the titers of the two viruses were comparable at day 7 p.i. in all other organs tested (data not shown). Furthermore, C57BL/6.IFN-γ−/− mice were as susceptible to infection with mutant virus as they were to ECTV-WT virus (Fig. 2D). Therefore, in the absence of host IFN-γ, vIFN-γbp does not affect virus replication, viral load, or susceptibility to infection, indicating that vIFN-γbp functions specifically through IFN-γ.
CD8 T-cell response in ECTV-IFN-γbpΔ-infected C57BL/6 mice.
CD8 T-cell determinants for VACV and ECTV, restricted by H-2b MHC class I molecules, have been recently identified (32, 45). Interestingly, the major determinant maps to VACV B8R and the corresponding ECTV ORF158. It was therefore of interest to determine whether deletion of ECTV ORF158 (vIFN-γbp) affected the CD8 T-cell response in C57BL/6 mice and whether loss of the specific immunodominant determinant is associated with compensation by other, subdominant determinants.
C57BL/6 mice were infected with ECTV-WT or ECTV-IFN-γbpΔ, and splenic CD8 T-cell cytolytic activity was measured ex vivo at day 8 p.i. using ECTV-infected or B8R20-27 peptide-pulsed target cells. Similar results were obtained using targets infected with either ECTV-WT or ECTV-IFN-γbpΔ; therefore, only data using ECTV-WT-infected target cells are shown. Figure 3A shows that in the absence of vIFN-γbp, CTL activity against ECTV-infected target cells was reduced by about threefold. Nevertheless, mice infected with ECTV-IFN-γbpΔ were able to control virus (Fig. 2A and B) and recovered from infection.
FIG. 3.
Effect of vIFN-γbp deletion on the CD8 T-cell response in virus-infected C57BL/6 mice. Groups of C57BL/6 mice were infected with 103 PFU of ECTV-WT, ECTV-IFN-γbpΔ, or ECTV-IFN-γbpΔR. At day 8 p.i., mice were sacrificed and splenic virus-specific CTL activity was measured ex vivo using (A) virus-infected and uninfected MC57G target cells or (B) B8R20-27 peptide-pulsed DC2.4 target cells. Splenocytes from infected mice at day 8 p.i. were also used to determine the proportion of IFN-γ-producing CD8 T cells by intracellular cytokine staining. To study the contribution of the B8R determinant in the context of the total anti-ECTV response (C), virus-infected DC2.4 cells were used as stimulator cells. To compare the contributions of other known determinants (D), splenocytes were stimulated with B8R20-27, A19L47-55, A47L138-146, A42R88-96, A3L270-277, A8R189-196, B2R54-62, A23R297-305, J3R289-296, L2R53-61, or a combination of all 10 peptides (P10). The data are represented as means ± standard errors of the mean for 9 to 12 mice per group. KO, knockout.
The absence of vIFN-γbp in ECTV-IFN-γbpΔ-infected mice completely abolished the CTL response to B8R20-27 peptide-pulsed target cells, consistent with the fact that it is a known CD8 T-cell determinant (Fig. 3B). The specificity of the CTL response was confirmed using a revertant virus, ECTV-IFN-γbpΔR, constructed by reinsertion of the vIFN-γbp gene in ECTV-IFN-γbpΔ. Splenic effector cells from ECTV-IFN-γbpΔR-infected mice lysed B8R20-27 peptide-pulsed targets as efficiently as effector cells from ECTV-WT-infected mice (Fig. 3B).
To investigate whether loss of the B8R determinant in ECTV-IFN-γbpΔ resulted in compensation by other determinants used by the host (32, 45), we studied the CD8 T-cell response to whole virus and nine other peptides by intracellular cytokine staining. Splenocytes from uninfected mice did not produce IFN-γ, whereas 20% of CD8 T cells from ECTV-WT-infected spleens responded to whole virus by producing IFN-γ (Fig. 3C). Interestingly, almost 16% of CD8 T cells from ECTV-IFN-γbpΔ-infected mice produced IFN-γ in response to whole virus. This reduction in IFN-γ response by CD8 T cells is consistent with a corresponding reduction in CTL activity (Fig. 3A) mediated by splenocytes from ECTV-IFN-γbpΔ-infected mice compared to those from the ECTV-WT-infected group.
The response to B8R20-27 peptide alone accounted for 4.1% of IFN-γ-positive CD8 T cells from ECTV-WT-infected mice, and this was about 20% of the total response to whole virus (Fig. 3D). As expected, the response to B8R20-27 peptide was diminished in ECTV-IFN-γbpΔ-infected animals. We then measured the response to the next 9 most significant, but subdominant, determinants, A19L47-55, A47L138-146, A42R88-96, A3L270-277, A8R189-196, B2R54-62, A23R297-305, J3R289-296, and L2R53-61, or a combination of all 10 peptides (32, 45). Individually, these determinants accounted for 2.6%, 3.3%, 1.1%, 9.6%, 8.2%, 5.0%, 7.3%, 4.2%, and 4.4% of the IFN-γ-producing CD8 T cells from ECTV-WT-infected spleens, respectively, (Fig. 3D). The response in splenic CD8 T cells from mutant virus-infected animals was roughly similar, and of the determinants tested, no single determinant compensated in any significant way for the loss of B8R20-27. However, when all 10 peptides were used together, the difference between the responses in ECTV-WT (6.3 ± 0.6%)- and ECTV-IFN-γbpΔ (4.5 ± 0.4%)-infected animals was less pronounced and consistent with the somewhat lower response to whole virus (Fig. 3C).
ECTV-IFN-γbpΔ is attenuated in susceptible mouse strains.
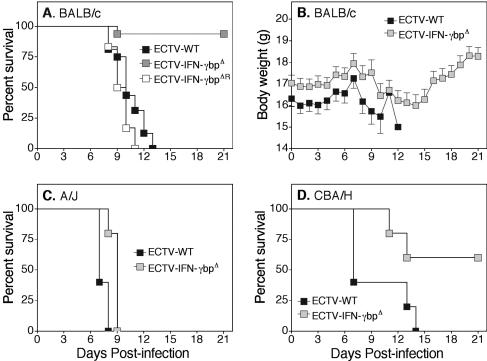
Having observed a small but significant attenuation of virus replication in the absence of vIFN-γbp in resistant C57BL/6 mice, which was dependent on host IFN-γ, we hypothesized that loss of vIFN-γbp may result in a more pronounced phenotype in a susceptible mouse strain. To assess this view, we used mouse strains known to be susceptible (A/J and BALB/c) and those that show intermediate resistance (CBA/H) to mousepox. Based on the degree of susceptibility to ECTV-WT and the 50% lethal dose for each strain (3), groups of mice were infected with various doses of ECTV-WT or ECTV-IFN-γbpΔ and were monitored for 21 days p.i. for signs of disease and weight loss. Data are shown for only selected doses of virus.
All BALB/c mice (n = 15) infected with ECTV-WT succumbed to disease between 8 and 13 days p.i., and this was coincident with a loss in body weight (Fig. 4A and B). In contrast, more than 90% of the animals infected with ECTV-IFN-γbpΔ recovered from infection and survived (log rank test; P < 0.0001). The crucial period was between days 8 and 14 p.i, when overt clinical signs of mousepox were observed and there was a reduction in body weight. Nevertheless, all animals recovered after this period. To verify that the absence of vIFN-γbp was responsible for the significant attenuation of ECTV in BALB/c mice, we tested the revertant virus. Figure 4A also shows that 100% of BALB/c mice infected with the revertant ECTV succumbed to infection at about the same time as the ECTV-WT-infected animals. The ECTV-IFN-γbpΔR strain maintained the ECTV-WT phenotype.
FIG. 4.
Outcomes of ECTV infection in BALB/c, A/J, and CBA/H mice. Groups of mice were infected with various doses of virus; however, data are shown for only selected doses. BALB/c (A and B) (n = 15) mice were infected with 500 PFU of ECTV-WT, ECTV-IFN-γbpΔ, or ECTV-IFN-γbpΔR. A/J (C) (n = 5) and CBA/H (D) (n = 5) mice were infected with 100 and 5,000 PFU, respectively. The mice were monitored daily for 21 days, and mean body weights ± standard errors of the mean for individual mice (B) and survival proportions (A, C, and D) were recorded. P values for survival proportions were obtained by the Kaplan-Meier log rank statistical test and were as follows: (A) P < 0.0001, (C) P = 0.0080, and (D) P = 0.0464.
A/J mice, known to be even more susceptible to mousepox, and CBA/H mice, which exhibit greater resistance to mousepox than BALB/c animals were inoculated with either ECTV-WT or ECTV-IFN-γbpΔ. Figure 4C and D shows data for A/J mice infected with 100 PFU and CBA/H mice infected with 5,000 PFU of virus, respectively. At these doses, all mice infected with ECTV-WT succumbed to mousepox. Although A/J mice infected with ECTV-IFN-γbpΔ were also susceptible, the mean time to death was significantly longer (log rank test; P = 0.0080) in the absence of vIFN-γbp. All CBA/H mice infected with ECTV-WT succumbed to the infection, whereas more than 50% of this strain infected with ECTV-IFN-γbpΔ survived, and those that succumbed had a significantly longer mean time to death (log rank test; P = 0.0464).
Host IFN-γ production correlates with resistance to mousepox.
Since IFN-γ is critical for recovery from mousepox and susceptibility to severe mousepox is correlated with deficiency in IFN-γ production (7, 25, 34), we hypothesized that the outcomes of infection in the four strains of mice used in this study were due to differences in production of the cytokine. To test this hypothesis, we measured IFN-γ production by splenocytes from ECTV-infected mice. Splenocytes from A/J, BALB/c, CBA/H, and C57BL/6 mice infected with ECTV-WT were isolated at day 5 p.i. and stimulated in vitro for either 24 or 48 h with syngeneic ECTV-WT-infected splenocytes. Clearly, the amount of IFN-γ produced by splenocytes from each mouse strain correlated with the level of resistance of that strain to mousepox (Fig. 5).
FIG. 5.
IFN-γ responses in inbred mouse strains. Groups of four A/J, BALB/c, CBA/H, and C57BL/6 mice were infected with 103 PFU of ECTV-WT and sacrificed at day 5 p.i. Splenocytes were stimulated in vitro with ECTV-WT-infected syngeneic spleen cells at a ratio of 5:1. At the times indicated, culture supernatants were harvested and IFN-γ levels were determined by ELISA. The data represent means plus standard errors of the mean.
Host response to ECTV-IFN-γbpΔ infection in BALB/c mice.
Having established that deletion of ECTV-IFN-γbp allowed a normally susceptible strain of mouse to recover from infection, we further investigated how BALB/c mice were able to effectively control infection with ECTV-IFN-γbpΔ but not ECTV-WT (Fig. 4A). We and others have previously shown that recovery from ECTV infection depends on the combined activities of IFN, NK, CTL, and antibody responses (6, 7, 12, 17, 24-26, 34, 35) and hypothesized that recovery of BALB/c mice from ECTV lacking vIFN-γbp was due to an enhanced immune response that restricted virus replication.
BALB/c mice were infected with either ECTV-WT or ECTV-IFN-γbpΔ, and virus titers were determined on various days p.i. Figure 6A shows that viral titers in the livers of ECTV-IFN-γbpΔ-infected mice were almost 2.5 log10 PFU lower than those from ECTV-WT-infected mice until day 8 p.i., after which the ECTV-WT-infected animals succumbed to infection. It is known that mice that succumb to mousepox have uncontrolled virus growth and die as a result of necrosis of the liver and spleen (5, 13, 18). Recovery of BALB/c mice infected with ECTV-IFN-γbpΔ, therefore, correlated with the ability of the host to better control viral replication early in infection.
FIG. 6.
Host response to infection in BALB/c mice. Groups of six BALB/c mice were infected with 500 PFU of either ECTV-WT or ECTV-IFN-γbpΔ. Mice infected with ECTV-WT did not survive beyond day 8 p.i. (A) At the times indicated, mice were sacrificed and tissue was collected for determination of virus titers. The data shown are from one representative of three separate animal experiments and are the means plus standard errors of the mean (SEM) for six mice per group. The viral loads in organs of mice infected with ECTV-IFN-γbpΔ were significantly lower than those in mice infected with ECTV-WT (P < 0.0001; Wilcoxon signed rank test). The sensitivity of the viral plaque assay was 2 log10 PFU, shown by a dotted line. ND, not done. (B) Splenocytes from infected mice at days 7 and 8 p.i. were stimulated with ECTV-WT-infected P815 cells for 6 h, and IFN-γ levels were measured in the culture supernatants. The data presented are means plus SEM for six mice per group. Splenocytes from day 5 p.i. (C) and day 7 p.i. (D) were used to measure NK cell cytotoxicity. Splenocytes from uninfected mice were used as controls. The data presented are means ± SEM for six mice per group. (E) Splenocytes from day 7 p.i. were used to determine antiviral CTL activity using virus-infected and uninfected P815 target cells. The data shown are from one representative of four separate animal experiments and are the means ± SEM for six mice per group. (F) Groups of mice were bled at the times indicated, and the sera were used for measurement of ECTV-specific IgG levels. The data shown are from one representative of two separate animal experiments and are the means plus SEM for six mice per group.
Since BALB/c mice are relatively poor producers of IFN-γ in response to infection with ECTV-WT (Fig. 5) (7), we investigated whether infection of this mouse strain with ECTV lacking vIFN-γbp influenced the levels of the cytokine produced. Splenocytes from either ECTV-WT- or ECTV-IFN-γbpΔ-infected BALB/c mice were isolated at the times indicated (Fig. 6B) and stimulated in vitro with ECTV-WT-infected P815 cells for 6 h. As expected, splenocytes from ECTV-WT-infected mice did not mount a good IFN-γ response (Fig. 6B), whereas those infected with ECTV-IFN-γbpΔ generated significantly larger (P < 0.031) amounts of IFN-γ.
Next, we performed ex vivo 51Cr release NK cell and antiviral CTL assays using splenocytes from infected mice. Although no difference was observed in the splenic NK cell cytolytic activity between the groups at day 5 (Fig. 6C), by day 7 p.i. it was at least 27-fold higher in ECTV-IFN-γbpΔ-infected mice than in the ECTV-WT-infected groups (Fig. 6D). IFN-γ levels in these 5-h cultures were significantly higher in supernatants from cells isolated from ECTV-IFN-γbpΔ-infected mice than in those from ECTV-WT-infected groups on days 5 (5.6 ± 0.3 U/ml and 2.9 ± 0.5 U/ml, respectively; P < 0.01 by two-way analysis of variance) and 7 (10.1 ± 0.2 U/ml and 4.1 ± 0.6 U/ml, respectively; P < 0.001) p.i. Significantly, ECTV-IFN-γbpΔ-infected mice generated a robust CTL response that was also at least 27-fold higher than the response to wild-type virus at day 7 p.i. (Fig. 6E). Mice infected with ECTV-WT mounted a poor CTL response, and this correlated with their susceptibility to infection and death by day 8 p.i.
A further immune parameter critical for recovery from ECTV infection is the generation of an antibody response (6, 17). Although BALB/c mice infected with ECTV-WT succumbed to infection before the host mounted a significant anti-ECTV IgG response, mice infected with the mutant virus generated a strong virus-specific IgG response that increased with time (Fig. 6F), concomitant with complete virus clearance by day 30 p.i. (Fig. 6A).
Therefore, increased IFN-γ production in the absence of vIFN-γbp allowed BALB/c mice to generate strong NK cell, CTL, and antibody responses that allowed virus clearance in an otherwise susceptible host. This conclusion is further supported by experiments using C57BL/6.Rag-1−/− mice (29), which are defective in both T- and B-cell responses. In these animals, there was no difference in viral growth between ECTV-IFN-γbpΔ and ECTV-WT (Fig. 7), indicating that even in vivo, there is no intrinsic difference in the abilities of these viruses to replicate. Figure 7 shows data for virus titers in the spleen and liver, with similar findings for other organs tested (data not shown). Thus, vIFN-γbp functions to dampen the host response, tipping the balance in favor of the virus.
FIG. 7.
Viral loads in infected C57BL/6.Rag-1−/− mice. Groups of three C57BL/6.Rag-1−/− mice were infected with 103 PFU of either ECTV-WT or ECTV-IFN-γbpΔ. At day 5 p.i., mice were sacrificed and organs were collected for determination of virus titers. The data shown are means plus standard errors of the mean. There was no significant difference between the viral loads in organs of mice infected with ECTV-IFN-γbpΔ and ECTV-WT. The sensitivity of the viral plaque assay was 2 log10 PFU, shown by a dotted line.
DISCUSSION
There is increased interest in understanding protective immunity to smallpox. Although mortality rates associated with smallpox were as high as 40%, a significant subset of those infected recovered. The basis of susceptibility or resistance is still being elucidated. It is becoming increasingly clear that the outcome of infection, recovery or death, is clearly dictated by several factors, including host and viral genes, both of which influence the immune response. Using a model in which a poxvirus infection can be studied in its natural host, we show that vIFN-γbp plays a critical role in pathogenesis and dampening of the immune response.
The importance of IFN-γ in the host response to poxvirus infections is well established (7, 22, 23, 25, 30, 34, 35). To counter this, orthopoxviruses, such as VARV, VACV, and ECTV, encode a molecule that mimics the host IFN-γ receptor. A number of studies have used VACV B8R deletion mutants to study the function of this protein in vivo, but with conflicting results (41, 43, 46). These discrepancies reflect the incompatibilities between the virus and host systems employed, for example, VACV vIFN-γbp binds with high affinity to rabbit, but not mouse, IFN-γ (31). In contrast, inactivation of the vIFN-γbp encoded by a leporipoxvirus, myxoma virus, resulted in diminished virulence in its natural host (30). The mechanism of action of the vIFN-γbp in this system, however, is confounded by the ability of the viral protein to also bind a number of chemokines (27). Since mouse models are the most versatile in which the roles of individual components of innate and adaptive immunity can be investigated, ECTV infection of mice provides an ideal system to elucidate the biological role of vIFN-γbp.
Using an ECTV deletion mutant, we have shown that absence of vIFN-γbp does not affect virus growth in vitro (Fig. 1) and that this viral protein is not required for replication per se. In contrast, it had a profound influence on replication, immune response, and disease outcome in vivo, which was dependent on host IFN-γ. In gene-targeted mice lacking IFN-γ, the presence or absence of ECTV vIFN-γbp did not affect the viral load in infected animals (Fig. 2C) or their susceptibility to infection (Fig. 2D). This finding indicates that ECTV vIFN-γbp must act through specific interactions with host IFN-γ. This is a significant finding, because many poxviruses encode immunomodulators that bind to multiple host proteins in vitro (37). The combination of natural pathogen and host plus the ability to specifically knock out both viral and host genes puts the ECTV model in an ideal position to study the biological function of viral immunomodulators in vivo.
To investigate the role of vIFN-γbp in the complex interplay between the host and virus, we studied a range of mouse strains with known differences in susceptibility to mousepox (3, 47). Our data show that although the magnitude of the effect, and the resultant outcome of infection, varied depending on the level of genetic susceptibility to mousepox, absence of vIFN-γbp resulted in an attenuation of virus in each strain studied (Fig. 2 and 4). The most pronounced effects were observed in susceptible BALB/c mice, where the absence of this single viral protein caused a normally lethal ECTV infection to be effectively cleared, with complete recovery of the host (Fig. 4). Even in highly resistant C57BL/6 mice, there was decreased viral load in organs from animals infected with mutant virus deficient in vIFN-γbp compared to those infected with the wild type (Fig. 2A and B). Interestingly, although all C57BL/6 mice eventually recovered from infection, the animals infected with ECTV-IFN-γbpΔ exhibited milder clinical symptoms of disease (data not shown).
It is significant that the abilities of different inbred mouse strains to produce IFN-γ correlate with their levels of resistance to mousepox (Fig. 5) (7). High producers of this cytokine, such as C57BL/6 mice, are highly resistant to mousepox and effectively controlled infection irrespective of whether vIFN-γbp was present or absent. For low producers of IFN-γ, such as BALB/c mice, the capacity of the virus to further reduce the availability of this cytokine via vIFN-γbp had a significant effect on the outcome of infection. Figure 6B shows that at the peak of the IFN-γ response, BALB/c mice infected with ECTV-IFN-γbpΔ produced significantly greater amounts of this cytokine than those infected with the wild-type virus. This correlated with reduced viral loads (Fig. 6A); correspondingly milder pathology in organs, especially the liver (data not shown); and recovery from infection (Fig. 4A).
BALB/c mice normally generate poor IFN-γ, NK cell, and antiviral CTL responses to ECTV infection (7). However, in the absence of vIFN-γbp and a concomitant increase in host IFN-γ, the mice were able to mount a sustained NK cell response (Fig. 6D) and a potent antiviral CTL response (Fig. 6E). In addition, since these animals survived beyond day 8 p.i., they were also able to generate a good anti-ECTV IgG antibody response (Fig. 6F). Thus, the absence of vIFN-γbp tipped the balance in favor of the host, which allowed it to mount an effective immune response to overcome an infection that was otherwise lethal.
The ability of the mutant virus to replicate was not impaired either in vitro (Fig. 1) or in vivo (Fig. 2C and 7). ECTV-WT and ECTV-IFN-γbpΔ replicated to equivalent titers in the absence of an appropriate immune response, that is, in mice lacking IFN-γ (Fig. 2C) and in mice deficient in T- and B-cell responses (Fig. 7). The vIFN-γbp encoded by the wild-type virus clearly functions to dampen the immune response; therefore, removing it allowed the response to be enhanced and virus to be controlled in immunocompetent animals.
The capacity of vIFN-γbp to interfere with the host's ability to control virus was evident as early as day 1 p.i. (Fig. 2A) and was dependent on host IFN-γ (Fig. 2C). The cellular source of this early production is likely to be γδ T cells, which are activated rapidly after ECTV infection (25). As the infection and the host response to the virus progress, NK cells and T cells become significant producers of this cytokine. In the absence of vIFN-γbp, the IFN-γ responses of both NK cells and CD8 T cells are enhanced. Indeed, it is well established that IFN-γ can upregulate its own production (4, 20), and therefore, an early advantage afforded to the host results in a snowballing effect in terms of being able to generate a robust immune response and to clear virus.
Although the importance of IFN-γ as part of a T helper type 1 and cell-mediated immune response to virus infection is well established, the molecular mechanisms by which it enhances either the numbers or the effector function of antiviral CTLs is not known. IFN-γ is known to augment adaptive immunity through increased antigen processing and presentation by (i) upregulation of MHC-I and MHC-II on antigen presenting cells (4) and (ii) induction of immunoproteosome formation (42). Some studies have also suggested that the maturation of CTLs is impaired in the absence of IFN-γ (36). The contribution of IFN-γ to the class of antibody produced is well established (4). Therefore, the influence of vIFN-γbp on several facets of the immune response is consistent with the multiple functions of IFN-γ in the host response to infection.
Intriguingly, an immunodominant CD8 T-cell determinant restricted by MHC class I Kb maps to vIFN-γbp in ECTV (45). Deletion of the vIFN-γbp gene, therefore, had significant effects on the antiviral CTL response in mice of the H-2b haplotype (Fig. 3A). However, despite the threefold reduction in the total CTL response in C57BL/6 mice infected with ECTV-IFN-γbpΔ, the animals effectively cleared virus and recovered from infection. The reduction in the CTL response did not correspond to an equivalent decrease in the number of IFN-γ-producing CD8 T cells responding to virus (Fig. 3C). In this specific case, vIFN-γbp also contains a major CD8 T-cell determinant. Thus, as expected, removal of a major CD8 T-cell determinant resulted in significant loss of cytolytic activity; however, the concomitant removal of IFN-γbp released the viral inhibition of the IFN-γ response. The net IFN-γ response in ECTV-IFN-γbpΔ-infected mice is therefore the sum of these two opposing influences, resulting in only a minimal decrease compared to ECTV-WT-infected animals. To gain a better understanding of how IFN-γ production and lytic activity are regulated in CD8 T cells, it should be possible to introduce mutations in ECTV vIFN-γbp that preserve the CD8 T-cell determinant but obliterate IFN-γ binding capacity. This approach is currently being pursued.
Little is known about the VARV-encoded immunomodulatory molecules, as there is no appropriate model for this virus. Our understanding of the functions of specific homologous molecules must therefore come from appropriate animal models, such as ECTV infection of mice. Given the similarities between smallpox and mousepox, the known functions of IFN-γ in both mice and humans, and a 91% sequence homology between the VARV and ECTV vIFN-γbp, we predict that the VARV orthologue functions in humans in a way analogous to that of ECTV vIFN-γbp in mice.
Our data clearly indicate that the deletion of vIFN-γbp results in an augmented immune response to virus. This has significant implications for the design of safer, new-generation vaccines for smallpox and for poxvirus-based vaccine vectors. There are significant concerns with the current smallpox vaccine, particularly for immunocompromised individuals. Some of the safety concerns can be addressed by the use of highly attenuated replication-deficient strains of VACV, for example, modified VACV Ankara. In this context, it is of interest that, in addition to other deficiencies, modified VACV Ankara encodes a truncated vIFN-γbp that is not functional (2). We have shown here that deletion of just one poxviral protein, vIFN-γbp, augmented the host's ability to mount a potent antiviral response. Through systematic analysis, it should be possible to determine the optimal combination of viral immunomodulatory genes that can be deleted to allow the generation of potent and long-lasting cell-mediated and antibody immune responses.
Further, immunomodulators that dampen functions of inflammatory cytokines are already being successfully utilized in the treatment of inflammatory conditions. An example, in clinical use for a range of diseases, is the tumor necrosis factor receptor Fc fusion protein ENBREL (etanercept) (44). In addition, there is emerging realization of the great therapeutic potential of immunomodulators, which viruses have evolved to regulate the immune response (16). In this regard, the myxoma virus-encoded serine protease inhibitor, Serp-1, is currently in phase II clinical trial for treatment of acute coronary syndrome (Viron Therapeutics Inc.). An understanding of the biological function of vIFN-γbp therefore has important implications for treatment of conditions in which IFN-γ is dysregulated.
Acknowledgments
This work was supported by grants from the Howard Hughes Medical Institute to G. Karupiah and National Health and the Medical Research Council of Australia to G. Karupiah and G. Chaudhri. I. G. Sakala is a recipient of an Australian National University Ph.D. scholarship. A. A. Nuara is a recipient of an American Heart Association Predoctoral Fellowship. R. M. Buller was supported by grant NO1-AI-15436 from the National Institutes of Health.
We thank David Tscharke for critically reading the manuscript.
Footnotes
Published ahead of print on 17 January 2007.
REFERENCES
- 1.Bai, H., R. M. Buller, N. Chen, M. Green, and A. A. Nuara. 2005. Biosynthesis of the IFN-gamma binding protein of ectromelia virus, the causative agent of mousepox. Virology 334:41-50. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard, T. J., A. Alcami, P. Andrea, and G. L. Smith. 1998. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J. Gen. Virol. 79:1159-1167. [DOI] [PubMed] [Google Scholar]
- 3.Blanden, R. V., B. D. Deak, and H. O. McDevitt. 1989. Strategies in virus-host interactions, p. 125-138. In R. V. Blanden (ed.), Immunology of virus diseases. Brolga Press, Canberra, Australia.
- 4.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15:749-795. [DOI] [PubMed] [Google Scholar]
- 5.Buller, R. M., and G. J. Palumbo. 1991. Poxvirus pathogenesis. Microbiol. Rev. 55:80-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhri, G., V. Panchanathan, H. Bluethmann, and G. Karupiah. 2006. Obligatory requirement for antibody in recovery from a primary poxvirus infection. J. Virol. 80:6339-6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhri, G., V. Panchanathan, R. M. Buller, A. J. van den Eertwegh, E. Claassen, J. Zhou, R. de Chazal, J. D. Laman, and G. Karupiah. 2004. Polarized type 1 cytokine response and cell-mediated immunity determine genetic resistance to mousepox. Proc. Natl. Acad. Sci. USA 101:9057-9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, N., R. M. Buller, E. M. Wall, and C. Upton. 2000. Analysis of host response modifier ORFs of ectromelia virus, the causative agent of mousepox. Virus Res. 66:155-173. [DOI] [PubMed] [Google Scholar]
- 9.Chen, N., M. I. Danila, Z. Feng, R. M. Buller, C. Wang, X. Han, E. J. Lefkowitz, and C. Upton. 2003. The genomic sequence of ectromelia virus, the causative agent of mousepox. Virology 317:165-186. [DOI] [PubMed] [Google Scholar]
- 10.Chen, W., R. Drillien, D. Spehner, and R. M. L. Buller. 1992. Restricted replication of ectromelia virus in cell culture correlates with mutations in virus-encoded host range gene. Virology 187:433-442. [DOI] [PubMed] [Google Scholar]
- 11.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 12.Delano, M. L., and D. G. Brownstein. 1995. Innate resistance to lethal mousepox is genetically linked to the NK gene complex on chromosome 6 and correlates with early restriction of virus replication by cells with an NK phenotype. J. Virol. 69:5875-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esteban, D. J., and R. M. Buller. 2005. Ectromelia virus: the causative agent of mousepox. J. Gen. Virol. 86:2645-2659. [DOI] [PubMed] [Google Scholar]
- 14.Falkner, F. G., and B. Moss. 1988. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J. Virol. 62:1849-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falkner, F. G., and B. Moss. 1990. Transient dominant selection of recombinant vaccinia viruses. J. Virol. 64:3108-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallon, P. G., and A. Alcami. 2006. Pathogen-derived immunomodulatory molecules: future immunotherapeutics? Trends Immunol. 27:470-476. [DOI] [PubMed] [Google Scholar]
- 17.Fang, M., and L. J. Sigal. 2005. Antibodies and CD8+ T cells are complementary and essential for natural resistance to a highly lethal cytopathic virus. J. Immunol. 175:6829-6836. [DOI] [PubMed] [Google Scholar]
- 18.Fenner, F. 1949. Mouse-pox (infectious ectromelia of mice): a review. J. Immunol. 63:341-373. [PubMed] [Google Scholar]
- 19.Fenner, F., D. A. Henderson, I. Arita, Z. Jezek, and I. D. Ladnyi. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland.
- 20.Hardy, K. J., and T. Sawada. 1989. Human gamma interferon strongly upregulates its own gene expression in peripheral blood lymphocytes. J. Exp. Med. 170:1021-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson, D. A. 1999. The looming threat of bioterrorism. Science 283:1279-1282. [DOI] [PubMed] [Google Scholar]
- 22.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 259:1742-1745. [DOI] [PubMed] [Google Scholar]
- 23.Karupiah, G., R. V. Blanden, and I. A. Ramshaw. 1990. Interferon gamma is involved in the recovery of athymic nude mice from recombinant vaccinia virus/interleukin 2 infection. J. Exp. Med. 172:1495-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karupiah, G., R. M. Buller, N. Van Rooijen, C. J. Duarte, and J. Chen. 1996. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J. Virol. 70:8301-8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karupiah, G., T. N. Fredrickson, K. L. Holmes, L. H. Khairallah, and R. M. Buller. 1993. Importance of interferons in recovery from mousepox. J. Virol. 67:4214-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karupiah, G., Q. W. Xie, R. M. Buller, C. Nathan, C. Duarte, and J. D. MacMicking. 1993. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science 261:1445-1448. [DOI] [PubMed] [Google Scholar]
- 27.Lalani, A. S., K. Graham, K. Mossman, K. Rajarathnam, I. Clark-Lewis, D. Kelvin, and G. McFadden. 1997. The purified myxoma virus gamma interferon receptor homolog M-T7 interacts with the heparin-binding domains of chemokines. J. Virol. 71:4356-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massung, R. F., L. I. Liu, J. Qi, J. C. Knight, T. E. Yuran, A. R. Kerlavage, J. M. Parsons, J. C. Venter, and J. J. Esposito. 1994. Analysis of the complete genome of smallpox variola major virus strain Bangladesh-1975. Virology 201:215-240. [DOI] [PubMed] [Google Scholar]
- 29.Mombaerts, P., J. Iacomini, R. S. Johnson, K. Herrup, S. Tonegawa, and V. E. Papaioannou. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68:869-877. [DOI] [PubMed] [Google Scholar]
- 30.Mossman, K., P. Nation, J. Macen, M. Garbutt, A. Lucas, and G. McFadden. 1996. Myxoma virus M-T7, a secreted homolog of the interferon-gamma receptor, is a critical virulence factor for the development of myxomatosis in European rabbits. Virology 215:17-30. [DOI] [PubMed] [Google Scholar]
- 31.Mossman, K., C. Upton, R. M. Buller, and G. McFadden. 1995. Species specificity of ectromelia virus and vaccinia virus interferon-gamma binding proteins. Virology 208:762-769. [DOI] [PubMed] [Google Scholar]
- 32.Moutaftsi, M., B. Peters, V. Pasquetto, D. C. Tscharke, J. Sidney, H. H. Bui, H. Grey, and A. Sette. 2006. A consensus epitope prediction approach identifies the breadth of murine TCD8+-cell responses to vaccinia virus. Nat. Biotechnol. 24:817-819. [DOI] [PubMed] [Google Scholar]
- 33.Nuara, A. A., H. Bai, N. Chen, R. M. Buller, and M. R. Walter. 2006. The unique C termini of orthopoxvirus gamma interferon binding proteins are essential for ligand binding. J. Virol. 80:10675-10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panchanathan, V., G. Chaudhri, and G. Karupiah. 2005. Interferon function is not required for recovery from a secondary poxvirus infection. Proc. Natl. Acad. Sci. USA 102:12921-12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panchanathan, V., G. Chaudhri, and G. Karupiah. 2006. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. J. Virol. 80:6333-6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puliaev, R., P. Nguyen, F. D. Finkelman, and C. S. Via. 2004. Differential requirement for IFN-gamma in CTL maturation in acute murine graft-versus-host disease. J. Immunol. 173:910-919. [DOI] [PubMed] [Google Scholar]
- 37.Seet, B. T., J. B. Johnston, C. R. Brunetti, J. W. Barrett, H. Everett, C. Cameron, J. Sypula, S. H. Nazarian, A. Lucas, and G. McFadden. 2003. Poxviruses and immune evasion. Annu. Rev. Immunol. 21:377-423. [DOI] [PubMed] [Google Scholar]
- 38.Seregin, S. V., I. N. Babkina, A. E. Nesterov, A. N. Sinyakov, and S. N. Shchelkunov. 1996. Comparative studies of gamma-interferon receptor-like proteins of variola major and variola minor viruses. FEBS Lett. 382:79-83. [DOI] [PubMed] [Google Scholar]
- 39.Shen, Z., G. Reznikoff, G. Dranoff, and K. L. Rock. 1997. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. 158:2723-2730. [PubMed] [Google Scholar]
- 40.Smith, V. P., and A. Alcami. 2002. Inhibition of interferons by ectromelia virus. J. Virol. 76:1124-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sroller, V., V. Ludvikova, L. Maresova, P. Hainz, and S. Nemeckova. 2001. Effect of IFN-gamma receptor gene deletion on vaccinia virus virulence. Arch. Virol. 146:239-249. [DOI] [PubMed] [Google Scholar]
- 42.Strehl, B., U. Seifert, E. Kruger, S. Heink, U. Kuckelkorn, and P. M. Kloetzel. 2005. Interferon-gamma, the functional plasticity of the ubiquitin-proteasome system, and MHC class I antigen processing. Immunol. Rev. 207:19-30. [DOI] [PubMed] [Google Scholar]
- 43.Symons, J. A., D. C. Tscharke, N. Price, and G. L. Smith. 2002. A study of the vaccinia virus interferon-gamma receptor and its contribution to virus virulence. J. Gen. Virol. 83:1953-1964. [DOI] [PubMed] [Google Scholar]
- 44.Taylor, P. C., R. O. Williams, and M. Feldmann. 2004. Tumour necrosis factor alpha as a therapeutic target for immune-mediated inflammatory diseases. Curr. Opin. Biotechnol. 15:557-563. [DOI] [PubMed] [Google Scholar]
- 45.Tscharke, D. C., G. Karupiah, J. Zhou, T. Palmore, K. R. Irvine, S. M. Haeryfar, S. Williams, J. Sidney, A. Sette, J. R. Bennink, and J. W. Yewdell. 2005. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J. Exp. Med. 201:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verardi, P. H., L. A. Jones, F. H. Aziz, S. Ahmad, and T. D. Yilma. 2001. Vaccinia virus vectors with an inactivated gamma interferon receptor homolog gene (B8R) are attenuated in vivo without a concomitant reduction in immunogenicity. J. Virol. 75:11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallace, G. D., R. M. Buller, and H. C. Morse III. 1985. Genetic determinants of resistance to ectromelia (mousepox) virus-induced mortality. J. Virol. 55:890-891. [DOI] [PMC free article] [PubMed] [Google Scholar]